Abstract
Liver regeneration is a well-orchestrated process in the liver that allows mature hepatocytes to re-enter the cell cycle in order to proliferate and replace lost or damaged cells. This process is often impaired in fatty or diseased livers, leading to cirrhosis and other deleterious phenotypes. Prior research has established the role of the complement system and its effector proteins in the progression of liver regeneration; however, a detailed mechanistic understanding of the involvement of complement in regeneration is yet to be established. In this study, we have examined the role of the complement system during the priming phase of liver regeneration through a systems level analysis using a combination of transcriptomic and metabolomic measurements. More specifically, we have performed partial hepatectomy on mice with genetic deficiency in complement component 3, the major component of the complement cascade, and collected their livers at various time points. Based on our analysis, we show that the complement component 3 cascade activates c-fos and promotes the tumor necrosis factor-α signaling pathway, which then activates acute phase genes such as serum amyloid proteins and orosomucoids. The complement activation also regulates the efflux and the metabolism of cholesterol, an important metabolite for cell cycle and proliferation. Based on our systems level analysis, we provide an integrated model for the complement-induced priming phase of liver regeneration.
Introduction
The liver is the second largest organ in the body with the unique ability to regenerate itself from as little as 25% of its original mass (1, 2). This regenerative property is essential in supporting the ongoing central role of the liver in many biological processes such as complex homeostasis and compound detoxification. However, liver regeneration is impaired in diseased, aged, or fatty livers (3–7). Therefore, a detailed understanding of the mechanisms underlying liver regeneration is necessary for the development of therapies to enhance or restore the regenerative property of livers in which it is impaired. Despite many efforts to unravel the mechanisms of liver regeneration over the past decades, the complex mechanisms still have not been fully mapped (8).
Liver regeneration occurs in three main phases: priming, proliferation, and termination (9). During the priming phase of liver regeneration, which lasts approximately 4 hours in mice, the majority of quiescent hepatocytes rapidly re-enter the cell cycle with the help of various cytokines such as tumor necrosis factor α (TNFα) and interleukin 6 (IL-6) (9, 10). These cytokines are mainly produced by nearby liver macrophages, also known as Kupffer cells, that are activated by lipopolysaccharide (LPS) and complement effector proteins such as complement 3a (C3a) and complement 5a (C5a) (11). The proliferation phase occurs when hepatocytes undergo mitosis, with the expansion of the remaining liver occurring with the help of growth factors and metabolic signaling (12). In rodents, most of the increase in the liver mass occurs by day 3 after surgical resection of two-thirds of the liver, called partial hepatectomy (PHx), with complete mass restoration achieved by 5 to 7 days post-surgery (12, 13). Finally, the termination phase occurs with the regulation of various pathways that can alter the hepatic mass (14). Of the main stages of liver regeneration, the priming phase is of great interest because the normally quiescent hepatocytes re-enter the cell cycle to proliferate in response to an injury or an infection (12). A better understanding of this phase of liver regeneration can provide key insights into the complex pathways that activate cellular proliferation and facilitate interventions to accelerate liver regeneration.
The complement system is part of the innate immune system and has recently been introduced as one of the key regulators of liver regeneration (11, 15–17). In prior studies, complement knockout mice were used to demonstrate the importance of the complement effector proteins, C3a and C5a, in mediating successful liver regeneration (16, 17). Mice deficient in complement component 3 (C3) and complement component 5 (C5) genes exhibited severe damage to parenchyma, increased necrosis and hepatocyte degeneration, and higher mortality than wild-type controls during liver regeneration (16–18). Various inflammatory cytokines and pathways were significantly affected in the complement knockout mice, especially during the first few hours after PHx (17, 18). Based on these studies, an overall mechanism of complement-induced liver regeneration with a focus on intercellular signaling has been proposed (11).
In this study we report additional insights into liver regeneration through a comparative time course analysis of transcriptional and metabolic changes in wild-type and C3 knockout mice during the priming phase. Our analysis shows the role of acute phase proteins and the modulation of regeneration by sterol metabolism.
Materials and Methods
Animal studies
PHx experiments, according to the method of Higgins et.al. (19), were performed to remove two-thirds of the liver from 13 to 16 weeks-old male mice of either C57BL/6 wild-type (WT) or C3-/- (KO) origin. Only male mice were chosen because of the reported findings that suggest sex differences can exist in liver regeneration (20, 21). Age- and sex-matched WT and KO mice underwent sham surgery to serve as negative controls. These sham experiments were necessary because surgical inflammation can influence the expression of genes related to the cell cycle or proliferation, which would interfere with the analysis of the priming phase of liver regeneration that is dependent on these biological processes. After the PHx experiments, the remaining parts of the liver were collected after 0.5, 1, and 3 hours to capture temporal changes during the priming phase of liver regeneration. Livers were also collected from WT and KO mice without surgery at 0 hours in order to assess baseline difference. Whole livers, instead of isolated hepatocytes, were analyzed in the study because the experimental process of separation is not only difficult but also known to cause stress signals to the cells, which would complicate the early inflammatory signals. Three biological replicates were used for each PHx and sham experiment for each group to account for biological variability.
RNAseq experiments
From the collected livers at various time points, RNA was extracted and purified with a Qiagen Allprep Kit for cDNA synthesis and gene expression analysis. A pooling scheme was devised to reduce the number of samples to 21 (Supplementary Table 1). The pooled RNA samples were run on a Bioanalyzer to check for their RNA integrity. An Illumina Truseq cDNA library construction kit was used to synthesize cDNA libraries after poly-A selection and fragmentation. Then, the cDNA fragments were size-selected and inserted into the flow cell of a Hiseq 2000 at the Biogem facility of the University of California, San Diego (UCSD). The sequencing option was single-end 50-bp with 7 samples in each of the 3 lanes.
RNAseq pipeline
An RNAseq pipeline was developed with existing tools to effectively analyze the transcriptomic data. First, Omicsoft Sequence Aligner (OSA) was used to align the RNAseq reads to the mouse genome and the transcriptome with the default parameters (22). Then, HTseq-count was used to quantify the number of aligned reads associated with each gene and transcript (23). All uniquely mapped reads were counted, but ambiguous reads that mapped to several different genes were ignored. DESeq, a popular R package for RNAseq analysis, was used to derive the list of differentially expressed genes across paired conditions (24). Different combinations of parameters and filtering schemes were optimized to produce the highest number of statistically relevant genes. For example, the genes in the lowest 40% quantile of the total read counts across all samples were removed to increase the power of the statistical analysis while minimizing the removal of differentially regulated genes. The counted reads were normalized with the DEseq’s default method, while the variance was estimated for each condition. The statistical tests were performed on each of the 7 core groups between PHx and sham: KO vs. WT at 0 hours, KO PHx vs. KO sham at 0.5 hours, KO PHx vs. KO sham at 1 hour, KO PHx vs. KO sham at 3 hours, WT PHx vs. WT sham at 0.5 hours, WT PHx vs. WT sham at 1 hour, and WT PHx vs. WT sham at 3 hours. Once the p-values and the false-discovery rate (FDR) values from the Benjamini-Hochberg method were calculated for each gene in the core groups, the genes under either the FDR of 0.1 or the p-value of 0.05 were chosen for further analyses.
In addition to the statistical tests performed on PHx vs. sham, the sequential fold change cutoff of 1.5 was applied to create the list of differentially up- or down-regulated genes in the KO mice with respect to the WT. More specifically, the union of differentially regulated genes from the statistical tests in the KO and the WT livers was obtained at each time point. Then, from the combined list of genes, only the genes with the KO/WT fold change difference of 1.5 or higher were selected. Both the KO and the WT PHx expression levels were normalized with respect to sham from the previous DESeq analysis. As a result, the final list of differentially regulated genes, which also show significant differences with respect to sham, was generated. For the differential analysis at 0 hours, only the DESeq results with the p-value cutoff of 0.05 were utilized between the KO and the WT samples. The complete workflow to derive the list of differentially regulated genes at each time point is shown in the Supplementary Table 2.
qPCR experiments
The genes, c-fos, c-jun, and TIS21, which showed the greatest changes over time with respect to sham control during the priming phase of liver regeneration from the study by Su et. al., were selected for qPCR validation (10). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as the housekeeping gene. The primer sequences for the genes were obtained from the published studies and Primerbank, Harvard online database for PCR primers that have been validated for mouse genes (25, 26). The primer sequences are listed as follows: 5’-CCTTCGGATTCTCCGTTTCTCT-3’ (forward) and 5’-TGGTGAAGACCGTGTCAGGA-3’ (reverse) for c-fos; 5’-CCTTCTACGACGATGCCCTC-3’ (forward) and 5’-GGTTCAAG GTCATGCTCTGTTT-3’ (reverse) for c-jun; 5’-ATGAGCCACGGGAAGAGAAC-3’ (forward) and 5’-GCCCTACTGAAAACCTTG AGTC-3’ (reverse) for TIS21; 5’- AGGTCGGTGTGAACGGATTTG-3’ (forward) and 5’- TGTAGACCATGTAGTTGAGGTCA -3’ (reverse) for GAPDH. Prior to the qPCR experiments, a set of validation experiments were performed to test the primers and check their PCR efficiencies for 4-log dilution. The qPCR experiments were performed in 2 steps. First, the cDNA library was created from the purified RNAs using a High Capacity cDNA Reverse Transcription Kit from Applied Biosystems. Then, the cDNA library was mixed with each of the 4 primers and Fast SYBR Green Master Mix from Applied Biosystems. Real-time fluorescent measurements were taken from the Eppendorf RealPlex qPCR machine. For each biological replicate, three technical replicates were used.
Enrichment analysis
DAVID analysis was performed to identify significantly enriched Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways and biological processes from Gene Ontology (GO) terms under the modified Fisher-exact p-value cutoff of 0.05 (27). A heatmap was derived for the enrichment results with Circos software (28). Negative log2 of enrichment p-value was used as the scale for the heatmap.
Network analysis
An integrative analysis using protein-protein interactions and gene expression data was performed by using a custom mouse interaction network derived in Cytoscape, a network visualization tool (29). This mouse interaction network was derived from the known mouse protein-protein interactions and transcription factor-to-target interactions using online databases such as STRING, BIOGRID, and TRANSFAC (30–33). From the STRING database that uses a scoring scheme between 0 and 1 based on both predicted and experimentally validated protein-protein interactions, a 0.9 cutoff was used to extract highly feasible protein-protein interactions. Autosome clustering from the Clustermaker plugin of Cytoscape was used to identify the clusters of genes that are well-connected within the mouse interaction network (34, 35). Autosome clustering uses the unsupervised training of a self-organizing map, a type of artificial neural network in machine learning (35). This algorithm used correlation from the gene expression data as node weights to find the clusters of genes that are different from each other. Among these clusters that are separated from one another, the largest clusters with enriched biological functions or KEGG pathways were selected as relevant networks for further analyses.
Metabolomics
Mass spectrometry measurements were made for 143 metabolites for all three biological replicates for each genotype, time point, and experiment (sham, PHx). The measured metabolites included cholesterol esters, phospholipids, and metabolites in the sterol pathway (Supplementary Material: List). The PHx values were first normalized with respect to their sham control. Then, the final fold change between the KO and the WT was calculated by comparing their normalized PHx values. The mass spectrometry measurements of important metabolites were used together with the transcriptomic data to identify novel mechanisms and derive mechanistic insights.
Sterol pathway metabolite analysis
Frozen tissues were extracted in a similar manner as described previously (36). Briefly, frozen tissue samples were pulverized using a liquid nitrogen-chilled mortar and pestle, then a 30 mg portion of the pulverized frozen tissue was extracted by sonication with 1 mL of 7:2:1 methanol:water:chloroform containing stable isotope labeled internal standards: 13C1015N5 adenosine monophosphate, 13C4 malate, and 13C6 citrate at 200, 200, and 20 µM, respectively. Following centrifugation, two 200 µL aliquots of supernatant were reserved for liquid chromatography–mass spectrometry (LC-MS) and gas chromatography–mass spectrometry (GC-MS) analysis of sterol synthesis intermediates. The LC-MS analysis was performed using an Agilent 1200 LC instrument connected to an Agilent 6410 tandem quadrupole mass spectrometer. Chromatography was performed using a Phenomenex Luna NH2 column. Mobile phase A was acetonitrile and mobile phase B was 5mM ammonium acetate in water, adjusted to pH 9.9 with ammonium hydroxide. The gradient consisted of a linear ramp from 20–100% B over 15 minutes, followed by a 5 minute hold and a 15 minute re-equilibration period at 100% B. The flow rate was 0.07 mL/min and the injection volume was 40 µL. Mass spectrometry was performed using negative ion electrospray ionization in multiple reaction monitoring mode. Metabolite identification in biological samples was performed based on matching retention times and MS/MS transitions determined using authentic standards (Supplementary Table 3). Quantitation was performed based on calibration curves generated using authentic standards, and all metabolite peak areas were normalized to the peak area of the internal standard with closest-matching retention time. Internal standards were included at identical concentrations in both samples and standards.
For GC-MS analysis, the 200 µL aliquot of muscle extract supernatant was dried in a glass vial under a gentle stream of nitrogen gas, then derivatized using a two-step procedure similar to one described previously (37). First, 30 µL of 20 mg/mL methoxyamine hydrochloride in pyridine was added to the dried sample and heated at 37°C for 1 hour. Then, 30 µL of N-Methyl-N-(trimethylsilyl)trifluoroacetamide with 1% trimethylchlorosilane were added to the sample and heated at 70°C for 1 additional hour. The resulting derivatized sample was analyzed by GC-MS on an Agilent 7890 GC with a 5975 mass spectrometer. The instrument was equipped with an Agilent HP-5MS column. The temperature program consisted of 1 min initial time at 80°C, a 10 min ramp from 80–320°C and a 1 min hold at 320°C for 12 min total run time. Other parameters were as follows: 250°C inlet temperature, 1 uL injection volume, splitless injection mode, helium carrier gas, 1.4 mL/min constant column flow rate, scan, m/z 40–650 MS mode. Peaks were putatively identified by searching against the NIST 2011 and Fiehn 2013 spectral libraries followed by manual confirmation of spectral similarity. Peak areas were used for relative quantitation of metabolite levels.
Lipid extraction and fatty acid profiling of total phospholipids and cholesterol esters
Lipids were extracted from frozen pulverized tissues (20–25 mg) following essentially the Bligh and Dyer’s method of solvent partition (38). A typical extraction procedure consists of suspending the tissues in 0.6 ml of water followed by adding 2.25 ml of a mixture of chloroform-methanol (1:2) containing 0.01 % of BHT and 10 µl of 4 mM heptadecanoic acid (C17:0) as an internal standard. The mixture was homogenized with a polytron homogenizer. The homogenized samples were further treated with 0.75 ml each of chloroform and NaCl (0.9%) solution followed by mixing and centrifugation at ~3000 rpm for 5–6 min on a table top centrifuge. The lower organic chloroform layer containing the lipids were separated out and saved at −20°C unless used as follows.
Isolation of total phospholipids and cholesterol esters by thin-layer chromatography (TLC)
The solvents from the lipid extracts were removed under nitrogen and re-dissolved in a small volume of chloroform for chromatography on TLC plate (silica gel 60, Merck, Germany). The samples were applied as a band on the plate applying side by side standard phospholipid and cholesterol ester as reference spots. The plate was developed with a solvent mixture of hexane-diethyl ether-acetic acid (80-20-1.5, v/v). Total phospholipids (PL) and cholesterol esters (CE) were then identified by comparing the retention flow of the authentic standards. The lipid contents from the TLC powders were extracted with chloroform followed by removal of the solvents under nitrogen and subjection to trans-methylation as follows.
Preparation of methyl ester with Boron trifluoride-methanol (BF3-methanol)
The fatty acid components of the lipids were derivatized into their methyl esters using BF3-methanol (39). To the dry residue, 2 ml of BF3-methanol, 14% solution from Sigma, was added; the tubes containing the mixture were closed under nitrogen and incubated at 60°C for about 3–3.5 hours. The methyl esters were extracted by adding 2 ml of hexane and 1 ml of water, with mixing and then centrifuging followed by collection in the upper hexane layers. A TLC purification step was necessary for the methyl ester derived from CE in order to remove liberated free cholesterol prior to GC analysis. A similar TLC procedure as described above was performed applying standard fatty acid methyl ester (FAME) for identification purposes. The identified FAMEs were extracted from the TLC powder with hexane, the volumes were concentrated appropriately, and the fatty acid compositions of CEs and total PLs were analyzed by GC.
Analysis of CE and PL-derived FAMEs by gas chromatography
Analysis of FAMEs was performed by GC on an Agilent GC model 6890N equipped with flame ionization detector, an autosampler, and Chemstation software for data analysis. The GC column used was Agilent HP 88, 30 meter, 0.25 mm I.D., and 0.20 µm film thickness. Hydrogen was used as a carrier gas as well as for the flame ionization detector and nitrogen was used as a makeup gas. Analyses were carried out with a temperature program of 125°C to 220°C. A calibration curve was prepared using proportional amounts of C17:0 methyl ester standard. A mixture of standard methyl esters was also run to identify the components in unknown samples by comparing their retention times. The fatty acids were quantified with respect to the amounts of C17:0 internal standard added and the calibration curve prepared. The coefficient of variation for GC analyses was found to be within 2.5 – 3.6%.
Correlational analysis
Pearson correlation analyses were performed in Excel. The first correlation analysis compared cytokine and transcriptomic profiles. Correlation between our previously-published cytokine data and the acute phase genes was performed with a time-delay; the cytokine measurements at 0, 0.5, and 1 hours were compared with the gene expression measurements at 0.5, 1, and 3 hours, respectively (18). The second correlation analysis compared the transcriptomic and the metabolic data in the sterol pathway at the same time points. The expanded pathway for sterol lipids was taken from LIPID Metabolites and Pathways Strategy (LIPID MAPS) (40, 41). In the sterol pathway, multiple genes regulated certain metabolites; among these genes, only the gene with the highest correlation with its paired metabolite was selected. The second correlation analysis used three time points, 0.5, 1, and 3 hours, for the KO and the WT and 4 time points, 0, 0.5, 1, and 3 hours, for the KO/WT fold change. The correlation results in the sterol pathway were visualized through a heat map.
Results
Transcriptional changes
To explore the molecular events in the priming phase of complement-induced liver regeneration, we first analyzed the time-series transcriptomic data in the C3 knockout (KO) and the C57BL/6 wild-type (WT) livers using RNAseq across multiple conditions (Supplementary Table 1). The alignment result from the RNAseq experiments produced the following statistics: aside from one outlier, the samples had an average count of 15 million uniquely mapped reads, 3 million of which were splice reads (Supplementary Table 4). Correlation between the RNAseq and the qPCR data for the selected genes, c-fos, c-jun, and TIS21, was high (r2=0.76) (Supplementary Figure 1).
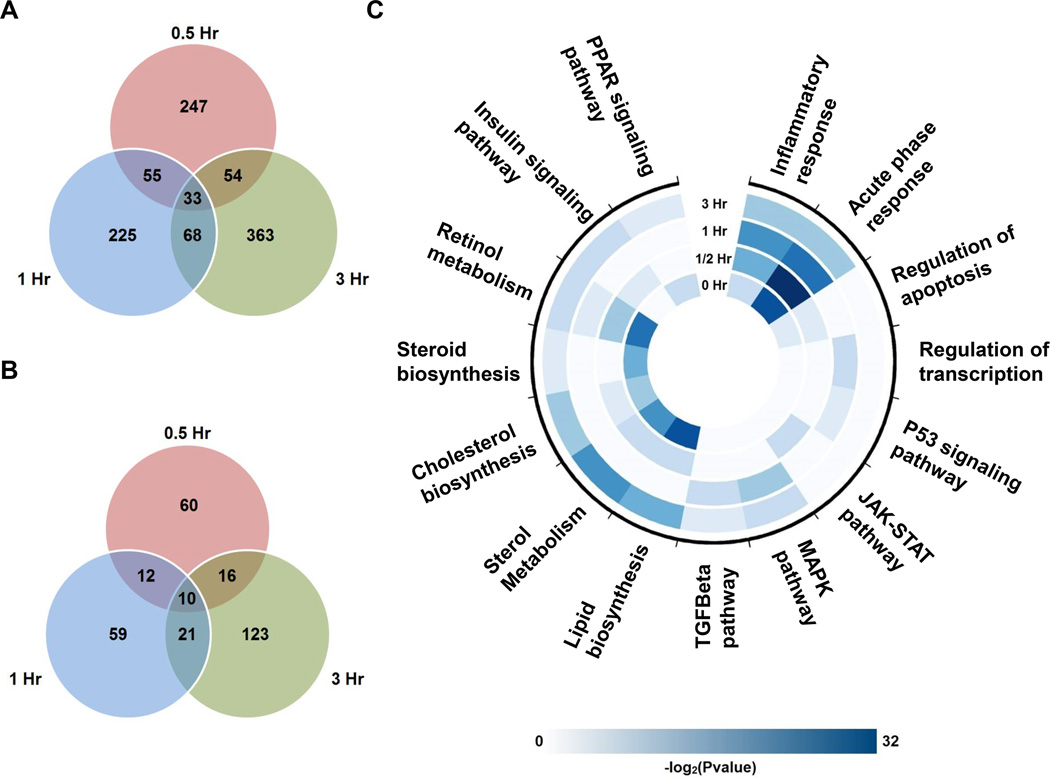
To identify differentially regulated genes, we first performed differential analysis using DEseq on PHx vs. sham for every genotype and time point to account for the inflammatory effect from the surgery. Then, a fold change cutoff of 1.5 was applied between the KO and the WT to produce the final list of differentially regulated genes. The total numbers of differentially regulated genes are shown in Figure 1A–B. The results showed an increase in the number of differentially regulated genes primarily at 3 hours following partial hepatectomy, suggesting that a small set of genes, such as immediate-early genes and transcription factors, are influenced first, with their downstream genes subsequently regulated.
Figure 1.
Venn diagrams of differentially regulated genes after PHx under the p-value cutoff of 0.05 (A) and the FDR cutoff of 0.1 (B) and over-represented biological functions and pathways during the priming phase (C). A and B, all data are shown as the number of differentially regulated genes between the KO and the WT at each time point (n=8 (0.5 hr) and n=6 (1–3 hr) mice) C, Enrichment analysis was performed at each time point using DAVID from the list of differentially regulated genes under the p-value cutoff of 0.05. Negative log2 of enrichment p-value was used as the scale for the heatmap (enrichment p≤0.05, a modified Fisher exact test from DAVID)
Activation of multiple signaling pathways
The complement system has been shown to increase the production of IL-6 and TNFα, which play a key role in liver regeneration by activating signaling pathways such as the NF-κB pathway in Kupffer cells (11). We confirmed that the signaling pathways activated by these cytokines in hepatocytes are deactivated in the KO mice. For example, differentially regulated genes showed enrichment in inflammatory and cell cycle-related pathways, such as janus kinase-signal transducer and activator of transcription (JAK-STAT), mitogen-activated protein kinases (MAPK), and TGFβ, demonstrating that the complement system activates an acute-phase, proliferative response between 1 and 3 hours following liver injury (Figure 1C). We also observed an indication of metabolic changes in the KO transcriptome; retinol metabolism, cholesterol biosynthetic processes, peroxisome proliferator-activated receptors (PPAR), and the insulin signaling pathways were significantly enriched at 0 and 3 hours after PHx (Figure 1C).
Temporal network analysis
To investigate the complex temporal changes in the transcriptome, we created networks in Cytoscape at each time point (Figure 2). These networks were derived from the gene expression data and a custom mouse interaction network composed of protein-protein and transcription factor interactions (see Materials and Methods). Autosome clustering was applied to create clusters, or networks of genes, that were different from each other based on transcriptomic co-expression values. The largest and the most statistically significant network of genes that is enriched with transcription factors and cell-cycle related genes (p<0.05) is shown at each time point (Figure 2). Other networks of genes from the clustering method were small in size and did not provide significant biological insights. The temporal network analysis revealed progressive downregulation of c-fos in the KO. It also revealed a significant, transient upregulation of SOCS3 and STAT3 in the first hour. Besides these clear changes in recognized genes, other changes in the transcriptional network across the multiple time points were complex, emphasizing the intricate regulation of multiple transcription factors and their target genes by the complement system during the priming phase of liver regeneration.
Figure 2.
Temporal network analysis in Cytoscape. The networks represent the biggest module derived from Autosome clustering at 4 time points. This particular network of 41 genes shows significant enrichment in transcription (FDR<0.1, DAVID). The red color indicates fold upregulation in the KO, whereas the green indicates fold downregulation, with respect to the WT. The size of a node is based on the number of directly connected genes. The solid line indicates protein-protein interaction (PPi), whereas the directed dashed line indicates transcription factor-target interaction (TFi). The diamond shape represents differentially regulated genes, either under the p-value of 0.05 or the FDR of 0.1, at that particular time point between the KO and the WT.
Complement and the acute phase response
The acute phase response was one of the most significantly over-represented biological processes in the KO across many time points from the enrichment analysis (Figure 1C). Therefore, we analyzed the transcriptional regulation of the acute phase genes from the acute phase response signaling pathway from QIAGEN’s Ingenuity Target explorer (42) (Figure 3). Among the acute phase genes, serum amyloid A (SAA) and orosomucoid (ORM) genes showed significant transcriptional regulation and similar temporal patterns with a progressive decline in expression in the KO livers from 0.5 to 3 hours, whereas their WT expression increased (Supplementary Figure 2).
Figure 3.
Acute phase genes after PHx. Heatmaps of transcriptional changes in the known acute phase genes are plotted for KO, WT, and KO/WT fold change. Log2 color scale is used. *differentially regulated at all three time points.
To investigate the source of the trends observed in the gene expression profiles of the acute phase proteins, we compared their PHx mRNA levels with the cytokine measurements from our previous study under the same experimental conditions (18). We incorporated a time-delay to compensate for the delay from the initiation of cytokine signaling to modulation of downstream gene expression. We compared the cytokine measurements at 0, 0.5, and 1 hours with the gene expression measurements at 0.5, 1, and 3 hours, respectively. The temporal profiles of TNFα levels showed time-delayed correlation with the temporal profiles of SAA1, representing the acute phase genes; the Pearson correlation coefficients for both the KO and the WT measurements between TNFα and SAA1 with the time-delay were both positive and very close to 1, indicating a strong linear relationship (Supplementary Figure 2). The temporal profiles of other cytokines, including IL-6, did not correlate with acute phase genes in either genotype. In addition to the strong positive correlation observed between TNFα and the acute phase genes, the correlation between c-fos and the acute phase genes was also high, with the Pearson correlation coefficients of 0.89 for the KO and 0.97 for the WT respectively (data not shown).
Complement and cholesterol metabolism and efflux
In addition to the changes in the early acute phase genes, the KO transcriptome was enriched for metabolic pathways related to cholesterol metabolism (Figure 1C). The baseline metabolic changes in the transcriptome also indicated the involvement of complement even before the priming phase of liver regeneration. In the liver, cholesterol homeostasis is closely monitored and regulated by liver X receptor (LXR) that is activated by oxysterols (43). Cholesterol 25-hydroxylase (CH25H), the gene responsible for the synthesis of the main agonist for LXR, 25-hydroxycholesterol, was significantly downregulated in the KO livers with respect to the WT (Supplementary Figure 3) (44). Other genes related to cholesterol efflux such as ATP-binding cassette sub-family G member 5 (ABCG5) and ATP-binding cassette sub-family G member 8 (ABCG8) also showed downregulation (Supplementary Figure 3) (45). Upregulation of HMG-CoA reductase (HMGCR) that results in increased cholesterol biosynthesis was observed at 3 hours (Supplementary Figure 3) (46). These results suggest that cellular conservation of cholesterol is regulated by the complement system during the priming phase of liver regeneration.
To observe the predicted metabolic changes based on the transcriptomic results, we analyzed 143 metabolites using mass spectrometry. Some notable metabolites related to cholesterol homeostasis were acetyl coenzyme A (acetyl-CoA), 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA), and cholesterol esters (CE) (Supplementary Figure 3). Although the changes in these metabolites at 3 hours were not statistically significantly different between the KO and the WT livers due to the high variance often observed in metabolic data, their KO/WT fold changes were greater than 1.5. Despite the metabolic and transcriptomic changes related to cholesterol metabolism and efflux, cholesterol levels were fairly consistent across both genotypes at all the time points, consistent with tight control of cellular cholesterol levels during liver regeneration (Supplementary Figure 3).
Overall metabolic demands
Cellular proliferation can cause significant remodeling of metabolic signals to meet the increased bioenergetic needs of the growing cells, like in cancer (47). Because hepatocytes undergo rapid proliferation during liver regeneration, we compared the metabolic levels between the two genotypes and observed an overall decrease in the KO mice during the late priming phase; 70% of the metabolites showed lower measurements in the KO at 3 hours compared to the WT measurements (Figure 4). Furthermore, 69% of the metabolites that were unrelated to cholesterol homeostasis also showed lower KO measurements (data not shown). These results suggest that metabolic demands in the KO are not being met during the later stage of the priming phase owing to the lack of C3 regulation.
Figure 4.
Metabolic fold changes at 3 hours. KO/WT fold changes at 3 hours are shown for all 143 metabolites. Each blue dot represents the fold change of a single metabolite.
To further evaluate metabolic changes, we analyzed the list of differentially regulated genes that may be related to lipid metabolism. The list of 1504 lipid-related mouse genes was taken from the LIPID MAPS Proteome database (40). A total of 43 genes were differentially regulated at either all three time points or only at 3 hours after PHx (Supplementary Figure 4). Among these genes was peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PPARGC1A), also known as PGC-1alpha, a key regulator of energy metabolism (48). In addition, solute carrier family 37 (glucose-6-phosphate transporter) member 1 (SLC37A1) may also regulate energy metabolism by transporting glycerol-3-phosphate between cellular compartments (49).
Transcriptomic and metabolic changes in the sterol pathway
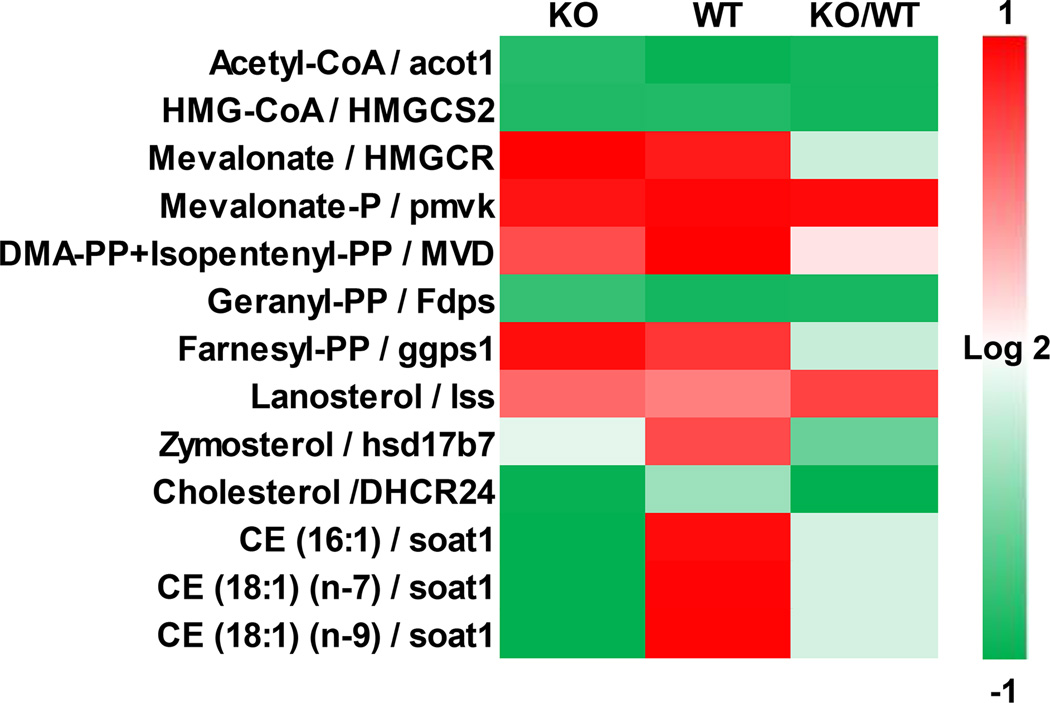
Correlation analysis between the transcriptomic and the metabolic data was performed for the gene-metabolite pair in the sterol pathway to observe if the transcriptomic changes would translate into metabolic changes. The majority of the measured gene-metabolite pairs in the sterol pathway showed high correlation (Figure 5). However, cholesterol esters showed significantly different correlation between the genotypes, which suggests that the mechanism for alterations in metabolite levels differ between KO and WT.
Figure 5.
Correlation heatmap of gene-metabolite pairs in the sterol pathway. Pearson correlation coefficient was calculated for the measured gene-metabolite pairs in the sterol pathway. If multiple genes are known to regulate a metabolite, the pair with the highest correlation was chosen. The numbers of time points used for correlation calculation were 3, 3, and 4 for KO, WT, and KO/WT categories, respectively.
Discussion
Transcriptional regulation of cell cycle-related pathways
The complement system, through activation of C3, regulates several significant genes and pathways related to cell cycle and proliferation, two major processes involved in liver regeneration (12). For example, the complement system activates the MAPK, p53, JAK-STAT, and TGFβ pathways across different time points. In addition, the network analysis shows progressive downregulation of c-fos, an immediate-early gene involved in proliferation, in the KO. Signal transducer and activator of transcription 3 (STAT3) and suppressor of cytokine signaling 3 (SOCS3), which are known regulators of liver regeneration, show brief but significant upregulation at one of the time points; STAT3 has been linked with cell survival and DNA synthesis during the acute phase, while SOCS3 negatively regulates liver regeneration by inhibiting the JAK-STAT pathway (50, 51). According to our previous measurements of cytokines on the first 6 hours of post-PHx, total STAT3 level increased while phosphorylation of STAT3 decreased (18). We also observed reduced DNA binding activity of STAT3 during the first 2 hours of post-PHx (17). These data, when combined together, suggest that reduced STAT3 phosphorylation activation and DNA binding activity may upregulate transcriptomic expression of STAT3 as a compensatory mechanism. Other genes in the transcriptional network did not show clear biological insights due to the observed complexity of the temporal transcriptomic changes.
Transcriptional regulation of the acute phase genes by TNFα
The gene expression profiles of the acute phase proteins such as SAA1, SAA2, ORM2, and ORM3 showed that C3 activation progressively promotes the acute phase response during the priming phase of liver regeneration. The increased transcriptional regulation of these acute phase responses at 0.5 hours post-PHx in the KO livers also suggest the existence of a compensatory mechanism for the less effective immune response from the complement knockout mice. TNFα, which is regulated by C3a and C5a of the complement system, is the most likely cytokine candidate for regulating the expression of these acute phase genes because the time-delayed correlation between TNFα and the acute phase genes was very high in both the WT and the KO livers (11). In addition, other cytokines, including IL-6, did not result in moderate or high correlation. Furthermore, several studies have shown that TNFα can regulate acute phase genes through the MAPK pathway (52, 53). Since the correlation between c-fos and the acute phase genes was also high in both the WT and the KO livers, TNFα may regulate the expression of the acute phase genes through the induction of c-fos and other immediate-early genes.
The acute phase genes and cholesterol efflux
The acute phase response has been linked with liver cirrhosis, a severe phenotype in advanced liver diseases with impaired liver regeneration (54–56). For example, ORMs and SAAs, the key acute phase proteins from our results, have been implicated as potential biomarkers for liver cirrhosis in humans (55, 56). These acute phase proteins showed significantly different transcriptional regulation during the priming phase. We further hypothesized that the acute phase proteins may regulate cholesterol that is required for cell cycle progression and cell growth. More specifically, the acute phase proteins may regulate cholesterol efflux through remodeling of high-density lipoprotein (HDL) (57). For example, SAA can remodel native HDL to acute-phase HDL by replacing apolipoprotein A1 as the major apolipoprotein during the acute phase response (58, 59). Acute-phase HDL with the dominant SAA proteins possesses a lower capacity to promote cholesterol efflux than native HDL; in other words, acute-phase HDL has a higher capacity to keep cholesterol within the cells than the native HDL (60, 61). Since the transcriptional regulations of SAA proteins steadily decreased over time in the KO mice, less SAA proteins were available to remodel native HDL to acute-phase HDL. Higher concentrations of native HDL during the first 3 hours is expected to promote higher cholesterol efflux and keep less cholesterol within the cells.
Further evidence for the relationship between SAA-rich acute-phase HDL and the change in cholesterol efflux is total cholesterol ester (CE) delivery. The result from the previous study showed that total CE delivery was significantly higher for acute-phase HDL than for native HDL (60). The metabolic data was consistent with this report; since the concentration of acute-phase HDL decreases over time in the KO, the total CE concentration also decreased significantly from 0.5 hours to 1 or 3 hours. Although the variance of total CE concentration at 0.5 hours was high, the majority of CEs with different saturation ratios showed similar profiles.
Regulation of cholesterol metabolism and efflux
Cholesterol is a well-known cell cycle regulator that is tightly controlled by the cells through LXR (62). We hypothesized that if the cholesterol level was to decrease as a result of higher cholesterol efflux during the first 3 hours after PHx, then hepatocytes would respond concomitantly by transcriptionally increasing cholesterol biosynthesis and reducing its catabolism and secretion. Several of our results support this hypothesis. For example, significant downregulation of cholesterol 25-hydroxylase (CH25H) that is responsible for synthesizing 25-hydroxycholesterol was observed at 3 hours. This oxysterol is the main agonist of LXR; it can deactivate the LXR pathway and downregulate genes related to cholesterol efflux such as ATP-binding cassette sub-family G member 5 (ABCG5) and ATP-binding cassette sub-family G member 8 (ABCG8) (44, 45). Hepatocytes also stimulated the expression of HMGCR at 3 hours post-PHx to promote cholesterol biosynthesis to further restore cholesterol availability (46). Upregulation of HMGCR can be explained by the reduced concentration of TNFα, an inhibitor of the insulin signaling pathway, because insulin can strongly stimulate HMGCR synthesis (63, 64). Further evidence for increased cholesterol availability within hepatocytes in the KO was enriched steroid and bile acid biosynthesis at 3 hours from the enrichment analysis of the transcriptomic data.
As a result of transcriptional regulation of cholesterol metabolism and efflux, which can restore the cholesterol availability lowered by the native HDL during the first 3 hours, the cholesterol level remained relatively stable in both genotypes across all time points. This highlights the tight regulation of cholesterol by hepatocytes to ensure successful progression of the cell cycle during the priming phase of liver regeneration. Although the resulting cholesterol level did not change much across the genotypes during the priming phase, it may change during the later stages of liver regeneration, such as in the proliferation phase, where significant metabolic changes are known to occur. Moreover, most of the metabolites and their associated genes in the sterol pathway showed high correlation, suggesting that the transcriptomic changes are being translated into metabolic changes.
Metabolic demands during early liver regeneration
Besides cholesterol metabolism, the complement system may help hepatocytes meet other metabolic demands. For example, the majority of the 143 measured metabolites showed lower measurements in KO livers compared to WT at 3 hours post-PHx. This result did not change when the metabolites that were related to cholesterol metabolism were excluded. Based on these results, we hypothesize that when KO hepatocytes are focused on synthesizing cholesterol and lowering its secretion, fewer resources are made available to meet the other metabolic needs in preparation for prolonged cellular proliferation. A similar phenomenon occurs in cancer cells when they dramatically alter the metabolic circuitry to meet the bioenergetic and biosynthetic demands of increased proliferation (47, 65). Our data also showed that PGC1A, the gene involved in energy metabolism, and SLC37A1, the gene involved in transporting glycerol-3-phosphate, are differentially regulated at all three time points. The regenerative process is highly dependent on increased proliferation, and liver regeneration is no exception.
A systems overview
In this study, we have performed systems analyses using diverse measurements across multiple time points to investigate the complex mechanism of the priming phase of complement-induced liver regeneration. Based on the significant results, we have proposed a mechanistic relationship between the complement activation at the level C3, acute phase proteins, and cholesterol metabolism during the priming phase of liver regeneration (Figure 6). Future studies including proteomic analysis and investigation of the role of the complement system using genetic and pharmacological perturbations on the later stages of liver regeneration can supplement the findings of this study.
Figure 6.
The proposed mechanism of the priming phase of complement-induced liver regeneration. Complement activation induced by liver injury or PHx increases the concentration of the complement effector proteins, C3a and C5a, which bind to the nearby Kupffer cells to release TNFα. TNFα then binds to the receptors on hepatocytes to initiate the MAPK pathway and its downstream immediate-early genes such as c-fos and SOCS3. This leads to activation of acute phase proteins such as SAA that can replace the lipoprotein of HDL to form acute-phase HDL. Acute-phase HDL promotes lower cholesterol efflux than native HDL, which causes hepatocytes to respond by activating LXR through oxysterols to reduce cholesterol biosynthesis for stable cholesterol levels. TNFα can also inhibit insulin signaling, potentially through the MAPK pathway, which then reduces the expression of HMGCR, a key enzyme in cholesterol biosynthesis. The reduced cholesterol biosynthesis allows hepatocytes to use their cellular resources to meet other metabolic demands required for upcoming prolonged proliferation of liver regeneration.
Supplementary Material
Acknowledgments
This work was supported by NIH grants: U01 DK097430, R01HL106579 and R01HL108735 (SS) and AI068730 (JL). This work also utilized Core Services supported by grant DK097153 of the NIH to the University of Michigan.
Abbreviations used in this article
- C3a
Complement component 3a
- C5a
Complement component 5a
- PHx
Partial hepatectomy
- KO
Knockout (C3-/-)
- WT
Wild-type
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- GO
Gene ontology
- LC-MS
Liquid chromatography-mass spectrometry
- GC-MS
Gas chromatography-mass spectrometry
- TLC
Thin-layer chromatography
- PL
Phospholipid
- CE
Cholesterol ester
- BF3 methanol
Boron trifluoride-methanol
- FAME
Fatty acid methyl ester
- SAA
Serum amyloid A
- ORM
Orosomucoid
- LXR
Liver X receptor
- ABCG
ATP-binding cassette sub-family G
- CH25H
Cholesterol 25-hydroxylase
- HMG
3-hydroxy-3-methylglutaryl
- PPARGC1A
Peroxisome proliferator-activated receptor gamma coactivator 1-alpha
- SLC37A1
Solute carrier family 37 (glucose-6-phosphate transporter) member 1
- HDL
High-density lipoprotein
Footnotes
Author Contributions
SS and JL conceived and supervised the project. Most of the analyses were carried out by JM with assistance from SG and MM. RD and ER carried out the animal experiments and RNA extraction while the mass spectrometric study was carried out in the University of Michigan Core by CE and AD with supervision by CB. The draft of the manuscript was written by JM and was reviewed by all authors, and revised by JL and SS.
References
- 1.Clavien PA. Liver regeneration: a spotlight on the novel role of platelets and serotonin. Swiss medical weekly. 2008;138:361–370. doi: 10.4414/smw.2008.12231. [DOI] [PubMed] [Google Scholar]
- 2.Clavien PA, Petrowsky H, DeOliveira ML, Graf R. Strategies for safer liver surgery and partial liver transplantation. The New England journal of medicine. 2007;356:1545–1559. doi: 10.1056/NEJMra065156. [DOI] [PubMed] [Google Scholar]
- 3.Gotohda N, Iwagaki H, Ozaki M, Kinoshita T, Konishi M, Nakagohri T, Takahashi S, Saito S, Yagi T, Tanaka N. Deficient response of IL-6 impaired liver regeneration after hepatectomy in patients with viral hepatitis. Hepato-gastroenterology. 2008;55:1439–1444. [PubMed] [Google Scholar]
- 4.Hines IN, Kremer M, Isayama F, Perry AW, Milton RJ, Black AL, Byrd CL, Wheeler MD. Impaired liver regeneration and increased oval cell numbers following T cell-mediated hepatitis. Hepatology. 2007;46:229–241. doi: 10.1002/hep.21674. [DOI] [PubMed] [Google Scholar]
- 5.Tanemura A, Mizuno S, Wada H, Yamada T, Nobori T, Isaji S. Donor age affects liver regeneration during early period in the graft liver and late period in the remnant liver after living donor liver transplantation. World journal of surgery. 2012;36:1102–1111. doi: 10.1007/s00268-012-1496-1. [DOI] [PubMed] [Google Scholar]
- 6.Torbenson M, Yang SQ, Liu HZ, Huang J, Gage W, Diehl AM. STAT-3 overexpression and p21 up-regulation accompany impaired regeneration of fatty livers. The American journal of pathology. 2002;161:155–161. doi: 10.1016/S0002-9440(10)64167-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang SQ, Lin HZ, Mandal AK, Huang J, Diehl AM. Disrupted signaling and inhibited regeneration in obese mice with fatty livers: implications for nonalcoholic fatty liver disease pathophysiology. Hepatology. 2001;34:694–706. doi: 10.1053/jhep.2001.28054. [DOI] [PubMed] [Google Scholar]
- 8.Michalopoulos GK. Liver regeneration after partial hepatectomy: critical analysis of mechanistic dilemmas. The American journal of pathology. 2010;176:2–13. doi: 10.2353/ajpath.2010.090675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zimmermann A. Regulation of liver regeneration. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2004;19(Suppl 4):iv6–iv10. doi: 10.1093/ndt/gfh1034. [DOI] [PubMed] [Google Scholar]
- 10.Su AI, Guidotti LG, Pezacki JP, Chisari FV, Schultz PG. Gene expression during the priming phase of liver regeneration after partial hepatectomy in mice. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:11181–11186. doi: 10.1073/pnas.122359899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeAngelis RA, Markiewski MM, Lambris JD. Liver regeneration: a link to inflammation through complement. Advances in experimental medicine and biology. 2006;586:17–34. doi: 10.1007/0-387-34134-X_2. [DOI] [PubMed] [Google Scholar]
- 12.Taub R. Liver regeneration: from myth to mechanism. Nature reviews. Molecular cell biology. 2004;5:836–847. doi: 10.1038/nrm1489. [DOI] [PubMed] [Google Scholar]
- 13.Grisham JW. A morphologic study of deoxyribonucleic acid synthesis and cell proliferation in regenerating rat liver; autoradiography with thymidine-H3. Cancer research. 1962;22:842–849. [PubMed] [Google Scholar]
- 14.Nygard IE, Mortensen KE, Hedegaard J, Conley LN, Kalstad T, Bendixen C, Revhaug A. The genetic regulation of the terminating phase of liver regeneration. Comparative hepatology. 2012;11:3. doi: 10.1186/1476-5926-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DeAngelis RA, Markiewski MM, Kourtzelis I, Rafail S, Syriga M, Sandor A, Maurya MR, Gupta S, Subramaniam S, Lambris JD. A complement-IL-4 regulatory circuit controls liver regeneration. Journal of immunology. 2012;188:641–648. doi: 10.4049/jimmunol.1101925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mastellos D, Papadimitriou JC, Franchini S, Tsonis PA, Lambris JD. A novel role of complement: mice deficient in the fifth component of complement (C5) exhibit impaired liver regeneration. Journal of immunology. 2001;166:2479–2486. doi: 10.4049/jimmunol.166.4.2479. [DOI] [PubMed] [Google Scholar]
- 17.Strey CW, Markiewski M, Mastellos D, Tudoran R, Spruce LA, Greenbaum LE, Lambris JD. The proinflammatory mediators C3a and C5a are essential for liver regeneration. The Journal of experimental medicine. 2003;198:913–923. doi: 10.1084/jem.20030374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Markiewski MM, DeAngelis RA, Strey CW, Foukas PG, Gerard C, Gerard N, Wetsel RA, Lambris JD. The regulation of liver cell survival by complement. Journal of immunology. 2009;182:5412–5418. doi: 10.4049/jimmunol.0804179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higgins G, R A. Experimental pathology of the liver 1. Restoration of the liver of the white rat following partial surgical removal. Arch Pathol. 1932:186–202. [Google Scholar]
- 20.Sun R, Park O, Horiguchi N, Kulkarni S, Jeong WI, Sun HY, Radaeva S, Gao B. STAT1 contributes to dsRNA inhibition of liver regeneration after partial hepatectomy in mice. Hepatology. 2006;44:955–966. doi: 10.1002/hep.21344. [DOI] [PubMed] [Google Scholar]
- 21.Desbois-Mouthon C, Wendum D, Cadoret A, Rey C, Leneuve P, Blaise A, Housset C, Tronche F, Le Bouc Y, Holzenberger M. Hepatocyte proliferation during liver regeneration is impaired in mice with liver-specific IGF-1R knockout. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2006;20:773–775. doi: 10.1096/fj.05-4704fje. [DOI] [PubMed] [Google Scholar]
- 22.Hu J, Ge H, Newman M, Liu K. OSA: a fast and accurate alignment tool for RNA-Seq. Bioinformatics. 2012;28:1933–1934. doi: 10.1093/bioinformatics/bts294. [DOI] [PubMed] [Google Scholar]
- 23.Anders S, Pyl PT, Huber W. HTSeq--a Python framework to work with high-throughput sequencing data. Bioinformatics. 2015;31:166–169. doi: 10.1093/bioinformatics/btu638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anders S, Huber W. Differential expression analysis for sequence count data. Genome biology. 2010;11:R106. doi: 10.1186/gb-2010-11-10-r106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spandidos A, Wang X, Wang H, Seed B. PrimerBank: a resource of human and mouse PCR primer pairs for gene expression detection and quantification. Nucleic acids research. 2010;38:D792–D799. doi: 10.1093/nar/gkp1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang X, Spandidos A, Wang H, Seed B. PrimerBank: a PCR primer database for quantitative gene expression analysis, 2012 update. Nucleic acids research. 2012;40:D1144–D1149. doi: 10.1093/nar/gkr1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nature protocols. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 28.Krzywinski M, Schein J, Birol I, Connors J, Gascoyne R, Horsman D, Jones SJ, Marra MA. Circos: an information aesthetic for comparative genomics. Genome research. 2009;19:1639–1645. doi: 10.1101/gr.092759.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome research. 2003;13:2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stark C, Breitkreutz BJ, Reguly T, Boucher L, Breitkreutz A, Tyers M. BioGRID: a general repository for interaction datasets. Nucleic acids research. 2006;34:D535–D539. doi: 10.1093/nar/gkj109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Szklarczyk D, Franceschini A, Kuhn M, Simonovic M, Roth A, Minguez P, Doerks T, Stark M, Muller J, Bork P, Jensen LJ, von Mering C. The STRING database in 2011: functional interaction networks of proteins, globally integrated and scored. Nucleic acids research. 2011;39:D561–D568. doi: 10.1093/nar/gkq973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.von Mering C, Huynen M, Jaeggi D, Schmidt S, Bork P, Snel B. STRING: a database of predicted functional associations between proteins. Nucleic acids research. 2003;31:258–261. doi: 10.1093/nar/gkg034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wingender E, Chen X, Hehl R, Karas H, Liebich I, Matys V, Meinhardt T, Pruss M, Reuter I, Schacherer F. TRANSFAC: an integrated system for gene expression regulation. Nucleic acids research. 2000;28:316–319. doi: 10.1093/nar/28.1.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morris JH, Apeltsin L, Newman AM, Baumbach J, Wittkop T, Su G, Bader GD, Ferrin TE. clusterMaker: a multi-algorithm clustering plugin for Cytoscape. BMC bioinformatics. 2011;12:436. doi: 10.1186/1471-2105-12-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Newman AM, Cooper JB. AutoSOME: a clustering method for identifying gene expression modules without prior knowledge of cluster number. BMC bioinformatics. 2010;11:117. doi: 10.1186/1471-2105-11-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Overmyer KA, Thonusin C, Qi NR, Burant CF, Evans CR. Impact of Anesthesia and Euthanasia on Metabolomics of Mammalian Tissues: Studies in a C57BL/6J Mouse Model. PloS one. 2015;10:e0117232. doi: 10.1371/journal.pone.0117232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Begley P, Francis-McIntyre S, Dunn WB, Broadhurst DI, Halsall A, Tseng A, Knowles J, Goodacre R, Kell DB. Development and Performance of a Gas Chromatography-Time-of-Flight Mass Spectrometry Analysis for Large-Scale Nontargeted Metabolomic Studies of Human Serum. Anal. Chem. (Washington, DC, US.) 2009;81:7038–7046. doi: 10.1021/ac9011599. [DOI] [PubMed] [Google Scholar]
- 38.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Canadian journal of biochemistry and physiology. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 39.Morrison WR, Smith LM. Preparation of Fatty Acid Methyl Esters and Dimethylacetals from Lipids with Boron Fluoride--Methanol. Journal of lipid research. 1964;5:600–608. [PubMed] [Google Scholar]
- 40.Subramaniam S, Fahy E, Gupta S, Sud M, Byrnes RW, Cotter D, Dinasarapu AR, Maurya MR. Bioinformatics and systems biology of the lipidome. Chemical reviews. 2011;111:6452–6490. doi: 10.1021/cr200295k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dennis EA, Deems RA, Harkewicz R, Quehenberger O, Brown HA, Milne SB, Myers DS, Glass CK, Hardiman G, Reichart D, Merrill AH, Jr, Sullards MC, Wang E, Murphy RC, Raetz CR, Garrett TA, Guan Z, Ryan AC, Russell DW, McDonald JG, Thompson BM, Shaw WA, Sud M, Zhao Y, Gupta S, Maurya MR, Fahy E, Subramaniam S. A mouse macrophage lipidome. The Journal of biological chemistry. 2010;285:39976–39985. doi: 10.1074/jbc.M110.182915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ingenuity Target Explorer. QIAGEN. 2015 https://targetexplorer.ingenuity.com.
- 43.Hu YW, Zheng L, Wang Q. Regulation of cholesterol homeostasis by liver X receptors. Clinica chimica acta; international journal of clinical chemistry. 2010;411:617–625. doi: 10.1016/j.cca.2009.12.027. [DOI] [PubMed] [Google Scholar]
- 44.Chen W, Chen G, Head DL, Mangelsdorf DJ, Russell DW. Enzymatic reduction of oxysterols impairs LXR signaling in cultured cells and the livers of mice. Cell metabolism. 2007;5:73–79. doi: 10.1016/j.cmet.2006.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Repa JJ, Berge KE, Pomajzl C, Richardson JA, Hobbs H, Mangelsdorf DJ. Regulation of ATP-binding cassette sterol transporters ABCG5 and ABCG8 by the liver X receptors alpha and beta. The Journal of biological chemistry. 2002;277:18793–18800. doi: 10.1074/jbc.M109927200. [DOI] [PubMed] [Google Scholar]
- 46.Jo Y, Debose-Boyd RA. Control of cholesterol synthesis through regulated ER-associated degradation of HMG CoA reductase. Critical reviews in biochemistry and molecular biology. 2010;45:185–198. doi: 10.3109/10409238.2010.485605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Phan LM, Yeung SC, Lee MH. Cancer metabolic reprogramming: importance, main features, and potentials for precise targeted anti-cancer therapies. Cancer biology & medicine. 2014;11:1–19. doi: 10.7497/j.issn.2095-3941.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liang H, Ward WF. PGC-1alpha: a key regulator of energy metabolism. Advances in physiology education. 2006;30:145–151. doi: 10.1152/advan.00052.2006. [DOI] [PubMed] [Google Scholar]
- 49.Bartoloni L, Wattenhofer M, Kudoh J, Berry A, Shibuya K, Kawasaki K, Wang J, Asakawa S, Talior I, Bonne-Tamir B, Rossier C, Michaud J, McCabe ER, Minoshima S, Shimizu N, Scott HS, Antonarakis SE. Cloning and characterization of a putative human glycerol 3-phosphate permease gene (SLC37A1 or G3PP) on 21q22.3: mutation analysis in two candidate phenotypes, DFNB10 and a glycerol kinase deficiency. Genomics. 2000;70:190–200. doi: 10.1006/geno.2000.6395. [DOI] [PubMed] [Google Scholar]
- 50.Moh A, Iwamoto Y, Chai GX, Zhang SS, Kano A, Yang DD, Zhang W, Wang J, Jacoby JJ, Gao B, Flavell RA, Fu XY. Role of STAT3 in liver regeneration: survival, DNA synthesis, inflammatory reaction and liver mass recovery. Laboratory investigation; a journal of technical methods and pathology. 2007;87:1018–1028. doi: 10.1038/labinvest.3700630. [DOI] [PubMed] [Google Scholar]
- 51.Riehle KJ, Campbell JS, McMahan RS, Johnson MM, Beyer RP, Bammler TK, Fausto N. Regulation of liver regeneration and hepatocarcinogenesis by suppressor of cytokine signaling 3. The Journal of experimental medicine. 2008;205:91–103. doi: 10.1084/jem.20070820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thorn CF, Lu ZY, Whitehead AS. Regulation of the human acute phase serum amyloid A genes by tumour necrosis factor-alpha, interleukin-6 and glucocorticoids in hepatic and epithelial cell lines. Scandinavian journal of immunology. 2004;59:152–158. doi: 10.1111/j.0300-9475.2004.01369.x. [DOI] [PubMed] [Google Scholar]
- 53.Westra J, Bijzet J, Doornbos-van der Meer B, van Rijswijk MH, Limburg PC. Differential influence of p38 mitogen activated protein kinase (MAPK) inhibition on acute phase protein synthesis in human hepatoma cell lines. Annals of the rheumatic diseases. 2006;65:929–935. doi: 10.1136/ard.2005.043232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lemmers A, Gustot T, Durnez A, Evrard S, Moreno C, Quertinmont E, Vercruysse V, Demetter P, Franchimont D, Le Moine O, Geerts A, Deviere J. An inhibitor of interleukin-6 trans-signalling, sgp130, contributes to impaired acute phase response in human chronic liver disease. Clinical and experimental immunology. 2009;156:518–527. doi: 10.1111/j.1365-2249.2009.03916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sasaki M, Yoneda N, Kitamura S, Sato Y, Nakanuma Y. A serum amyloid A-positive hepatocellular neoplasm arising in alcoholic cirrhosis: a previously unrecognized type of inflammatory hepatocellular tumor. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2012;25:1584–1593. doi: 10.1038/modpathol.2012.114. [DOI] [PubMed] [Google Scholar]
- 56.Ryden I, Pahlsson P, Lindgren S. Diagnostic accuracy of alpha(1)-acid glycoprotein fucosylation for liver cirrhosis in patients undergoing hepatic biopsy. Clinical chemistry. 2002;48:2195–2201. [PubMed] [Google Scholar]
- 57.O'Brien KD, Chait A. Serum amyloid A: the "other" inflammatory protein. Current atherosclerosis reports. 2006;8:62–68. doi: 10.1007/s11883-006-0066-0. [DOI] [PubMed] [Google Scholar]
- 58.Coetzee GA, Strachan AF, van der Westhuyzen DR, Hoppe HC, Jeenah MS, de Beer FC. Serum amyloid A-containing human high density lipoprotein 3. Density, size, and apolipoprotein composition. The Journal of biological chemistry. 1986;261:9644–9651. [PubMed] [Google Scholar]
- 59.de Beer MC, Webb NR, Wroblewski JM, Noffsinger VP, Rateri DL, Ji A, van der Westhuyzen DR, de Beer FC. Impact of serum amyloid A on high density lipoprotein composition and levels. Journal of lipid research. 2010;51:3117–3125. doi: 10.1194/jlr.M005413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Artl A, Marsche G, Lestavel S, Sattler W, Malle E. Role of serum amyloid A during metabolism of acute-phase HDL by macrophages. Arteriosclerosis, thrombosis, and vascular biology. 2000;20:763–772. doi: 10.1161/01.atv.20.3.763. [DOI] [PubMed] [Google Scholar]
- 61.Jahangiri A, de Beer MC, Noffsinger V, Tannock LR, Ramaiah C, Webb NR, van der Westhuyzen DR, de Beer FC. HDL remodeling during the acute phase response. Arteriosclerosis, thrombosis, and vascular biology. 2009;29:261–267. doi: 10.1161/ATVBAHA.108.178681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim KH, Lee GY, Kim JI, Ham M, Won Lee J, Kim JB. Inhibitory effect of LXR activation on cell proliferation and cell cycle progression through lipogenic activity. Journal of lipid research. 2010;51:3425–3433. doi: 10.1194/jlr.M007989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Peraldi P, Hotamisligil GS, Buurman WA, White MF, Spiegelman BM. Tumor necrosis factor (TNF)-alpha inhibits insulin signaling through stimulation of the p55 TNF receptor and activation of sphingomyelinase. The Journal of biological chemistry. 1996;271:13018–13022. doi: 10.1074/jbc.271.22.13018. [DOI] [PubMed] [Google Scholar]
- 64.Osborne AR, Pollock VV, Lagor WR, Ness GC. Identification of insulin-responsive regions in the HMG-CoA reductase promoter. Biochemical and biophysical research communications. 2004;318:814–818. doi: 10.1016/j.bbrc.2004.04.105. [DOI] [PubMed] [Google Scholar]
- 65.Jones RG, Thompson CB. Tumor suppressors and cell metabolism: a recipe for cancer growth. Genes & development. 2009;23:537–548. doi: 10.1101/gad.1756509. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.