Abstract
Introduction
The aim of this study was to investigate the effect of aerobic exercise (AE) in reducing bleomycin-induced fibrosis in mice of a Th2-dominant immune background (BALB/c).
Methods
BALB/c mice were distributed into: sedentary, control (CON), Exercise-only (EX), sedentary, bleomycin-treated (BLEO) and bleomycin-treated+exercised (BLEO+EX); (n = 8/group). Following treadmill adaptation, 15 days following a single, oro-tracheal administration of bleomycin (1.5U/kg), AE was performed 5 days/week, 60min/day for 4 weeks at moderate intensity (60% of maximum velocity reached during a physical test) and assessed for pulmonary inflammation and remodeling, and cytokine levels in bronchoalveolar lavage (BAL).
Results
At 45 days post injury, compared to BLEO, BLEO+EX demonstrated reduced collagen deposition in the airways (p<0.001) and also in the lung parenchyma (p<0.001). In BAL, a decreased number of total leukocytes (p<0.01), eosinophils (p<0.001), lymphocytes (p<0.01), macrophages (p<0.01), and neutrophils (p<0.01), as well as reduced pro-inflammatory cytokines (CXCL-1; p<0.01), (IL-1β; p<0.001), (IL-5; p<0.01), (IL-6; p<0.001), (IL-13; p<0.01) and pro-fibrotic growth factor IGF-1 (p<0.001) were observed. Anti-inflammatory cytokine IL-10 was increased (p<0.001).
Conclusion
AE attenuated bleomycin-induced collagen deposition, inflammation and cytokines accumulation in the lungs of mice with a predominately Th2-background suggesting that therapeutic AE (15–44 days post injury) attenuates the pro-inflammatory, Th2 immune response and fibrosis in the bleomycin model.
Introduction
Idiopathic pulmonary fibrosis (IPF) affects primarily men in the 5th decade of life at a rate of 4-12/100,000, and has a prognosis of 3–5 years following diagnoses [1]. Patients present dyspnea due to expanding fibrotic lesions caused by the accumulation of extracellular matrix proteins in the lung parenchyma, which gradually destroys alveoli leading to insufficient gas exchange. While surfactant protein folding defects are responsible for a small percentage of pulmonary fibrosis [2], most cases are idiopathic. Studies suggest that repetitive epithelial injury caused by environmental or endoplasmic reticulum (ER) stress, combined with an aberrant wound repair mechanism may be partly to blame but the exact mechanisms remain unknown [3,4]. While some drugs slightly reduce the rate of lung function decline, treatment options remain palliative [5]. Though the role of inflammation in IPF, specially from Th2 background, including the treatment of IPF with anti-inflammatories is hotly debated [6], the expression of Th2 cytokines, specifically IL-4 and IL-5, predominate over Th1 cytokine, IFN-gamma, as accredited to play an important role for progression of pulmonary fibrosis [7].
Aerobic exercise (AE) has been found to reduce Th2-mediated inflammation in murine allergic asthma models [8,9] and some clinical studies with allergic asthma patients show that exercise may be beneficial [10–12]. In the context of IPF, recent clinical studies have highlighted that while exercise does not cure IPF, pulmonary rehabilitation programs that incorporate physical training improve the patient’s six-minute walk scores, body composition, and quality of life [13–15] with some patients responding more positively than others.
Though bleomycin was originally used as an anti-cancer drug, given its DNA cleaving and anti-tumorigenic activity, it’s systemic use was repealed due to the occurrence of a lethal, bleomycin-induced pulmonary fibrosis side effect [16,17]. Oro-tracheal administration of bleomycin in rodents is currently the most utilized animal model of IPF as the lesions formed due to bleomycin are histologically similar to those observed in IPF [18]. However, the model is limited, as mice are able to repair the lesions over time. The rate of repair however is often used to indicate whether a particular molecule, pathway or treatment could potentially be beneficial to IPF patients. While two studies have shown that chronic, moderate AE attenuates bleomycin-induced fibrosis [19,20], this study uses mice of a Th2-dominant immune-responsive background (BALB/c) to test for the first time, the hypothesis that AE accelerates the resolution of bleomycin-induced airway fibrosis in part by attenuating the Th2 immune response.
Methods
We also state that all experiments were approved by the ethical committees of the University of Sao Paulo School of Medicine and Nove de Julho University (375/13). Experiments were carried out in accordance to the Guide for the Care and Use of Laboratory Animals, published by the U.S. National Institutes of Health (NIH publication no. 85–23, revised 1996). During the whole experiment, the animals did not present any alteration in health status, which was monitored 5x/week prior the physical training sessions. Also, no mice died during the experiments.
Animals and experimental groups
BALB/c, male mice (20–25 g) were obtained from the Central Animal Facility of School of Medicine of the University of Sao Paulo and distributed into Control (Con), Exercise (Ex), Bleomycin (Bleo) and Bleomycin+Exercise (Bleo+Ex) groups (n = 8/group).
Bleomycin-induced pulmonary fibrosis protocol
Bleomycin sulfate (1.5UI/kg; Meizler Biopharma, SP, Brazil) was administered oro-tracheally under anesthesia (ketamine 100mg/kg and xylazine 10mg/kg).
Exercise test and aerobic training protocol
The aerobic training protocol began 15 days after bleomycin administration. Adaptation to treadmill training was performed as previously described [8,21]. Following 3 days of adaptation (15 min/day, 25° incline, 0.2 Km/h), animals were submitted to a physical test (beginning at 0.2 Km/h, increasing 0.1 Km/h every 2.5 minutes) until animals were exhausted. Exhaustion was defined as failure to run following 10 gentle, mechanical stimuli [8,22]. Treadmill training was performed at 60% of the maximum velocity reached in the physical test during 4 weeks, 5x/week, 60 minutes/session. Twenty-four hours before euthanasia (ketamine 200mg/kg and xylazine 20mg/kg), the final physical test was performed [8,22].
Quantification of collagen fibers in the airways wall and in the lung parenchyma
Following transcardiac de-sanguination with phosphate buffered saline (PBS), left lobe were excised, fixed in 4% formalin solution, embedded in paraffin and sectioned in five micrometers slices. The serial sections were done in approximately 2mm deep into the lung tissue to assure that central to distal airways would be reached in the sections. Picro Sirius Red staining for collagen fibers was performed as previously described [9,22–24]. Collagen fibers were detectable via light microscopy (red staining) and the extent of collagen content was determined in the walls of five airways per mouse, in all 8 mice of each group and also in fifteen lung parenchyma fields per mouse (at 20X magnification) using the Image Pro Plus 4.5 software.
First, to assess collagen content within the airway wall, the airway area (defined as the area between the epithelial basement membrane and airway adventitia) was delimited; vessels were excluded. Next, a constant color threshold was calculated for the analyses of all samples by subtracting the difference of staining intensity between controls and fibrotic animals. This threshold was calculated by the software and was used consistently to measure the areas of red staining. Airway collagen content was calculated as the relation between the area of red staining within the airway wall and the total airway area [9,22–24] and results expressed as percentage. Airway collagen content in each animal was calculated as mean values of 5 airways.
For quantification of collagen content in the lung parenchyma, first the total area of lung parenchyma in each field was determined and also the area of air spaces. The parenchymal tissue area was determined in each field by subtracting the air spaces area from the total parenchymal area. Then, using a constant color threshold, the red staining area was determined within the lung parenchyma (15 fields per animal). Parenchyma collagen content in each field was then calculated as the relation between the parenchymal area of collagen fibers and the total parenchyma area in the field and expressed as percentage.
Collection and analyses of bronchial alveolar lavage (BAL) fluid
Following anesthetization, a cannula was inserted into the trachea and lungs were washed with 2x 500ml of PBS. BAL fluid was centrifuged at 900g for 10 minutes at 4°C. Supernatant was stored at -80°C for further ELISA experiments, and the cell pellet was resuspended in 1ml of PBS for total cell count (Neubauer chamber) and differential cell count analyses (cytospin preparation). Cytospins were stained with Diff Quick and differential cell counts were performed based on hematological criteria, considering 300 cells [25].
Cytokines Measurements in BAL Fluid
The levels ofIL-1β (432603; Biolegend, CA, USA), IL-5 (431203; Biolegend, CA, USA), IL-6 (431303; Biolegend, CA, USA), CXCL-1/KC (DY453; RD Systems, MN USA), IL-10 (431413; Biolegend, CA, USA), IL-13 (DY413; RD Systems, MN, USA) and IGF-1 (DY791; RD Systems, MN USA), were measured in BAL fluid using ELISA kits according to the manufactures’ recommendations.
Statistical analysis
The software Graph Pad Prism 5.0 was used to perform statistical analysis and also for graphs. The normality of the data was tested by Shapiro-Wilk test and the data was analyzed by one-way analysis of variance (ANOVA) followed by the Bonferroni post-hoc test. Significance levels were considered for p<0.05. Values were expressed as mean ± SD.
Results
Collagen deposition in the airways and in the lung parenchyma was reduced by aerobic exercise
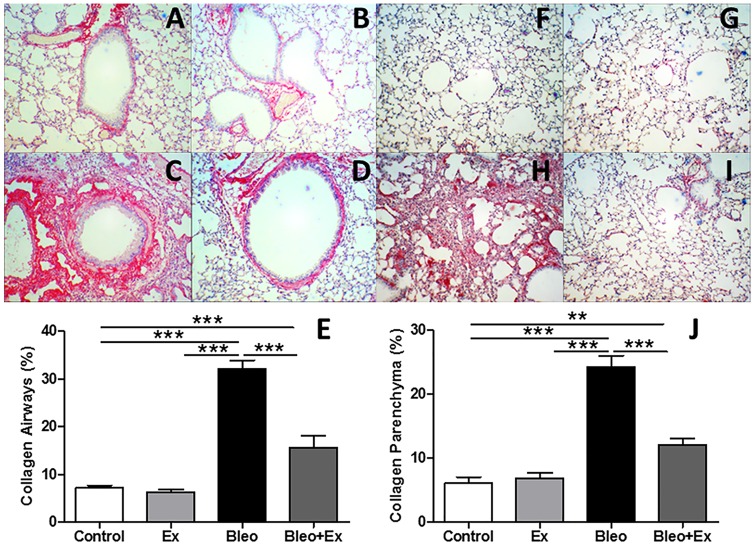
Picro Sirius Red staining (red indicates collagen expression) was used to visualize the extent of collagen deposition in the airways wall in sedentary (CON) animals (Fig 1A), exercise only (EX) (Fig 1B), sedentary bleomycin (BLEO) (Fig 1C), and exercised bleomycin animals (BLEO+EX) (Fig 1D). An increase in collagen fibers was observed in BLEO mice compared to CON and EX while BLEO+EX mice showed less collagen than the BLEO group. In summary, these observations (n = 8/group) were quantified by image analysis software (Fig 1E) and showed that aerobic exercise (AE) decreased the extent of bleomycin-induced collagen deposition. In addition, parenchymal remodeling was also evaluated in sedentary (CON) animals (Fig 1F), exercise only (EX) (Fig 1G), sedentary bleomycin (BLEO) (Fig 1H), and exercised bleomycin animals (BLEO+EX) (Fig 1I). An increase in collagen fibers in the lung parenchyma was observed in BLEO mice compared to CON and EX while BLEO+EX mice showed less collagen than the BLEO group. In summary, these observations (n = 8/group) were quantified by image analysis software (Fig 1J) and showed that aerobic exercise (AE) decreased the extent of bleomycin-induced collagen deposition.
Fig 1. Collagen deposition in the airways and in the lung parenchyma.
(A–D) Representative light microscopy images (20X) of Picro Sirius Red staining (red indicates collagen expression) in the airways wall of: Control, Ex, Bleo, Bleo+Ex groups, respectively) and in the (F–I) lung parenchyma; Control, Ex, Bleo, Bleo+Ex groups, respectively). (F) Quantitative analysis of collagen depositon in the airways and (J) in the lung parenchyma. These observations (n = 8/group) were quantified by image analysis software. ***p<0.001 and **p<0.01. Scale bars: 100um.
Aerobic exercise decreased bleomycin-induced inflammatory cells in BAL
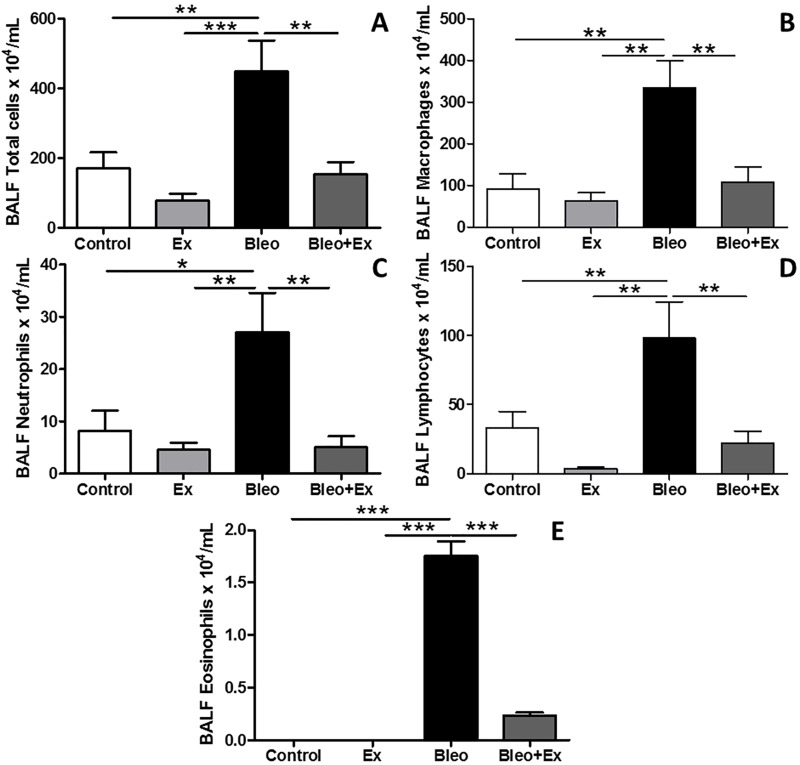
In Fig 2, total and differential cell counts of bronchoalveolar lavage (BAL) fluid (n = 8 animals/group) were analyzed. Total cell count was slightly reduced in EX group compared to CON animals (Fig 2A) but not to a statistically significant extent. An increase in the total number of cells in the BAL fluid of BLEO mice was detected (Fig 2A). In BLEO+EX, this number was reduced to the levels of the CON group (Fig 2A). Differential cell counts for macrophages (Fig 2B), neutrophils (Fig 2C) and lymphocytes (Fig 2D) showed the same pattern: compared to CON, EXE only mice showed a trend towards a decrease in immune cells, while cell counts were significantly augmented in BLEO only mice and reduced in BLEO+EX compared to BLEO only. Very few eosinophils were detected in CON and EX mice compared to BLEO mice (Fig 2B). Eosinophil number was decreased in BLEO+EX mice compared to BLEO mice (Fig 2E). In summary, AE reduced the number of bleomycin-induced inflammatory cells in BAL.
Fig 2. Total and differential cell counts of inflammatory cells in BAL.
Total and differential analyses of immune cells (n = 8/group) in bronchoalveolar lavage (BAL) fluid in Sedentary (CON), exercise-only (EX), sedentary bleomycin (BLEO), and exercised bleomycin animals (BLEO+EX). (A) Total cell count, (B) macrophages, (C) neutrophils, (D) lymphocytes, and (E) eosinophils. ***p<0.001; **p<0.01 and *p<0.05.
Aerobic exercise diminished the expression of bleomycin-induced pro-inflammatory cytokines and IGF-1 in BAL
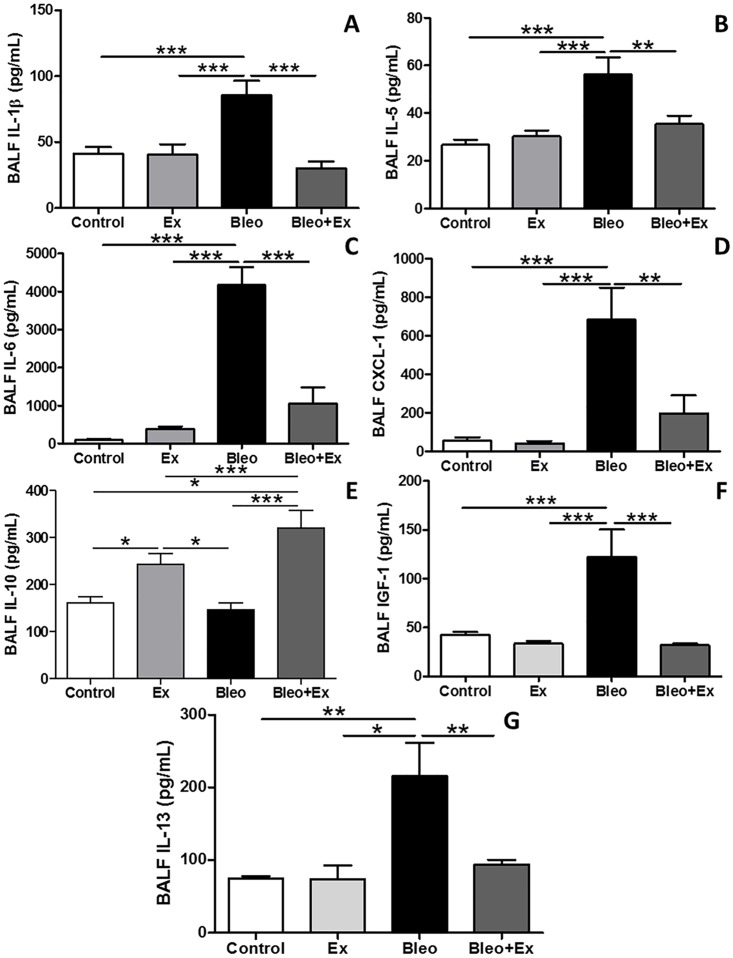
The pro-inflammatory cytokine IL-1β, was increased in BLEO and decreased in BLEO+EX (Fig 3A). Pro-inflammatory cytokines IL-5, IL-6, and IL-13 showed were also increased in BLEO and decreased in BLEO+EX (Fig 3B, 3C and 3G respectively). The neutrophil chemo-attractant CXCL-1/KC was increased in BLEO mice and reduced in BLEO+EX group (Fig 3D). Anti-inflammatory cytokine IL-10 was increased in EX compared to CON and further increased in BLEO+EX compared to all groups (Fig 3E). Insulin-like growth factor 1 (IGF-1), implicated as a potent, pro-fibrotic, fibroblast growth factor in the context of IPF and bleomycin-induced fibrosis, was increased in the BLEO group, and reduced in the BLEO+EX group (Fig 3F).
Fig 3. Cytokine and growth factor levels in BAL.
Cytokines and a growth factor in BAL were analyzed by ELISA (n = 8/group); Sedentary (CON), exercise-only (EX), sedentary bleomycin (BLEO), and exercised bleomycin animals (BLEO+EX). (A) IL-1β, (B) IL-5, (C) IL-6, (D) CXCL-1, (E) IL-10, (F) IGF-1 and (G) IL-13. ***p<0.001; **p<0.01 and *p<0.05.
Discussion
Using a Th2-dominant immune-responsive background (BALB/c), this study tested for the first time the hypothesis that AE accelerates the resolution of bleomycin-induced airway fibrosis in part by attenuating the Th2 response, as demonstrated by reduced levels of IL-5 and IL-13. While other strains of mice also mount a mild Th2 inflammatory cytokine response to bleomycin treatment, the Th2 reaction is significantly stronger in BALB/c mice than in other strains [26–28]. Though the role of Th2 cytokines in IPF is debated; as patients present with very little overt inflammation at the time of diagnosis, inflammation is suspected to play a role in the early stages of disease [23]. Though mere overexpression of IL-13 in mice lead to increased fibrosis [24], and a clinical study using anti-IL-13 therapy is currently underway (NCT01872689), it is important to note that so far, therapies attenuating either Th1 [29] or Th2 inflammation [30,31] do not generally lead to better overall outcomes for IPF patients [32]. Therefore, if increased Th2 inflammation indeed contributes to the onset of IPF, exercise-induced attenuation of Th2 inflammation may be more beneficial as a preventative therapy. However, the anti-fibrotic effects of exercise are not limited to the immune system as demonstrated in a recent study which implicated decreased 5-serotonin and Akt-signaling in the reduction of fibrosis [33]. In summary, further studies are needed in order to fully understand the extent and mechanisms by which aerobic exercise attenuates fibrosis.
In a more recent study, C57Bl/6 (Th1-dominant background) mice were subjected to the same treadmill protocol used in the present study (moderate intensity running at 60% maximal capacity, for 4 weeks, beginning at 15 days post-injury) [20]. C57Bl/6 (BLEO+EX) also showed decreased inflammation and fibrosis, and attenuated Akt and serotonin signaling compared to BLEO. Both Akt and serotonin pathways promote and sustain fibroblast growth. While a reduction in collagen fibers in the lung parenchyma (p<0.01) was reported in the C57Bl/6 study, they were reduced to a lesser extent than in this study (p<0.001). However, the accuracy of collagen content measurement reported by this study is limited due to the low proportion of actual 2D areas measured versus total lung area and the lack of stereological methods [34].
In addition, the study of Pereira et. al., also observed that AE reduced the influx of inflammatory cells, except for eosinophils, a classical inflammatory cell involved in allergic inflammation. Furthermore, pro-inflammatory cytokines: CXCL1/KC, IL-1beta, and IL-6 were reduced. However, the reduction in inflammation also occurred to a lesser extent than in BALB/c mice despite the use of the same treatment protocols. Pereira et. al., observed an increase in anti-inflammatory cytokine IL-10 due to AE as well, but also to a lesser extent than in BALB/c mice used in this study. These differences highlight not only the potent anti-inflammatory effect of AE in a Th2 dominant background vs. a Th1 dominant background but also the significant affect that heterogeneous immune compositions may have on IPF patient treatment responses to pulmonary rehabilitation therapy. As observed in recent, preliminary clinical studies with exercise and IPF patients, some individuals respond more positively than others to exercise [13,35]. Therefore, it may be prudent for future clinical studies that combine IPF and therapeutic exercise to examine the levels of Th2 cytokines in blood or BAL.
A precedent has already been set in the literature for the ability of exercise to dampen Th2-mediated immune responses, especially in the context of allergic asthma. For example, in ovalbumin-treated mice, just a single bout of exercise decreased ovalbumin-induced Th2 cytokines IL-5 and IL-13 [36]. Extended protocols have shown a reduction in eosinophils, CD3+ and CD4+ lymphocytes, CXCL-1, IL-4, IL-5, IL-6, IL-13, resulting in a decrease in airway remodelling, mucus synthesis, smooth muscle thickness, and airway resistance [25,37,38]. Therefore, not surprisingly, in the present study, pro-inflammatory IL-13 and IL-5, the neutrophil chemo-attractant CXCL-1, as well as the total number of eosinophils, lymphocytes and neutrophils, were all reduced in BLEO+EX compared to BLEO alone.
While eosinophilia and IL-4 expression may be expendable in rodent fibrosis models [39] IL-4 itself is neither particularly elevated in sera [40], nor in BAL of end-stage IPF patients [41]. However, receptors IL-4Rα, as well as IL-13 receptors: IL-13Rα2, and IL-13Rα1, are upregulated in fibroblastic foci of IPF patients [42]. IL-13, is a pro-fibrotic, Th2 cytokine that increases collagen synthesis in fibroblasts and its expression may correlate with the severity of IPF [43,44]. IL-13-/- mice are in fact protected from FITC-induced fibrosis [45]. Clinical studies using Tralokinumab, a human recombinant monoclonal antibody against IL-13 are in progress [46,47]. Therefore, attenuation of IL-13 by moderate AE may be an important therapeutic benefit of pulmonary rehabilitation for IPF patients.
This study also looked for the first time at the expression of insulin-like growth factor 1 (IGF-1), a pro-fibrotic growth factor, in the context of AE and bleomycin injury. Low intensity exercise has been shown to decrease IGF-1 levels in low-intensity exercisers after a period of six-weeks and is associated with decreased risk of cardiovascular disease [48]. A small trend towards a reduction was observed in EX compared to CON and a significant reduction was observed in BLEO+EX compared to BLEO. In non-IPF patients, IGF-1 localized exclusively to alveolar macrophages. In contrast, in IPF-patients, the domain of IGF-I expression is expanded to interstitial macrophages, alveolar epithelial cells, and ciliated columnar epithelial cells. IGF-1 expression by interstitial macrophages was found to correspond positively to the level of fibrosis in IPF patients [49]. In this model, AE not only reduced IGF-1 but also IL-4 and IL-13 which have been shown to stimulate IGF-1 in macrophages and myofibroblasts [50]. Furthermore, AE also resulted in a decrease in the number of macrophages in BLEO+EX lungs thus reducing a potential source of pro-fibrotic IGF-1.
In addition, this study also evaluated pro-inflammatory cytokine IL-1β expression as genetic over-expression models can cause a pulmonary fibrotic phenotype [51] similar to bleomycin-induced fibrosis. In both IL-1β and bleomycin-induced fibrosis in rodents, the development of fibrosis was IL-17 dependent [52]. Both IL-17 and IL-1β expression are increased in IPF patient BAL [53]. Interestingly, Wilson et. al., also demonstrated that IL-10 inhibits the pro-inflammatory, pro-fibrotic IL-23–IL-17A pathway rather than the IL-12–IFN-γ (Th1) axis. Although IL-17 was not measured in the current study, taken together, the increase in IL-10 in the BLEO+EX group likely had a strong anti-inflammatory and anti-fibrotic effect due to inhibition of IL-1β by IL-10. AE-dependent increases in IL-10 may also be responsible for the reduction of IL-6 in the BLEO+EX group as IL-6 is known to induce IL-10, and IL-10 in turn inhibits IL-6. While there was significant variability found in the expression of IL-1β and IL-6 in alveolar macrophages isolated from IPF patients [54] increased levels of these cytokines in BAL fluid are associated with IPF [53,55]. Interestingly, IPF patients with polymorphisms in IL-10 which may affect the efficiency of IL-10 translocation and signal peptide cleavage have been identified [56] which may contributing to a pro-inflammatory environment. Thus, in IPF patients with these IL-10 polymorphisms, it would be unlikely that an AE-induced increase in IL-10 alone could attenuate an aberrant Th2 immune response. Furthermore, though genetic IL-10 overexpression was found to attenuate inflammation and fibrosis in mice [57,58], lung-specific overexpression of IL-10 was found to cause fibrosis in a STAT-independent manner [59]. Therefore, a tight regulation of Th2 cytokines is likely important for fibrosis repair.
Conclusions
These results support the significance of the individual immune response in the context of a complicated, heterogeneous pulmonary disease: IPF. These findings suggest that future IPF and exercise studies should more closely examine not only the Th2 cytokine levels in patients who respond to exercise, but also additional potential mechanisms by which exercise may have a more positive outcome in particular individuals. Finally, although increased inflammation in IPF surgical lung biopsies predict poorer disease outcome [60] and many studies suggest that inflammation may play a role in acute exacerbations of IPF [61], an occurrence which often ends in death [31], corticosteroids are not an effective treatment for IPF patients [30]. Therefore, although moderate exercise decreased the Th2 immune response in this model, whether or not inhibition of Th2 cytokines was the most important exercise-induced anti-fibrotic effect remains unknown. Future studies should investigate alternative, beneficial exercise-induced modulations in growth factors pathways, hormones, apoptosis, and cell survival pathways, which may have a central role in exercise-mediated anti-fibrotic affects.
Supporting Information
Acknowledgments
This study was supported by Sao Paulo Research Foundation (FAPESP), grants 2012/15165-2 and 2012/51464-4. ASAS holds a PhD fellowship from CAPES. BM holds a postdoctoral fellowship from FAPESP (2014/23196-0). MCOJ holds a PhD fellowship from FAPESP (2014/14604-8).
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was supported by Sao Paulo Research Foundation (FAPESP), grants 2012/15165-2 and 2012/51464-4. ASAS holds a PhD fellowship from CAPES. BM holds a postdoctoral fellowship from FAPESP (2014/23196-0). MCOJ holds a PhD fellowship from FAPESP (2014/14604-8).
References
- 1.Nalysnyk L, Cid-Ruzafa J, Rotella P, Esser D (2012) Incidence and prevalence of idiopathic pulmonary fibrosis: review of the literature. Eur Respir Rev 21: 355–361. Available: http://err.ersjournals.com/content/21/126/355.full. Accessed 27 February 2013. 10.1183/09059180.00002512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Korfei M, Ruppert C, Mahavadi P, Henneke I, Markart P, et al. (2008) Epithelial endoplasmic reticulum stress and apoptosis in sporadic idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 178: 838–846. Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2566794&tool=pmcentrez&rendertype=abstract. Accessed 10 March 2013. 10.1164/rccm.200802-313OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glasser SW, Hagood JS, Wong S, Taype CA, Madala SK, et al. (2016) Mechanisms of Lung Fibrosis Resolution. Am J Pathol. Available: http://www.ncbi.nlm.nih.gov/pubmed/27021937. Accessed 7 April 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Günther A, Korfei M, Mahavadi P, von der Beck D, Ruppert C, et al. (2012) Unravelling the progressive pathophysiology of idiopathic pulmonary fibrosis. Eur Respir Rev 21: 152–160. Available: http://www.ncbi.nlm.nih.gov/pubmed/22654088. Accessed 11 March 2013. 10.1183/09059180.00001012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kreuter M, Bonella F, Wijsenbeek M, Maher TM, Spagnolo P (2015) Pharmacological Treatment of Idiopathic Pulmonary Fibrosis: Current Approaches, Unsolved Issues, and Future Perspectives. Biomed Res Int 2015: 329481 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4686637&tool=pmcentrez&rendertype=abstract. Accessed 7 April 2016. 10.1155/2015/329481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Behr J, Kolb M, Cox G (2009) Treating IPF—all or nothing? A PRO-CON debate. Respirology 14: 1072–1081. Available: http://www.ncbi.nlm.nih.gov/pubmed/19909457. Accessed 7 April 2016. 10.1111/j.1440-1843.2009.01645.x [DOI] [PubMed] [Google Scholar]
- 7.Wallace WA, Ramage EA, Lamb D, Howie SE (1995) A type 2 (Th2-like) pattern of immune response predominates in the pulmonary interstitium of patients with cryptogenic fibrosing alveolitis (CFA). Clin Exp Immunol 101: 436–441. Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1553239&tool=pmcentrez&rendertype=abstract. Accessed 6 April 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vieira RP, de Andrade VF, Duarte ACS, Dos Santos ABG, Mauad T, et al. (2008) Aerobic conditioning and allergic pulmonary inflammation in mice. II. Effects on lung vascular and parenchymal inflammation and remodeling. Am J Physiol Lung Cell Mol Physiol 295: L670–9. Available: http://www.ncbi.nlm.nih.gov/pubmed/18757522. Accessed 31 July 2015. 10.1152/ajplung.00465.2007 [DOI] [PubMed] [Google Scholar]
- 9.MacKenzie B, Andrade-Sousa AS, Oliveira-Junior MC, Assumpção-Neto E, Alves-Rangel MB, et al. (2016) Dendritic Cells Are Involved in the Effects of Exercise in a Model of Asthma. Med Sci Sport Exerc: 1 Available: http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00005768-900000000-97559. [DOI] [PubMed] [Google Scholar]
- 10.Fanelli A, Cabral ALB, Neder JA, Martins MA, Carvalho CRF (2007) Exercise training on disease control and quality of life in asthmatic children. Med Sci Sports Exerc 39: 1474–1480. Available: http://www.ncbi.nlm.nih.gov/pubmed/17805077. Accessed 22 January 2016. [DOI] [PubMed] [Google Scholar]
- 11.Moreira A, Delgado L, Haahtela T, Fonseca J, Moreira P, et al. (2008) Physical training does not increase allergic inflammation in asthmatic children. Eur Respir J 32: 1570–1575. Available: http://www.ncbi.nlm.nih.gov/pubmed/18684843. Accessed 4 December 2015. 10.1183/09031936.00171707 [DOI] [PubMed] [Google Scholar]
- 12.França-Pinto A, Mendes FAR, de Carvalho-Pinto RM, Agondi RC, Cukier A, et al. (2015) Aerobic training decreases bronchial hyperresponsiveness and systemic inflammation in patients with moderate or severe asthma: a randomised controlled trial. Thorax 70: 732–739. Available: http://www.ncbi.nlm.nih.gov/pubmed/26063507. Accessed 22 November 2015. 10.1136/thoraxjnl-2014-206070 [DOI] [PubMed] [Google Scholar]
- 13.Nishiyama O, Kondoh Y, Kimura T, Kato K, Kataoka K, et al. (2008) Effects of pulmonary rehabilitation in patients with idiopathic pulmonary fibrosis. Respirology 13: 394–399. Available: http://www.ncbi.nlm.nih.gov/pubmed/18399862. Accessed 17 March 2016. 10.1111/j.1440-1843.2007.01205.x [DOI] [PubMed] [Google Scholar]
- 14.Vainshelboim B, Fox BD, Oliveira J, Kramer MR (2016) Exercise training in idiopathic pulmonary fibrosis. Expert Rev Respir Med 10: 69–77. Available: http://www.ncbi.nlm.nih.gov/pubmed/26567878. Accessed 7 April 2016. 10.1586/17476348.2016.1121104 [DOI] [PubMed] [Google Scholar]
- 15.Kenn K, Gloeckl R, Behr J (2013) Pulmonary rehabilitation in patients with idiopathic pulmonary fibrosis—a review. Respiration 86: 89–99. Available: http://www.ncbi.nlm.nih.gov/pubmed/23942353. Accessed 17 March 2016. 10.1159/000354112 [DOI] [PubMed] [Google Scholar]
- 16.D’Andrea AD, Haseltine WA (1978) Sequence specific cleavage of DNA by the antitumor antibiotics neocarzinostatin and bleomycin. Proc Natl Acad Sci U S A 75: 3608–3612. Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=392834&tool=pmcentrez&rendertype=abstract. Accessed 10 March 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McLeod BF, Lawrence HJ, Smith DW, Vogt PJ, Gandara DR (1987) Fatal bleomycin toxicity from a low cumulative dose in a patient with renal insufficiency. Cancer 60: 2617–2620. Available: http://www.ncbi.nlm.nih.gov/pubmed/2445461. Accessed 10 March 2013. [DOI] [PubMed] [Google Scholar]
- 18.Degryse AL, Lawson WE (2011) Progress toward improving animal models for idiopathic pulmonary fibrosis. Am J Med Sci 341: 444–449. Available: /pmc/articles/PMC3103078/?report=abstract. Accessed 13 March 2013. 10.1097/MAJ.0b013e31821aa000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prata LO, Oliveira FMS, Ribeiro TMS, Almeida PWM, Cardoso JA, et al. (2012) Exercise attenuates pulmonary injury in mice with bleomycin-induced pulmonary fibrosis. Exp Biol Med (Maywood) 237: 873–883. Available: http://www.ncbi.nlm.nih.gov/pubmed/22903133. Accessed 23 September 2015. [DOI] [PubMed] [Google Scholar]
- 20.Pereira PR, Oliveira-Junior MC, MacKenzie B, Chiovatto JED, Matos Y, et al. (2016) Exercise Reduces Lung Fibrosis Involving Serotonin/Akt Signaling. Med Sci Sport Exerc: 1 Available: http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00005768-900000000-97579. [DOI] [PubMed] [Google Scholar]
- 21.Vieira RP, Duarte ACS, Claudino RC, Perini A, Santos ABG, et al. (2007) Creatine supplementation exacerbates allergic lung inflammation and airway remodeling in mice. Am J Respir Cell Mol Biol 37: 660–667. Available: http://www.ncbi.nlm.nih.gov/pubmed/17641295. Accessed 14 September 2015. [DOI] [PubMed] [Google Scholar]
- 22.Vieira RP, Claudino RC, Duarte ACS, Santos ABG, Perini A, et al. (2007) Aerobic exercise decreases chronic allergic lung inflammation and airway remodeling in mice. Am J Respir Crit Care Med 176: 871–877. Available: http://www.ncbi.nlm.nih.gov/pubmed/17690332. Accessed 3 August 2015. [DOI] [PubMed] [Google Scholar]
- 23.Keane MP (2008) The role of chemokines and cytokines in lung fibrosis. Eur Respir Rev 17: 151–156. Available: http://err.ersjournals.com/content/17/109/151. Accessed 4 April 2016. [Google Scholar]
- 24.Lee CG, Homer RJ, Zhu Z, Lanone S, Wang X, et al. (2001) Interleukin-13 Induces Tissue Fibrosis by Selectively Stimulating and Activating Transforming Growth Factor β 1. J Exp Med 194: 809–822. Available: http://www.jem.org/lookup/doi/10.1084/jem.194.6.809. Accessed 2 July 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brüggemann TR, Ávila LCM, Fortkamp B, Greiffo FR, Bobinski F, et al. (2015) Effects of Swimming on the Inflammatory and Redox Response in a Model of Allergic Asthma. Int J Sports Med. Available: http://www.ncbi.nlm.nih.gov/pubmed/25958938. Accessed 23 November 2015. [DOI] [PubMed] [Google Scholar]
- 26.Shin YS, Takeda K, Gelfand EW (2009) Understanding asthma using animal models. Allergy Asthma Immunol Res 1: 10–18. Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2831565&tool=pmcentrez&rendertype=abstract. Accessed 4 August 2015. 10.4168/aair.2009.1.1.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walkin L, Herrick SE, Summers A, Brenchley PE, Hoff CM, et al. (2013) The role of mouse strain differences in the susceptibility to fibrosis: a systematic review. Fibrogenesis Tissue Repair 6: 18 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3849643&tool=pmcentrez&rendertype=abstract. Accessed 27 January 2014. 10.1186/1755-1536-6-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Belperio JA, Dy M, Burdick MD, Xue YY, Li K, et al. (2012) Interaction of IL-13 and C10 in the Pathogenesis of Bleomycin-Induced Pulmonary Fibrosis. http://dx.doi.org/101165/rcmb2002-0009OC. [DOI] [PubMed] [Google Scholar]
- 29.Raghu G, Brown KK, Bradford WZ, Starko K, Noble PW, et al. (2004) A placebo-controlled trial of interferon gamma-1b in patients with idiopathic pulmonary fibrosis. N Engl J Med 350: 125–133. Available: http://www.ncbi.nlm.nih.gov/pubmed/14711911. Accessed 4 April 2016. [DOI] [PubMed] [Google Scholar]
- 30.Collard HR, Ryu JH, Douglas WW, Schwarz MI, Curran-Everett D, et al. (2004) Combined corticosteroid and cyclophosphamide therapy does not alter survival in idiopathic pulmonary fibrosis. Chest 125: 2169–2174. Available: http://www.ncbi.nlm.nih.gov/pubmed/15189938. Accessed 10 March 2013. [DOI] [PubMed] [Google Scholar]
- 31.Papiris SA, Manali ED, Kolilekas L, Kagouridis K, Triantafillidou C, et al. (2010) Clinical review: idiopathic pulmonary fibrosis acute exacerbations—unravelling Ariadne’s thread. Crit Care 14: 246 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3220036&tool=pmcentrez&rendertype=abstract. Accessed 11 April 2016. 10.1186/cc9241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bringardner BD, Baran CP, Eubank TD, Marsh CB (2008) The Role of Inflammation in the Pathogenesis of Idiopathic Pulmonary Fibrosis. Antioxid Redox Signal 10: 287–302. Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2737712&tool=pmcentrez&rendertype=abstract. Accessed 23 September 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pereira PR, Oliveira-Junior MC, MacKenzie B, Chiovatto JED, Matos Y, et al. (2016) Exercise Reduces Lung Fibrosis Involving Serotonin/Akt Signaling. Med Sci Sports Exerc. Available: http://www.ncbi.nlm.nih.gov/pubmed/26895395. Accessed 23 February 2016. [DOI] [PubMed] [Google Scholar]
- 34.Hsia CCW, Hyde DM, Ochs M, Weibel ER (2010) An official research policy statement of the American Thoracic Society/European Respiratory Society: Standards for quantitative assessment of lung structure. Am J Respir Crit Care Med 181: 394–418. 10.1164/rccm.200809-1522ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vainshelboim B, Fox BD, Kramer MR, Izhakian S, Gershman E, et al. (2016) Short-term Improvement in Physical Activity and Body Composition Following Supervised Exercise Training Program in Idiopathic Pulmonary Fibrosis. Arch Phys Med Rehabil. Available: http://www.ncbi.nlm.nih.gov/pubmed/26869288. Accessed 17 March 2016. [DOI] [PubMed] [Google Scholar]
- 36.Hewitt M, Creel A, Estell K, Davis IC, Schwiebert LM (2009) Acute exercise decreases airway inflammation, but not responsiveness, in an allergic asthma model. Am J Respir Cell Mol Biol 40: 83–89. Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2606949&tool=pmcentrez&rendertype=abstract. Accessed 14 September 2015. 10.1165/rcmb.2008-0172OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Silva RA, Vieira RP, Duarte ACS, Lopes FDTQS, Perini A, et al. (2010) Aerobic training reverses airway inflammation and remodelling in an asthma murine model. Eur Respir J 35: 994–1002. Available: http://www.ncbi.nlm.nih.gov/pubmed/19897558. Accessed 31 July 2015. 10.1183/09031936.00049509 [DOI] [PubMed] [Google Scholar]
- 38.Olivo CR, Vieira RP, Arantes-Costa FM, Perini A, Martins MA, et al. (2012) Effects of aerobic exercise on chronic allergic airway inflammation and remodeling in guinea pigs. Respir Physiol Neurobiol 182: 81–87. Available: http://www.ncbi.nlm.nih.gov/pubmed/22633937. Accessed 4 December 2015. 10.1016/j.resp.2012.05.004 [DOI] [PubMed] [Google Scholar]
- 39.Izbicki G, Breuer R (2003) IL-4 Is Not a Key Profibrotic Cytokine in Bleomycin-Induced Lung Fibrosis Model. J Immunol 171: 2767–2768. Available: http://www.jimmunol.org/content/171/6/2767.1.full. Accessed 7 April 2016. [DOI] [PubMed] [Google Scholar]
- 40.Tsoutsou PG, Gourgoulianis KI, Petinaki E, Germenis A, Tsoutsou AG, et al. (2006) Cytokine levels in the sera of patients with idiopathic pulmonary fibrosis. Respir Med 100: 938–945. Available: http://www.ncbi.nlm.nih.gov/pubmed/16236490. Accessed 7 April 2016. [DOI] [PubMed] [Google Scholar]
- 41.Taniguchi H, Katoh S, Kadota J, Matsubara Y, Fukushima K, et al. (2000) Interleukin 5 and granulocyte-macrophage colony-stimulating factor levels in bronchoalveolar lavage fluid in interstitial lung disease. Eur Respir J 16: 959–964. Available: http://www.ncbi.nlm.nih.gov/pubmed/11153599. Accessed 7 April 2016. [DOI] [PubMed] [Google Scholar]
- 42.Jakubzick C, Choi ES, Kunkel SL, Evanoff H, Martinez FJ, et al. (2004) Augmented pulmonary IL-4 and IL-13 receptor subunit expression in idiopathic interstitial pneumonia. J Clin Pathol 57: 477–486. Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1770295&tool=pmcentrez&rendertype=abstract. Accessed 11 April 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murray LA, Argentieri RL, Farrell FX, Bracht M, Sheng H, et al. (2008) Hyper-responsiveness of IPF/UIP fibroblasts: interplay between TGFbeta1, IL-13 and CCL2. Int J Biochem Cell Biol 40: 2174–2182. Available: http://www.ncbi.nlm.nih.gov/pubmed/18395486. Accessed 7 April 2016. 10.1016/j.biocel.2008.02.016 [DOI] [PubMed] [Google Scholar]
- 44.Park S-W, Ahn M-H, Jang HK, Jang AS, Kim D-J, et al. (2009) Interleukin-13 and its receptors in idiopathic interstitial pneumonia: clinical implications for lung function. J Korean Med Sci 24: 614–620. Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2719183&tool=pmcentrez&rendertype=abstract. Accessed 7 April 2016. 10.3346/jkms.2009.24.4.614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kolodsick JE, Toews GB, Jakubzick C, Hogaboam C, Moore TA, et al. (2004) Protection from fluorescein isothiocyanate-induced fibrosis in IL-13-deficient, but not IL-4-deficient, mice results from impaired collagen synthesis by fibroblasts. J Immunol 172: 4068–4076. Available: http://www.ncbi.nlm.nih.gov/pubmed/15034018. Accessed 7 April 2016. [DOI] [PubMed] [Google Scholar]
- 46.Murray LA, Zhang H, Oak SR, Coelho AL, Herath A, et al. (2014) Targeting interleukin-13 with tralokinumab attenuates lung fibrosis and epithelial damage in a humanized SCID idiopathic pulmonary fibrosis model. Am J Respir Cell Mol Biol 50: 985–994. Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4068948&tool=pmcentrez&rendertype=abstract. Accessed 7 April 2016. 10.1165/rcmb.2013-0342OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brightling CE, Chanez P, Leigh R, O’Byrne PM, Korn S, et al. (2015) Efficacy and safety of tralokinumab in patients with severe uncontrolled asthma: a randomised, double-blind, placebo-controlled, phase 2b trial. Lancet Respir Med 3: 692–701. Available: http://www.ncbi.nlm.nih.gov/pubmed/26231288. Accessed 7 April 2016. 10.1016/S2213-2600(15)00197-6 [DOI] [PubMed] [Google Scholar]
- 48.Nishida Y, Matsubara T, Tobina T, Shindo M, Tokuyama K, et al. (2010) Effect of low-intensity aerobic exercise on insulin-like growth factor-I and insulin-like growth factor-binding proteins in healthy men. Int J Endocrinol 2010 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2946576&tool=pmcentrez&rendertype=abstract. Accessed 7 April 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Uh ST, Inoue Y, King TE, Chan ED, Newman LS, et al. (1998) Morphometric analysis of insulin-like growth factor-I localization in lung tissues of patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 158: 1626–1635. Available: http://www.ncbi.nlm.nih.gov/pubmed/9817718. Accessed 4 April 2016. [DOI] [PubMed] [Google Scholar]
- 50.Wynes MW, Frankel SK, Riches DWH (2004) IL-4-induced macrophage-derived IGF-I protects myofibroblasts from apoptosis following growth factor withdrawal. J Leukoc Biol 76: 1019–1027. Available: http://www.ncbi.nlm.nih.gov/pubmed/15316031. Accessed 7 April 2016. [DOI] [PubMed] [Google Scholar]
- 51.Kolb M, Margetts PJ, Anthony DC, Pitossi F, Gauldie J (2001) Transient expression of IL-1beta induces acute lung injury and chronic repair leading to pulmonary fibrosis. J Clin Invest 107: 1529–1536. Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=200196&tool=pmcentrez&rendertype=abstract. Accessed 10 March 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wilson MS, Madala SK, Ramalingam TR, Gochuico BR, Rosas IO, et al. (2010) Bleomycin and IL-1beta-mediated pulmonary fibrosis is IL-17A dependent. J Exp Med 207: 535–552. Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2839145&tool=pmcentrez&rendertype=abstract. Accessed 7 April 2016. 10.1084/jem.20092121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Whittington HA, Armstrong L, Uppington KM, Millar AB (2004) Interleukin-22: a potential immunomodulatory molecule in the lung. Am J Respir Cell Mol Biol 31: 220–226. Available: http://www.ncbi.nlm.nih.gov/pubmed/15039135. Accessed 7 April 2016. [DOI] [PubMed] [Google Scholar]
- 54.Losa García JE, Rodríguez FM, Martín de Cabo MR, García Salgado MJ, Losada JP, et al. (1999) Evaluation of inflammatory cytokine secretion by human alveolar macrophages. Mediators Inflamm 8: 43–51. Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1781780&tool=pmcentrez&rendertype=abstract. Accessed 11 April 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Park CS, Chung SW, Ki SY, Lim GI, Uh ST, et al. (2000) Increased levels of interleukin-6 are associated with lymphocytosis in bronchoalveolar lavage fluids of idiopathic nonspecific interstitial pneumonia. Am J Respir Crit Care Med 162: 1162–1168. Available: http://www.atsjournals.org/doi/full/10.1164/ajrccm.162.3.9906007#.VwwIVXB95ow. Accessed 11 April 2016. [DOI] [PubMed] [Google Scholar]
- 56.Whittington HA, Freeburn RW, Godinho SIH, Egan J, Haider Y, et al. (2003) Analysis of an IL-10 polymorphism in idiopathic pulmonary fibrosis. Genes Immun 4: 258–264. Available: http://www.ncbi.nlm.nih.gov/pubmed/12761561. Accessed 29 March 2016. [DOI] [PubMed] [Google Scholar]
- 57.Kradin RL, Sakamoto H, Jain F, Zhao L-H, Hymowitz G, et al. (2004) IL-10 inhibits inflammation but does not affect fibrosis in the pulmonary response to bleomycin. Exp Mol Pathol 76: 205–211. Available: http://www.ncbi.nlm.nih.gov/pubmed/15126102. Accessed 29 March 2016. [DOI] [PubMed] [Google Scholar]
- 58.Nakagome K, Dohi M, Okunishi K, Tanaka R, Miyazaki J, et al. (2006) In vivo IL-10 gene delivery attenuates bleomycin induced pulmonary fibrosis by inhibiting the production and activation of TGF-beta in the lung. Thorax 61: 886–894. Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2104751&tool=pmcentrez&rendertype=abstract. Accessed 23 September 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee CG, Homer RJ, Cohn L, Link H, Jung S, et al. (2002) Transgenic overexpression of interleukin (IL)-10 in the lung causes mucus metaplasia, tissue inflammation, and airway remodeling via IL-13-dependent and -independent pathways. J Biol Chem 277: 35466–35474. Available: http://www.ncbi.nlm.nih.gov/pubmed/12107190. Accessed 11 April 2016. [DOI] [PubMed] [Google Scholar]
- 60.Parambil JG, Myers JL, Ryu JH (2005) Histopathologic features and outcome of patients with acute exacerbation of idiopathic pulmonary fibrosis undergoing surgical lung biopsy. Chest 128: 3310–3315. Available: http://www.ncbi.nlm.nih.gov/pubmed/16304277. Accessed 11 April 2016. [DOI] [PubMed] [Google Scholar]
- 61.Selman M, Carrillo G, Estrada A, Mejia M, Becerril C, et al. (2007) Accelerated variant of idiopathic pulmonary fibrosis: clinical behavior and gene expression pattern. PLoS One 2: e482 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1868965&tool=pmcentrez&rendertype=abstract. Accessed 11 April 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.