Abstract
Assessing glucocorticoid levels in free-ranging nonhuman primates provides a means to determine the social and environmental stress load for individuals. We investigated the effect of four proximate variables—reproductive state, season, male rank stability, and dominance rank—on the level of fecal glucocorticoids (cortisol metabolites) in eight adult female white-faced capuchin monkeys in Costa Rica. Reproductive state, season, and male rank stability significantly affected fecal glucocorticoids while female dominance rank did not. Cortisol levels were significantly higher in pregnant females as compared with lactating or other reproductive states. Cortisol levels were higher among females during the dry season compared with the wet season, suggesting a metabolic adaptation to maintain homeostasis in drier, hotter conditions. Although unfamiliar males present a greater infanticidal threat than do familiar ones, we found that females experienced higher glucocorticoid levels during male rank instability events, regardless of whether the alpha male role was taken over by a familiar or an unfamiliar male. Our findings provide important benchmark and comparative data for future studies on the variables that affect glucocorticoid levels in this species and other mammals.
Keywords: reproductive state, dry season, male stability, glucocorticoids, stress
INTRODUCTION
Studying the factors that affect glucocorticoid levels in wild primates can be difficult because there are many variables that cannot be controlled as they might be in captive studies. In the wild, significant social disruptions, such as changes to the dominance hierarchy, or infanticides can occur at the same time that individuals are challenged by the availability of resources and/or intragroup feeding competition; however, all these variables affect the way an individual metabolizes energy, and therefore affects their glucocorticoids levels. As such, it seems necessary to incorporate a variety of these stressors when studying the factors that affect glucocorticoids levels, which a few studies have recently done [e.g. Crockford et al., 2008; Emery Thompson et al., 2010; Engh et al., 2006; Foerster & Monfort, 2010; Weingrill et al., 2004].
During a stressful event, many hormones are stimulated and released into the blood stream that helps various body systems and organs cope with the incident. Cortisol is the major glucocorticoid hormone secreted in human and nonhuman primates [Sapolsky, 2002] and is produced by the adrenal glands via the hypothalamus–pituitary–adrenal (HPA) axis [Sapolsky, 2002]. The instant release of cortisol into the blood stream functions to quickly mobilize energy from storage to, for example, increase breathing rates and pain thresholds, whereas at the same time suppressing nonessential body functions, such as digestion, growth, and reproduction [Sapolsky, 2002]. Because the stress response is fundamentally an energetic response (i.e. energy is released for short term use while inhibiting long term energy storage), measuring the level of glucocorticoids can provide an indication of the energetic “stress” load an individual experiences [Emery Thompson et al., 2010].
White-faced capuchins (Cebus capucinus), living in Sector Santa Rosa (SSR), Costa Rica, live in a highly seasonal tropical dry forest and their behavioral ecology has been closely studied for many years [Fedigan & Jack, in press], which allows us to assess the effects of social stressors (e.g. rank and male stability) and environmental stressors temperature, resources), as well as the effects of reproduction on the level of excreted cortisol metabolites.
Reproduction
It is known that cortisol increases during pregnancy in humans [Zakar & Mitchell, 1998] and in many nonhuman primates [e.g. chacma baboons: Engh et al., 2006; Weingrill et al., 2004; mandrills, Mandrillus sphinx: Setchell et al., 2008]. The rise in cortisol during gestation is owing to the stimulation of the HPA axis in conjunction with fetal estrogen and the placental production of corticotrophin releasing hormone, which stimulates cortisol production in the mother [Smith & McLean, 1998; Zakar & Mitchell, 1998]. Additionally, the maternal HPA axis continues to respond to stressors by secreting cortisol, as necessary, to maintain homeostasis [Smith & McLean, 1998]. This rise in cortisol is not immediate, but increases over the course of gestation as the fetus and the placenta develop. For these reasons, lactating and nonpregnant/nonlactating females are thought to have lower cortisol levels than pregnant females; we predicted that pregnant white-faced capuchin females, during the latter half of pregnancy, will have higher fecal cortisol levels compared with nonpregnant females (i.e. lactating and females in other reproductive states).
Dominance Rank
The allostatic load model for social status [Goymann & Wingfield, 2004; McEwen & Steller, 1993] predicts that if the costs to high ranked individuals of attaining and maintaining their rank is the same as the costs to subordinate individuals of obtaining food and reproducing while coping with threats, then there should be little or no difference in cortisol levels between dominant and subordinate individuals. In white-faced capuchins, food competition is thought to be low because individuals disperse widely during foraging and consume a flexible diet of fruit, insects, and vertebrate prey [Fragaszy et al., 2004; Hall & Fedigan, 1997; Phillips, 1995]. Because we do not yet have evidence of significant costs associated with being either high and/or low ranking, we predicted that there would be no difference in fecal cortisol levels between dominant and subordinate females in this population of capuchins.
Social Instability
Changes in the social environment have been shown to increase glucocorticoid levels in primates. Cortisol levels increased in cotton-top tamarins when females were removed from their natal group and exposed to a novel male [Ziegler et al., 1995]. In baboons, when a potentially infanticidal male took over the male hierarchy, cortisol levels rose in females [Beehner et al., 2005], and in chimpanzees, the strongest predictor of increased cortisol levels among estrous females was the amount of aggression they received from males [Emery Thompson et al., 2010]. When new capuchin males move into groups, they usually do so aggressively [Jack & Fedigan, 2004]— wounding adults, killing the youngest infants, and creating an obvious disruption to group dynamics. We tested whether or not females’ fecal cortisol levels were affected during the change in the male hierarchy, and predicted that there would be no change in cortisol levels when the familiar male took over the hierarchy, but there would be an increase in cortisol levels among females when the unfamiliar (potentially infanticidal) male took over the alpha position.
Environmental
When homeothermic animals experience ambient temperatures above or below their thermal neutral zone, cortisol is secreted to activate appropriate coping mechanisms, such as sweating, panting, or shivering [Follenius et al., 1982; Randall et al., 2001]. For instance, colder temperatures were found to contribute to higher cortisol levels in chacma baboons [Weingrill et al., 2004] and geladas [Theropithecus gelada; Beehner & McCann, 2008]; extreme heat was found to be the cause of elevated cortisol levels, which contributed to reduced conception rates in buffalos (Bubalus bubalis) and disrupted circulating ovarian hormones in cottontopped tamarins [Megahed et al., 2008; Ziegler et al., 1995].
Capuchins in Santa Rosa live in an extreme environment with a distinct wet season (from mid-May to mid-December) and a distinct dry season (from mid-December to mid-May), during which temperatures can exceed 37°C and little-to-no precipitation falls. As the dry season progresses, water holes dry up and deciduous trees lose their leaves, reducing the availability of shade. Thus, groups have been found to alter both their activity patterns and their home ranges [Campos & Fedigan, 2009] during this season. Food availability is likely not a factor affecting capuchins because fruit abundance actually starts to increase during the dry season and peaks just when the rains begin [Carnegie et al., in press; Melin et al., submitted]. As such, we predicted that female capuchins would exhibit higher cortisol levels in the dry season, as a result of energetic responses to adapt to the drier and more exposed (i.e. hotter) conditions compared with the wet season, and that cortisol levels would increase over the course of the dry season as these conditions persisted.
The purpose of this study was to investigate some of the proximate factors that may affect fecal glucocorticoid levels in wild female capuchin monkeys (C. capucinus) in SSR, Costa Rica. Specifically, we predicted that cortisol levels among adult females would be affected by reproductive state, male stability, and season, but not by dominance rank.
METHODS
Study Site and Subjects
The study took place between June 2005 and November 2006 in SSR, the original sector of the Area de Conservación Guanacaste (ACG). SSR is located in northwestern Costa Rica (10.82049 Lat; −85.62813 Long), the boundaries of which encompass 108 km2 of dry deciduous forest fragments of semi-evergreen riparian and regenerating pasture land (“tropical dry forest”) [Janzen, 1983].
The eight females in our analyses ranged in age from 5 to 22 years of age and were part of two different habituated groups: Los Valles (four females) and Cerco de Piedra (CP: four females). Females start to show hormonal activity and ovarian cycling at 5 years of age [Carnegie et al., 2005] and can produce their first infant at 6 years; thus, we classified them as “adult” at 6 years old. Additionally, young female white-faced capuchins attain a similar rank to that of their mother soon after reaching reproductive maturity [~5–6 years; Bergstrom & Fedigan, 2010; Carnegie et al., 2005] and take only 1–2 years to attain an established position in the hierarchy, which they usually maintain throughout their lifetime in the group [Bergstrom & Fedigan, 2010; Perry, 1996]. All females’ ranks remained stable and linear over the duration of the study period. Capuchins give birth year round, but 44% of births occur between May and July and 80% occur between February and July [Carnegie et al., in press]. This population of capuchins has been studied continually since the early 1980’s, by Dr. LM Fedigan and her students, so detailed information is known about maternal relatedness and the demographics of each group. All behavioral data and fecal collection protocols complied with the Life and Environmental Science Animal Care Committee (University of Calgary; certification ]BIO8R-03), the American Society of Primatologists Principles for the Ethical Treatment of Nonhuman Primates, and met the legal requirements of Costa Rica and the ACG.
Behavioral and Fecal Sampling
Two capuchin groups were followed simultaneously by the first author and another observer from dawn to dusk (06:00–18:00 hr) for an average of 21 days per month (2969.60 contact hr). Ten-minute continuous focal follow samples were collected on each of the subject females to record agonistic interactions for calculation of dominance hierarchies [Altmann, 1974], resulting in 746.97 hr of focal animal data (range: 85.69–99.17 hr/female). We used a software program called “Behavior” (Syscan International Inc., Montreal, QC) to enter coded behavioral data into a PSION Workabout MX portable computer (Psion Teklogix Inc., Mississauga, ON).
Every day that behavioral data collection occurred, fecal samples were also collected from each of the subject females. We obtained 1,352 fecal samples from the eight females (mean = 169 samples/female; mean samples female/week = 2.4). Fecal samples were collected consistently between 06:00 and 00:00 h, although no evidence of diurnal variation in the level of excreted cortisol has been found (paired t-test: t(29) = 1.47, P = 0.152 [Carnegie, unpublished], and within 5 min of defecation, marked with the female ID, date, time, and placed on an ice pack until transferred to a freezer at end of day.
Hormone Extraction and Analysis
For the initial field extraction of the steroids, we followed the techniques described in Carnegie et al. [2005] [but also see, Strier & Ziegler, 1997]: 2.5 ml distilled water and 2.5 ml ethanol was added to 0.1 g wet weight fecal matter (homogenized sample) in a 15 ml centrifuge tube. The tube was vortexed for 10 min and then centrifuged for 10 min at 2000 × g (IEC Medilite centrifuge), and then 2 ml of the aliquot was pushed through a Prevail C-18 Solid Phase Extraction (SPE) cartridge (Alltech Associates, Inc., Deerfield, IL). The SPE cartridges were stored in sealed plastic bags at ambient temperatures and in a dark environment for between 259 and 747 days (first samples were collected in May 16, 2005; last samples were collected in November 30, 2006), until the immunoassays could be run at the Assay Services Unit at the National Primate Research Center in Madison (NPRC), University of Wisconsin-Madison.
Samples were analyzed between June and August, 2007, at the NPRC by S.D.C. Cortisol metabolites (F) were analyzed using an in-house enzyme immunoassay (EIA), using methods developed by Munro and Stabenfeldt [1984]; progesterone metabolites (P) were assayed using an EIA method described by Saltzman et al. [1994]; and estradiol metabolites (E2) were assayed using an in-house tritium-based radio immunoassay, following the methods described by Ziegler et al. [1987]. All assays used antibodies raised in rabbits and are competitive binding assays. Concentrations of metabolites were measured in ng/ml.
Mean intra- and interassay coefficient of variation (CV) for F were 7.4 and 13.8% for the low pool and 3.4 and 9.6% for the high pool (Table I). Mean percent accuracy (the degree to which the assay actually measures the steroid in a known pool) was high for the F assays (F: 115.48±2.27, N = 6). The percent bound values from the pooled samples paralleled the percent bound values of the standard curves for F, and there were no differences in the slopes (F: t(21) = 0.066, N = 7, P>0.05). Pooled samples are the quality control samples used to calculate the intra- and interassay CV in each assay, which are created from pooled fecal samples from several individuals.
TABLE I.
Assay Results for Ovarian Steroids and Glucocorticoid Analysis
| Intra-CV1 (%) |
Inter-CV (%) |
|||||
|---|---|---|---|---|---|---|
| Hormone | Low | High | Low | High | Accuracy2 | Parallelism3 |
| Progesterone | 7.7 | 3.2 | 27.7 | 11.5 | 107.25±2.11 | t(26) = 0.29, P>0.05 |
| Estradiol | 9.9 | 4.8 | 18 | 8.6 | 101.28±2.29 | t(42) = 1.74, P>0.05 |
| Cortisol | 7.4 | 13.8 | 3.4 | 9.6 | 115.48±2.27 | t(21) = 0.07, P>0.05 |
Coefficient of variation;
the percent of expected value vs. the observed value can vary over the eight points of the curve; the mean±SE can be anywhere from 85 to 115% to be accurate;
slopes do not differ.
Concentrations of fecal hormone metabolites can be affected by diet by altering the amount of fecal bulk and/or the concentration of excreted hormones [von der Ohe & Servheen, 2002; Wasser et al., 1993]. In this study, details on the type of foods consumed were not recorded nor were total fecal bolus weights measured. Fecal consistency (e.g. a sample with many seeds) varied throughout the day, depending on what was being eaten and regardless of the season. Additionally, water content has been found to have little effect on the concentration of excreted hormones [Shideler et al., 1993; Ziegler et al., 1996]. Data analysis consisted of the average concentration of metabolites from samples collected over 1 week (up to 3 samples/female/week) and values were analyzed relative to each other and never considered as a true measure of the concentration of metabolites excreted.
Interpretation of Fecal Hormone Profiles
The concentrations of P and E2 were used to determine the reproductive state of each female. The first day of the rise in P was considered the day of conception, because the LH surge is associated with the rise in plasma progesterone [Nagle & Denari, 1983], and minimal lag time has been found between plasma secretion and fecal excretion of metabolites [Carosi et al., 1999]. Gestation lengths were calculated as the time interval between the estimated date of conception and the parturition date and an average gestation length was calculated to be 157.83±8.13 days (N = 6; range 145–166 days; median = 160.5 days).
Definitions of Variables
The four categorical variables considered in this analysis are described below:
1. Reproductive state
The category “pregnant” was defined as starting from the day of estimated conception to the day before parturition (N = 7). The second category, “lactating,” was defined as starting from the day of parturition to 12 months (N = 7). The third category, which we called “other,” consisted of both cycling and noncycling females (N = 8). We combined these latter two groups because we had very few data points from females in a “cycling” state. One female from the eight did not cycle, and of the seven females that conceived, there were data gaps for two females during their presumed cycling periods. Three females conceived on their first ovulation, and therefore did not experience a complete ovarian cycle. Finally, there were three females who showed complete ovarian cycles, but less than 3 weeks of data were collected per female (on average). As such, we decided to combine noncycling females (postlactation to conception) with the few females that displayed some ovarian cycling activity.
2. Season
Seasons were split into two groups: “dry” and “wet.” We defined the dry season as starting when weekly mean rainfall was less than 10 mm per week and the wet season defined as starting when the mean weekly rainfall was consistently greater than 10 mm each week. In this 18-month study, there were 5 months of dry season (from December, 2005, to April, 2006) and 13 months of wet season (from June to November, 2005, and from May to November, 2006). Females in this data set were pregnant during both the dry and wet seasons, so reproductive state was not dependent on season.
3. Dominance rank
Rank was divided into two categories—high and low—with four females in each group. The “high” ranked females were the two top ranked females of each group and the “low” ranked females were the two bottom ranked females in each group. Dominance was based on the number of approach–retreat interactions (i.e. supplantations) and the direction of submissive signals received in dyadic interactions. Females were then arranged in a matrix based on the order that produced the fewest number of reversals.
4. Male rank stability
During this study, there were two periods of male rank instability in one of our study groups (N = 4 females), one in which a familiar male took over the alpha position and one in which an unfamiliar male took over the alpha position. In the first event, which occurred during the transition in seasons, the two top ranked males left the group and the third ranked male succeeded to the alpha position. During this event, three females were pregnant and one was noncycling. In the second event, which occurred during the wet season, a new male immigrated into the group, the resident alpha left, and the new male succeeded to the alpha position. During the second event, two females were lactating and two were cycling (one of the pregnant females from the first event lost her infant at birth). We compared weekly averages of cortisol for 5 weeks pre-event and 5 weeks postevent in both cases. Male rank stability remained constant in the second group (CP) for the duration of the study.
Statistical Analyses
We used linear mixed models (LMMs) to assess the effect of the four categorical variables on cortisol levels. This method was used because it accounts for repeated measures on the same individual over time. Individual identity was entered as the random factor, weekly averages of cortisol concentrations (log transformed to reach a normal distribution) were entered as the dependent variable, and the “focal week” was entered as the repeated measure. All variables of interest were entered as fixed effects in the models along with their interaction with reproductive state to test for any two-way interaction effects. We then used a backward stepwise method to remove variables that explained the least amount of variation until the remaining variables had P values of less than 0.05 and the explanatory power of the model no longer increased (i.e. the AIC score did not decrease) [see also, Crockford et al., 2008; Starling et al., 2010]. The covariance structure used was first-order autocorrelation (ar1). We used restricted maximum likelihood method for model estimation and Satterwaite’s F tests to gauge fixed effects because these provided the best fit model for the data (as indicated by the AIC score). The male rank replacements occurred in only one group, so additional LMMs (3) were run using a subgroup of females to analyze the effect of male stability (overall, in event 1 and 2) on fecal cortisol and any interaction with reproductive state and season. All tests were two-tailed and the analyses were performed using the MIXED method in PASW® 17.0 (SPSS Inc., Chicago, IL).
RESULTS
In the final models, reproductive state, season, and male stability significantly predicted fecal cortisol levels in female capuchins (Table II). Female rank did not significantly predict cortisol levels (although female rank neared significance) nor were there any two-way interaction effects between any of the main effects and reproductive state.
TABLE II.
Effects of Physiological, Environmental, and Social Factors on Fecal Cortisol Levels in Female Capuchins
| Variables | Num (df ) |
Dem (df ) | F | P |
|---|---|---|---|---|
| Model I | ||||
| ReproSt. | 2 | 154.110 | 48.088 | < 0.001* |
| Season | 1 | 160.586 | 20.064 | < 0.001* |
| Rank | 1 | 109.451 | 3.468 | 0.065 |
| ReproSt. * Season | 2 | 216.180 | 0.569 | 0.567 |
| ReproSt.*Rank | 2 | 143.383 | 0.253 | 0.777 |
| Model II (pregnant females excluded) | ||||
| Season | 1 | 115.292 | 16.548 | < 0.001* |
| Repro. State | 1 | 97.251 | 0.328 | 0.568 |
| Rank | 1 | 78.017 | 2.084 | 0.153 |
| Model III | ||||
| Male Stability Overall | 1 | 38.976 | 4.649 | 0.037* |
| ReproSt. *Male Stability | 2 | 39.036 | 0.209 | 0.081 |
| Model IV | ||||
| Male Stability One | 1 | 15.983 | 17.361 | 0.001* |
| ReproSt. *Male Stability One |
2 | 15.953 | 0.068 | 0.797 |
| Model V | ||||
| Male Stability Two | 1 | 19.146 | 6.876 | 0.017* |
| ReproSt. *Male Stability Two |
2 | 27.652 | 0.798 | 0.379 |
Focal ID was entered as the random variable; focal week was entered as the repeated measure. ReproSt., reproductive state; asterisk denotes significance at P<0.05; Num, numerator; Dem, denominator. “Male Stability One” is the first event with familiar male, “Male Stability Two” is the second event with the unfamiliar male. Effects included in the final models are shown in bold. F and P values shown are the values in model before that effect was removed (if applicable).
Reproductive State
Reproductive state had a significant effect on mean weekly fecal cortisol levels in female capuchins (LMM: F(2, 154.110) = 48.09, P<0.001) (Table II; Fig. 1). Pregnant females had significantly higher cortisol levels than lactating females (P<0.05) and females in other reproductive conditions (P<0.001), and there was no difference in fecal cortisol levels between lactating and females in other reproductive conditions (P = 0.385). Additionally, there was a significant relationship between the level of fecal cortisol and gestation phase—as gestation progressed, cortisol levels increased (LMM: F(23, 80.676) = 1.723, P = 0.039). On average, mean weekly cortisol levels (raw values) were about 23% higher in pregnant females than in lactating or among females in other reproductive states.
Fig. 1.
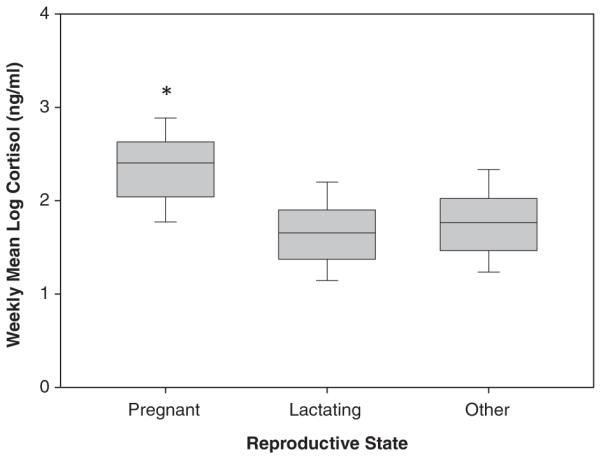
Fecal cortisol levels in female capuchins of different reproductive states. Pregnant females have significantly higher fecal cortisol levels than lactating or females in other reproductive states (weekly mean±SEM). There is no difference between lactating and females in other reproductive states.
Season
Season also had a significant effect on female fecal cortisol levels (LMM: F(1, 160.586) = 20.06, P<0.001) (Table II; Fig. 2). Females had higher cortisol levels (~25% higher) in the dry season compared with the wet season. However, there was no relationship between cortisol levels and length into the dry season.
Fig. 2.
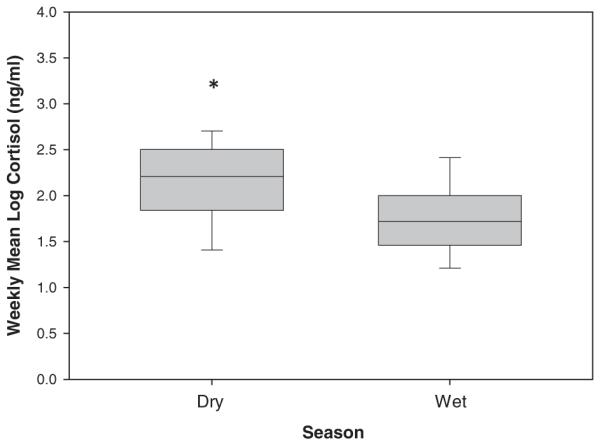
Female fecal cortisol levels between the dry and wet seasons (weekly means±SEM). Female capuchins had significantly higher fecal cortisol levels in the dry season compared with the wet season.
Male Rank Stability
Separate LMMs were conducted on a subgroup of females (N = 4) to investigate if fecal cortisol levels changed from a period of male rank stability to a period of male rank instability, and if there was any interaction with reproductive state and season. Overall, we found that male rank stability significantly affected fecal cortisol levels (LMM: F(1, 38.98) = 4.649, P = 0.037; Table II); females had significantly higher cortisol levels in the 5 weeks after a male rank replacement compared with the previous 5 weeks. We found no two-way interactions between male stability and reproductive state or season, meaning that all females showed an increase in fecal cortisol regardless of their reproductive state or the season.
In considering each event separately, we found that fecal cortisol levels increased significantly in both the male instability events (Fig. 3). In the first event, when the familiar male took over the alpha male position, females had significantly higher cortisol levels in the 5 weeks postevent compared with the 5 weeks pre-event (LMM: F(1, 15.983) = 17.361, P = 0.001; increase of 20%). Females also had significantly higher cortisol levels in the second event when the unfamiliar male took over the alpha position (F(1, 19.146) = 6.876, P = 0.017; increase of 18%).
Fig. 3.
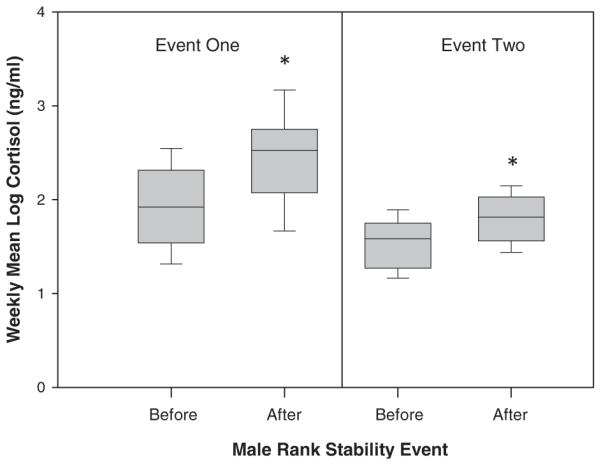
Female fecal cortisol levels during two male rank replacement events. Cortisol levels increased significantly after a familiar male (event one) and an unfamiliar male (event two) took over the alpha positions.
Female Dominance Rank
Female rank did not seem to have a significant effect on female fecal cortisol, and there were no two-way interaction effects between rank and reproductive state (P = 0.065). Because high levels of HPA activity during pregnancy could mask the effects of other variables (e.g. rank), we ran a separate LMM and excluded pregnant females. We found that there was no effect of rank on cortisol levels among lactating and females in other reproductive states (LMM: F(1, 78.02) = 2.084, P = 0.153; Table II, Model II). Regardless, we did find a trend toward higher cortisol levels among lower ranked females, suggesting that with a larger data set (i.e. <8 individuals) this trend may become significant.
DISCUSSION
Female capuchins show changes in glucocorticoid levels in response to certain reproductive and environmental variables. Additionally, changes in fecal glucocorticoid levels are related to social disruptions, such as male rank replacements, but are not necessarily related to female dominance rank.
As predicted, pregnant females were found to have significantly higher fecal cortisol levels than lactating females and females in other reproductive states, and cortisol levels increased as gestation progressed, with higher levels in the latter half of pregnancy compared with the first half. This result is not surprising and instead acts as a confirmation that pregnancies in white-faced capuchin females are also affected by the stimulus of the HPA axis and the fetal and placental production of cortisol, as documented in human and other nonhuman primates [e.g. Setchell et al., 2008; Zakar & Mitchell, 1998; Ziegler et al., 1995]. In this study, we were unable to compare fecal cortisol levels in pregnant and lactating females to levels in cycling females because of the very few complete ovarian cycles we observed. However, in other nonhuman primates, cortisol levels have been found to be high in pregnant females as compared with lactating and cycling females, and no difference has been found between lactating and cycling females [Engh et al., 2006; Setchell et al., 2008]. As such, we would predict the same to apply to white-faced capuchins, but further research with a greater number of reproductively active females will be needed to test this prediction.
Females had higher fecal cortisol levels during the dry season (from December to May) than they did in the wet season (from June to November), which supports our prediction that greater exposure to high temperatures combined with the lack of rainfall affects glucocorticoid levels in this species. However, fecal cortisol levels did not increase over time as the dry season progressed. During the dry, hot season, capuchins become noticeably more lethargic (i.e. they rest more), travel less, and modify their home ranges to accommodate more permanent water sources [Campos & Fedigan, 2009; see also Scholz & Kappeler, 2004]. Campos and Fedigan [2009] also reported that capuchins stick out their tongues (“tongue-out” behavior) more often in the dry season, which was suggested as a means to help them keep cool by evaporation off the tongue. These behavioral changes may be sufficient to maintain their body temperature within their thermal neutral zone during this season.
Lower food abundance during the dry season [Janson & Chapman, 2000] could instead be argued to be the more direct “cause” of the higher cortisol levels in the dry season. However, fruit abundance in Santa Rosa actually starts to increase in the dry season [Carnegie et al., in press; Melin et al., submitted] and, during the times of lower fruit abundance (late wet and early dry season), capuchins supplement their diet with insects and small vertebrates [Chapman & Fedigan, 1990]; thus, these may be a few reasons why cortisol levels did not increase as the dry season progressed. A more detailed study is needed to understand the full effect of food supply on cortisol levels, but our evidence to date suggests that exposure to higher temperatures and reduced rainfall affect cortisol levels more so than food abundance in this species.
Female capuchins had increased fecal cortisol levels after male rank replacement events regardless of the “type” of male that took over the alpha position. Although our sample size is admittedly small for this rarely occurring but influential event of resident male replacement, our finding supports the prediction that social disruptions can affect the glucocorticoid levels; but refutes our specific prediction that cortisol levels would change only when an unfamiliar, potentially infanticidal, male took over the male hierarchy. Infanticide in this species, and specifically in this population, occurs almost every time a new male (or males) aggressively takes over a group [Fedigan, 2003]. The familiar male in this study had been a member of the group for 2 years and attained the alpha position by default; therefore, the risk to females and their infants should have been minimal. Additionally, the youngest infant during the first event was already 1 year old and previous studies have shown that the mean age for infant victims of infanticide is 4.4 months [Fedigan, 2003]. In the second event, the male was new to the group and there were two 3-month old infants present. Even though he came in peacefully, he still posed a strong infanticidal risk as it was highly unlikely that he had sired any of the infants.
Our findings contrast with Setchell et al. [2008] and Beehner et al. [2005] who found no relationship between female cortisol levels and male rank instability when familiar males attained new ranks. However, Engh et al. [2006] suggested that there is a differential anticipation of risk by females who are in different reproductive states during male instability events—that is, lactating females with young, vulnerable offspring would likely experience more anxiety than females with older, independent offspring or no offspring. In fact, Beehner et al. [2005] did find that the more “vulnerable” females (i.e. those with young offspring or were pregnant) had higher cortisol levels when an unfamiliar male entered the group. Unfortunately, our data set lacked sufficient numbers of females in each of the reproductive states to test this hypothesis, but it would be worth investigating in future studies.
We found that fecal cortisol levels are less strongly related to dominance rank in this population of female capuchins compared with some other species [Sapolsky, 1982], which supports our prediction (and that of the allostatic load model) of the relationship between social status and stress. Goymann and Wingfield’s [2004] meta-analysis found that the concentration of cortisol is not necessarily determined by the absolute rank of an individual, but instead by the method needed to acquire and maintain that rank. A juvenile capuchin female tends to obtain the rank position of her mother soon after reaching sexual maturity and holds this position throughout her lifetime in the group [Bergstrom & Fedigan, 2010; Perry, 1996]; thus, it seems that the costs or the amount of stress experienced with rank positioning are minimal. However, stress associated with dominance or subordinance is not absent in this species, because dominant positions can be threatened when females immigrate or leave a group via emigration or death [Fragaszy et al., 2004; Jack & Fedigan, 2009], and positions are contested until a new rank order can be established. Also, rank-related disputes over clumped resources (e.g. fruit) can constitute a cost to subordinate females because higher ranked females tend to defend resources aggressively [Vogel, 2004]. These aspects of capuchin social organization, along with our findings, warrant further research into the relationship between female capuchin dominance rank and glucocorticoid levels.
Various physiological, environmental, and social variables can differentially affect the glucocorticoids levels of an individual. This study suggests that, in female white-faced capuchin monkeys, reproductive state, season, and male rank stability affect fecal glucocorticoid levels and that dominance rank may not. Our findings provide benchmark data for future studies on the variables that affect glucocorticoid levels in this species and important comparative data for future research among primates and other mammals.
ACKNOWLEDGMENTS
We are grateful to the Costa Rican National Park Service and the administrators of the ACG (especially R. Blanco-Seguro) for allowing us to work in Santa Rosa National Park. Many thanks to A. Biasin, G. McCabe, and A. Petrosoniak for their assistance in the field. Thanks to J. Addicott and T. Fung for statistical advice, and to D. Wittwer and S. Jacoris for assistance with laboratory methods. This manuscript was improved by the helpful comments of two anonymous reviewers. This research was funded by a National Sciences and Engineering Research of Canada (NSERCC) Ph.D. scholarship, an Alberta Ingenuity Scholarship, an International Primatology Society Research Grant, and a Sigma-Xi grant to S.D.C., an operating grant from NSERCC and Canada Research Chair funds to L.M.F., and a base grant (RR000167, the Wisconsin National Primate Research Center) to T.E.Z. All behavioral data and fecal collection protocols complied with the Life and Environmental Science Animal Care Committee (University of Calgary; certification no. BIO8R-03) and the Area de Conservación Guanacaste, Costa Rica.
Contract grant sponsors: National Sciences and Engineering Research of Canada (NSERCC) Ph.D. Scholarship; Alberta Ingenuity Scholarship; International Primatology Society Research Grant; Sigma-Xi; NSERCC; Canada Research Chair; The Wisconsin National Primate Research Center; Contract grant number: RR000167.
REFERENCES
- Altmann J. Observational study of behavior: sampling methods. Behaviour. 1974;49:227–265. doi: 10.1163/156853974x00534. [DOI] [PubMed] [Google Scholar]
- Beehner JC, McCann C. Seasonal and altitudinal effects on glucocorticoid metabolites in a wild primate (Theropithecus gelada) Physiology and Behavior. 2008;95:508–514. doi: 10.1016/j.physbeh.2008.07.022. [DOI] [PubMed] [Google Scholar]
- Beehner JC, Bergman TJ, Cheney DL, Seyfarth RM, Whittens PL. The effect of new alpha males on female stress in free-ranging baboons. Animal Behaviour. 2005;69:1211–1221. [Google Scholar]
- Bergstrom ML, Fedigan LM. Dominance among female white-faced capuchin monkeys (Cebus capucinus): hierarchical linearity, nepotism, strength, and stability. Behaviour. 2010;147:899–931. [Google Scholar]
- Campos F, Fedigan LM. Behavioral adaptations to heat stress and water scarcity in white-faced capuchins (Cebus capucinus) in Santa Rosa National Park, Costa Rica. American Journal of Physical Anthropology. 2009;138:101–111. doi: 10.1002/ajpa.20908. [DOI] [PubMed] [Google Scholar]
- Carnegie SD, Fedigan LM, Ziegler TE. Behavioral indicators of ovarian phase in white-faced capuchins (Cebus capucinus) American Journal of Primatology. 2005;67:51–68. doi: 10.1002/ajp.20169. [DOI] [PubMed] [Google Scholar]
- Carnegie SD, Fedigan LM, Melin AD. Reproductive seasonality in female capuchins (Cebus capucinus) in Santa Rosa (Area de Conservacion) Costa Rica. In press. [Google Scholar]
- Carosi M, Heistermann M, Visalberghi E. Display of proceptive behaviors in relation to urinary and fecal progestin levels over the ovarian cycle in female tufted capuchins. Hormones and Behavior. 1999;36:252–265. doi: 10.1006/hbeh.1999.1545. [DOI] [PubMed] [Google Scholar]
- Chapman CA, Fedigan LM. Dietary differences between neigbouring Cebus capucinus groups: local traditions, food availability or response to food profitability? Folia Primatologica. 1990;54:177–186. doi: 10.1159/000156442. [DOI] [PubMed] [Google Scholar]
- Crockford C, Wittig RM, Whitten P, Seyfarth RM, Cheney DL. Social stressors and coping mechanisms in wild female baboons (Papio hamadryas ursinus) Hormones and Behavior. 2008;53:254–265. doi: 10.1016/j.yhbeh.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Emery Thompson M, Muller MN, Kahlenberg SM, Wrangham RW. Dynamics of social and energetic stress in wild female chimpanzees. Hormones and Behavior. 2010;58:440–449. doi: 10.1016/j.yhbeh.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engh A, Beehner JC, Bergman T, Whitten P, Hoffmeier RR, Seyfarth RM, Cheney DL. Female hierarchy instability, male immigration and infanticide increase glucocorticoid levels in female chacma baboons. Animal Behaviour. 2006;71:1227–1237. [Google Scholar]
- Fedigan LM. Impact of male take-overs on infant deaths, births and conceptions in Cebus capucinus at Santa Rosa, Costa Rica. International Journal of Primatology. 2003;24:723–741. [Google Scholar]
- Fedigan LM, Jack K. Tracking monkeys in Santa Rosa: long-term lessons from a regenerating Costa Rican dry forest. In: Kappeler PM, Watts DP, editors. Long-term field studies of primates. Springer Press; New York: In press. [Google Scholar]
- Foerster S, Monfort S. Fecal glucocorticoids as indicators of metabolic stress in female Sykes’ monkeys (Cercopithecus mitis albogularis) Hormones and Behavior. 2010;58:685–697. doi: 10.1016/j.yhbeh.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Follenius M, Brandenberger G, Oyono S, Candas V. Cortisol as a sensitive index of heat-tolerance. Physiology and Behavior. 1982;29:509–513. doi: 10.1016/0031-9384(82)90274-8. [DOI] [PubMed] [Google Scholar]
- Fragaszy DM, Visalberghi E, Fedigan LM. The complete capuchin: the biology of the genus cebus. Cambridge University Press; Cambridge: 2004. [Google Scholar]
- Goymann W, Wingfield J. Allostatic load, social status and stress hormones: the costs of social status matters. Animal Behaviour. 2004;67:591–602. [Google Scholar]
- Hall C, Fedigan L. Spatial benefits afforded by high rank in white-faced capuchins (Cebus capucinus) Animal Behaviour. 1997;53:1069–1082. [Google Scholar]
- Jack K, Fedigan L. Male dispersal patterns in white-faced capuchins, Cebus capucinus Part 1: patterns and causes of natal emigration. Animal Behaviour. 2004;67:761–769. [Google Scholar]
- Jack K, Fedigan LM. Female dispersal in a female philopatric system, Cebus capucinus. Behaviour. 2009;146:437–470. [Google Scholar]
- Janson C, Chapman CA. Resources and primate community structure. In: Fleagle J, Janson C, Reed K, editors. Primate communities. Cambridge University; Cambridge: 2000. pp. 237–267. [Google Scholar]
- Janzen D. Costa Rican natural history. University of Chicago Press; Chicago: 1983. [Google Scholar]
- McEwen B, Steller E. Stress and the individual. Archives of Internal Medicine. 1993;153:2093–2101. [PubMed] [Google Scholar]
- Megahed G, Anwar M, Wasfy S, Hammadeh M. Influence of heat stress on the cortisol and oxidant-antioxidants balance during oestrous phase in buffalo-cows (Bubalus bubalis): thermo-protective role of antioxidant treatment. Reproduction in Domestic Animals. 2008;43:672–677. doi: 10.1111/j.1439-0531.2007.00968.x. [DOI] [PubMed] [Google Scholar]
- Munro C, Stabenfeldt G. Development of a microtitle plate enzyme immunoassay for the determination of progesterone. Journal of Endocrinology. 1984;101:41–49. doi: 10.1677/joe.0.1010041. [DOI] [PubMed] [Google Scholar]
- Nagle C, Denari J. The cebus monkey (Cebus apella) In: Hearn J, editor. Reproduction in New World primates. MTP Press; Lancaster: 1983. pp. 39–67. [Google Scholar]
- Perry S. Female-female social relationships in wild white-faced capuchin monkeys, Cebus capucinus. American Journal of Primatology. 1996;40:167–182. [Google Scholar]
- Phillips K. Resource patch size and flexible foraging in white-faced capuchins (Cebus capucinus) International Journal of Primatology. 1995;16:259–261. [Google Scholar]
- Randall D, Burggren W, French K. Eckert animal physiology: mechanisms and adaptations. W.H. Freeman & Company; New York: 2001. p. 752. [Google Scholar]
- Saltzman W, Schultz-Darken N, Scheffler G, Wegner F. Social and reproductive influences on plasma cortisol in female marmoset monkeys. Physiology and Behavior. 1994;56:801–810. doi: 10.1016/0031-9384(94)90246-1. [DOI] [PubMed] [Google Scholar]
- Sapolsky R. The endocrine stress-response a social status in the wild baboon. Hormones and Behavior. 1982;16:279–292. doi: 10.1016/0018-506x(82)90027-7. [DOI] [PubMed] [Google Scholar]
- Sapolsky R. Endocrinology of the stress response. In: Becker L, Breedlove S, Crews D, McCarthy M, editors. Behavioural endocrinology. The MIT Press; Cambridge MA: 2002. pp. 409–450. [Google Scholar]
- Scholz F, Kappeler PM. Effects of seasonal water scarcity on the ranging behavior of Eulemur fulvus rufus. International Journal of Primatology. 2004;25:599–613. [Google Scholar]
- Setchell JM, Smith T, Wickings EJ, Knapp LA. Factors affecting fecal glucocorticoid levels in semi-free-ranging female mandrills (Mandrillus sphinx) American Journal of Primatology. 2008;70:1–10. doi: 10.1002/ajp.20594. [DOI] [PubMed] [Google Scholar]
- Shideler SE, Ortuno AM, Moran FM, Moorman EA, Lasley BL. Simple extraction and enzyme immunoassays for estrogen and progesterone metabolites in the feces of Macaca fascilaris during the non-conceptive and conceptve ovarian cycles. Biology of Reproduction. 1993;48:1290–1298. doi: 10.1095/biolreprod48.6.1290. [DOI] [PubMed] [Google Scholar]
- Smith R, McLean M. Reproductive endocrinology and biology. JAI Press Inc.; Greenwich: 1998. The endocrinology of pregnancy; pp. 155–165. [Google Scholar]
- Starling AP, Charpentier MJE, Fitzpatrick C, Scordato ES, Drea CM. Seasonality, sociality, and reproduction: long-term stressors of ring-tailed lemurs (Lemur catta) Hormones and Behavior. 2010;57:76–85. doi: 10.1016/j.yhbeh.2009.09.016. [DOI] [PubMed] [Google Scholar]
- Strier K, Ziegler T. Behavioral and endocrine characteristics of the reproductive cycle in wild muriqui monkeys, Brachyteles arachnoides. American Journal of Physical Anthropology. 1997;42:299–310. doi: 10.1002/(SICI)1098-2345(1997)42:4<299::AID-AJP5>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Vogel E. The ecological basis of aggression in white-faced capuchins, Cebus capucinus, in a Costa Rican dry forest [Ph.D.] Stony Brook; NY: 2004. p. 256. [Google Scholar]
- von der Ohe CG, Servheen C. Measuring stress in mammals using fecal glucocorticoids: opportunities and challenges. Wildlife Society Bulletin. 2002;30:1215–1225. [Google Scholar]
- Wasser S, Thomas R, Nair P, Guidry C, Southers J, Lucas J, Wildt D, Monfort S. Effects of dietary fibre on faecal steroid measurements in baboons (Papio cynocephalus cynocephalus) Journal of Reproduction and Fertility. 1993;97:569–574. doi: 10.1530/jrf.0.0970569. [DOI] [PubMed] [Google Scholar]
- Weingrill T, Gray D, Barrett L, Henzi S. Fecal cortisol levels in free ranging female chacma baboons: relationship to dominance, reproductive state and environmental factors. Hormones and Behavior. 2004;45:259–269. doi: 10.1016/j.yhbeh.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Zakar T, Mitchell B. Reproductive endocrinology and biology. JAI Press; Greenwich: 1998. The endocrinology of late pregnancy and parturition; pp. 167–182. [Google Scholar]
- Ziegler T, Bridson W, Snowdon C, Eman S. Urinary gonadotropin and estrogen excretion during the postpartum estrus, conception, and pregnancy in the cotton-top tamarin (Saguinus oedipus oedipus) American Journal of Primatology. 1987;12:127–140. doi: 10.1002/ajp.1350120202. [DOI] [PubMed] [Google Scholar]
- Ziegler T, Scheffler G, Snowdon C. The relationship of cortisol levels to social environment and reproductive functioning in female cotton-top tamarins, Saguinus oedipus. Hormones and Behavior. 1995;29:407–424. doi: 10.1006/hbeh.1995.1028. [DOI] [PubMed] [Google Scholar]
- Ziegler T, Scheffler G, Wittwer D, Schultz-Darken N, Snowdon C, Abbott D. Metabolism of reproductive steroids during the ovarian cycle in two species of Calltrichids, Saguinus oedipus and Callithrix jacchus, and estimation of the ovualtory period from fecal steroids. Biology of Reproduction. 1996;54:91–99. doi: 10.1095/biolreprod54.1.91. [DOI] [PubMed] [Google Scholar]


