Abstract
Background: In this study, the efficacy of nitazoxanide in the treatment of Helicobacter pylori isolates, which were resistant to metronidazole, was examined.
Methods: One hundred twenty two patients who underwent endoscopy examinations at Kasra and Laleh hospitals in Tehran from November 2014 to July 2015 were enrolled. Helicobacter pylori strains were isolated from the patients’ endoscopy biopsies by bacteriological culture. Those bacterial isolates resistant to metronidazole were examined for susceptibility to nitazoxanide. Serial agar dilution method was utilized to determine the minimum inhibitory concentrations for the antibiotics.
Results: From 122 gastric biopsy specimens, 55 H. pylori isolates were recovered (45%); of which, 40 (72.7%) were resistant to metronidazole. Comparing the MIC values of nitazoxanide with metronidazole revealed significant differences (p<0.05). The MIC50 and MIC90 values for nitazoxanide and metronidazole were 8 and ≥8μg/ml, and 32 and 64μg/ml, respectively.
Conclusion: The high levels of metronidazole resistance suggest that this medication may not be beneficial for first-line therapy in Iran. However, considering the relative effectiveness of nitazoxanide, it may be considered a suitable alternative for patients in Iran.
Keywords: Helicobacter pylori, Antibiotic Resistance, Metronidazole, Nitazoxanide, Minimum Inhibitory Concentration
Introduction
Helicobacter pylori (H. pylori) is a Gram negative, non-spore forming microaerophilic spiral resident of gastric mucosa in more than half of the world’s population. The World Health Organization introduced this bacterium as a carcinogen class 1 agent in 1994 (1). Based on epidemiological studies, H. pylori is one of the most common pathogens that causes chronic inflammation of the gastrointestinal tract in humans and is an important risk factor in chronic gastritis, gastric ulcer, duodenal ulcer and gastric cancer (2). H. pylori antibiotic resistance is the main factor affecting the effectiveness of current therapeutic regimens. Prevalence of bacterial resistance varies in different geographic areas; and it is correlated with the consumption of antibiotics in the general population (3). Triple therapy to eradicate H. pylori in which a bismuth preparation or proton pump inhibitor is combined with two drugs from clarithromycin, metronidazole, amoxicillin and tetracycline, is the most widely used regimen. However, H. pylori rapidly acquires resistance to many classes of antibiotics after repeated exposure. Resistance is prevalent worldwide, especially in developing countries, and is considered the primary reason for failure to eradicate infection (4). Various researches have shown that resistance to metronidazole reduces the adequacy of treatment by 50% (5). Resistance to antibiotics is so high that in many countries it has led to removal of many first line antibiotic choices such as clarithromycin (6). Nitazoxanide is a benzamide thiazolide compound (7). Antimicrobial properties of nitazoxanide have been shown against helminthes, protozoa (cryptosporidia, microsporidia, trichomonas, entamoeba, and giardia) and many bacteria (8,9).
A treatment method called LOAD therapy, which includes levofloxacin, omeprazole, alinia (nitazoxanide) and doxycycline, was used for seven days at doses of 100- 500- 40- 250mg per day, respectively; this method is the preferred treatment choice for H. pylori infections in the United States (10). According to the high prevalence of H. pylori infection and its complications such as peptic ulcers, gastric adenocarcinoma and increasing resistance to metronidazole and the emergence of MDR strains that cause treatment failure, we aimed to examine the effectiveness of nitazoxanide against metronidazole resistant isolates. H. pylori strains were isolated from patients who referred to the endoscopy ward in Laleh and Kasra hospitals in Tehran from November 2014 to July 2015. Following bacterial culture, the resistance pattern and minimal inhibitory concentrations (MICs) toward metronidazole and nitazoxanide were determined.
Methods
This study was conducted at Kasra and Laleh hospitals in Tehran from November 2014 to July 2015. H. pylori strains were isolated from 122 biopsy specimens of patients with gastrointestinal complaints who underwent endoscopy. Exclusion criteria included treatment with proton pump inhibitor and use of antibiotics within the two weeks prior to sample collection. Two biopsies per patient were obtained from antrum of stomach by a gastroenterologist, which were used for pathology and rapid urease test. The samples for culture were kept in transfer medium (thioglycolate broth), which were subsequently processed for bacterial culture. Biopsy specimens were cultured in Brucella agar medium containing 10% horse blood, 10% fetal bovine serum and antibiotic supplement. The plates were incubated in microaerophilic atmosphere (concentrations of 8-10% by volume CO2 and 5-7% by volume oxygen) and placed in a Gas Pak jar (Anaerocult C sachets, Merck, Germany)) for up to 10 days. Colonies were identified by positive catalase, urease and oxidase tests as well as morphologic examinations. The strains were maintained frozen at -20oC before testing.
Determination of MICs
Minimal inhibitory concentrations were determined by agar dilution method according to the CLSI guidelines. A suspension with a turbidity equivalent to that of a McFarland No.2 standard (approximately 6×108cfu/ml) was prepared from a 48-h culture on agar. Mueller Hinton agar, which contained horse blood, was used as a medium. An appropriate dilution of metronidazole was added at concentrations ranging from 0.5 to 64µg/ml. Following the preparation of microbial suspension, inoculation took place in plates containing Mueller Hinton agar by spotting 10µl of bacterial suspension with different concentrations of metronidazole. Within a short time after the absorption of the samples, plates were incubated under microaerophilic conditions at 37oC for two days. The MIC was determined as the lowest concentration of antimicrobial agent inhibiting the total growth of bacteria. Resistance was considered when the metronidazole MIC was greater than 4µg/ml. After identifying metronidazole resistant isolates, they were tested to determine the MICs of nitazoxanide. The MIC determination protocol towards nitazoxanide was similar to that metronidazole, except for the bacterial turbidity, which was set at McFarland No.3 standard (approximately 6×109cfu/ml). Nitazoxanide concentrations ranged from 0.03 to 32µg/ml. The solvent DMSO was used for nitazoxanide dissolution. Metronidazole and nitazoxanide were obtained from Sigma Company (USA). Nitazoxanide is not yet available commercially in Iran. Since there is no determined breakpoint MIC value for nitazoxanide against H. pylori, the nitazoxanide MIC values were reported as such. Chi-square test was used for data analysis.
Results
From the 122 gastric biopsy specimens, 55 H. pylori isolates (45%) were collected; of which, 40 isolates (72.7%) were shown to be resistant to metronidazole using agar dilution method. The time required for rapid urease test in the positive biopsy samples based on the severity of the infection varied from 20 minutes to 24 hours. Bacterial colonies grown on Brucella agar were all clear in color. All the colonies were positive for urease test. The time required for RUT (Rapid Urease Test) in these colonies varied from 30 seconds to 10 minutes. Additionally, all isolates were positives for oxidase test. The MIC values for H. pylori isolates resistant to metronidazole are demonstrated in Table 1. The MIC50 and MIC90 values for metronidazole were 32, 64 µg/ml, respectively. The MIC values for nitazoxanide in the H. pylori isolates resistant to metronidazole are presented in Table 2. The MIC50 and MIC90 values for nitazoxanide reported to be 8 and ≥8µg/ml, respectively. Comparison of the MIC values of nitazoxanide and metronidazole revealed significant differences (p<0.05). The MIC values of nitazoxanide for all the metronidazole resistant isolates are shown in Figure 1.
Table 1. MIC Values for H. Pylori Isolates Resistant to Metronidazole .
| MICs Metronidazole (µg/ml) | n | % |
| 8 | 7 | 17.5 |
| 16 | 10 | 25.0 |
| 32 | 14 | 35.0 |
| 64 | 9 | 22.5 |
| Total | 40 | 100 |
Table 2. MIC Values of Nitazoxanide for H. pylori Isolates Resistant to Metronidazole .
| MICs Nitazoxanide (µg/ml) | n | % |
| 0.03-0.5 | ||
| 1 | 1 | 2.5 |
| 2 | 3 | 7.5 |
| 4 | 9 | 22.5 |
| 8 | 11 | 27.5 |
| 16 | 4 | 10.0 |
| 32 | 2 | 5.0 |
| >32 | 10 | 25.0 |
| Total | 40 | 100 |
Fig. 1 .
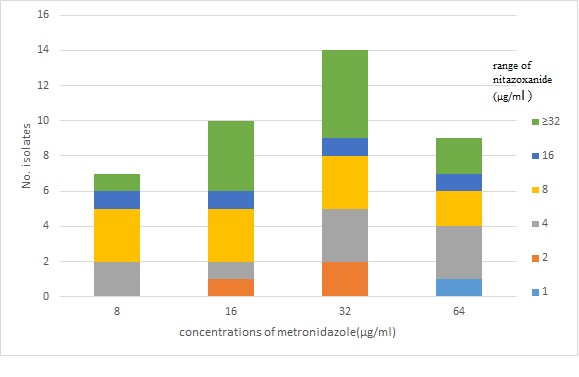
The MIC Values for Nitazoxanide in the Metronidazole Resistant H. pylori Isolates
Discussion
The world around us is full of infectious agents. Gastrointestinal disorders are very common in developing countries with poor health indicators (11). Antibiotic resistance is a main reason for treatment failure. The increasing resistance of microorganisms to antibiotics is commonly due to the lack of proper administration, inappropriate dosing, arbitrary use of drugs, and mutations in microorganisms (11). H. pylori has been recognized as an important human pathogen worldwide. The prevalence of H. pylori in Iran, similar to other developing countries, is higher than in developed countries (12,13). The prevalence of H. pylori infection varies in different areas of Iran (from 30.6% to 93.0%) (14-16). In this study, the estimated infection rate was 45% in two hospitals of Tehran.
H. pylori resistance to metronidazole has been on the rise in Iran: 73.4% in 2009 and 88.2% in 2011 (17). Metronidazole resistant isolates of H. pylori in this study was significantly high (72.7%). Due to the increasing use of metronidazole for various parasitic and oral cavity infections, the emergence of resistant strains is becoming very common (18). Metronidazole is a common antibiotic used for H. pylori treatment in Iran; and drug resistant strains are becoming a major problem for therapeutic regiment (18). Nitazoxanide is a derivative of nitrothiazole. This drug works by disrupting the enzyme pyruvate oxidation and energy metabolism, eventually causing death of microorganisms (19). The American College of Gastroenterology in its 2009 annual conference proposed a quadruple therapy for the treatment of ulcers caused by H. pylori infections. This four-drug regimen (LOAD) includes levofloxacin, omeprazole, alinia (nitazoxanide) and doxycycline (10).
In this study, increased levels of MICs to values up to ≥32µg/ml were confirmed for metronidazole. Significant differences were detected in the levels of MICs values towards nitazoxanide. Although most of H. pylori strains showed MIC values higher than ≥32µg/ml, lower MIC values were found for nitazoxanide at the same concentrations.
Conclusion
Considering the very high levels of metronidazole resistance observed in this study, this medication might not be appropriate as the first-line therapy in Iran. However, considering the relative effectiveness of nitazoxanide, this drug could be an acceptable and satisfactory alternative for patients in Iran.
Acknowledgements
This study was financially supported by a grant (No.26419-30-04-94) from Iran University of Medical Sciences (Tehran, Iran), for which we are very grateful. Our special thanks go to the distinguished gastroenterologist Dr. N. Ebrahimi Daryani who kindly provided the gastric biopsies.
Cite this article as: Baradaran Moghaddam A, Mansouri Sh, Alebouyeh M, Farzi N, Bayati S, Amirmozafari N. Sensitivity to nitazoxanide among metronidazole resistant Helicobacter pylori strains in patients with gastritis. Med J Islam Repub Iran 2016 (8 August). Vol. 30:405.
References
- 1.Haag S, Talley N, Holtmann G. Symptom patterns in functional dyspepsia and irritable bowel syndrome: relationship to disturbances in gastric emptying and response to a nutrient challenge in consulters and non-consulters. Gut. 2004;53(10):1445–51. doi: 10.1136/gut.2003.030049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wotherspoon MR, Path AC. Gastric lymphoma of mucosa-associated lymphoid tissue and Helicobacter pylori. Annual Review of Medicine. 1998;49(1):289–99. doi: 10.1146/annurev.med.49.1.289. [DOI] [PubMed] [Google Scholar]
- 3.Covacci A, Telford JL, Del Giudice G, Parsonnet J, Rappuoli R. Helicobacter pylori virulence and genetic geography. Science. 1999;284(5418):1328–33. doi: 10.1126/science.284.5418.1328. [DOI] [PubMed] [Google Scholar]
- 4.Öztürk E. Öztürk EDiagnostic methods of Helicobacter pylori infectionGulhane Med. J. 2008;50(1):060–4. [Google Scholar]
- 5.Wu H, Shi X, Wang H, Liu J. Resistance of Helicobacter pylori to metronidazole, tetracycline and amoxycillin. Journal of Antimicrobial Chemotherapy. 2000;46(1):121–3. doi: 10.1093/jac/46.1.121. [DOI] [PubMed] [Google Scholar]
- 6.Zullo A, De Francesco V, Hassan C, Morini S, Vaira D. The sequential therapy regimen for Helicobacter pylori eradication: a pooled-data analysis. Gut. 2007;56(10):1353–7. doi: 10.1136/gut.2007.125658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramos-Soriano AG, Black J. Nitazoxanide use as part of an empiric multi-drug regimen in treating children with suspected Helicobacter pylori infection. Case Reports in Gastroenterology. 2015;9(1):36–42. doi: 10.1159/000375116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doumbo O, Traore JF, Diallo AD. Nitazoxanide in the treatment of cryptosporidiosis in 24 AIDS patients with chronic diarrhea in Mali. The American Journal of Tropical Medicine and Hygiene. 1997;26(56):637–39. doi: 10.4269/ajtmh.1997.56.637. [DOI] [PubMed] [Google Scholar]
- 9.Dubreuil LU, Houcke I, Mouton Y, Rossignol JF. In vitro evaluation of activities of nitazoxanide and tizoxanide against anaerobes and aerobic organisms. Antimicrobial Agents and Chemotherapy. 1996;40(10):2266–70. doi: 10.1128/aac.40.10.2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramos-Soriano AG, Black J. Nitazoxanide use as part of an empiric multi-drug regimen in treating children with suspected Helicobacter pylori infection. Case Reports in Gastroenterology. 2015;9(1):36–42. doi: 10.1159/000375116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bohr UR, Primus A, Zagoura A, Glasbrenner B, Wex T, Malfertheiner P. A group‐specific PCR assay for the detection of Helicobacteraceae in human gut. Helicobacter. 2002;7(6):378–83. doi: 10.1046/j.1523-5378.2002.00113.x. [DOI] [PubMed] [Google Scholar]
- 12.Hunt RH, Xiao SD, Megraud F, Leon-Barua R, Bazzoli F, Van der Merwe S. et al. Helicobacter pylori in developing countries. Journal of Gastrointestinal & Liver Diseases. 2011;20(3):299–304. [PubMed] [Google Scholar]
- 13.Novis BH, Gabay G, Naftali T. Helicobacter pylori: the Middle East scenario. The Yale Journal of Biology and Medicine. 1998;71(2):135–41. [PMC free article] [PubMed] [Google Scholar]
- 14.Khedmat H, Karbasi-Afshar R, Agah S, Taheri S. Helicobacter pylori Infection in the general population: A Middle Eastern perspective. Caspian Journal of Internal Medicine. 2013;4(4):745–53. [PMC free article] [PubMed] [Google Scholar]
- 15.Moradi A, Rashidipour A. Seroepidemiology of Helicobacter pylori infection in Semnan. Journal of Koomesh. 2000;1(3):53–7. [Google Scholar]
- 16.Mokhtari M. Evaluation of antibody of Helicobacter pylori infection in preschool children in Isfahan. Iranian Journal of Gastroentrology. 2002;36(6):33–8. [Google Scholar]
- 17.Tomatari F, Mobarez A, Amini M, Hosseini D, Abadi A. Helicobacter pylori resistance to metronidazole and clarithromycin in dyspeptic patients in Iran. Iranian Red Crescent Medical Journal. 2010;12(4):409–12. [Google Scholar]
- 18.Rafeey M, Ghotaslou R, Nikvash S, Hafez A. Primary resistance in Helicobacter pylori isolated in children from Iran. Journal of Infection and Chemotherapy. 2007;13(5):291–5. doi: 10.1007/s10156-007-0543-6. [DOI] [PubMed] [Google Scholar]
- 19.Guttner Y, Windsor HM, Viiala CH, Dusci L, Marshall BJ. Nitazoxanide in treatment of Helicobacter pylori: a clinical and in vitro study. Antimicrobial Agents & Chemotherapy. 2003;47(12):3780–3. doi: 10.1128/AAC.47.12.3780-3783.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]


