Abstract
Exogenous and endogenous opioids have been shown to modulate the immune system. Morphine-induced immunosuppression has been investigated extensively. However, the immune-regulating function of endogenous opioid peptides is unclear. The present study aimed to evaluate the difference in effects on cellular immune function between recombinant rat β-endorphin (β-EP; 50 µg/kg) and plant source morphine (10 mg/kg) via intraperitoneal injection treatment in a rat model of bone cancer pain. Walker 256 cells were injected into a tibial cavity injection to establish the bone cancer pain model. The paw withdrawal thresholds and body weights were measured prior to surgery, at 6 days after surgery, and following 1, 3,6 and 8 treatments. The spleen cells were harvested for detection of T cell proliferation, natural killer (NK) cell cytotoxicity, and the relative quantities of T cell subtypes (CD3+, CD4+ and CD8+ cells). Plasma levels of interleukin-2 (IL-2) were also determined. It was found that single or multiple treatments with β-EP (a homogenous opioid peptide) and morphine (a heterogenous opioid) had good analgesic effects on bone cancer pain, while the analgesia provided by morphine was stronger than that of β-EP. Treatment with β-EP 3, 6 and 8 times increased the body weight gain in the rat model of bone cancer pain, while morphine treatment had on effect on it. With regard to immunomodulatory functions, β-EP treatment increased T cell proliferation and NK cell cytotoxicity, and increased the relative quantities of T cell subtypes, but no effect on T cell secretion. However, morphine treatment decreased T cell proliferation and the levels of T cell subtypes. These data indicate that opioids from different sources have different effects on cellular immune function in vivo. A small dose of homogenous opioid peptide exhibited positive effects (analgesia and immune enhancement) on cancer pain. These results provide experimental evidence supporting the exploitation of human opioids for the treatment of cancer pain.
Keywords: bone cancer pain, cellular immune, heterogenous, homogenous, opioid peptide
Introduction
In patients with cancer, pain is a common symptom and the major factor responsible for decreasing the quality of life (1,2). A number of studies concerning the prevalence of pain in cancer patients have shown that 24–60% of patients undergoing active anticancer treatment (3,4) and 62–86% of terminal cancer patients (5,6) suffer from burdensome pain symptoms. The unique pathophysiology of cancer pain causes it to exceed that of a combination of inflammatory and neuropathic pain (7). In addition, there is evidence suggesting that patients with chronic pain always exhibit immune suppression symptoms (8). It has been suggested that the levels of CD4+ T cells in the serum of patients with cancer pain are decreased (9). Therefore, cancer pain and immune suppression are two main symptoms in cancer patients.
Opioids are used widely to treat acute pain following extensive surgery and many kinds of chronic pain, particularly cancer pain (10–13). It is known that opioids not only result in analgesia but also modulate the immune system (14). Opioids include endogenous opioid peptides and exogenous opiates. There is growing evidence that acute and long-term administration of exogenous opiates, especially morphine, which is a heterogenous opioid, mediates immunosuppression (15). However, the effects of endogenous opioids on the immune system remain a subject of debate, with some reports that endogenous opioids promote the immune function and others supporting the opposite view (15,16).
Although numerous studies have observed the effects of opioid drugs on immune responses, the clinical relevance of these observations for heterogenous and homogenous opioids remains uncertain. Few studies have analyzed the association between opioids and the immune system in vivo. To address this, in the present study, Walker 256 cells were injected into a tibial cavity in rats to establish a bone cancer pain model. Recombinant rat β-endorphin (β-EP; 50 µg/kg) and plant-derived morphine (10 mg/kg) were administered by intraperitoneal injection and the analgesic effects were compared. In addition, the effects of the opioids on cellular immune function, specifically T lymphocyte proliferation, natural killer (NK) cell cytotoxicity and the levels of T cell subgroups in the bone cancer pain models were examined, and the differences between the effects on cellular immune function were compared between the heterogenous and homogenous opioid treatment groups. The aim of this study was to provide scientific evidence useful in the development of human opioids to treat cancer pain.
Materials and methods
Animals
A total of 40 adult female Sprague-Dawley (SD) rats weighing (150–170 g; Shanghai SLAC Laboratory Animal Co., Ltd, Shanghai, China) and 10 female SD rats (weight, 70–80 g; Shanghai SLAC Laboratory Animal Co., Ltd.) were raised in a 12-h light/dark cycle with access to plentiful amounts of food and water. They were housed five per cage and were acclimatized for 1 week prior to behavioral studies. Efforts were made to minimize animal discomfort and reduce the numbers of animals used. The animal protocols were approved by the Animal Ethics Committee at Zhejiang Chinese Medical University (Hangzhou, China).
Surgery
Walker 256 cells (1×107; The Cell Bank of Type Culture Collection of Chinese Academy of Sciences, Shanghai, China) were administered by intraperitoneal injection into the abdominal cavity of juvenile rats (70–80 g). After 7 days, ascites were generated in the peritoneal cavity and carcinoma cells were harvested through sterile syringes. The percentage of cellular activity was checked to ensure that it was >95%, as measured using a TC10™ Automated Cell Counter (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Female SD rats were anesthetized by the administration of 10% chloral hydrate (0.35 ml/100 g) intraperitoneally, and then placed in a supine position. The left leg of the rat was shaved and the skin sterilized with iodophor and 75% ethanol. A 1-cm rostro-caudal incision was then made in the skin in the upper half of the tibia. The tibia was carefully exposed with minimal damage to the muscle and blood vessels. A 21-gauge needle was inserted at the site of intercondylar eminence at a 30–45° angle and pierced 5 mm below the knee joint into the medullary cavity of the tibia. The needle was then removed and replaced with a 10-µl syringe (Hamilton Co., Bonaduz, Switzerland) containing the carcinoma cells (3×105) to be injected into the tibial cavity. The syringe was kept in position for 2 min prior to removal from the tibial cavity to prevent cells from leaking out along the injection hole. The injection site was quickly sealed using bone wax and the wound was closed with stitches. Penicillin (20,000 units, intramuscular injection) was given to avoid infection.
Rats of the sham surgery group were injected with the same volume of phosphate-buffered saline (PBS) into the tibial cavity, and the other protocols were the same as those used in the surgery group.
Experimental groups
The rats were separated randomly into four groups: i) Sham surgery group (n=10); ii) surgery group (n=10); iii) morphine group (n=10); and iv) β-EP group (n=10).
Paw withdrawal thresholds (PWTs)
The PWTs were observed at six time points: Baseline (prior to surgery), at 6 days after surgery and following 1, 3, 6, and 8 treatments (as described below). As in a previous study (17), rats were adapted to the new environment by being placed on a metal mesh table. A mechanical stimulus (force 0–50 g over a 20 sec time period) was delivered to the plantar surface of the left hind paw using a Dynamic Plantar Aesthesiometer (37450; Ugo Basile, Monvalle, Italy). When the animal withdrew its hind paw, the mechanical stimulus was automatically stopped, and the force at which the animal withdrew its paw was recorded as the PWT. Withdrawal responses were taken from four consecutive trials with ≥3 min between trials and averaged.
Administration of treatments
Immediately after finishing the measurement of PWTs on 6 day, rats in the morphine group were intraperitoneally injected once every other day with 10 mg/kg morphine hydrochloride injection (C81004-2; Northeast Pharmaceutical Group Shenyang No. 1 Pharmaceutical Co., Ltd., Shenyang, China) and rats in the β-EP group were injected intraperitoneally with 50 µg/kg β-EP (H-284; Bachem AG, Hauptstrasse, Switzerland) for 15 days, once every other day. Sham surgery and surgery groups did not received any treatment.
Body weight measurements
The body weights of the rats were measured at baseline, at 6 days after surgery and following 1, 3, 6, and 8 treatments. The increase in body weight was calculated as follows: Body weight growth rate (%) = measured value/basal value × 100.
Extraction of splenic monocytes
Rats were sacrificed by cervical dislocation after the last PWT had been measured. The dead rats were soaked into 75% alcohol, and then moved onto a super clean bench. The spleen was excised, soaked in RPMI-1640 medium, HEPES [RPMI-1640 supplemented with 10% fetal bovine serum (FBS), 10,000 U/ml penicillin G and 10,000 µg/ml streptomycin; Thermo Fisher Scientific, Inc., Waltham, MA, USA] for 20 min. The spleen was placed on a 200-mesh stainless steel screen, cut into pieces and then ground, using PBS (sterile) to keep the tissue moist during the whole experiment. The obtained cell suspension was combined with 3–5 volumes of red blood cell lysis buffer (Beyotime Institute of Biotechnology, Haimen, China), and mixed gently. After standing for 2 min, the suspension was centrifuged at room temperature for 10 min at 1,000 g, and the supernatant was discarded. The obtained cell suspension was combined with 5 volumes of PBS (sterile), mixed gently, then centrifuged at room temperature for 10 min at 1,000 × g, twice. Cells were suspended in RPMI-1640 (10% FBS, 100 U/ml penicillin G and 100 µg/ml streptomycin) after cleaning, and the concentration of the cell suspension was adjusted to 1×106/ml.
T lymphocyte proliferation assays
Splenic monocytes were seeded into 96-well plates at 2×105 cells/well in triplicate. For the test samples, 20 µl concanavalin A (ConA; 10 µg/ml; Sigma-Aldrich, St. Louis, MO, USA) was added to each well. For the control, 20 µl RPMI-1640 was added to three wells. The plates were incubated at 37°C for 72 h. Cell viability was assessed using Cell Counting kit-8 (CCK-8; Beyotime Institute of Biotechnology) and according to the manufacturer's protocol. WTS-8 (20 µl) was added to each well and plates were incubated at 37°C for 4 h. The absorbance of each sample was measured at 450 nm using a microtiter plate reader (SpectraMax M4; Molecular Devices, LLC, Sunnyvale, CA, USA). The reference wavelength was >650 nm. The T lymphocyte proliferation function was determined using the following equation: T lymphocyte activity (%) = absorbance of test sample/absorbance of control × 100.
NK cell cytotoxicity assays
YAC-1, a mouse lymphoma cell line, was purchased from Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences (No. TCM28; Shanghai, China). Cells were grown in suspension in a culture bottle (Corning Incorporated, Corning, NY, USA), with RPMI-1640 medium, HEPES (RPMI-1640 supplemented with 10% FBS, 100 U/ml penicillin G and 100 µg/ml streptomycin). Only cells in the exponential growth phase were used for cytotoxicity assays. The YAC-1 cells were used as sensitive target cells for the evaluation of NK cell cytotoxicity in vitro (18). Determination of NK cell function was implemented using an enzymatic colorimetric technique involving lactate dehydrogenase (LDH) release (LDH-cytotoxicity assay kit; BioVision, Inc., Milpitas, CA, USA).
Splenic monocytes as effector cells were incubated in RPMI-1640 medium, HEPES (RPMI-1640 supplemented with 10% FBS, 100 U/ml penicillin G and 100 µg/ml streptomycin). Viability of effector and target cells was determined by the trypan blue dye exclusion test prior to the cytotoxicity test to confirm that the viability was >95%. In the test sample, the effector cells at a concentration of 1×106 in 100 µl culture medium were mixed with 100 µl YAC-1 cells at a concentration of 2×104, resulting in an effector cell:target cell ratio of 50:1. As the background control, 200 µl medium/well was added to triplicate wells (the background value was subtracted from all other values). As the low control, 1×106 cells/well in 200 µl culture medium were added to triplicate wells. As the high control, 1×106 cells/well in 200 µl culture medium containing 1% Triton X-100 were added to triplicate wells. Each test sample and all controls were evaluated in triplicate in 96-micro-well plates, and incubated at 37°C in a thermostatic incubator with 5% CO2 for 4 h. The micro-well plates were centrifuged at 250 × g for 10 min, and the supernatant was isolated. The LDH reaction mixture was added and maintained for 30 min at room temperature with the absence of light. The absorbance was measured using the SpectraMax M4 reader at 490 nm and the percentage of cytotoxicity was determined using the following equation: Cytotoxicity (%) = [absorbance (test sample - background control - low control)]/[absorbance (high control - low control)] × 100.
Flow cytometry assay
Spleen cell suspensions were collected from the cell culture bottles, and were adjusted to a concentration of 1–5×106 cells/ml. Fluorescein isothiocyanate (FITC)-conjugated anti-rat CD3 monoclonal antibody (cat. no. E00051-1631; eBioscience, Inc., San Diego, CA, USA), allophycocyanin (APC)-conjugated anti-rat CD4 monoclonal antibody ((E07034-1632; eBioscience, Inc.) and phycoerythrin (PE)-conjugated anti-rat CD8a monoclonal antibody (cat. no. E01045-1633; eBioscience, Inc.) were used for T cell, helper T cell and cytotoxic T cell detection respectively. Spleen cell suspensions were added to the 100 µl paraformaldehyde solution and were incubated with the above-mentioned antibodies for 15 min at 4°C. The cells were then washed three times with PBS, and 500 µl PBS was added to resuspend the cells. CD3+, CD4+ and CD8a+ cell analysis was performed within the lymphocyte cell range. The data were analyzed using BD FACSCanto II software (BD Biosciences, Franklin Lakes, NJ, USA). PBS was substituted for the antibody to serve as the control. Monoclonal mouse IgG3 Isotype Control FITC (cat. no. E11772-1632; eBioscience, Inc.), mouse IgG2a K Isotype Control APC (cat. no. E11418-1633; eBioscience, Inc.) and mouse IgG1 K Isotype Control PE (cat. no. E11418-1633; eBioscience, Inc.) were used as the respective isotype controls.
Enzyme linked immunosorbent assay (ELISA) for plasma interleukin (IL)-2
Plasma was analyzed to determine IL-2 levels using a Quantikine® ELISA kit (R&D Systems Europe, Ltd., Abingdon, UK) according to manufacturer's protocol. All samples were run on one 96-well plate for each variable. Plasma IL-2 levels were measured using the SpectraMax M4 microplate reader. Each sample was measured in triplicate.
Statistical analysis
All data are expressed as the mean ± standard error of the mean. The PWTs were analyzed using a two-way analysis of covariance (ANOVA) with repeated measures. One-way ANOVA with post hoc multiple comparisons was applied to identify differences between experimental groups. P<0.05 was considered to indicate a statistically significant difference.
Results
Effects of opioids on PWTs in a rat model of bone cancer pain
As shown in Fig. 1, there were no differences of basal PWTs among the sham surgery, surgery, morphine and β-EP groups (P>0.05). Compared with the sham surgery group, the injection of Walker 256 cells into the tibial cavity in the surgery group induced a marked reduction of the PWT in the ipsilateral hind paw at 6 days (P<0.01). After receiving treatment 1, 3, 6 and 8 times, the PWTs of the β-EP and morphine group were markedly increased compared with those of the surgery group (P<0.01). The PWTs of the morphine group were the most increased, being higher than those of the β-EP group at any time of treatment (P<0.01).
Figure 1.
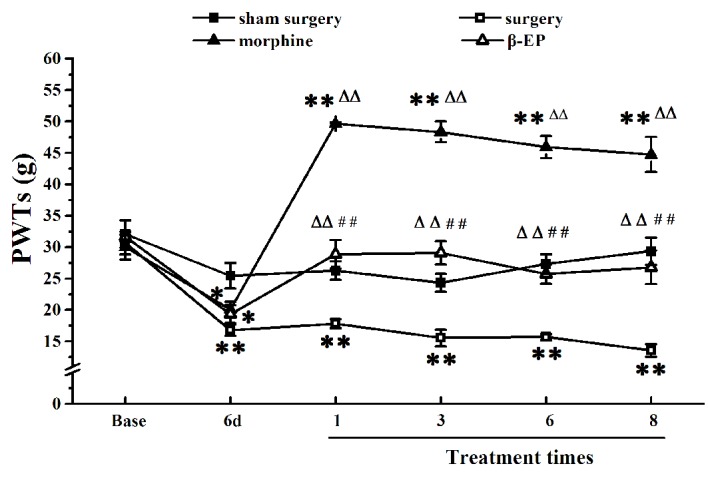
Effect of opioid treatment on the PWTs in an animal model of bone cancer pain. The analgesic effect of morphine on allodynia was stronger than that of β-EP. *P<0.05 and **P<0.01 vs. the sham surgery group; ΔΔP<0.01 vs. the surgery group; ••P<0.01 vs. the morphine group. n=8–10. PWTs, paw withdrawal thresholds; β-EP, β-endorphin; 6d, 6 day.
Effects of opioids on body weight increase in a rat model of bone cancer pain
The percentage increase in body weight of the rats in each group was determined. As shown in Fig. 2, the weight increase in the sham surgery, surgery, morphine and β-EP groups were not different prior to surgery, on 6 day after surgery, and following one treatment. After treatment 3 and 6 times, the percentage increase in body weight of the β-EP group was higher than that of the sham surgery (P<0.05), surgery (P<0.01) and morphine groups (P<0.01). After 8 treatments, the percentage increase of body weight in the β-EP group was higher than that of the morphine group only (P<0.05).
Figure 2.
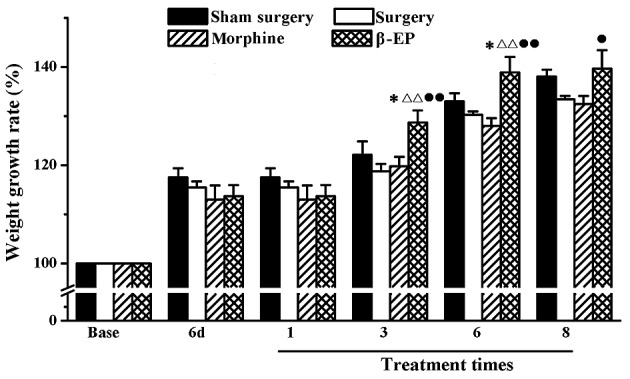
Effect of opioid treatment on percentage weight increase in a rat model of bone cancer pain. Intraperitoneal injection of β-EP increased the rate of weight gain of bone cancer pain model rats (surgery group), however, intraperitoneal morphine did not have a similar effect. *P<0.05 vs. the sham surgery group; ΔΔP<0.01 vs. the surgery group; •P<0.05 and ••P<0.01 vs. the morphine group. n=8–10. β-EP, β-endorphin; 6d, 6 day.
Effects of opioids on T cell proliferation in the splenic lymphocytes of a rat model of bone cancer pain
A Cell Counting kit-8 assay was conducted to observe the T cell growth rate of the spleen, with measurement of the optical density for splenic lymphocytes incubated with WST-8 for 4 h. As shown in Fig. 3, compared with the sham surgery group, the T cell growth rates of the surgery group and morphine group were significantly decreased (P<0.05 and P<0.01, respectively), and there was no significant difference between the surgery and morphine groups (P>0.05). Compared with morphine group, the T cell growth rate of the β-EP group was significantly increased (P<0.05).
Figure 3.
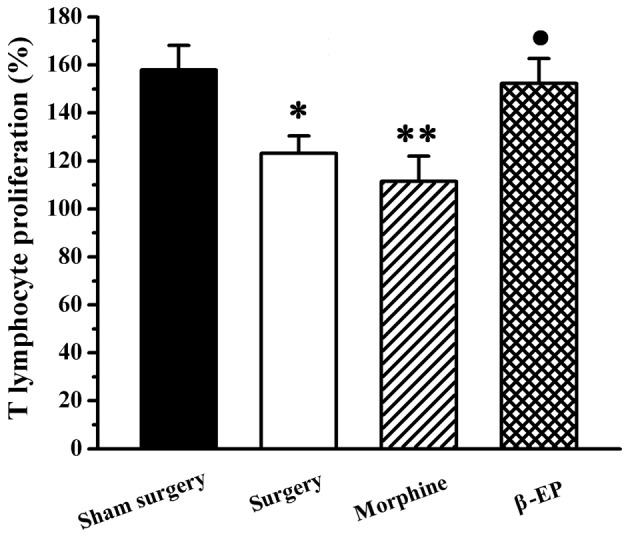
Effect of treatment with two different opioids on T lymphocyte proliferation in a rat model of bone cancer pain. T lymphocyte proliferation in the β-EP group was stronger than that of the morphine group. *P<0.05 and **P<0.01, vs. the sham surgery group; •P<0.05 vs. the morphine group. n=8–10. β-EP, β-endorphin.
Effects of opioids on NK cell cytotoxicity in splenic lymphocytes of a rat model of bone cancer pain
An assay involving LDH release was used to measure the cytotoxicity of NK cells in the spleen. As shown in Fig. 4, there were no significant differences of spleen NK cell cytotoxicity among the sham surgery, surgery and morphine groups. The NK cell cytotoxicity of the spleen in the β-EP group was stronger than that of the morphine group (P<0.05).
Figure 4.
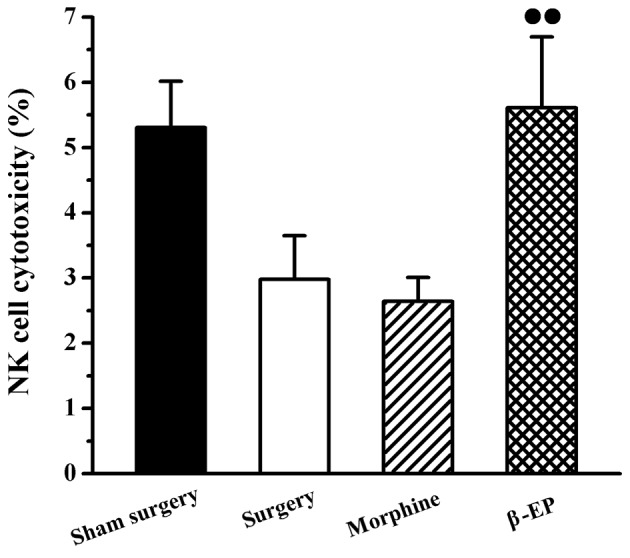
Effect of opioid treatment on NK cell cytotoxicity in a rat model of bone cancer pain. NK cell cytotoxicity of the β-EP group was stronger than that of the morphine group. ••P<0.01 vs. the morphine group. n=8–10. β-EP, β-endorphin; NK, natural killer.
Effects of opioids on levels of T cell subtypes (CD3+, CD4+ and CD8+ cells) in splenic lymphocytes of a rat model of bone cancer pain
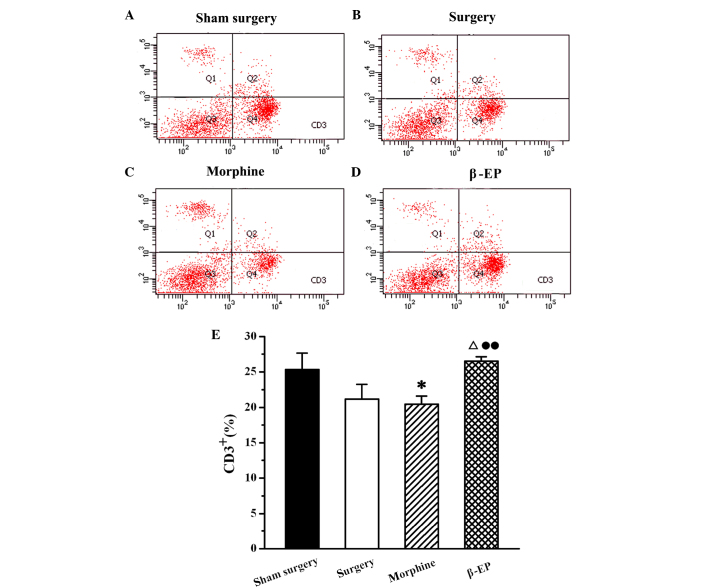
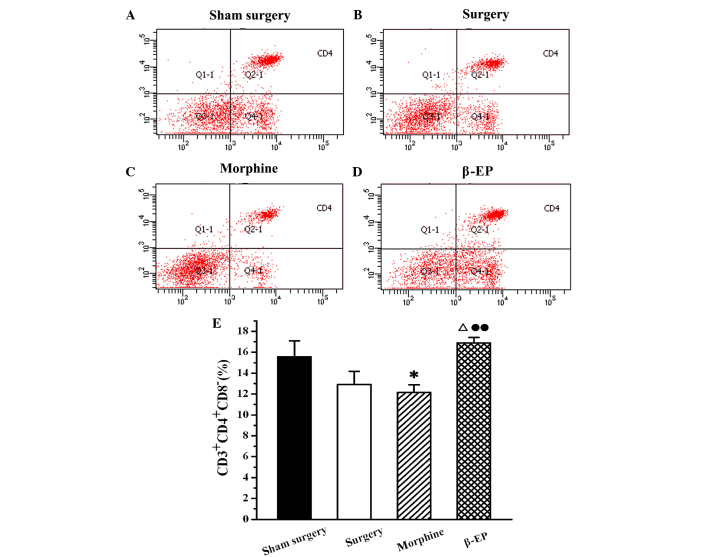
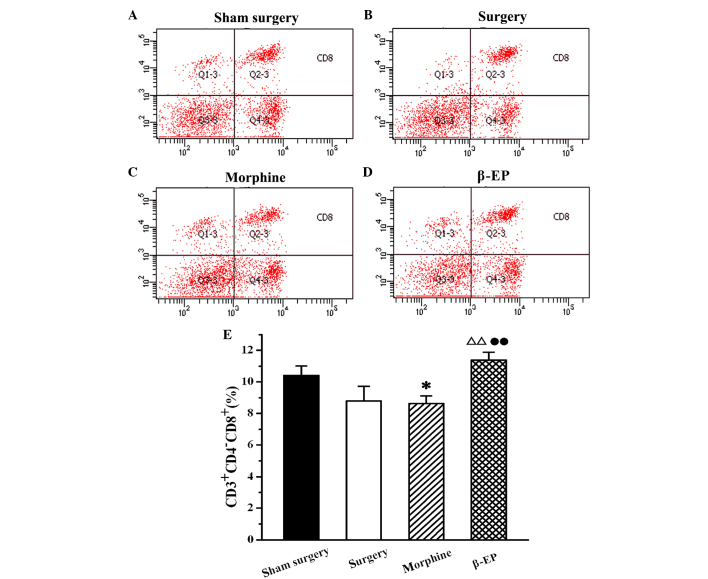
Flow cytometry was used to assay the content of CD3+, CD4+ and CD8+ cells in splenic lymphocytes among the sham surgery, surgery, morphine and β-EP groups. As shown in Fig. 5, the percentage of CD3+ cells in the splenocytes of the surgery group was decreased compared with that in the sham surgery group, but the reduction was not statistically significant. When the surgery and morphine groups were compared with the β-EP group, the differences in CD3+ cell percentages were significant (P<0.05 and P<0.01, respectively). The percentage of CD3+ cells in the β-EP group was greater than that in the surgery and morphine groups, and there was no significant difference between the surgery and morphine groups. The results for CD4+ and CD8+ percentages in the splenic lymphocytes were similar to those for CD3 (Figs. 6 and 7).
Figure 5.
Effect of opioid treatment on CD3+ cells in a rat model of bone cancer pain. Morphine treatment induced a reduction of the content of CD3+ cells. However, β-EP treatment caused a significant elevation of the CD3+ cell content. Flow cytometry shows CD3+ cells in the spleen of the (A) Sham surgery, (B) Surgery, (C) Morphine and (D) β-EP. (E) Quantification of (A-D). *P<0.05 vs. the sham surgery group; ΔP<0.05 vs. the surgery group; ••P<0.01 vs. the morphine group. n=8–10. β-EP, β-endorphin.
Figure 6.
Effect of opioid treatment on CD4+ cells in a rat model of bone cancer pain. β-EP treatment increased the content of CD4+ cells, but morphine treatment decreased them. Flow cytometry shows CD4+ cells in the spleen of the (A) Sham surgery, (B) Surgery, (C) Morphine and (D) β-EP. (E) Quantification of (A-D). *P<0.05 vs. the sham surgery group; ΔP<0.05 vs. the surgery group; ••P<0.01 vs. the morphine group. n=8–10. β-EP, β-endorphin.
Figure 7.
Effect of treatment with two different opioids on CD8+ cells in a rat model of bone cancer pain. β-EP treatment increased the content of CD8+ cells, but morphine treatment decreased them. Flow cytometry shows CD3+ cells in the spleen of the (A) Sham surgery, (B) Surgery, (C) Morphine and (D) β-EP. (E) Quantification of (A-D). *P<0.05 vs. the sham surgery group; ΔΔP<0.01 vs. the surgery group; ••P<0.01 vs. the morphine group. n=8–10. β-EP, β-endorphin.
Effects of opioids on plasma IL-2 levels in a rat model of bone cancer pain
No significant difference was found among the sham surgery, surgery, morphine and β-EP groups with regard to plasma IL-2 level (P>0.05; Fig. 8).
Figure 8.
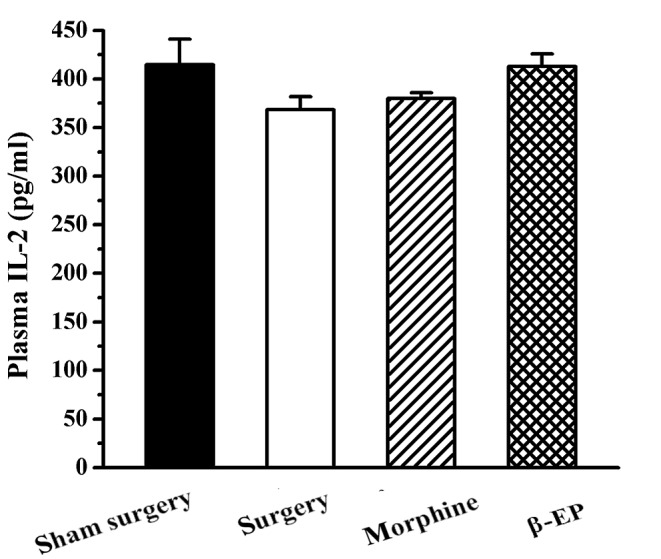
Effect of opioid treatment on plasma IL-2 levels in a rat model of bone cancer pain. No significant differences of plasma IL-2 level were observed among the sham surgery, surgery, morphine and β-EP groups. n=8–9. β-EP, β-endorphin; IL, interleukin.
Discussion
In the present study, the analgesic effect and immune modulating function of a homogenous opioid peptide (rat source β-EP) and heterogenous opioid (plant source morphine) were compared in a bone cancer pain model. It was found that both β-EP and morphine have good analgesic effects in this bone cancer pain model, and that the analgesia provided by of morphine was stronger than that of β-EP. Morphine treatment reduced the spleen T cell growth rate and the content of T cell subtypes (CD3+, CD4+ and CD8+ cells), whereas β-EP administration had the opposite effects on those indices as well as the NK cell cytotoxicity. Morphine and β-EP each had no effect on plasma IL-2 levels. These results demonstrate that the homogenous opioid had a positive effect on cancer pain.
Bone cancer pain in the most commonly used model of cancer pain, and many researchers have successfully established calcaneus, tibial and femur bone cancer pain models. Injection of Walker 256 cells into a tibial cavity can be used to study the mechanism of bone cancer pain. Mao-Ying et al reported that bone cancer developed from intra-tibial Walker 256 cells induced ambulatory pain and mechanical allodynia, and also reduced weight bearing, but that thermal hyperalgesia was not observed after Walker 256 cell inoculation (19). A previous study by the present team had similar results; it found that in a bone cancer model induced by the injection of Walker 256 cells into the tibial cavity, the rats had mechanical allodynia and spontaneous pain on days 4–22, and thermal hyperalgesia appeared in the intermediate stage (20). It was also found that the tibial cavity injection of Walker 256 cells in the bone cancer model induced a reduction in PWTs.
A number of studies have demonstrated that NK cells are able to kill tumor target cells in vitro and in vivo in animal models (21–23). Studies have shown that decreased NK cell activity is associated with the growth and development of a variety of cancers in humans (24) and animals (25), and NK cells protect against the metastatic spread of tumor cells (26). Multiple studies have provided evidence that morphine, a typical exogenous opiate, is involved in inhibiting the innate immune response (27). It has been reported that NK cell immune function is downregulated by morphine in vivo (28). NK cells are the first line of defense of the immune system, with a key role in the host defense against tumor cells (29). NK cells represent a unique subset of lymphocytes that have no restriction by major histocompatibility complex (MHC) antigens, are important in the initiation of tumor development and have the ability to lyse certain tumor cells without the requirement for prior immune sensitization of the host (30,31). Not all kinds of opioids share the same immunosuppressive effects. It has been found that β-EP, an endogenous opioid peptide, promotes innate immune function and reduces the incidence of cancer in rat models (32). β-EP can also increase peripheral NK cell activities; in vitro differentiated β-EP cells when transplanted into the paraventricular nucleus improved NK cell cytolytic function in model rats (33). In the present study, intraperitoneal morphine and β-EP administration had different effects on NK cell cytotoxic activity. The systemic injection of rat source β-EP, a homogenous opioid peptide, increased the NK cell cytotoxicity of the rats with bone cancer pain, while the systemic injection of morphine, a heterogenous opiate, did not have such an effect.
In the present study, in addition to reducing the immune function of NK cells, morphine treatment also inhibited T cell function. It has been suggested that the immune system is able to detect and reject incipient tumors, and that total T cells (CD3+), helper T cells (CD4+) and cytotoxic T cells (CD8+) play key roles in tumor immunology (34). CD8+ cells are traditionally considered to be the major mediators of an effective antitumor response by T cells. This is based on the pronounced cytotoxic activity of CD8+ T cells exhibited in vitro, and the observation that tumors capable of evading attack by CD8+ T cells may have altered or downregulated expression of MHC class I antigens (35–37). Furthermore, in a study involving a transgenic mouse with MHC class I-restricted T cell receptors, it was found that CD8+ T cells maintained an antitumor effect when CD4+ T cells were absent (38). Conflicting with these observations, other studies have indicated that the antitumor effects of CD8+ T cells alone are limited (39,40). CD4+ cells that were limited by MHC II of themselves recognized exogenous antigen peptides (about 13–17 amino acids long). Thus, the MHC class II status of tumor cells is of importance in the immune response of CD4+ T cells to tumors. However, a number of studies have indicated that CD4+ T cells contribute to the eradication of tumors even in the absence of CD8+ T cells (41,42). Cytotoxic CD4+ T cells have been shown to be capable of directly eliminating tumor cells that are MHC class II positive, in addition to indirectly killing tumor cells that lack MHC class II expression (43,44). In the present study, it was found that the systemic injection of rat-derived β-EP, a homogenous opioid peptide, increased CD3+, CD4+ and CD8+ T cell subtype expression in a rat model of bone cancer pain, while the systemic injection of morphine, a heterogenous opioid compound, reduced their expression.
In conclusion, morphine and β-EP exhibited good analgesic effects in the rat model of bone cancer pain, and the analgesia provided by morphine was stronger than that of β-EP. Morphine and β-EP administration in vivo have no significant effect on the secretion of IL-2 by T cells. With regard to T cell proliferation rate, the effects of the two different types of opioids differed; morphine suppressed T cell proliferation and β-EP increased it. The opioid compounds from different sources exhibited different effects on the adaptive cell immune; the homogenous opioid peptide β-EP increased the adaptive cell immune function.
Acknowledgements
This study was supported by the National Natural Science Fund of China (grant no. 81102643) and the State Administrative Bureau of Traditional Chinese Medicine (Acupuncture) of Key Subjects Construction Funding [grant no. (2009) 30].
References
- 1.Mercadante S, Fulfaro F. Management of painful bone metastases. Curr Opin Oncol. 2007;19:308–314. doi: 10.1097/CCO.0b013e3281214400. [DOI] [PubMed] [Google Scholar]
- 2.Marcus DA. Epidemiology of cancer pain. Curr Pain Headache Rep. 2011;15:231–234. doi: 10.1007/s11916-011-0208-0. [DOI] [PubMed] [Google Scholar]
- 3.Pignon T, Fernandez L, Ayasso S, Durand MA, Badinand D, Cowen D. Impact of radiation oncology practice on pain: A cross-sectional survey. Int J Radiat Oncol Biol Phys. 2004;60:1204–1210. doi: 10.1016/j.ijrobp.2004.04.040. [DOI] [PubMed] [Google Scholar]
- 4.Rietman JS, Dijkstra PU, Debreczeni R, Geertzen JH, Robinson DP, De Vries J. Impairments, disabilities and health related quality of life after treatment for breast cancer: A follow-up study 2.7 years after surgery. Disabil Rehabil. 2004;26:78–84. doi: 10.1080/09638280310001629642. [DOI] [PubMed] [Google Scholar]
- 5.Wilson KG, Graham ID, Viola RA, Chater S, de Faye BJ, Weaver LA, Lachance JA. Structured interview assessment of symptoms and concerns in palliative care. Can J Psychiatry. 2004;49:350–358. doi: 10.1177/070674370404900603. [DOI] [PubMed] [Google Scholar]
- 6.van den Beuken-van Everdingen MH, de Rijke JM, Kessels AG, Schouten HC, van Kleef M, Patijn J. Prevalence of pain in patients with cancer: A systematic review of the past 40 years. Ann Oncol. 2007;18:1437–1449. doi: 10.1093/annonc/mdm056. [DOI] [PubMed] [Google Scholar]
- 7.Honore P, Rogers SD, Schwei MJ, Salak-Johnson JL, Luger NM, Sabino MC, Clohisy DR, Mantyh PW. Murine models of inflammatory, neuropathic and cancer pain each generates a unique set of neurochemical changes in the spinal cord and sensory neurons. Neuroscience. 2000;98:585–598. doi: 10.1016/S0306-4522(00)00110-X. [DOI] [PubMed] [Google Scholar]
- 8.Schwab JM, Zhang Y, Kopp MA, Brommer B, Popovich PG. The paradox of chronic neuroinflammation, systemic immune suppression, autoimmunity after traumatic chronic spinal cord injury. Exp Neurol. 2014;258:121–129. doi: 10.1016/j.expneurol.2014.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen HY, Li SG, Cho WC, Zhang ZJ. The role of acupoint stimulation as an adjunct therapy for lung cancer: A systematic review and meta-analysis. BMC Complement Altern Med. 2013;13:362. doi: 10.1186/1472-6882-13-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sacerdote P. Opioid-induced immunosuppression. Curr Opin Support Palliat Care. 2008;2:14–18. doi: 10.1097/SPC.0b013e3282f5272e. [DOI] [PubMed] [Google Scholar]
- 11.Smith RJ, Rhodes K, Paciotti B, Kelly S, Perrone J, Meisel ZF. Patient perspectives of acute pain management in the era of the opioid epidemic. Ann Emerg Med. 2015;66:246–252. doi: 10.1016/j.annemergmed.2015.03.025. e1. [DOI] [PubMed] [Google Scholar]
- 12.Kostev K, Wartenberg F, Richter H, Reinwald M, Heilmaier C. Persistence with opioid treatment in Germany in patients suffering from chronic non-malignant or cancer pain. Curr Med Res Opin. 2015;31:1157–1163. doi: 10.1185/03007995.2015.1034095. [DOI] [PubMed] [Google Scholar]
- 13.Deyo RA, Von Korff M, Duhrkoop D. Opioids for low back pain. BMJ. 2015;350:g6380. doi: 10.1136/bmj.g6380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stefano GB, Fricchione G, Goumon Y, Esch T. Pain, immunity, opiate and opioid compounds and health. Med Sci Monit. 2005;11:MS47–MS53. [PubMed] [Google Scholar]
- 15.Vallejo R, de Leon-Casasola O, Benyamin R. Opioid therapy and immunosuppression: A review. Am J Ther. 2004;11:354–365. doi: 10.1097/01.mjt.0000132250.95650.85. [DOI] [PubMed] [Google Scholar]
- 16.Sacerdote P. Opioids and the immune system. Palliat Med. 2006;20(Suppl 1):S9–S15. [PubMed] [Google Scholar]
- 17.Fang JQ, Du JY, Liang Y, Fang JF. Intervention of electroacupuncture on spinal p38 MAPK/ATF-2/VR-1 pathway in treating inflammatory pain induced by CFA in rats. Mol Pain. 2013;9:13. doi: 10.1186/1744-8069-9-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poli A, Brons NH, Ammerlaan W, Michel T, Hentges F, Chekenya M, Zimmer J. Novel method for isolating untouched rat natural killer cells with higher purity compared with positive selection and fluorescence-activated cell sorting. Immunology. 2010;131:386–394. doi: 10.1111/j.1365-2567.2010.03312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mao-Ying QL, Zhao J, Dong ZQ, Wang J, Yu J, Yan MF, Zhang YQ, Wu GC, Wang YQ. A rat model of bone cancer pain induced by intra-tibia inoculation of Walker 256 mammary gland carcinoma cells. Biochem Biophys Res Commun. 2006;345:1292–1298. doi: 10.1016/j.bbrc.2006.04.186. [DOI] [PubMed] [Google Scholar]
- 20.Jun-Ying D, Yi L, Yi-Tian C, et al. Functional evaluation of spleen T lymphocytes in the rat model of Walker-256 bone cancer pain. Zhong Guo Bi Jiao Yi Xue Za Zhi. 2014;24:8-13MS. (In Chinese) [Google Scholar]
- 21.Wennerberg E, Kremer V, Childs R, Lundqvist A. CXCL10-induced migration of adoptively transferred human natural killer cells toward solid tumors causes regression of tumor growth in vivo. Cancer Immunol Immunother. 2015;64:225–235. doi: 10.1007/s00262-014-1629-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ardolino M, Azimi CS, Iannello A, Trevino TN, Horan L, Zhang L, Deng W, Ring AM, Fischer S, Garcia KC, Raulet DH. Cytokine therapy reverses NK cell anergy in MHC-deficient tumors. J Clin Invest. 2014;124:4781–4794. doi: 10.1172/JCI74337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zitvogel L, Kroemer G. Cytokines reinstate NK cell-mediated cancer immunosurveillance. J Clin Invest. 2014;124:4687–4689. doi: 10.1172/JCI78531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shereck E, Satwani P, Morris E, Cairo MS. Human natural killer cells in health and disease. Pediatr Blood Cancer. 2007;49:615–623. doi: 10.1002/pbc.21158. [DOI] [PubMed] [Google Scholar]
- 25.Saijo N, Ozaki A, Beppu Y, Takahashi K, Fujita J, Sasaki Y, Nomori H, Kimata M, Shimizu E, Hoshi A. Analysis of metastatic spread and growth of tumor cells in mice with depressed natural killer activity by anti-asialo GM1 antibody or anticancer agents. J Cancer Res Clin Oncol. 1984;107:157–163. doi: 10.1007/BF01032600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sacerdote P. Opioid-induced immunosuppression. Curr Opin Support Palliat Care. 2008;2:14–18. doi: 10.1097/SPC.0b013e3282f5272e. [DOI] [PubMed] [Google Scholar]
- 27.Chang MC, Fan SZ, Hsiao PN, Cheng WF, Sun WZ. Influence of morphine on host immunity. Acta Anaesthesiol Taiwan. 2011;49:105–108. doi: 10.1016/j.aat.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 28.Brown JN, Ortiz GM, Angel TE, Jacobs JM, Gritsenko M, Chan EY, Purdy DE, Murnane RD, Larsen K, Palermo RE, et al. Morphine produces immunosuppressive effects in nonhuman primates at the proteomic and cellular levels. Mol Cell Proteomics. 2012;11:605–618. doi: 10.1074/mcp.M111.016121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kheav VD, Busson M, Scieux C, de Latour R Peffault, Maki G, Haas P, Mazeron MC, Carmagnat M, Masson E, Xhaard A, et al. Favorable impact of natural killer cell reconstitution on chronic graft-versus-host disease and cytomegalovirus reactivation after allogeneic hematopoietic stem cell transplantation. Haematologica. 2014;99:1860–1867. doi: 10.3324/haematol.2014.108407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jacobs B, Ullrich E. The interaction of NK cells and dendritic cells in the tumor environment: how to enforce NK cell & DC action under immunosuppressive conditions? Curr Med Chem. 2012;19:1771–1779. doi: 10.2174/092986712800099857. [DOI] [PubMed] [Google Scholar]
- 31.Zhao JX. Advances of enhancing cytotoxicitiy of NK cells. Chinese Journal of Cancer Prevention & Treatment. 2009;16:1267–1271. [Google Scholar]
- 32.Schweizerhof M, Stösser S, Kurejova M, Njoo C, Gangadharan V, Agarwal N, Schmelz M, Bali KK, Michalski CW, Brugger S, et al. Hematopoietic colony-stimulating factors mediate tumor-nerve interactions and bone cancer pain. Nat Med. 2009;15:802–807. doi: 10.1038/nm.1976. [DOI] [PubMed] [Google Scholar]
- 33.Sarkar DK, Murugan S, Zhang C, Boyadjieva N. Regulation of cancer progression by β-endorphin neuron. Cancer Res. 2012;72:836–840. doi: 10.1158/0008-5472.CAN-11-3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koebel CM, Vermi W, Swann JB, Zerafa N, Rodig SJ, Old LJ, Smyth MJ, Schreiber RD. Adaptive immunity maintains occult cancer in an equilibrium state. Nature. 2007;450:903–907. doi: 10.1038/nature06309. [DOI] [PubMed] [Google Scholar]
- 35.Antony PA, Piccirillo CA, Akpinarli A, Finkelstein SE, Speiss PJ, Surman DR, Palmer DC, Chan CC, Klebanoff CA, Overwijk WW, et al. CD8+ T cell immunity against a tumor/self-antigen is augmented by CD4+ T helper cells and hindered by naturally occurring T regulatory cells. J Immunol. 2005;174:2591–2601. doi: 10.4049/jimmunol.174.5.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fujii H, Arakawa A, Utsumi D, Sumiyoshi S, Yamamoto Y, Kitoh A, Ono M, Matsumura Y, Kato M, Konishi K, et al. CD8+ tumor-infiltrating lymphocytes at primary sites as a possible prognostic factor of cutaneous angiosarcoma. Int J Cancer. 2014;134:2393–2402. doi: 10.1002/ijc.28581. [DOI] [PubMed] [Google Scholar]
- 37.Chen Y, Ayaru L, Mathew S, Morris E, Pereira SP, Behboudi S. Expansion of anti-mesothelin specific CD4+ and CD8+ T cell responses in patients with pancreatic carcinoma. PLoS One. 2014;9:e88133. doi: 10.1371/journal.pone.0088133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hanson HL, Donermeyer DL, Ikeda H, White JM, Shankaran V, Old LJ, Shiku H, Schreiber RD, Allen PM. Eradication of established tumors by CD8+ T cell adoptive immunotherapy. Immunity. 2000;13:265–276. doi: 10.1016/S1074-7613(00)00026-1. [DOI] [PubMed] [Google Scholar]
- 39.Arancibia-Carcamo CV, Osawa H, Arnett HA, Háskova Z, George AJ, Ono SJ, Ting JP, Streilein JW. A CIITA-independent pathway that promotes expression of endogenous rather than exogenous peptides in immune-privileged sites. Eur J Immunol. 2004;34:471–480. doi: 10.1002/eji.200324195. [DOI] [PubMed] [Google Scholar]
- 40.Boon T, Coulie PG, Van den Eynde BJ, van der Bruggen P. Human T cell responses against melanoma. Annu Rev Immunol. 2006;24:175–208. doi: 10.1146/annurev.immunol.24.021605.090733. [DOI] [PubMed] [Google Scholar]
- 41.Segal BM, Glass DD, Shevach EM. Cutting Edge: IL-10-producing CD4+ T cells mediate tumor rejection. J Immunol. 2002;168:1–4. doi: 10.4049/jimmunol.168.1.1. [DOI] [PubMed] [Google Scholar]
- 42.Quezada SA, Simpson TR, Peggs KS, Merghoub T, Vider J, Fan X, Blasberg R, Yagita H, Muranski P, Antony PA, et al. Tumor-reactive CD4(+) T cells develop cytotoxic activity and eradicate large established melanoma after transfer into lymphopenic hosts. J Exp Med. 2010;207:637–650. doi: 10.1084/jem.20091918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Noyan F, Lieke T, Taubert R, Sievers M, Dywicki J, Hapke M, Falk CS, Manns MP, Jaeckel E, Hardtke-Wolenski M. Naive tumour-specific CD4+ T cells were efficiently primed in acute lymphoblastic leukaemia. Scand J Immunol. 2014;80:161–168. doi: 10.1111/sji.12198. [DOI] [PubMed] [Google Scholar]
- 44.Snook AE, Magee MS, Schulz S, Waldman SA. Selective antigen-specific CD4(+) T-cell, but not CD8(+) T- or B-cell, tolerance corrupts cancer immunotherapy. Eur J Immunol. 2014;44:1956–1966. doi: 10.1002/eji.201444539. [DOI] [PMC free article] [PubMed] [Google Scholar]