Abstract
The objective of the present study was to investigate the usefulness of the maximum standardized uptake value (SUVmax) of the primary tumor on preoperative 18F-fluoro-2-deoxy-D-glucose (FDG) positron emission tomography and computed tomography (PET/CT) as a prognostic indicator in patients with endometrial neoplasms. A total of 75 patients with endometrial cancer or uterine carcinosarcoma who underwent surgical treatment were included in the present study. All patients underwent preoperative PET/CT, and the correlation between the SUVmax of the primary tumor and clinical outcomes was analyzed. The SUVmax was significantly higher in patients with stage II/III disease, a histology of grade 3 endometrioid adenocarcinoma and carcinosarcoma, a positive lymph node (LN) status, positive lymph-vascular space involvement (LVSI), and deep (≥1/2) myometrial invasion. Receiver operating characteristic curve analysis revealed that the optimal cut-off values of SUVmax for predicting a positive LN, LVSI and deep myometrial invasion were 7.49, 6.45 and 6.45, respectively. The overall survival (OS) and progression-free survival (PFS) of patients with a high SUVmax were significantly lower compared with those of patients with a low SUVmax using the cut-off value of 7.30. However, no significant difference was observed in the OS or PFS between the high and low SUVmax groups when analyzed in carcinosarcoma patients alone. Finally, multivariate analyses demonstrated that the SUVmax of the primary tumor was an independent prognostic factor for impaired PFS in 55 endometrioid adenocarcinoma patients; however, not in all patients, including those with carcinosarcoma. The present findings demonstrated that the SUVmax of the primary tumor may be a useful biomarker for predicting clinical outcomes of patients with endometrial cancer, although its prognostic impact appears to be limited in patients with uterine carcinosarcoma.
Keywords: FDG-PET/CT, SUVmax, endometrial cancer, carcinosarcoma, prognosis
Introduction
Endometrial cancer is the most common gynecological cancer type in developed countries (1). According to the pathological, hormonal and molecular characteristics, it is classified into two types: Type 1 includes grade 1 (G1) and grade 2 (G2) endometrioid adenocarcinomas, while type 2 includes grade 3 (G3) endometrioid adenocarcinoma and other types with a specific histology, including serous and clear cell carcinoma (2). In addition, carcinosarcoma is currently defined as one particular subtype of epithelial endometrial carcinoma that shares similar behavior with type 2 endometrial cancer (3) and, thus, carcinosarcoma must be staged according to the International Federation of Gynecology and Obstetrics (FIGO) staging system for carcinoma of the endometrium, not for uterine sarcoma.
While endometrial cancer patients with stage I–II disease achieve a favorable outcome with surgery alone, patients with advanced disease or recurrence show poor survival. Several clinicopathological factors are used for the classification of relapse risks, including the histological type, grade, depth of myometrial invasion, lymph node metastasis and lymph-vascular space involvement (LVSI) (2). Furthermore, previous studies revealed that carcinosarcoma, even with early-stage disease, is associated with a much lower survival rate compared with those of endometrioid adenocarcinoma (4,5). For patients belonging to high-risk groups defined by clinicopathological parameters, either adjuvant chemotherapy or radiotherapy has been applied. However, the criteria for selecting patients that should receive adjuvant therapy remain controversial (2,6). Therefore, the identification of additional prognostic markers may be helpful for the individualization of adjuvant therapy and for improving the survival of patients with endometrial cancer and carcinosarcoma.
18F-fluoro-2-deoxy-D-glucose-positron emission tomography (FDG-PET) with computed tomography (CT) is a well-established imaging modality for diagnosing and staging numerous types of cancer. In gynecological malignancies, previous studies revealed that the maximum standardized uptake value (SUVmax), a quantitative measurement of the tissue deoxyglucose metabolic rate measured on FDG-PET/CT, may be a useful parameter, not only for evaluating malignancy, but also for assessing the prognosis of patients with ovarian (7,8) and cervical cancer (9,10). In endometrial neoplasms, several previous reports demonstrated the usefulness of SUVmax for preoperative risk stratification (11,12), although its prognostic impact remains controversial (13–16), and it remains to be sufficiently studied in patients with uterine carcinosarcoma.
The present study investigated the SUVmax of primary tumors measured by preoperative FDG-PET/CT in patients with endometrial cancer and uterine carcinosarcoma, and attempted to clarify whether the SUVmax is an indicator for risk stratification and prognosis determination in these patients.
Patients and methods
Patient selection
A total of 75 patients with endometrial neoplasms who underwent preoperative FDG-PET/CT at Wakayama Minami Radiology Clinic (Wakayama, Japan), followed by the surgical resection of tumors at Wakayama Medical University Hospital (Wakayama, Japan) between January 2008 and January 2013, were included in the present study. All patients underwent a total abdominal hysterectomy and bilateral salpingo-oophorectomy, followed by surgical staging, including peritoneal washing cytology and pelvic and/or para-aortic lymph-node dissection. The median age of the patients was 59 years (range, 37–85 years). All patients were staged according to FIGO 2008 criteria: 50 were stage I (39 were IA, 11 were IB), 8 were stage II, 13 were stage III and 4 were stage IV. The postoperative pathological diagnosis was assigned according to the criteria of the World Health Organization classification: 31 were G1, 19 were G2 and 5 were G3 endometrioid adenocarcinoma, 8 were specific types of histology including 3 serous, 3 clear cell and 2 mucinous carcinomas, and 12 were carcinosarcoma. The evaluation of the pathological factors, including lymph node metastasis, LVSI and the depth of myometrial invasion, was also performed by pathologists. In the present study, patients with FIGO stage IA with specific histological types or carcinosarcoma and all patients with FIGO stage IB or more advanced-stage disease received postoperative adjuvant chemotherapy with six cycles of paclitaxel plus carboplatin. Patients who underwent any form of radiation therapy were excluded from the present study. The present study was approved by the Ethics Committee of Wakayama Medical University (approval no. 1792).
FDG-PET/CT and imaging analysis
PET studies were performed with a PET scanner (SET-3000BCT/L; Shimadzu, Kyoto, Japan) with an axial resolution of 3.9 mm and a 20 cm field of view, as described in our previous study (8). At the time of the tracer injection, all patients had fasted for at least 5 h and had blood glucose levels <150 mg/dl. Images were captured from the top of the head to the mid-thigh 50 min after the intravenous injection of 18F-FDG (2.6 MBq/kg body weight). Following the completion of PET, CT images were obtained using a multidetector row CT scanner (Brilliance 64; Philips Medical Systems, Best, The Netherlands). Fusion images of PET and CT were made using a Workstation (EV Insite; PSP Corporation, Tokyo, Japan). FDG-PET/CT images were evaluated by a nuclear medicine physician/radiologist. For each study, the SUVmax of the primary tumor was measured. SUV is a semi-quantitatively analyzed value of radiotracer uptake and is defined as the ratio of radiotracer activity per milliliter of tissue to the activity in the injected dose corrected for decay and the patient's body weight.
Data analysis
The SUVmax among the groups were compared using the Mann-Whitney U test. Receiver operating characteristic (ROC) curve analysis was performed in order to determine the cut-off values of the SUVmax. The overall survival (OS) was calculated from the date of surgery until the patient succumbed to mortality, and the progression-free survival (PFS) was calculated from the date of surgery until that of recurrence. The median follow-up period was 26.4 months, ranging between 3 and 59 months. Survival analyses were performed according to the Kaplan-Meier method. A comparison of the survival between groups was performed using the log-rank test. The Cox proportional-hazard regression model was used for multivariate analyses to explore the impact of individual variables on survival. P<0.05 was considered to indicate a statistically significant difference.
Results
Correlation between the SUVmax of the primary tumor and clinicopathological factors
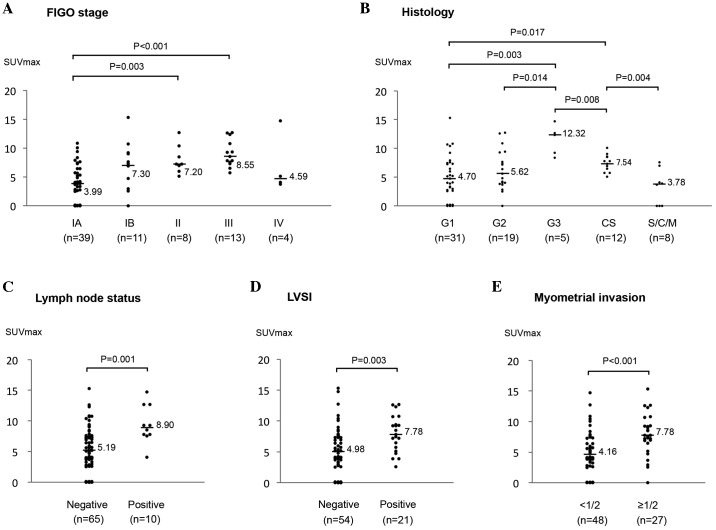
Clinicopathological characteristics of the 75 patients and the median SUVmax of the primary tumor in each group are shown in Table I. As shown in Fig. 1A, the SUVmax values for stage II and III were significantly higher compared with those for stage IA (P=0.003 and P<0.001, respectively), however, not those for stage IB. No significant difference in the SUVmax was observed between patients with stage IV and the others, although the number of stage IV patients was only 4.
Table I.
Clinicopathological characteristics and the median SUVmax in 75 patients with endometrial cancer and uterine carcinosarcoma.
| Characteristic | Patient number (%) | Median SUVmax |
|---|---|---|
| Total | 75 | 5.71 |
| Age | ||
| <60 | 41 (54.7) | 5.33 |
| ≥60 | 34 (45.3) | 6.70 |
| FIGO stage | ||
| IA | 39 (52.0) | 3.99 |
| IB | 11 (14.7) | 7.30 |
| II | 8 (10.7) | 7.20 |
| III | 13 (17.3) | 8.55 |
| IV | 4 (5.3) | 4.59 |
| Histology | ||
| G1 | 31 (41.3) | 4.70 |
| G2 | 19 (25.3) | 5.62 |
| G3 | 5 (6.7) | 12.32 |
| S/C/M | 8 (10.7) | 3.78 |
| CS | 12 (16.0) | 7.54 |
| LN status | ||
| Negative | 65 (86.7) | 5.19 |
| Positive | 10 (13.3) | 8.90 |
| LVSI | ||
| Negative | 54 (72.0) | 4.98 |
| Positive | 21 (28.0) | 7.78 |
| Myometrial invasion | ||
| <1/2 | 48 (64.0) | 4.16 |
| ≥1/2 | 27 (36.0) | 7.78 |
S/C/M, serous adenocarcinoma (n=3)/clear cell adenocarcinoma (n=3)/mucinous adenocarcinoma (n=2); CS, carcinosarcoma, LN, lymph node; LVSI, lymph-vascular space involvement; FIGO, International Federation of Gynecology and Obstetrics; SUVmax, maximum standardized uptake value.
Figure 1.
Association between the SUVmax and clinicopathological factors in 75 patients with endometrial cancer. The association between (A) FIGO Stage, (B) histology, (C) lymph node status, (D) LVSI and (E) myometrial invasion, and the SUVmax was assessed. S/C/M, serous adenocarcinoma (n=3)/clear cell adenocarcinoma (n=3)/mucinous adenocarcinoma (n=2); CS, carcinosarcoma; LVSI, lymph-vascular space involvement; FIGO, International Federation of Gynecology and Obstetrics; SUVmax, maximum standardized uptake value.
Next, the correlation between the SUVmax and histology was investigated (Fig. 1B). The SUVmax for G3 endometrioid adenocarcinoma was significantly higher compared with those for stage G1 and G2 (P=0.003 and P=0.014, respectively). The SUVmax for carcinosarcoma was also significantly higher compared with those for stage G1 (P=0.017), and, it was significantly lower compared with those for G3 (P=0.008). The SUVmax for patients with specific histological types, including serous, clear cell and mucinous carcinoma, was lower (median, 3.78), although the number of patients in this group was low (n=8).
The SUVmax was significantly higher in patients with a pathologically positive lymph node status (P=0.001; Fig. 1C) and a positive LVSI (P=0.003; Fig. 1D). In addition, the SUVmax in patients with deep (≥1/2) myometrial invasion was significantly higher compared with those in patients with <1/2 (P<0.001; Fig. 1E).
Cut-off values of the SUVmax for predicting the presence of risk factors
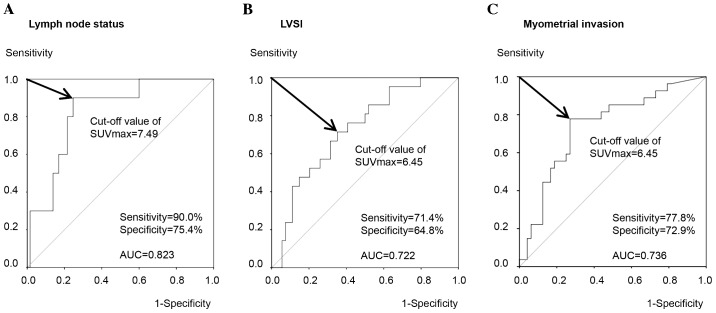
The present study attempted to determine the optimal cut-off values of the SUVmax for risk stratification. ROC curve analysis demonstrated that the optimal cut-off value of the SUVmax for predicting a pathologically positive lymph node status was 7.49, with a sensitivity of 90.0%, specificity of 75.4% and AUC of 0.823 (Fig. 2A). By contrast, the cut-off value of the SUVmax for predicting a positive LVSI was 6.45, with a sensitivity of 71.4%, specificity of 64.8% and AUC of 0.722 (Fig. 2B). Similarly, ROC curve analysis revealed that the optimal cut-off values of the SUVmax for myometrial invasion of ≥1/2 was 6.45 with a sensitivity of 77.8%, specificity of 72.9% and AUC of 0.736 (Fig. 2C). These results indicated that the primary tumor SUVmax may be predictive for the presence of these clinicopathological risk factors using optimal cut-off values with relatively high sensitivity and specificity.
Figure 2.
Receiver operating characteristic curve analysis of the SUVmax for determining the optimal cut-off values in order to predict the presence of clinicopathological risk factors. The (A) lymph node status, (B) LVSI and (C) myometrial invasion were assessed. AUC, area under the curve; LVSI, lymph-vascular space involvement; SUVmax, maximum standardized uptake value.
Correlation of the SUVmax of the primary tumor with patient survival
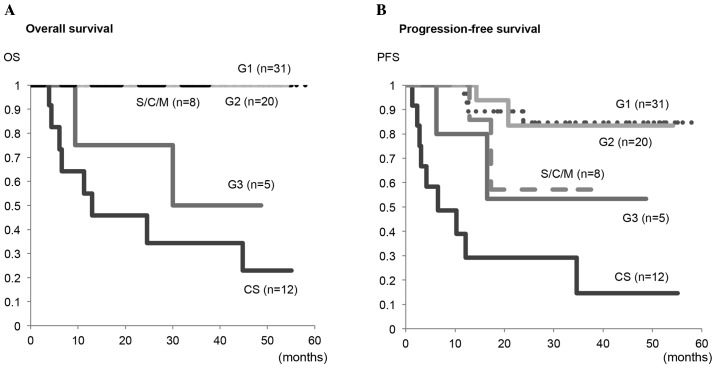
Firstly, the present study compared the OS and PFS rates among the five groups with G1, G2, G3 endometrioid adenocarcinoma, a specific histology, and carcinosarcoma (Fig. 3). The OS in patients with carcinosarcoma was significantly lower compared with in those with G1 (P<0.001), G2 (P<0.001) or a specific histology (P=0.024), however, not those with G3 (P=0.307). Similarly, the PFS in patients with carcinosarcoma was significantly lower compared with that in those with G1 (P<0.001), G2 (P<0.001) or a specific histology (P=0.028), and there was a trend toward a lower PFS in carcinosarcoma as compared with that in G3, although it did not reach a significant difference (P=0.134).
Figure 3.
Kaplan-Meier plots for (A) OS and (B) PFS in G1, G2 and G3 endometrioid adenocarcinomas, S/C/M and CS. S/C/M included serous (n=3), clear cell (n=3) and mucinous adenocarcinomas (n=2). Significant differences in the OS were observed in CS vs. G1 (P<0.001), CS vs. G2 (P<0.001) and CS vs. S/C/M (P=0.024), however, not in CS vs. G3 (P=0.307). Significant differences in PFS were observed in CS vs. G1 (P<0.001), CS vs. G2 (P<0.001) and CS vs. S/C/M (P=0.028), however, not in CS vs. G3 (P=0.134). OS, overall survival; PFS, progression-free survival; S/C/M, serous/clear cell/mucinous adenocarcinoma; CS, carcinosarcoma.
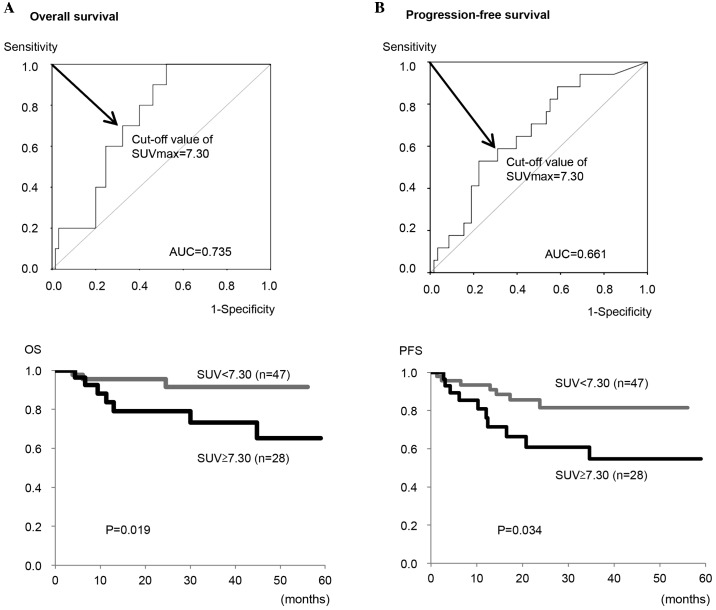
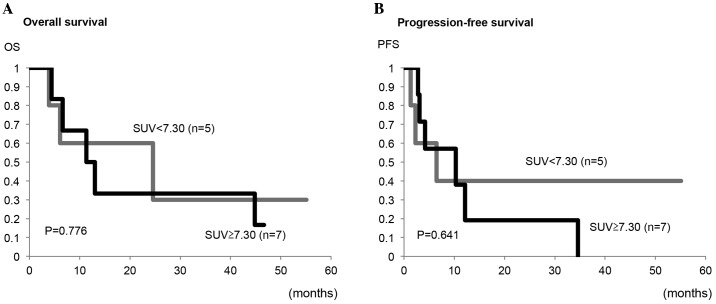
Next, the optimal cut-off value of the SUVmax for predicting OS and PFS in all patients was determined to be 7.30 from the ROC curve analysis (Fig. 4). Using this cut-off value, the OS rate of patients with a high SUVmax (≥7.30) was significantly lower compared with patients with a low SUVmax (<7.30) (P=0.019; Fig. 4A). Similarly, the PFS rate of the patients with a high SUVmax (≥7.30) was significantly lower compared with the patients with a low SUVmax (<7.30) (P=0.034; Fig. 4B). Furthermore, the present study analyzed the impact of the preoperative SUVmax on the prognosis of patients with carcinosarcoma alone. As shown in Fig. 5, no significant differences in the OS or PFS rates between patients with a high SUVmax and those with a low SUVmax were observed (P=0.776 and P=0.641, respectively).
Figure 4.
Receiver operating characteristic curve analysis and Kaplan-Meier plots for (A) OS and (B) PFS in all 75 patients. Significant differences were observed between the high and low SUVmax groups (P=0.019 for OS and P=0.034 for PFS by log-rank test). OS, overall survival; PFS, progression-free survival; AUC, area under the curve; SUVmax, maximum standardized uptake value.
Figure 5.
Kaplan-Meier plots for (A) OS and (B) PFS in 12 carcinosarcoma patients. No significant differences were observed between the high and low SUVmax groups (P=0.776 for OS and P=0.641 for PFS by log-rank test). OS, overall survival; PFS, progression-free survival; SUVmax, maximum standardized uptake value.
Finally, to clarify whether the SUVmax can be an independent prognostic factor, multivariate analyses were performed. As shown in Table II, multivariate analysis demonstrated that the FIGO stage, histology and myometrial invasion, however, not SUVmax, were independent prognostic factors for impaired PFS in all 75 patients, including any adenocarcinomas and carcinosarcomas. By contrast, when analyzed in 55 endometrioid adenocarcinoma (G1, G2 and G3) patients alone (Table III), a high SUVmax was an independent prognostic factor for predicting impaired PFS (hazard ratio=12.453; P=0.002).
Table II.
Univariate and multivariate analyses of the clinicopathological factors and SUVmax for progression-free survival in all 75 patients.
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| Characteristic | P-value | Hazard ratio | 95% CI | P-value |
| Age | ||||
| <60 vs. ≥60 | 0.242 | 2.092 | 0.679–6.446 | 0.198 |
| FIGO stage | ||||
| I/II vs. III/IV | <0.001 | 4.969 | 1.699–14.527 | 0.003 |
| Histology | ||||
| G1/G2 vs. G3/SCM/CS | <0.001 | 2.956 | 1.004–8.703 | 0.049 |
| LN status | ||||
| Negative vs. positive | <0.001 | 1.283 | 0.320–5.139 | 0.725 |
| LVSI | ||||
| Negative vs. positive | 0.029 | 1.107 | 0.356–3.445 | 0.860 |
| Myometrial invasion | ||||
| <1/2 vs. ≥1.2 | <0.001 | 3.070 | 1.114–8.457 | 0.030 |
| SUVmax | ||||
| <7.30 vs. ≥7.3 | 0.034 | 0.984 | 0.311–3.118 | 0.978 |
| ≥7.30 | ||||
G1, endometrioid adenocarcinoma grade 1; G2, endometrioid adenocarcinoma grade 2; G3, endometrioid adenocarcinoma grade 3; SCM, serous, clear cell and mucinous adenocarcinomas; CS, carcinosarcoma; LN, lymph node; LVSI, lymph-vascular space involvement; CI, confidence interval; FIGO, International Federation of Gynecology and Obstetrics; SUVmax, maximum standardized uptake value.
Table III.
Univariate and multivariate analyses of the clinicopathological factors and SUVmax for progression-free survival in 55 patients with endometrioid adenocarcinoma.
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| Characteristic | P-value | Hazard ratio | 95% CI | P-value |
| Age | ||||
| <60 vs. ≥60 | 0.600 | 3.234 | 0.438–23.892 | 0.250 |
| FIGO stage | ||||
| I/II vs. III/IV | <0.001 | 1.961 | 0.115–33.412 | 0.642 |
| Histology | ||||
| G1/G2 vs. G3/SCM/CS | 0.050 | 0.876 | 0.082–9.405 | 0.913 |
| LN status | ||||
| Negative vs. positive | <0.001 | 2.288 | 0.245–21.376 | 0.468 |
| LVSI | ||||
| Negative vs. positive | <0.005 | 2.515 | 0.208–30.412 | 0.468 |
| Myometrial invasion | ||||
| <1/2 vs. ≥1/2 | <0.017 | 2.090 | 0.168–26.049 | 0.567 |
| SUVmax | ||||
| <7.30 vs. ≥7.30 | <0.001 | 12.453 | 2.501–62.016 | 0.002 |
G1, endometrioid adenocarcinoma grade 1; G2, endometrioid adenocarcinoma grade 2; G3, endometrioid adenocarcinoma grade 3; LN, lymph node; LVSI, lymph-vascular space involvement; CI, confidence interval; FIGO, International Federation of Gynecology and Obstetrics; SUVmax, maximum standardized uptake value.
Discussion
Previous studies demonstrated that a high FDG uptake within the primary tumors evaluated by the SUVmax on PET-CT can be associated with clinicopathological factors and aggressive biological characteristics in endometrial cancer (11,12), although its impact on disease recurrence or OS remains controversial (13–16). Nakamura et al (11) reported that the SUVmax of the primary tumor was correlated with the histological grade. Antonsen et al (12) showed that a high SUVmax was predictive of risk factors, including deep myometrial invasion, an advanced FIGO stage and lymph node metastasis in patients with endometrial cancer. In terms of the prognostic impact, Nakamura et al (13) reported that the SUVmax was an independent prognostic factor for OS, based on multivariate analysis, however, not for disease-free survival (DFS), in 106 patients with endometrial cancer, including 9 carcinosarcomas. Similarly, Walentowicz-Sadlecka et al (14) revealed that the preoperative SUVmax was an independent prognostic factor of OS in 101 endometrial cancer patients. By contrast, Kitajima et al (15) demonstrated that the SUVmax was an independent factor for DFS on multivariate analysis in 57 patients with G1-G3 endometrioid carcinoma. Ghooshkhanei et al (16) showed in their review that the usefulness of the SUVmax for classifying patients with endometrial cancer into pre-defined risk groups appears to be limited. To further clarify the clinical significance of the primary tumor SUVmax on preoperative PET/CT not only in endometrioid adenocarcinomas, but also in specific histological types, including carcinosarcoma, the present study investigated its clinicopathological and prognostic impacts on these patients.
These results demonstrated that a high SUVmax of the primary tumor was significantly correlated with the presence of conventional clinicopathological risk factors, including histology of G3 or carcinosarcoma, positive lymph node metastasis, positive LVSI and deep myometrial invasion. Furthermore, it demonstrated the individual cut-off values of the SUVmax for predicting the presence of each risk factor with high sensitivity and specificity based on the ROC curve analysis. These findings suggested that the preoperative SUVmax of the primary tumor may be useful for predicting clinicopathological risk factors and tumor aggressiveness using optimal cut-off values. In the present study, the SUVmax in patients with specific histological types, including serous, clear cell and mucinous carcinomas, was rather low despite their aggressive characteristics and less favorable prognosis, although the number of patients in this group was very low (n=8). Our previous study demonstrated that SUVmax was lower in patients with ovarian cancer with a clear cell or mucinous histology (8). Further studies are required to clarify the FDG uptake and its clinicopathological significance in specific histological types.
In the present study, based on the results of ROC curve analysis, it was demonstrated that both the OS and PFS in patients with a higher (≥7.30) SUVmax were significantly lower compared with those with a lower (<7.30) SUVmax. This cut-off value may be easy to use and helpful for preoperative risk stratification in each patient as an index, although it may vary among the institutions due to its dependence on the setting of PET conditions and method of imaging analysis in each institution. Notably, the multivariate analyses demonstrated that a high SUVmax was an independent prognostic factor for impaired PFS when analyzed in G1-G3 endometrioid adenocarcinomas without a specific histology or carcinosarcoma, however, not when analyzed in all 75 patients including those specific types. Furthermore, no significant differences were observed between high and low SUVmax groups with carcinosarcoma, although the total number of carcinosarcoma patients eligible for our study was low (n=12). These findings suggested that the SUVmax of the primary tumor can be a prognostic indicator of endometrioid adenocarcinoma, and may be a promising non-invasive biomarker to evaluate the risk of recurrence, as shown in other previous studies (15,16), while its prognostic impact on patients with carcinosarcoma or a specific histology remains to be confirmed. Both the OS and PFS rates in carcinosarcoma were markedly lower, even in patients with early-stage disease, as shown in the present study (Fig. 3) and previous studies (4–6). This may be the reason why the SUVmax was difficult to use to stratify the recurrence risk in this disease. Further studies on the prognostic impact of the primary tumor SUVmax in a large number of carcinosarcoma patients are required.
Previously, several novel metabolic parameters of FDG-PET/CT, in addition to the SUVmax, were shown to be useful in endometrial cancer. Kitajima et al (17) demonstrated that the metabolic tumor volume (MTV) and total lesion glycolysis (TLG) of the primary tumors were correlated with clinicopathological features and useful for differentiating high- from low-risk endometrial cancer. Consistently, Chung et al (18) reported that MTV was an independent prognostic factor for disease recurrence in endometrial cancer and Husby et al (19) also showed that MTV was useful for the identification of patients with high-risk endometrial carcinoma. Lee et al (20) revealed that preoperative TLG was associated with recurrence in 28 patients with carcinosarcoma. As the primary tumors have intratumoral FDG metabolic heterogeneity (21), the present study, focusing only on the simple and easy to measure SUVmax, may have limitations. Further studies using multi-metabolic parameters of FDG-PET/CT, including the SUVmax, MTV and TLG, in combination with other non-invasive biomarkers, are required to clarify the optimal prognostic parameter for patients with endometrial cancer and uterine carcinosarcoma.
In conclusion, the present study demonstrated that a high SUVmax on preoperative PET/CT correlates with clinicopathological risk factors and less favorable clinical outcomes in patients with endometrial cancer. These findings suggested that the SUVmax of the primary tumor may be a promising prognostic indicator for risk stratification in patients with this disease, although its usefulness in specific histological types, including carcinosarcoma, requires clarification by further studies.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Morice P, Leary A, Creutzberg C, AbuRustum N, Darai E. Endometrial cancer. Lancet. 2016;387:1094–1108. doi: 10.1016/S0140-6736(15)00130-0. [DOI] [PubMed] [Google Scholar]
- 3.Singh R. Review literature on uterine carcinosarcoma. J Cancer Res Ther. 2014;10:461–468. doi: 10.4103/0973-1482.138197. [DOI] [PubMed] [Google Scholar]
- 4.Zhang C, Hu W, Jia N, Li Q, Hua K, Tao X, Wang L, Feng W. Uterine carcinosarcoma and high-risk endometrial carcinomas: A clinicopathological comparison. Int J Gynecol Cancer. 2015;25:629–636. doi: 10.1097/IGC.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 5.Zhu J, Wen H, Bi R, Wu X. Clinicopathological characteristics, treatment and outcomes in uterine carcinosarcoma and grade 3 endometrial cancer patients: A comparative study. J Gynecol Oncol. 2016;27:e18. doi: 10.3802/jgo.2016.27.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cantrell LA, Havrilesky L, Moore DT, O'Malley D, Liotta M, Secord AA, Nagel CI, Cohn DE, Fader AN, Wallace AH, et al. A multi-institutional cohort study of adjuvant therapy in stage I–II uterine carcinosarcoma. Gynecol Oncol. 2012;127:22–26. doi: 10.1016/j.ygyno.2012.06.020. [DOI] [PubMed] [Google Scholar]
- 7.Nakamura K, Hongo A, Kodama J, Hiramatsu Y. The pretreatment of maximum standardized uptake values (SUVmax) of the primary tumor is predictor for poor prognosis for patients with epithelial ovarian cancer. Acta Med Okayama. 2012;66:53–60. doi: 10.18926/AMO/48081. [DOI] [PubMed] [Google Scholar]
- 8.Tanizaki Y, Kobayashi A, Shiro M, Ota N, Takano R, Mabuchi Y, Yagi S, Minami S, Terada M, Ino K. Diagnostic value of preoperative SUVmax on FDG-PET/CT for the detection of ovarian cancer. Int J Gynecol Cancer. 2014;24:454–460. doi: 10.1097/IGC.0000000000000074. [DOI] [PubMed] [Google Scholar]
- 9.Kidd EA, Siegel BA, Dehdashti F, Grigsby PW. The standardized uptake value for F-18 fluorodeoxyglucose is a sensitive predictive biomarker for cervical cancer treatment response and survival. Cancer. 2007;110:1738–1744. doi: 10.1002/cncr.22974. [DOI] [PubMed] [Google Scholar]
- 10.Lee YY, Choi CH, Kim CJ, Kang H, Kim TJ, Lee JW, Lee JH, Bae DS, Kim BG. The prognostic significance of the SUVmax (maximum standardized uptake value for F-18 fluorodeoxyglucose) of the cervical tumor in PET imaging for early cervical cancer: Preliminary results. Gynecol Oncol. 2009;115:65–68. doi: 10.1016/j.ygyno.2009.06.022. [DOI] [PubMed] [Google Scholar]
- 11.Nakamura K, Kodama J, Okumura Y, Hongo A, Kanazawa S, Hiramatsu Y. The SUVmax of 18F-FDG PET correlates with histological grade in endometrial cancer. Int J Gynecol Cancer. 2010;20:110–115. doi: 10.1111/IGC.0b013e3181c3a288. [DOI] [PubMed] [Google Scholar]
- 12.Antonsen SL, Loft A, Fisker R, Nielsen AL, Andersen ES, Høgdall E, Tabor A, Jochumsen K, Fagö-Olsen CL, Asmussen J, et al. SUVmax of 18FDG PET/CT as a predictor of high-risk endometrial cancer patients. Gynecol Oncol. 2013;129:298–303. doi: 10.1016/j.ygyno.2013.01.019. [DOI] [PubMed] [Google Scholar]
- 13.Nakamura K, Hongo A, Kodama J, Hiramatsu Y. The measurement of SUVmax of the primary tumor is predictive of prognosis for patients with endometrial cancer. Gynecol Oncol. 2011;123:82–87. doi: 10.1016/j.ygyno.2011.06.026. [DOI] [PubMed] [Google Scholar]
- 14.WalentowiczSadlecka M, Malkowski B, Walentowicz P, Sadlecki P, Marszalek A, Pietrzak T, Grabiec M. The preoperative maximum standardized uptake value measured by 18F-FDG PET/CT as an independent prognostic factor of overall survival in endometrial cancer patients. Biomed Res Int. 2014;2014:234813. doi: 10.1155/2014/234813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kitajima K, Kita M, Suzuki K, Senda M, Nakamoto Y, Sugimura K. Prognostic significance of SUVmax (maximum standardized uptake value) measured by [18F]FDG PET/CT in endometrial cancer. Eur J Nucl Med Mol Imaging. 2012;39:840–845. doi: 10.1007/s00259-011-2057-9. [DOI] [PubMed] [Google Scholar]
- 16.Ghooshkhanei H, Treglia G, Sabouri G, Davoodi R, Sadeghi R. Risk stratification and prognosis determination using (18)F-FDG PET imaging in endometrial cancer patients: A systematic review and meta-analysis. Gynecol Oncol. 2014;132:669–676. doi: 10.1016/j.ygyno.2014.01.039. [DOI] [PubMed] [Google Scholar]
- 17.Kitajima K, Suenaga Y, Ueno Y, Maeda T, Ebina Y, Yamada H, Okunaga T, Kubo K, Sofue K, Kanda T, et al. Preoperative risk stratification using metabolic parameters of (18)F-FDG PET/CT in patients with endometrial cancer. Eur J Nucl Med Mol Imaging. 2015;42:1268–1275. doi: 10.1007/s00259-015-3037-2. [DOI] [PubMed] [Google Scholar]
- 18.Chung HH, Lee I, Kim HS, Kim JW, Park NH, Song YS, Cheon GJ. Prognostic value of preoperative metabolic tumor volume measured by 18F-FDG PET/CT and MRI in patients with endometrial cancer. Gynecol Oncol. 2013;130:446–451. doi: 10.1016/j.ygyno.2013.06.021. [DOI] [PubMed] [Google Scholar]
- 19.Husby JA, Reitan BC, Biermann M, Trovik J, Bjørge L, Magnussen IJ, Salvesen ØO, Salvesen HB, Haldorsen IS. Metabolic tumor volume on 18F-FDG PET/CT improves preoperative identification of high-risk endometrial carcinoma patients. J Nucl Med. 2015;56:1191–1198. doi: 10.2967/jnumed.115.159913. [DOI] [PubMed] [Google Scholar]
- 20.Lee JW, Heo EJ, Moon SH, Lee H, Cheon GJ, Lee M, Kim HS, Chung HH. Prognostic value of total lesion glycolysis on preoperative 18F-FDG PET/CT in patients with uterine carcinosarcoma. Eur Radiol. 2016 Feb 16; doi: 10.1007/s00330-016-4264-z. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 21.Kidd EA, Grigsby PW. Intratumoral metabolic heterogeneity of cervical cancer. Clin Cancer Res. 2008;14:5236–5241. doi: 10.1158/1078-0432.CCR-07-5252. [DOI] [PubMed] [Google Scholar]