Abstract
An enantioselective redox-relay oxidative Heck arylation of 1,1-disubstituted alkenes to construct β-stereocenters was developed using a new pyridyloxazoline ligand. Various 1,2-diaryl carbonyl compounds were readily obtained in moderate yield and good to excellent enantioselectivity. Additionally, analysis of the reaction outcomes using multidimensional correlations revealed that enantioselectivity is tied to specific electronic features of the 1,1-disubstituted alkenol and the extent of polarizability of the ligand.
Graphical Abstract
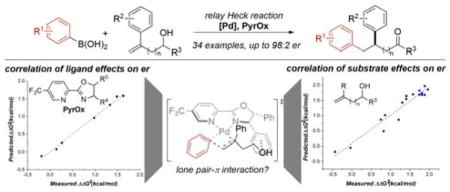
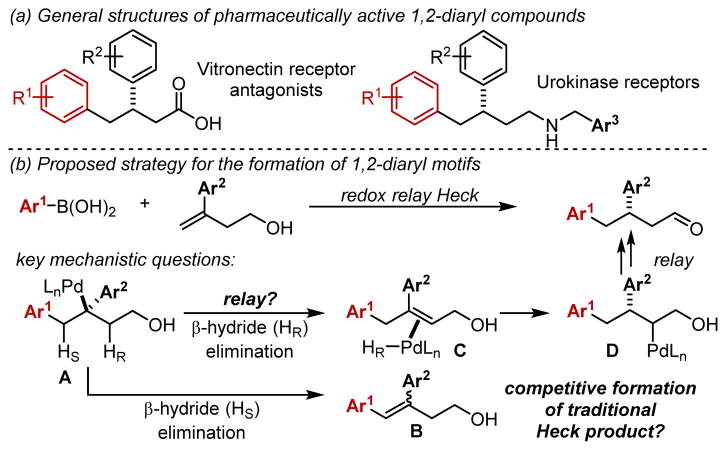
Molecules containing a 1,2-diaryl motif are a common scaffold in various medicinally relevant molecules. For example, several 1,2-diaryl compounds target vitronectin receptor antagonists, implicated in the treatment of osterporosis and breast cancer, and the urokinase receptor, an important cell surface GPI-anchored protein suggested to promote tumor metastasis (Figures 1a).1 Accordingly, various synthetic methods have been developed, including benzylation of cinnamyl compounds,2 diarylation of alkenes,3 isomerization of trisubstituted styrene alcohols,4 and arylation of tert-cyclobutanols via C–C bond cleavage.5 Despite these advances, catalytic enantioselective variants to access 1,2-diaryl carbonyl compounds are rare and suffer from limited substrate scope.6 We saw this dearth of examples as an opportunity to explore our recently developed enantioselective redox-relay Heck reaction of multi-substituted alkenols on 1,1-disubstituted alkenes (Figure 1b).7,8 Successful development of such a protocol would provide direct and modular access to enantiomerically enriched 1,2-diaryl carbonyl structures.
Figure 1.
Effective drug candidates containing 1,2-diaryl motifs and proposed strategy.
In contrast to our previous reports, initial migratory insertion of the Pd-Ar species into 1,1-disubstituted alkenols forms a new C–C bond at the terminal position of the alkene, and the chiral center is established β-to this site (A, Figure 1b).9 A central concern for this substrate class involves selective β-hydride elimination. Specifically, β-hydride elimination of HS (Hstyrenyl) would yield the traditional Heck product, trisubstituted styrene B, or β-hydride elimination of HR (Hrelay) would ultimately lead to the desired aldehyde compound. The styrenyl product is not only thermodynamically more stable but is also historically formed using terminal alkenes under similar conditions.10 Herein, we present the development of a successful enantioselective redox-relay Heck reaction of 1,1-homoallylic alcohols forming the desired 1,2-diaryl structures. This outcome required the use of a new pyridyl-oxazoline (PyrOx) ligand to overcome the propensity of trisubstituted-styrene formation and achieve high enantioselectivity with this new alkene class.
On the basis of our previous success, chiral PyrOx ligand L1 was first examined with Pd(CH3CN)4(OTf)2 as a precatalyst under standard redox-relay oxidative Heck conditions7c using 1,1-disubstituted alkene 1a and p-chlorophenylboronic acid as model coupling partners. When DMF was used as solvent, the desired relay product (2e) was only produced in 24% yield and a disappointing 69.5:30.5 er (entry 1). The mass balance was a combination of unreacted starting material and the traditional Heck product. To improve the reaction outcome, we evaluated several PyrOx ligands with modifications (entries 2–4) to the oxazoline portion. By incorporating aryl groups into the ligand structure, potential involvement of noncovalent π interactions between the ligand substituent and the substrate became evident. A considerable enhancement was observed using ligand L4 (89:11 er) in comparison to L1 (69.5:30.5 er), which lacks an aryl group (a putative explanation is discussed below). In further optimization of the reaction conditions, solvent proved the most important variable as illustrated with initial identification of MeOH as the reaction solvent (entries 7–8). Ultimately, a combination of MeOH and MTBE provided the best results in terms of both yield and enantioselectivity, yielding product 2e in 63% and 95.5:4.5 er (entry 9).
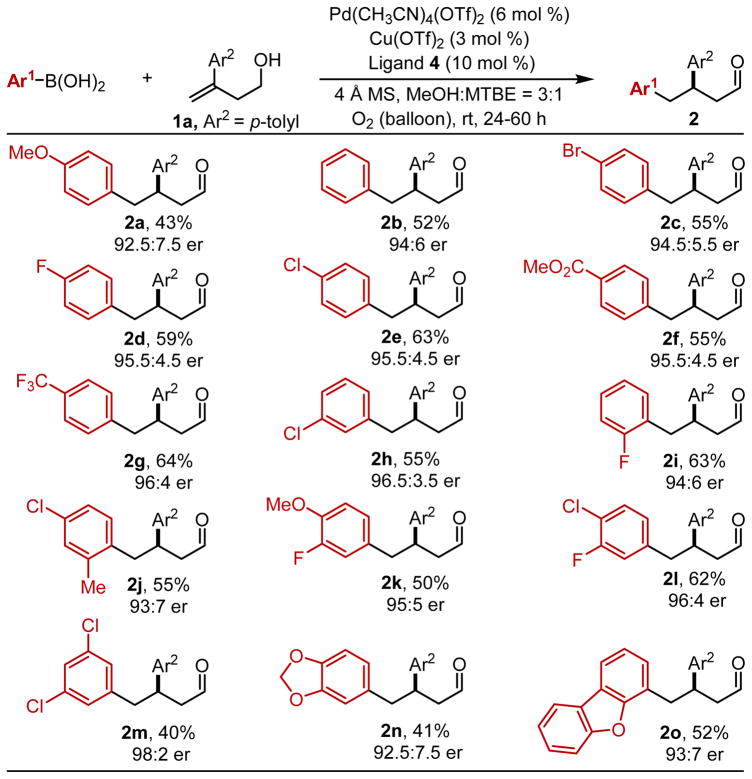
Using these optimal conditions, the reaction scope was explored with a number of arylboronic acids (Table 2). In general, the desired 1,2-diaryl carbonyl compounds were obtained with moderate yields and good to excellent enantiomeric ratios. Again, the mass balance was mainly composed of the traditional Heck product. Compared with p-chlorophenylboronic acid (2e), the use of electron-rich arylboronic acids decreased the yield and the enantioselectivity (2a, 2b), while electron-withdrawing groups in the meta- or para-position did not have a significant effect (2c–h). This is consistent with a greater propensity for β-hydride elimination at HS with adjacent electron-rich arenes observed previously in our lab.4c,10 Substituents at the ortho-position led to a modest reduction in enantioselectivity (2i, 2j). Finally, multi-substituted aryl-boronic acids were tested in this reaction (2j–o). In particular, the use of 3,5-dichlorophenylboronic acid afforded the desired product 2m in 98:2 er. It should also be noted that a simple heteroaromatic boronic acid, 2-dibenzofuranyl, was successfully incorporated, providing the corresponding product 2o in 52% yield and 93:7 er. However, other Lewis basic boronic acids were incompatible under these reaction conditions.11
Table 2.
Arylboronic Acid Scopea
Ar2 = p-tolyl.
Each entry represents the isolated yield on 0.3 mmol scale and used 3.0 equivs of arylboronic acid. Er values were determined by SFC.
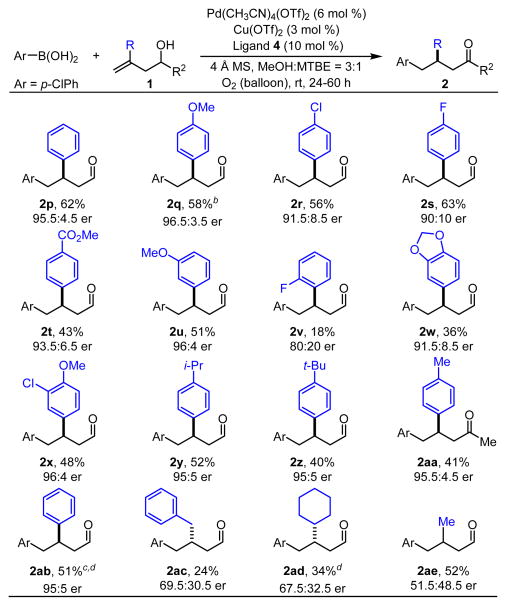
Next, substituent effects on the alkenol (1) were evaluated under the standard reaction conditions (Table 3). The electronic nature of para-substituents on the styrenyl moiety had a greater influence on the enantioselectivity than was observed with substituted arylboronic acids. In this case, electron-donating groups led to higher enantioselectivity (2q–t). Unfortunately, an ortho-fluorine was not well-tolerated, resulting in a significant decrease in yield and enantioselectivity (2v). It is noteworthy that a substrate containing a secondary alcohol gave 95.5:4.5 er, although in lower yield than observed with a primary alcohol (2aa compared to 2e).
Table 3.
Scope of the Homoallylic Alcoholsa
Ar = p-chlorophenyl.
Each entry represents the isolated yield on 0.3 mmol scale and used 3.0 equivs of arylboronic acid. Er values were determined by SFC.
On 3 mmol scale, 2q was isolated in 55% yield and 95.5:4.5 er.
Phenylboronic acid was used.
The absolute configuration was determined to be (R) and thus the rest of the products, except 2ac and 2ad, were assigned as (R) by analogy. The absolute configuration of 2ad was determined to be (S) and thus 2ac was assigned as (S) by analogy.12
As the enantiodetermining step is likely migratory insertion of the alkene (C to D, Figure 1b),9a we hypothesized that the reaction of an 1,1-disubstituted alkene with two similar alkyl substituents would lead to low enantioselectivity due to a difficult differentiation of the substituents by the catalyst. Indeed, when alkyl-substituted alkenols were explored, the corresponding products were obtained with poor er and opposite face selection (2ad–ae). Additionally, if the aryl group is not in conjugation with the alkene, low enantioselectivity was observed (2ac).
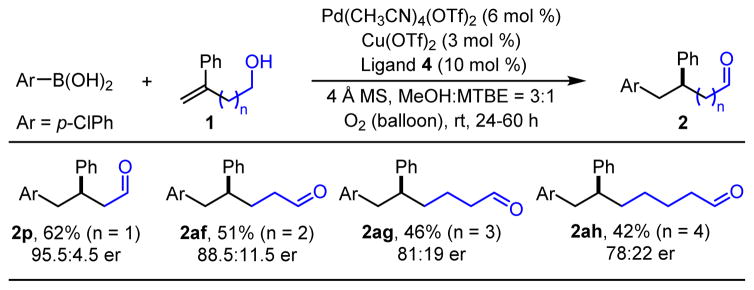
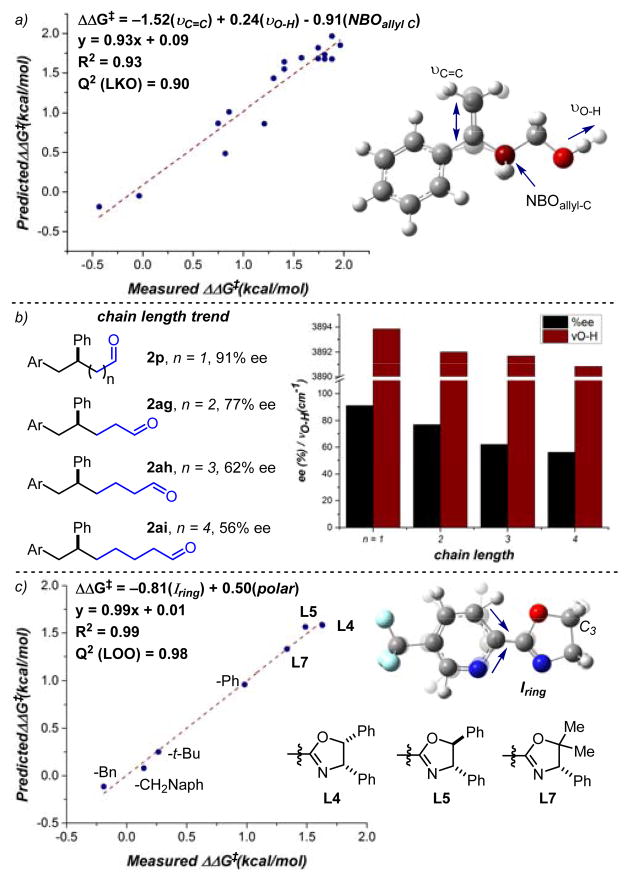
In our previous redox-relay Heck reports, the chain length between the alcohol and the alkene minimally influenced the enantioselectivity, albeit none of these examples were styrenyl in nature and used ligand 1 exclusively. Therefore, the profound effect of chain length on enantioselectivity in this system was surprising (Table 4). The bis-homoallylic alcohol substrate 1af delivered desired product 2af in 51% yield and 88.5:11.5 er, a significantly diminished result as compared to the model homoallylic substrate (1p). The yields and enantioselectivities continued to decrease as a function of increasing chain length (2ag, 2ah).
Table 4.
Chain-Length Effecta
Ar = p-chlorophenyl.
Each entry represents the isolated yield on 0.3 mmol scale and used 3.0 equivs of p-chlorophenylboronic acid. Er values were determined by SFC.
As the primary goal was to synthesize 1,2-diaryl compounds as described in Figure 1, we focused on the functionalization of homoallylic alcohols. Nonetheless, the observed product enantioselectivities with longer chain lengths are disappointing from the perspective of broadening the scope of the reaction. In order to facilitate a deeper understanding of the limitations of this system and potentially lend insight to future solutions, analysis of the data sets in terms of both the substrate and ligand effects on enantioselectivity was performed.
A multidimensional modeling strategy developed within our group was executed.13 Through this iterative process, relevant molecular descriptors are related to the reaction output, enantioselectivity, through linear regression modeling techniques. When analyzing the effect of substrate identity on the enantioselectivity in this reaction, a correlation was identified with three terms: the alkene C=C vibration (νC=C), the O–H stretching frequency (νO-H), and the NBO charge of the allylic carbon (NBOallyl-C, Figure 2a). The electronic nature of the alkene, modulated by the variable substituent, is likely described using the alkene stretching frequency, νC=C, and thus plays a crucial role in the alkene face selection and enantiodetermining step. The chain length effect can be expressed using νO-H, as demonstrated by the univariate correlation of this parameter to the enantioselectivities of products 2p and 2ag–ai (R2 = 0.91, Figure 2b). As this stretching frequency decreases based on the distance between the alcohol and the alkene, the observed enantioselectivities also decrease. A similar trend between the enantioselectivities of these products with varying chain lengths and NBOallyl-C was also identified with increasingly negative charge of the allylic carbon correlating with higher enantioselectivity (R2 = 0.92, Figure S6). Both of these trends suggest that the polarization of the alkyl chain due to the distance between the alcohol and the alkene is partially responsible for face discrimination of the substrate that is required for an enantioselective migratory insertion. We proposed a similar hypothesis in rationalizing site selectivity trends in the redox-relay Heck reaction of 1,2-disubstituted alkenols.7b,9a Further, these trends could also indicate that the electronic and geometric nature of the alcohol itself is an important element for catalyst recognition. Overall, identifying these trends revealed several inherent properties of the alkenol, including the electronic character of the alkene and the alcohol, that are required for high er.
Figure 2.
a) Multidimensional correlation of substrate properties and enantioselectivities (Leave-K-Out (LKO), K=6). b) Univariate trend between the chain length and the alcohol stretching frequency. c) Multidimensional correlation of ligand effects and enantioselectivies (Leave-One-Out (LOO)).
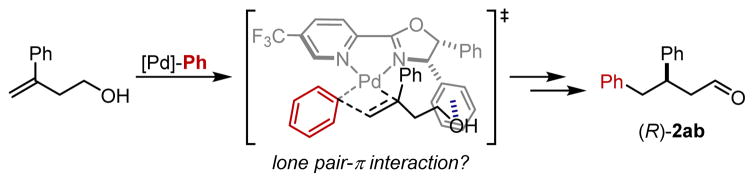
To gain further insight into this process, the focus shifted to identifying the subtle structural effects of the ligand on the enantiodetermining step in this reaction. Using the same modeling strategy, properties of seven ligands with changes to the oxazoline were related to the measured enantioselectivities. An excellent correlation was identified with two terms: an IR intensity of a specific ring vibration on the pyridine (Iring 3) and the ligand’s computed polarizability (polar, Figure 2c). Upon closer examination of the corresponding ring stretch, it was observed that the C–C bond between heterocycles and the hydrogens or substituents at C3 are also affected by this stretch. Consequently, the intensity of this frequency is likely accounting for steric effects as well as the electronic contributions from the substituents on the oxazoline. Additionally, the polarizability, or the extent of electron cloud distortion in the presence of an external electric field, could represent the ability of the ligand’s substituents to interact in an attractive manner with the substrate.14 As the electronic natures of the alkene and the alcohol were identified as significant influences in enabling a highly enantioselective method, a positive interaction between the aryl substituent or alcohol of the substrate and the ligand, such as a π-stacking or lone pair-π interaction, is plausible.15 Certain geometric constraints are also observed within these data, including the requirement of a styrenyl substrate, homoallylic alcohol and a Ph-substituted oxazoline for high er values. Since attractive interactions are more sensitive to the distance between the two interacting species,16 these geometric requisites support the presence of an attractive noncovalent interaction between the ligand and the substrate. By combining the insight gained from the substrate and ligand effects as well as our previous computational report, 9a a working hypothesis on the origin of enantioselectivity is proposed (Figure 3). The proposed transition state, which would lead to the observed product enantiomer, features a lone pair-π interaction between the alcohol and the phenyl group on the ligand.15c This interaction is consistent with the requirements described above.
Figure 3.
Proposed lone pair-π interaction as a controlling element in the transition state.
In summary, we have successfully developed a method to synthesize enantiomerically enriched 1,2-diaryl carbonyl compounds, a common pharmacophore, in good to high enantioselectivity. The insight gained through uni- and multivariate correlations has revealed key properties in both the substrate and ligand, leading to a proposed attractive interaction responsible for achieving a highly selective process. Detailed mechanistic studies are in progress to investigate the unexpected effect of chain length on enantioselectivity and the potential role of noncovalent π interactions as controlling elements in the enantiodetermining step.
Supplementary Material
Table 1.
Optimization.a
| ||||
|---|---|---|---|---|
| entry | ligand | solvent | % yield | er |
| 1 | L1 | DMF | 24 | 69.5:30.5 |
| 2 | L2 | DMF | 30 | 80:20 |
| 3 | L3 | DMF | 35 | 51:49 |
| 4 | L4 | DMF | 30 | 89:11 |
| 5 | L4 | acetone | 24 | 86:14 |
| 6 | L4 | dioxane | 20 | 90:10 |
| 7 | L4 | MeOH | 57 | 95.5:4.5 |
| 8b | L4 | MeOH | 49 | 96.5:3.5 |
| 9 | L4 | MeOH:MTBE = 3:1 | 63 | 95.5:4.5 |
|
| ||||
|
| ||||
Each entry represents the isolated yield on 0.3 mmol scale at rt and used 3.0 equivs of p-chlorophenylboronic acid unless otherwise noted. Er values were determined by SFC.
The reaction was performed at 10°C.
Acknowledgments
The synthetic aspects were supported by NIH (R01GM063540) and the modeling was supported by NSF (CHE-1361296). We gratefully acknowledge the Center for High Performance Computing (CHPC) at the University of Utah. Z.-M.C. would like to thank Shanghai Jiao Tong University for a postdoctoral fellowship.
Footnotes
Supporting Information Available: Experimental details, analytical data for products, NMR spectra of products, computations and modeling details can be found in the supporting information. The Supporting Information is available free of charge via the Internet at http://pubs.acs.org/.
References
- 1.(a) Manley PJ, Miller WH, Uzinskas IN SmithKline Beecham Corporation. 1218005 A2. Patent EP. 2002 Jul 3;; (b) Millera WH, Manley PJ, Cousins RD, Erhard KF, Heerding DA, Kwon C, Ross ST, Samanen JM, Takata DT, Uzinskas IN, Yuan CCK, Haltiwanger RC, Gress CJ, Lark MW, Hwang SM, James IE, Rieman DJ, Willette RN, Yue TL, Azzarano LM, Salyers KL, Smith BR, Ward KW, Johanson KO, Huffman WF. Bioorg Med Chem Lett. 2003;13:1483. doi: 10.1016/s0960-894x(03)00102-1. [DOI] [PubMed] [Google Scholar]; (c) Wang F, Li J, Sinn AL, Knabe WE, Khanna M, Jo I, Silver JM, Oh K, Li L, Sandusky GE, Sledge GW, Nakshatri H, Jones DR, Pollok KE, Meroueh SO. J Med Chem. 2011;54:7193. doi: 10.1021/jm200782y. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Hasui T, Matsunaga N, Ora T, Ohyabu N, Nishigaki N, Imura Y, Igata Y, Matsui H, Motoyaji T, Tanaka T, Habuka N, Sogabe S, Ono M, Siedem CS, Tang TP, Gauthier CG, De Meese LA, Boyd SA, Fukumoto S. J Med Chem. 2011;54:8616. doi: 10.1021/jm2011645. [DOI] [PubMed] [Google Scholar]; (e) Kim M, Ryu J, Hah J. Bioorg Med Chem Lett. 2013;23:1639. doi: 10.1016/j.bmcl.2013.01.082. [DOI] [PubMed] [Google Scholar]; (f) Irannejad H, Unsal Tan O, Ozadali K, Dadashpour S, Tuylu Kucukkilinc T, Ahangar N, Ahmadnejad M, Emami S. Chem Biol Drug Des. 2015;85:494. doi: 10.1111/cbdd.12435. [DOI] [PubMed] [Google Scholar]; (g) Sun J, Chen L, Liu C, Wang Z, Zuo D, Pan J, Qi H, Bao K, Wu Y, Zhang W. Chem Biol Drug Des. 2015;86:1541. doi: 10.1111/cbdd.12617. [DOI] [PubMed] [Google Scholar]; (h) Koo KA, Lorent K, Gong W, Windsor P, Whittaker SJ, Pack M, Wells RG, Porter JR. Chem Res Toxicol. 2015;28:1519. doi: 10.1021/acs.chemrestox.5b00227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.(a) Lenz GR. J Org Chem. 1988;53:5791. [Google Scholar]; (b) Mandal SK, Jana S, Roy SC. Tetrahedron Lett. 2005;46:6115. [Google Scholar]; (c) Franzoni I, Guénée L, Mazet C. Org Biomol Chem. 2015;13:6338. doi: 10.1039/c5ob00702j. [DOI] [PubMed] [Google Scholar]
- 3.(a) Trejos A, Fardost A, Yahiaoui S, Larhed M. Chem Commun. 2009:7587. doi: 10.1039/b918358b. [DOI] [PubMed] [Google Scholar]; (b) Lundin MP, Esquivias J, Fu CG. Angew Chem, Int Ed. 2009;48:154. doi: 10.1002/anie.200804888. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Urkalan BK, Sigman MS. Angew Chem, Int Ed. 2009;48:3146. doi: 10.1002/anie.200900218. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) René O, Lapointe D, Fagnou K. Org Lett. 2009;11:4560. doi: 10.1021/ol901799p. [DOI] [PubMed] [Google Scholar]; (e) Stokes BJ, Liao L, de Andrade AM, Wang Q, Sigman MS. Org Lett. 2014;16:4666. doi: 10.1021/ol502279u. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) You W, Brown MK. J Am Chem Soc. 2014;136:14730. doi: 10.1021/ja509056j. [DOI] [PubMed] [Google Scholar]; (g) Tang C, Xie Z. Angew Chem, Int Ed. 2015;54:7662. doi: 10.1002/anie.201502502. [DOI] [PubMed] [Google Scholar]; (h) You W, Brown MK. J Am Chem Soc. 2015;137:14578. doi: 10.1021/jacs.5b10176. [DOI] [PubMed] [Google Scholar]
- 4.(a) Li H, Mazet C. Org Lett. 2013;15:6170. doi: 10.1021/ol403023x. [DOI] [PubMed] [Google Scholar]; (b) Larionov E, Lin L, Guénée L, Mazet C. J Am Chem Soc. 2014;136:16882. doi: 10.1021/ja508736u. [DOI] [PubMed] [Google Scholar]
- 5.Ziadi A, Martin R. Org Lett. 2012;14:1266. doi: 10.1021/ol300119u. [DOI] [PubMed] [Google Scholar]
- 6.(a) Weisenburger GA, Beak P. J Am Chem Soc. 1996;118:12218. [Google Scholar]; (b) Bull SD, Davies SG, Nicholson RL, Sanganee HJ, Smith AD. Org Biomol Chem. 2003;1:2886. doi: 10.1039/b305623f. [DOI] [PubMed] [Google Scholar]; (c) Li T, Zhu J, Wu D, Li X, Wang S, Li H, Li J, Wang W. Chem–Eur J. 2013;19:9147. doi: 10.1002/chem.201300304. [DOI] [PubMed] [Google Scholar]; (d) Dell’Amico L, Companyó X, Naicker T, Bräuer TM, Jørgensen KA. Eur J Org Chem. 2013:5262. [Google Scholar]; (e) Alonso DA, Kitagaki S, Utsumi N, Barbas CF. Angew Chem, Int Ed. 2008;47:4588. doi: 10.1002/anie.200801088. [DOI] [PubMed] [Google Scholar]; (f) Seo WS, Kim S. Tetrahedron Lett. 2012;53:2809. [Google Scholar]; (g) Izquierdo J, Orue A, Scheidt AK. J Am Chem Soc. 2013;135:10634. doi: 10.1021/ja405833m. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Lu P, Jackson JJ, Eickhoff JA, Zakarian A. J Am Chem Soc. 2015;137:656. doi: 10.1021/ja512213c. [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Nishimura T, Matsumura S, Maeda Y, Uemura S. Chem Commun. 2002:50. doi: 10.1039/b107736h. [DOI] [PubMed] [Google Scholar]; (j) Matsumura S, Maeda Y, Nishimura T, Uemura S. J Am Chem Soc. 2003;125:8862. doi: 10.1021/ja035293l. [DOI] [PubMed] [Google Scholar]; (k) Zhang C, Yun J. Org Lett. 2013;15:3416. doi: 10.1021/ol401468v. [DOI] [PubMed] [Google Scholar]
- 7.For selected our group works, see: Werner EW, Mei TS, Burckle AJ, Sigman MS. Science. 2012;338:1455. doi: 10.1126/science.1229208.Mei TS, Werner EW, Burckle AJ, Sigman MS. J Am Chem Soc. 2013;135:6830. doi: 10.1021/ja402916z.Mei TS, Patel HH, Sigman MS. Nature. 2014;508:340. doi: 10.1038/nature13231.
- 8.For selected other group works, see: Larock RC, Leung WY, Stolz-Dunn S. Tetrahedron Lett. 1989;30:6629.Bouquillon S, Ganchegui B, Estrine B, Hénin F, Muzart J. J Organomet Chem. 2001;634:153.Berthiol F, Doucet H, Santelli M. Tetrahedron. 2006;62:4372.Correia CRD, Oliveira CC, Salles AG, Jr, Santos EAF. Tetrahedron Lett. 2012;53:3325.Oliveira CC, Angnes RA, Correia CRD. J Org Chem. 2013;78:4373. doi: 10.1021/jo400378g.Angnes RA, Oliveira JM, Oliveira CC, Martins NC, Correia CRD. Chem–Eur J. 2014;20:13117. doi: 10.1002/chem.201404159.Oliveira CC, Pfaltz A, Correia CRD. Angew Chem, Int Ed. 2015;54:14036. doi: 10.1002/anie.201507927.
- 9.(a) Xu LP, Hilton MJ, Zhang X, Norrby PO, Wu YD, Sigman MS, Wiest O. J Am Chem Soc. 2014;136:1960. doi: 10.1021/ja4109616. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Hilton MJ, Xu LP, Norrby PO, Wu YD, Wiest O, Sigman MS. J Org Chem. 2014;79:11841. doi: 10.1021/jo501813d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.For selected recent works, see: Parrish JP, Jung YC, Shin S, Jung KW. J Org Chem. 2002;6:7127. doi: 10.1021/jo020159p.Werner EW, Sigman MS. J Am Chem Soc. 2010;132:13981. doi: 10.1021/ja1060998.Werner EW, Sigman MS. J Am Chem Soc. 2011;133:9692. doi: 10.1021/ja203164p.
- 11.No product was observed using 3-pyridinylboronic acid, 3-furanylboronic acid and 3-thienylboronic acid.
- 12.For details on the assignment of absolute configuration, please see the supporting information.
- 13.Sigman MS, Harper KC, Bess EN, Milo A. Acc Chem Res. 2016;49:1292. doi: 10.1021/acs.accounts.6b00194. [DOI] [PubMed] [Google Scholar]
- 14.(a) Guckian KM, BA, Ren RX, Sheils CJ, Tahmassebi DC, Kool ETJ. J Am Chem Soc. 2000;122:2213. doi: 10.1021/ja9934854. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Watt M, Hardebeck LKE, Kirkpatrick CC, Lewis M. J Am Chem Soc. 2011;133:3854. doi: 10.1021/ja105975a. [DOI] [PubMed] [Google Scholar]
- 15.(a) Raju RK, Bloom JWG, An Y, Wheeler SE. Chem-PhysChem. 2011;12:3116. doi: 10.1002/cphc.201100542. [DOI] [PubMed] [Google Scholar]; (b) Sherrill CD. Acc Chem Res. 2013;46:1020. doi: 10.1021/ar3001124. [DOI] [PubMed] [Google Scholar]; (c) Singh SK, Das A. Phys Chem Chem Phys. 2015;17:9596. doi: 10.1039/c4cp05536e. [DOI] [PubMed] [Google Scholar]
- 16.Biedermann F, Schneider HJ. Chem Rev. 2016;116:5216. doi: 10.1021/acs.chemrev.5b00583. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.