Abstract
Asthma is a chronic bronchial inflammation that results to reversible incidence of airway obstruction and shortness of breath. Under normal circumstances, the lung immune system is maintained in a state of controlled inflammation, where balance exists between protective immunity mediated by effector cells and tolerance mediated by cells with regulatory function. Therefore, the inflammation observed in asthma patients may be caused by an imbalance between regulatory T (Treg) cells (CD4-positive with high expression of CD25 surface markers) and forkhead box P3 (FOXP3)-positive pro-inflammatory T helper 17 (Th17) cells. The aim of the present study was to evaluate whether reduced Treg cells and increased Th17 cells could be observed in the peripheral blood samples of asthma patients. As important markers of Treg cells, the expression levels of FOXP3 and interleukin (IL)-17a were analyzed via reverse trancription-quantitative polymerase chain reaction. The results indicated that the levels of cytokines that promote Th17 cells, including IL-6, IL-23 and TGF-β, were found to increase in the bronchoalveolar lavage fluid sample of asthma patients. However, the IL-10 level in the corresponding sample was much lower compared with that in control individuals. In conclusion, these results suggest that asthma associated with a reduced proportion of Treg and Th17 cells in the blood is characterized by the expression of pro-inflammatory cytokines that may be beneficial for the continuous generation of Th17 cells.
Keywords: asthma, regulatory T cells, T helper 17 effector cells, FOXP3, bronchoalveolar lavage
Introduction
Asthma is a disease that features chronic bronchial inflammation, exaggerated bronchial response and airway obstruction (1). Immune tolerance has been demonstrated to be mediated by regulatory T (Treg) cells, defined as a sub-population of CD4+ T cells that mediates the immune reaction in the lungs through the prohibition of the proliferation and activities of effecting factors secreted by other T cells (2). T helper 17 (Th17) cells consist of a definitive lineage of pro-inflammatory Th cells, which serve a major role in autoallergic diseases (3). Experimental data have suggested an association between the pathology induced by Th17 cells and the regulatory role of Treg cells (4). Murine models have demonstrated that Th17 cells induce autoimmune response by promoting tissue inflammation and activating the innate immune system (5). Treg cells, a subgroup of T lymphocytes with features of immune repression on effector T cells, can be recognized by the expression of the high affinity α-chain receptor of interleukin (IL)-2, namely CD25. However, CD25 is also an upregulated marker of the activated CD4+ T cells, and is thus considered as a non-exclusive marker. In addition, further distinction of CD4+ CD25high Treg cells from other T cells can be achieved based on the selective expression of the forkhead box P3 (FOXP3) transcription factor, since this expression has a central role in the growth and function of Treg cells (6). Although the underlying mechanism through which the proliferation of other T cells is suppressed by Treg cells has not been elucidated, previous evidence has revealed that Treg cells are involved in the prevention of autoimmune response and the control of pulmonary and gastric inflammation in vivo (7,8).
Increasing evidence has demonstrated that Th17 and Treg cells are essential T cell subsets and are critical in maintaining the homeostasis of the immune system. The native T cell precursor pool responsible for the generation of Treg cells can also generate CD4+ Th17 cells that may secrete IL-17 cytokines (9). In previous animal models, numerous inflammation cytokines were found to be involved in the differentiation of T cells, which are responsible for the activation of adaptive immune response. The potential role of IL-6, transforming growth factor (TGF)-β, IL-10 and IL-23 in the promotion of human Th17 cell differentiation has been revealed in a previous study (10), although the exact cytokine combination requires further verification. It has been suggested that IL-6 or IL-23 are indispensable for the ability of activated T cells to secrete IL-17, while IL-23 along with TGF-β are essential for Th17 cell differentiation from primitive T cells (11,12). Cytokine levels in have been shown to orchestrate the development of these cells in a temporal and spatial manner (13–15). Th17 cells are potent activators of neutrophils to enable the eradication of pathogens (16,17). Although Th17 cells are considered to be biologically crucial in eradicating extracellular pathogens, the abnormal expression of IL-17a by these cells is considered as a factor participating in the pathogenesis of various airway inflammatory disorders. For instance, elevated levels of IL-17a have been identified in rheumatoid arthritis, multiple sclerosis and systemic lupus erythematosus (18). IL-10 is upregulated in the lungs of asthma patients, indicating that an immune response driven by Th17 cells contributes to the pathogenesis of asthma (19). The balance between Th17 and Treg cells may be necessary to maintain the immune homeostasis (20); however, no previous studies have analyzed this balance in individual asthma patients to date.
The aim of the present study was to examine whether the imbalance of Th17 and Treg cells had an important role in asthma patients. A stringent flow cytometry-based staining and gating strategy was developed to accurately enumerate the Treg and Th17 cells using FOXP3 and IL-17a, respectively, as the defining markers. Each cell type was investigated individually, and was compared in the same subgroup of patients simultaneously. It was hypothesized that the imbalance observed in cell types may be caused by the expression of FOXP3+ and pro-inflammatory cytokines. Therefore, the expression levels of IL-6, IL-10, IL-23 and TGF-β were detected in the bronchoalveolar lavage fluid (BALF) specimens of asthma patients and controls. The present study focused on the regulation of the Treg cells ratio on the cytokine environment in an inflammatory condition.
Patients and methods
Patients
Asthma patients treated at the Department of Respiratory Medicine of the Shandong Provincial Hospital (Jinan, China) between March 2014 and September 2014 were retrospectively analyzed in the present study. Informed consent was acquired from all patients prior to sample collection. Ethical approval for the present study was granted by the Ethical Committee of Wuxi People's Hospital of Nanjing Medical University, and was performed according to the National Statement on Ethical Conduct in Research Involving Humans (1999) issued by the National Health and Medical Research Council of China. In total, 25 allergy asthma patients (female, 11; male, 14) and 18 control individuals (female, 7; male, 11) were enrolled into the study, and blood samples were collected. All asthma patients did not present clinical exacerbation at the time of blood sample collection, based on the evaluation of clinical symptoms, signs and serum C-reactive protein levels (<10 mg/l). Subjects in the control group were all non-allergic healthy volunteers. The blood sample was collected and stored in the EDTA tubes. Patients were included if they fulfilled the following criteria: i) Positive diagnosis (ii) no traumatic and inflammatory treatment; and iii) no surgical intervention. Patients exhibited mild (n=11), moderate (n=10) and severe (n=4) severe asthma.
Flow cytometry analysis
Peripheral blood mononuclear cells (PBMCs) were separated via density gradient centrifugation in lymphocyte separation medium (Ficoll-Paque Plus; GE Healthcare, Chalfont, UK) at 600 × g for 20 min at 4°C. In order to recognize the Treg cells, 1×106 PBMCs were surface stained with anti-CD4 monoclonal antibodies (1:50; 564419) labeled with fluorescein isothiocynate, anti-CD8 (1:50; RPA-T8) and anti-CD25 antibodies (1:50; 561399) labeled with phycoerythrin (PE)-Cyanine 5 (Cy5; all from BD Biosciences, Sydney, Australia). Next, permeabilization in the FOXP3 fix/perm solution (eBioscience, Inc., San Diego, CA, USA) was performed, and then intracellular labeling was conducted with an anti-FOXP3 antibody conjugated with PE (1:50; PCH101; eBioscience, Inc.), according to the manufacturer's protocol. For IL-17a assays, PBMCs (2×106) were stimulated for 5 h using 50 ng/ml phorbol 12-myristate 13-acetate (PMA) and 1 g/ml ionomycin in the presence of 5 g/ml brefeldin A solution (all from Sigma-Aldrich; Merck Millipore, Darmstadt, Germany), incubated with 5% CO2 at 37°C. Cells were washed twice in phosphate-buffered saline (PBS) and surface labeled with CD3-PE-Cy5, prior to fixing with 4% w/v paraformaldehyde and permeabilizing with 0.1% w/v saponin (Sigma-Aldrich; Merck Millipore). Subsequent to permeabilization, all samples were washed with PBS. Cells were blocked with 5% w/v separated milk powder in PBS/0.1% w/v saponin for 30 min, followed by intracellular labeling with anti-IL17a-PE (clone ebio64DEC17; eBioscience, Inc.). Flow cytometry was conducted with the BD FACScan system (BD Biosciences), with 300,000–500,000 events gathered in each experiment and lymphocytes gated based on the properties of their forward and side light scatter. Stained cells were all analyzed by a BD LSR II flow cytometer (BD Biosciences), and the flow cytometry data were analyzed by FlowJo 7.6 software (FlowJo, LLC, Ashland, OR, USA).
RNA isolation and analysis
RNA from whole blood collected in EDTA tubes was isolated via an organic extraction technique, using TRIzol LS (Thermo Fisher Scientific, Inc., Waltham, MA, USA) according to the manufacturer's protocols. Further purification of DNA was performed on a QIAamp spin column (Qiagen, Hilden, Germany) and on-column DNase digestion. mRNA expression levels of FOXP3 and IL-17a in the blood were determined by reverse transcription-quantitative polymerase chain reaction (RT-qPCR) with the use of a QuantiTect RT kit (Qiangen) and a StepOnePlus Real-Time PCR system (Applied Biosystems; Thermo Fisher Scientific, Inc.). qPCR was performed with the following thermal cycles: 95°C for 10 min, followed by 95°C for 15 sec, 60°C for 30 sec and 72°C for 30 sec for 40 cycles using cDNA (2 µl), forward primer (0.4 µl), reverse primer (0.4 µl), SYBR Green solution (10 µl), and ddH2O (7.2 µl). Specific primers for FOXP3, IL-17a and β-actin used were as previously reported (21). To quantify the results, 2−ΔΔCq was calculated for every sample (22), and the mRNA expression levels were normalized to β-actin.
IL-6, IL-23, IL-10 and TGF-β expression levels determined by ELISA
Expression levels of IL-6 (EH2IL62), IL-23 (KHC0231; both Thermo Fisher Scientific, Inc.), IL-10 (RAB1060; Sigma-Aldrich; Merck Millipore)and TGF-β (E-EL-H0110c; Elabscience Bioengineering Co., Ltd., Wuhan, China) were detected in BALF samples obtained from the patients and control subjects using respective ELISA kits according to the manufacturer's protocols. All samples were measured in duplication. Optical densities were determined at 450 nm using a microplate reader.
Statistical analysis
All analyses were performed with the SPSS statistical software for Windows (version 10.1.4; SPSS, Inc., Chicago, IL, USA). Data are expressed as the mean ± standard error. In paired comparison, Student t test and Wilcoxon rank-sum (Mann-Whitney U) test were used for normally or non-normally distributed data, respectively. One-way analysis of variance and Kruskal-Wallis tests were used in the comparison of multiple groups. For all the analyses, two-sided P-values of <0.05 were considered to indicate statistically significant differences.
Results
Patients and sampling
Blood samples were collected from 25 asthma patients (female, 11; male, 14) and 18 controls without lung diseases (female, 7; male, 11), with mean ages of 38.7±3.1 and 45.8±2.8 years, respectively. In addition, BALF samples were collected from 15 allergy asthma patients and 10 controls, with mean ages of 30.9±7.63 and 46.8±15.1 years, respectively. There were no significant differences in age, gender and other clinical data among the patients and healthy subjects.
Reduced number of CD4+ CD25bright FOXP3+ Treg cells in the peripheral blood of asthma patients
In the present study, the highest intensity of CD25+ staining was ranked as the brightest 0.5% CD4+ CD25+cells for all patient samples. As shown in Fig. 1, the flow cytometric analysis demonstrated that patients with asthma generally exhibited a statistically decreased absolute number of Treg cells in each ml of peripheral blood, compared with the controls. The mean number of Treg cells/ml was found to be significantly lower in asthma patients (5.56±3.3×103/ml) in comparison with the healthy controls (8.98±4.3×103/ml; P=0.0093). In addition, the proportion of CD4+ CD25bright FOXP3+ Treg cells among PBMCs was 0.012–0.51% in asthma patients and 0.121–0.55% in the control group (data not shown).
Figure 1.
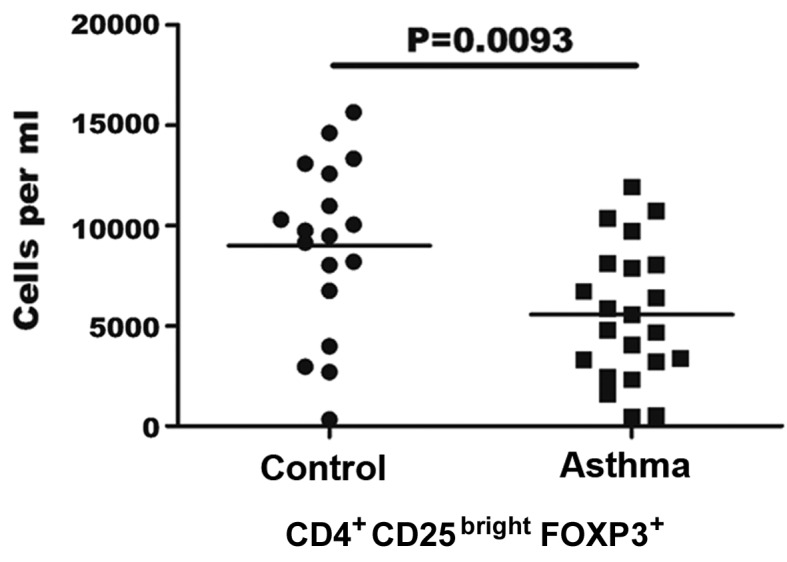
Quantification of regulatory T (Treg) cells in asthma patients. Absolute number of Treg cells was calculated using lymphocyte counts and the frequency of CD4+ CD25bright FOXP3+ cells determined by flow cytometry. Each point represents an individual patient sample. The horizontal line represents the mean values for each group. FOXP3, forkhead box P3.
Elevated number of Th17 cells in the peripheral blood of asthma patients
The count of Th17 cells in the blood was assessed by flow cytometry in order to determine whether it was altered in asthma patients compared with the controls. As the stimulation of PMA/ionomycin can lead to decreased human CD4 expression, CD3+CD8− was selected as a marker for CD4+ Th17 cells, according to a previous study (23). CD3+CD8− gating was performed in PBMCs to identify IL-17+ cells by distinguishing Th17 cells from the cluster of T cells in PBMCs. The results indicated that the percentage of Th17 cells in the sample of PBMCs in asthma patients was 0.36–1.25%, while this percentage was 0.10–0.49% in the control group (data not shown). As shown in Fig. 2, the number of Th17 cells per ml was significantly higher in the blood of asthma patients when compared with the control group (16.45±7.0×103/ml vs. 7.44±4.60×103/ml, respectively; P=0.007).
Figure 2.
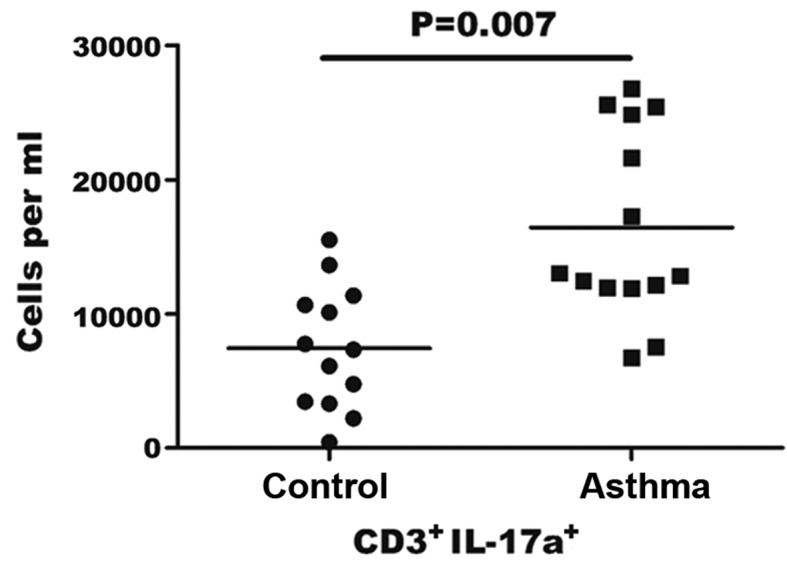
Quantification of T helper 17 (Th17) cells in asthma patients. Absolute number of Th17 cells was calculated using patient lymphocyte counts and the frequency of CD3+ IL-17a+ cells determined by flow cytometry. Each point represents an individual patient sample. The horizontal line represents the mean values for each group. IL, interleukin.
Imbalance between Treg and Th17 cells in the blood of asthma patients
As shown earlier, the number of Treg cells was decreased, while that of Th17 cells was elevated in asthma patients. This association was further investigated to determine the association of Treg and Th17 cell counts in the asthma patients and corresponding control individuals. The number of Treg and Th17 cells in the peripheral blood specimens of asthma patients was directly measured simultaneously for comparison, and the balance of Treg and Th17 cells was assessed in 14 controls and 14 asthma patients. As shown in Fig. 3, the ratio of Th17/Treg was significantly elevated in patients with asthma in comparison with that in healthy individuals (P=0.003), whereas the numbers of Treg and Th17 cells were comparable in the control group. Thus, an imbalance between Treg and Th17 cells was observed in the peripheral blood of asthma patients (Fig. 3), due to the observed reduction in the number of Treg cells and increase in the number of Th17 cells. No significant differences in the number of Treg cells were detected between the asthma and control groups. However, a significant increase in the number of Th17 cells was noted in asthma patients, when compared with healthy control subjects (P=0.0045).
Figure 3.

Balance of Th17 and regulatory T cell numbers is disrupted in asthma patients. Absolute number of CD4+ CD25bright FOXP3+ Treg and Th17 cells was determined using the same patient lymphocyte samples. Each point represents an individual patient sample. The horizontal line represents the mean values for each group. Th, T helper; Treg, regulatory T; FOXP3, forkhead box P3; NS, not significant.
Upregulated gene expression levels of FOXP3 and IL-17a in the peripheral blood of asthma patients
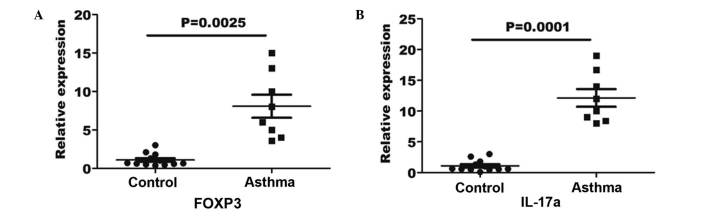
In order to verify the Treg and Th17 cell numbers in the asthma patients and healthy controls, RT-qPCR was performed for the measurement of FOXP3 and IL-17a gene expression in the blood specimens. An 8-fold elevation in FOXP3 gene expression was observed in the asthma blood samples in comparison with the expression in the controls (P=0.0025; Fig. 4A). Furthermore, a 12-fold elevation in IL-17a expression was determined in asthma patients in comparison with the controls (P=0.0001; Fig. 4B).
Figure 4.
(A) FOXP3 and (B) IL-17a gene expression levels in the peripheral blood of asthma patients and controls. RNA was extracted from the peripheral blood, quantified by reverse transcription-quantitative polymerase chain reaction and normalized to β-actin. The horizontal line represents the mean values for each group. FOXP3, forkhead box P3; IL, interleukin.
Increased levels of Th17-associated cytokines within the BALF of asthma patients
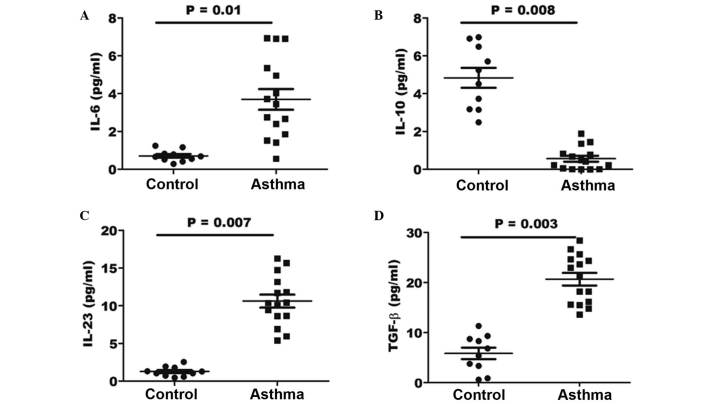
In order to determine whether the effect of cytokines in the lungs of asthma patients may be beneficial for the generation of pathological Th17 cells, the expression levels of various associated cytokines were investigated by ELISA. The concentration of the IL-6, IL-10, IL-23 and TGF-β cytokines was determined in the BALF specimens of asthma and control patients. As shown in Fig. 5, the results indicated a significantly elevated concentration of IL-6, IL-23 and TGF-β in the BALF of asthma patients (P=0.01, 0,007 and 0.003, respectively) when compared with that in healthy controls. However, the expression of IL-10 in the BALF samples of asthma patients was significantly lower when compared with that of control individuals (P=0.008).
Figure 5.
Expression levels of cytokines (A) IL-6, (B) IL-10, (C) IL-23 and (D) TGF-β, as determined by ELISA. The horizontal line represents the mean values for each group. IL, interleukin; TGF, transforming growth factor.
Discussion
The present study reported that CD4+ CD25high T cells are able to suppress T-cell responses. Immunotherapy with peptides targeting CD4 T cell epitopes has been under extensive investigation in animal models for the prevention and amelioration of inflammatory responses with antigen specificity, and is therefore currently the focus of clinical trials in the allergy and autoimmunity areas (24). Asthma is characterized by a decline in Treg cells along with an elevation in Th17 cells in the peripheral blood of patients in disease remission (25). In the different pathological stages of asthma, an increase in the expression levels of FOXP3 and IL-17a, which are alternative markers of Treg and Th17 cells, respectively, has been observed in the peripheral blood (26). The data reported in the present study indicated significantly increased expression levels of FOXP3 and IL-17a in asthma patients, suggesting that Treg may be activated and released into the peripheral blood in order to suppress the pro-inflammatory Th17 immune responses. In addition, the current results identified a strong correlation between the amount of FOXP3 transcript and the T cell number. However, although Treg cells isolated from the peripheral blood of asthma patients were demonstrated to be functionally suppressive in vitro, their ability to regulate Th17 proliferation and its effector activity may be limited in vivo due to the levels of pro-inflammatory cytokines.
For the inflammatory cytokines participating in the maintenance the inflammatory response, prolonged exposure of Treg cells to various cytokines (particularly IL-10 and TGF-β)may impair their suppressive properties, as well as prevent their conversion into Th17 cells (27). This process may account for the reduced in Treg cell count reported in the blood samples of asthma patients in the present study, which was accompanied by an increase in the number of Th17 cells. The imbalance of circulating Treg and Th17 cells in the blood of asthma patients may account for the relapsing and remitting nature of airway inflammation, and methods that aim to restore and maintain this balance may provide an effective therapeutic strategy.
In mice, it has been reported that TGF-β together with IL-6 promote the generation of Th17 cells, with TGF-β being a key cytokine for the initiation of Th17 expression and IL-6 acting as a crucial co-factor for Th17 differentiation; in addition, IL-23 enhances the expansion of Th17 cells (10). However, the results of the majority of previous studies demonstrated that a range of additional cytokines are necessary and able to induce Th17 differentiation in humans, including combinations of IL-13, IL-6, IL-21, IL-10 and TGF-β (28,29). The results revealed that there was no evident difference in IL-10 cytokine expression between the control and asthma adults; however, IL-10 is known to serve an important role in the pathogenesis of asthma (30). An alternative pathway of Th17 differentiation involves IL-23 and TGF-β, and may be a mechanism counteracting the resolution of inflammation that is promoted by TGF-β in asthma. In the present study, the level of IL-6 was found to be significantly higher in the peripheral blood of asthma patients, compared with the controls. Notably, the expression of IL-17a was closely correlated with IL-6 expression in asthma patients in a previous study (31), suggesting that these cytokines are involved in driving Th17 production in asthma patients. The protective role of TGF-β in asthma patients may also be important, since elevated TGF-β has been implicated in the differentiation of Treg cells, although these patients concurrently experienced a low FOXP3 level in the peripheral blood, suggesting that low levels of Treg cells were present (32). Thus, TGF-β may only have a protective effect in the absence of pro-inflammatory cytokines and may promote Th17 cell growth in the presence of IL-6.
In conclusion, the present study revealed that asthma is characterized by an imbalance between Th17 effector cells and Treg cells, with elevated numbers of Th17 cells and increased levels of proinflammatory cytokines that promote Th17 growth. The observed deficit in Treg cells in the asthma patients may impair the ability of the immune system to limit excessive pathogenic Th17-driven immune responses in the lungs. Accumulating data has indicated that the imbalance between the counts of Treg and Th17 cells is a characteristic feature of inflammatory disorders (33). Therefore, re-establishing the balance by upregulating the number of Treg cells in asthma patients and by specifically targeting Th17 cells in a disease environment may be an effective therapeutic strategy for asthma.
References
- 1.Anderson GP. Endotyping asthma: New insights into key pathogenic mechanisms in a complex, heterogeneous disease. Lancet. 2008;372:1107–1119. doi: 10.1016/S0140-6736(08)61452-X. [DOI] [PubMed] [Google Scholar]
- 2.Tucci M, Stucci S, Strippoli S, Silvestris F. Cytokine overproduction, T-cell activation, and defective T-regulatory functions promote nephritis in systemic lupus erythematosus. J Biomed Biotechnol. 2010;2010:457146. doi: 10.1155/2010/457146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ristich V, Liang S, Zhang W, Wu J, Horuzsko A. Tolerization of dendritic cells by HLA-G. Eur J Immunol. 2005;35:1133–1142. doi: 10.1002/eji.200425741. [DOI] [PubMed] [Google Scholar]
- 4.Hong M, Zheng J, Ding ZY, Chen JH, Yu L, Niu Y, Hua YQ, Wang LL. Imbalance between Th17 and Treg cells may play an important role in the development of chronic unpredictable mild stress-induced depression in mice. Neuroimmunomodulation. 2013;20:39–50. doi: 10.1159/000343100. [DOI] [PubMed] [Google Scholar]
- 5.O'Connor RA, Taams LS, Anderton SM. Translational mini-review series on Th17 cells: CD4+ T helper cells: Functional plasticity and differential sensitivity to regulatory T cell-mediated regulation. Clin Exp Immunol. 2010;159:137–147. doi: 10.1111/j.1365-2249.2009.04040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miyara M, Yoshioka Y, Kitoh A, Shima T, Wing K, Niwa A, Parizot C, Taflin C, Heike T, Valeyre D, et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009;30:899–911. doi: 10.1016/j.immuni.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 7.Schreiber TH, Wolf D, Tsai MS, Chirinos J, Deyev VV, Gonzalez L, Malek TR, Levy RB, Podack ER. Therapeutic Treg expansion in mice by TNFRSF25 prevents allergic lung inflammation. J Clin Invest. 2010;120:3629–3640. doi: 10.1172/JCI42933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, Nomura T, Sakaguchi S. CTLA-4 control over FOXP3+ regulatory T cell function. Science. 2008;322:271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 9.Chauhan SK, El Annan J, Ecoiffier T, Goyal S, Zhang Q, Saban DR, Dana R. Autoimmunity in dry eye is due to resistance of Th17 to Treg suppression. J Immunol. 2009;182:1247–1252. doi: 10.4049/jimmunol.182.3.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sadhu S, Khaitan BK, Joshi B, Sengupta U, Nautiyal AK, Mitra DK. Reciprocity between regulatory T Cells and Th17 cells: Relevance to polarized immunity in leprosy. PLoS Negl Trop Dis. 2016;10:e0004338. doi: 10.1371/journal.pntd.0004338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maddur MS, Sharma M, Hegde P, Lacroix-Desmazes S, Kaveri SV, Bayry J. Inhibitory effect of IVIG on IL-17 production by Th17 cells is independent of anti-IL-17 antibodies in the immunoglobulin preparations. J Clin Immunol. 2013;33(Suppl 1):S62–S66. doi: 10.1007/s10875-012-9752-6. [DOI] [PubMed] [Google Scholar]
- 12.Su Z, Shotorbani SS, Jiang X, Ma R, Shen H, Kong F, Xu H. A method of experimental rheumatoid arthritis induction using collagen type II isolated from chicken sternal cartilage. Mol Med Rep. 2013;8:113–117. doi: 10.3892/mmr.2013.1476. [DOI] [PubMed] [Google Scholar]
- 13.Volpe E, Servant N, Zollinger R, Bogiatzi SI, Hupé P, Barillot E, Soumelis V. A critical function for transforming growth factor-beta, interleukin 23 and proinflammatory cytokines in driving and modulating human TH-17 responses. Nat Immunol. 2008;9:650–657. doi: 10.1038/ni.1613. [DOI] [PubMed] [Google Scholar]
- 14.Bettelli E, Korn T, Oukka M, Kuchroo VK. Induction and effector functions of T(H)17 cells. Nature. 2008;453:1051–1057. doi: 10.1038/nature07036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McGeachy MJ, Chen Y, Tato CM, Laurence A, Joyce-Shaikh B, Blumenschein WM, McClanahan TK, O'Shea JJ, Cua DJ. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat Immunol. 2009;10:314–324. doi: 10.1038/ni.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fontao L, Brembilla NC, Masouyé I, Kaya G, Prins C, Dupin N, Saurat JH, Chizzolini C, Piguet V. Interleukin-17 expression in neutrophils and Th17 cells in cutaneous T-cell lymphoma associated with neutrophilic infiltrate of the skin. Br J Dermatol. 2012;166:687–689. doi: 10.1111/j.1365-2133.2011.10647.x. [DOI] [PubMed] [Google Scholar]
- 17.Kurimoto E, Miyahara N, Kanehiro A, Waseda K, Taniguchi A, Ikeda G, Koga H, Nishimori H, Tanimoto Y, Kataoka M, et al. IL-17A is essential to the development of elastase-induced pulmonary inflammation and emphysema in mice. Respir Res. 2013;14:5. doi: 10.1186/1465-9921-14-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 cells. Ann Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 19.Movahedi M, Mahdaviani SA, Rezaei N, Moradi B, Dorkhosh S, Amirzargar AA. IL-10, TGF-beta, IL-2, IL-12, and IFN-gamma cytokine gene polymorphisms in asthma. J Asthma. 2008;45:790–794. doi: 10.1080/02770900802207261. [DOI] [PubMed] [Google Scholar]
- 20.Littman DR, Rudensky AY. Th17 and regulatory T cells in mediating and restraining inflammation. Cell. 2010;140:845–858. doi: 10.1016/j.cell.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 21.Eastaff-Leung N, Mabarrack N, Barbour A, Cummins A, Barry S. FOXP3+ regulatory T cells, Th17 effector cells, and cytokine environment in inflammatory bowel disease. J Clin Immunol. 2010;30:80–89. doi: 10.1007/s10875-009-9345-1. [DOI] [PubMed] [Google Scholar]
- 22.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCt method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 23.Saurer L, Mueller C. T cell-mediated immunoregulation in the gastrointestinal tract. Allergy. 2009;64:505–519. doi: 10.1111/j.1398-9995.2009.01965.x. [DOI] [PubMed] [Google Scholar]
- 24.Vanderlugt CL, Miller SD. Epitope spreading in immune-mediated diseases: Implications for immunotherapy. Nat Rev Immunol. 2002;2:85–95. doi: 10.1038/nri724. [DOI] [PubMed] [Google Scholar]
- 25.Wilke CM, Bishop K, Fox D, Zou W. Deciphering the role of Th17 cells in human disease. Trends Immunol. 2011;32:603–611. doi: 10.1016/j.it.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lim HW, Lee J, Hillsamer P, Kim CH. Human Th17 cells share major trafficking receptors with both polarized effector T cells and FOXP3+ regulatory T cells. J Immunol. 2008;180:122–129. doi: 10.4049/jimmunol.180.1.122. [DOI] [PubMed] [Google Scholar]
- 27.Fantini MC, Rizzo A, Fina D, Caruso R, Becker C, Neurath MF, Macdonald TT, Pallone F, Monteleone G. IL-21 regulates experimental colitis by modulating the balance between Treg and Th17 cells. Eur J Immunol. 2007;37:3155–3163. doi: 10.1002/eji.200737766. [DOI] [PubMed] [Google Scholar]
- 28.Li H, Li D, Sun J, Li Y, Yang W, Li Y. Autoimmune regulator-overexpressing dendritic cells induce T helper 1 and T helper 17 cells by upregulating cytokine expression. Mol Med Rep. 2016;13:565–571. doi: 10.3892/mmr.2015.4530. [DOI] [PubMed] [Google Scholar]
- 29.Geri G, Terrier B, Rosenzwajg M, Wechsler B, Touzot M, Seilhean D, Tran TA, Bodaghi B, Musset L, Soumelis V, et al. Critical role of IL-21 in modulating TH17 and regulatory T cells in Behçet disease. J Allergy Clin Immunol. 2011;128:655–664. doi: 10.1016/j.jaci.2011.05.029. [DOI] [PubMed] [Google Scholar]
- 30.Umetsu DT, McIntire JJ, Akbari O, Macaubas C, DeKruyff RH. Asthma: An epidemic of dysregulated immunity. Nat Immunol. 2002;3:715–720. doi: 10.1038/ni0802-715. [DOI] [PubMed] [Google Scholar]
- 31.Charrad R, Berraïes A, Hamdi B, Ammar J, Hamzaoui K, Hamzaoui A. Anti-inflammatory activity of IL-37 in asthmatic children: Correlation with inflammatory cytokines TNF-α, IL-β, IL-6 and IL-17A. Immunobiology. 2016;221:182–187. doi: 10.1016/j.imbio.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 32.Zhou L, Lopes JE, Chong MM, Ivanov II, Min R, Victora GD, Shen Y, Du J, Rubtsov YP, Rudensky AY, et al. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature. 2008;453:236–240. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yan C, Zhang BB, Hua H, Li B, Zhang B, Yu Q, Li XY, Liu Y, Pan W, Liu XY, Tang RX, Zheng KY. The dynamics of Treg/Th17 and the imbalance of Treg/Th17 in Clonorchis sinensis-infected mice. PLoS One. 2015;10:e0143217. doi: 10.1371/journal.pone.0143217. [DOI] [PMC free article] [PubMed] [Google Scholar]