Abstract
Introduction
Improvements in risk stratification for sudden cardiac arrest (SCA) will require discovery of markers that extend beyond the LV ejection fraction (LVEF). The frontal QRS-T angle has been shown to predict risk of SCA but the value of this marker independent of the LVEF has not been investigated.
Methods and Results
Cases of adult SCA with an archived electrocardiogram (12-lead ECG) available before the event, with a computable frontal QRS-T angle, were identified from the Oregon Sudden Unexpected Death Study (Oregon SUDS) ongoing in the Portland, Oregon metro area. A total of 666 SCA cases (mean age 67.2 years; 95% CI, 52.3 to 82.1 years; 68.6% males) were compared to 863 controls (mean age 66.6 years, 55.2 to 78.0 years; 68.1% males; 75.0% had CAD) from the same geographical region. The mean frontal QRS-T angle was wider in cases (74°; 95% CI, 17°–131°) compared to controls (51°; 95% CI, 5°–97° p<0.0001). A frontal QRS-T angle of more than 90° remained associated with increased risk of SCD after adjusting for age, gender, heart rate, prolonged intraventricular conduction, electrocardiographic left ventricular hypertrophy (ECG LVH), baseline comorbidities and left ventricular ejection fraction (LVEF) (OR 2.2; 95% CI, 1.60–3.09; p<0.0001).
Conclusion
A wide QRS-T angle greater than 90° is associated with an increased risk of SCA independent of the left ventricular ejection fraction.
Keywords: electrocardiogram, QRS-T angle, sudden cardiac arrest, sudden death
Introduction
Sudden cardiac arrest (SCA) is a major public health challenge, representing approximately 20% of all mortality in industrialized countries and, more importantly, close to 50% of these victims are not previously diagnosed to have cardiac disease.1 Based on current guidelines, only one-third of SCA patients would have been eligible for an implantable cardioverter-defibrillator (ICD)2 and even in the subgroup with an ICD implanted, only a small proportion are destined to receive appropriate therapies.3,4 Hence, there is a need to look beyond the left ventricular ejection fraction (LVEF) in order to enhance SCA risk stratification. Markers of abnormal ventricular repolarization identified from the standard 12-lead electrocardiogram (ECG) have garnered considerable interest in this regard.5 One of these is the frontal QRS-T angle, defined as the absolute difference between the QRS and T-wave axis.6,7 Several studies have identified an abnormally wide QRS-T angle as a predictor of cardiovascular morbidity and mortality but the majority of these have used the spatial rather than frontal QRS-T angle.8–10 Since the frontal QRS-T angle is a reasonable substitute for the spatial QRS-T angle for risk prediction11 and does not require additional software for calculation, it makes it an attractive tool for the everyday clinician.
A recent Finnish population-based study established that a frontal QRS-T angle of ≥100° conferred over a two-fold increase in the risk of arrhythmic death but echocardiographic measures were not available, so the relationship to the left ventricular ejection fraction (LVEF) could not be evaluated.12 Given the importance of extending risk markers beyond the LVEF, we evaluated the potential independent association of the abnormally wide frontal QRS-T angle with SCA risk, from the ongoing Oregon Sudden Unexpected Death Study (Oregon SUDS).
Methods
The Oregon Sudden Unexpected Death Study (Oregon SUDS) prospectively identifies cases of SCA from out-of-hospital cardiac arrests in the Portland, Oregon, metropolitan region (population of approximately 1 million). Detailed methods have been published previously.2,3,13–18 Briefly, SCA cases were identified using multiple sources, including the emergency medical services (EMS) response system, the medical examiner’s office and local emergency departments, following a comprehensive process of in-house adjudication by 3 physicians carried out to determine cases meeting pre-specified criteria for SCA. SCA was defined as a sudden, unexpected, pulseless condition of likely cardiac origin, including survivors. If unwitnessed, subjects had to be last seen alive in a normal state of health within the preceding 24 hours. Subjects with terminal illnesses such as cancer, traumatic deaths, drug overdoses and noncardiac causes of cardiac arrest such as pulmonary embolism were excluded.
In the present study, SCA cases from 2002 till 2015 were compared to controls, with and without coronary artery disease (CAD), from the same geographical area in the same time frame. The analysis was restricted to cases and controls aged ≥18 years with a resting 12-lead ECG available. For cases, this ECG had to be archived prior and unrelated to the SCA event; and for controls, the ECG was obtained at the time of study enrollment visit or from clinic or hospital visits unrelated to the study. Since ≥80% of subjects with SCD were previously demonstrated to have significant CAD on autopsy,19 we chose a control group with a majority proportion of CAD but without history of SCA. Subjects were categorized as having CAD if they had a ≥50% stenosis of an epicardial coronary artery or history of myocardial infarction (MI), coronary bypass graft surgery or percutaneous coronary intervention. Controls were identified from patients undergoing coronary angiography at one of the region’s major participating health systems or from patients transported by the region’s EMS system with symptoms suggestive of coronary ischemia. Subjects with paced rhythm or missing values of the QRS or T-wave axis were excluded from this study. The study was approved by the Institutional Review Boards of Cedars-Sinai Medical Center, Oregon Health and Science University and all participating hospitals and health systems.
Electrocardiographic measurements
A standard 12-lead ECG tracing at 25 mm/s paper speed and 10 mm/mV amplitude was used for analysis. The frontal QRS-T angle was calculated as the absolute difference in value between the frontal plane QRS axis and T-wave axis. If the difference between the QRS axis and T-wave axis was >180°, the resultant QRS-T angle would be calculated as 360° minus the absolute angle to obtain a value between 0° to 180°. Individual ECGs with a narrow and wide QRS-T angle along with a schematic illustrating how the QRS-T angles are derived are shown in Figures 1 and 2. A normal QRS axis was defined as −30° to 90° inclusive20 and a normal T-wave axis as −15° to 105° inclusive in accordance to previous literature.21,22 Subjects with abnormal QRS axes were further categorized into left-axis deviation (−31° to −90°), right-axis deviation (91° to 180°) or extreme QRS axis (181° to 269°)20. Measurements of ECG intervals were conducted manually using digital onscreen software (Datainf Measure: DataInf GmbH; Tübingen, Germany). Prolonged intraventricular conduction was defined as QRS duration (QRSD) >110ms including right and left bundle branch blocks and nonspecific intraventricular conduction disturbance (IVCD) defined in accordance to published recommendations.20 Subjects were categorized as having electrocardiographic left ventricular hypertrophy (ECG LVH) if they met either Sokolow-Lyon criteria (RV1 +SV5,6 ≥35mm) or Cornell criteria (RaVL + SV3 >20mm in females and >28mm in males).23,24.
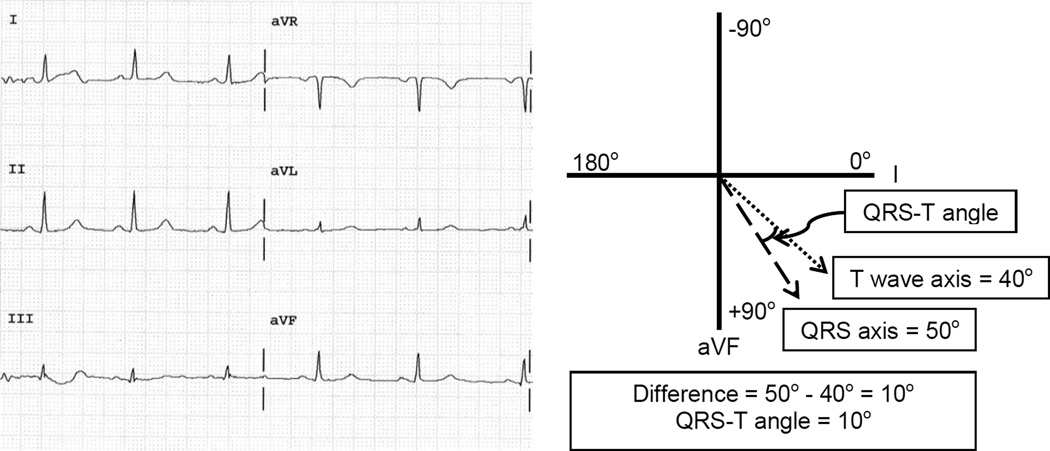
Figure 1.
Electrocardiogram of a subject with a narrow QRS-T angle with a schematic depicting how the QRS-T angle is derived. The QRS axis measures 50° while the T wave axis measures 40° giving a QRS-T angle of 10°.
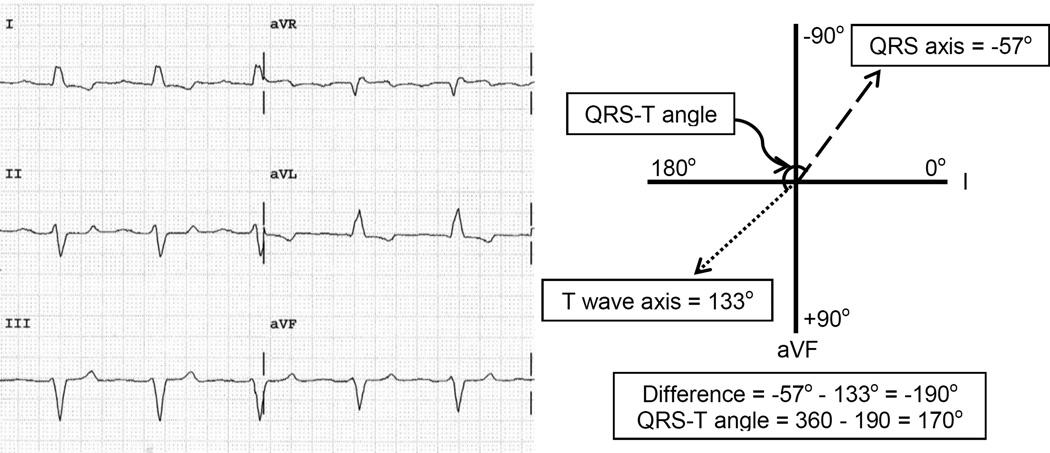
Figure 2.
Electrocardiogram of a subject with a wide QRS-T angle with a schematic depicting how the QRS-T angle is derived. The QRS axis measures −57° while the T wave axis measures 133° giving a QRS-T angle of 170°. When the difference between the QRS axis and T wave axis is >180°, the QRS-T angle is taken to be the 360 minus the absolute difference to obtain a value between 0° and 180°.
LVEF was assessed by echocardiograms, angiograms or multi-gated acquisition scans that were performed before and unrelated to the SCA event, and severely reduced LVEF was defined as LVEF ≤35%.
Statistical analysis
All analyses were performed using SPSS version 21.0 (SPSS Inc., Chicago, IL, USA). Independent-samples t tests and Pearson χ2 tests were used for univariate case-control comparisons of continuous and categorical variables, respectively. For variables with missing values, proportions and P values were calculated using the non-missing data as the denominator. Multiple logistic regression models were used to estimate the odds ratios for SCA. Two models including all potentially associated variables were analyzed. Values are presented as n (%) or mean (95% CI) and a P value of ≤0.05 was considered significant for all analyses. A receiver-operating characteristics (ROC) curve analysis was done to measure the predictive ability of QRS-T angle as a continuous variable. The Youden index, calculated as sensitivity + specificity – 1, is a statistical index often used in conjunction with the ROC analysis to compare the diagnostic performance of test values.25 This index was used to evaluate several QRS-T angle cut-offs.
Results
A total of 1573 subjects were identified. Of these, 39 (2.5%) subjects were excluded due to missing values on QRS axis or T-wave axis and 5 (0.3%) were excluded due to a paced rhythm. Hence, 1529 subjects met inclusion criteria for this analysis, comprising of 666 SCA cases (mean age 67.2 years; 95% CI, 52.3 to 82.1 years; 68.6% males; 84.1% whites) and 863 controls with and without CAD (mean age 66.6 years, 55.2 to 78.0 years; 68.1% males; 92.8% whites; 75.0% had CAD) from the same geographical region. Their baseline characteristics, comorbidities, ECG parameters and LVEF are shown in Table 1. Cases were older with more comorbidities compared to controls. They were also more likely to have prolonged intraventricular conduction, abnormal QRS axis, ECG LVH, higher heart rate and severely impaired LVEF versus controls. The mean frontal QRS-T angle was wider in cases (74°; 95% CI, 17°–131°) compared to controls (51°; 95% CI, 5°–97° p<0.0001), but the proportion of subjects with an abnormal T-wave axis did not differ among cases and controls (11.0% vs 10.1%, respectively; p=0.577).
Table 1.
Baseline Characteristics, ECG parameters and LVEF Among Cases and Controls
| Total Subjects (n=1529) | |||
|---|---|---|---|
| Variable | Cases (n=666) | Controls (n=863) | P value |
| Age, yrs | 67.2 (52.3–82.1) | 66.6 (55.2–78.0) | 0.403 |
| Male gender | 457 (68.6) | 588 (68.1) | 0.84 |
| Hypertension | 495 (74.3) | 590 (68.6) | 0.015 |
| Diabetes mellitus | 269 (40.4) | 228 (26.5) | <0.0001 |
| Total cholesterol, mg/dL* | 176.7 (126.9–226.5) | 173.1 (128.3–217.9) | 0.231 |
| Previous MI | 280 (42.0) | 303 (35.2) | 0.007 |
| Heart rate, bpm | 78.5 (59.9–97.1) | 68.2 (53.2–83.2) | <0.0001 |
| QRS duration, ms | 102.8 (77.9–127.7) | 98.1 (77.1–119.1) | <0.0001 |
| Prolonged intraventricular conduction |
176 (26.4) | 163 (18.9) | <0.0001 |
| LBBB | 44 (25.0) | 38 (23.3) | |
| RBBB | 50 (28.4) | 59 (36.2) | |
| Others | 82 (46.6) | 66 (40.5) | |
| ECG LVH | 114 (17.1) | 103 (11.9) | 0.004 |
| Abnormal QRS axis | 168 (25.2) | 153 (17.7) | 0.0004 |
| Left-axis deviation | 115 (17.3) | 115 (13.3) | |
| Right-axis deviation | 37 (5.6) | 33 (3.8) | |
| Extreme QRS axis | 16 (2.4) | 5 (0.6) | |
| Abnormal T axis | 73 (11.0) | 87 (10.1) | 0.577 |
| QRS-T angle, ° | 74.0 (17.4–130.6) | 51.0 (4.8–97.2) | <0.0001 |
| LVEF, %† | 49.2 (32.9–65.5) | 53.9 (40.7–67.1) | <0.0001 |
| Severely impaired LVEF† | 82 (24.8) | 54 (13.4) | <0.0001 |
Data are presented as n (%) or mean (95% CI). Bpm denotes beats per minute; LBBB, left bundle branch block; LVEF, left ventricular ejection fraction; ECG LVH, electrocardiographic left ventricular hypertrophy; MI, myocardial infarction; RBBB, right bundle branch block.
Prolonged intraventricular conduction defined as QRS duration (QRSD) >110ms; LBBB and RBBB, in accordance to published recommendations;19 Others include incomplete LBBB, incomplete RBBB and non-specific intraventricular disturbance; ECG LVH, satisfying either Sokolow-Lyon (RV1 +SV5,6 ≥35mm) or Cornell criteria (RaVL + SV3 >20mm in females and >28mm in males)23,24; Abnormal QRS axis, <−30° or >90°; Left-axis deviation, −31° to −90°; Right-axis deviation, 91° to 180°; Extreme QRS axis, 181° to 269°; Abnormal T-wave axis, <−15° or >105°; Severely impaired LVEF, ≤35%.
Information on total cholesterol levels was available for 402 cases and 710 controls. Proportions and P values were calculated using the non-missing data as the denominator.
Information on LVEF was available for 330 cases and 402 controls. Proportions and P values were calculated using the non-missing data as the denominator.
Univariate analysis
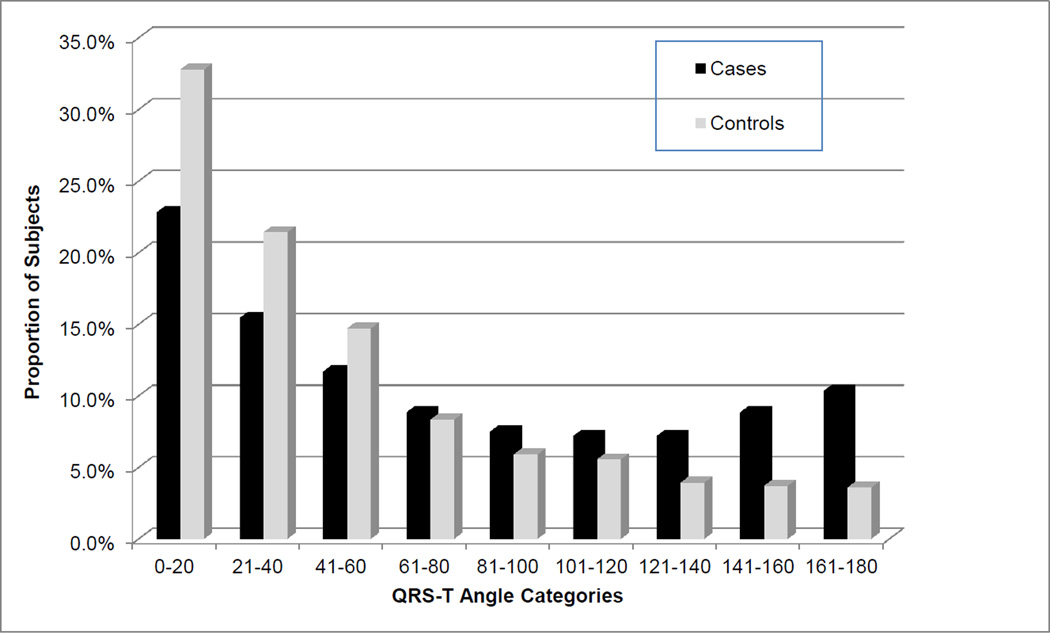
As presented in Table 2, the QRS-T angle was significantly associated with SCA (OR 1.009 per degree increase in QRS-T angle; 95% CI, 1.007 to 1.011; p<0.0001). As shown in Figure 3, the proportion of SCA cases increases with widening QRS-T angle and with every 10° increase in QRS-T angle the odds of SCA increase by 9% (OR 1.09; 95% CI, 1.07–1.11; p<0.0001). Taking the highest quartile of QRS-T angle at 97° as a cut-off, the risk of SCD increased by 2.5 times (OR 2.49; 95% CI, 1.96 to 3.15; p<0.0001) compared to the rest of the subjects. Subjects with an abnormal QRS axis had an increased risk of SCA (n=321; OR 1.57; 95% CI, 1.22–2.00; p<0.0001) and those with an extreme QRS axis had the highest risk of SCA compared to those with a normal QRS axis (OR 4.22; 95% CI, 1.54–11.6; p=0.005). Among the subjects with an abnormal QRS axis, a QRS-T angle >90° remained predictive of SCA independent of prolonged intraventricular conduction (n=321, OR 1.91; 95% CI, 1.18–3.09; p=0.008). Using a receiver-operating characteristics (ROC) curve analysis, the predictive ability of QRS-T angle for SCA was found to be 0.727 compared to that of LVEF at 0.702. Combining the QRS-T angle with LVEF modestly improved the predictive ability to 0.731. Evaluating several cut-off values for QRS-T angle in association with SCA, presented in Table 3, the odds of SCA increased with a widening QRS-T angle and correspondingly there was an expected drop in sensitivity and rise in specificity. The Youden index was only marginally different between the various cut-offs of QRS-T angles presented.
Table 2.
Univariate SCA odds ratios for QRS-T angle, and for abnormal QRS axis and T-wave axis.
| OR (95% CI) | P value | |
|---|---|---|
| QRS-T Angle* | 1.009 (1.007–1.011) | <0.0001 |
| 10°increase | 1.09 (1.07–1.11) | <0.0001 |
| QRS-T Angle in the highest quartile (>97°) † | 2.49 (1.96–3.15) | <0.0001 |
| Abnormal QRS axis | 1.57 (1.22–2.00) | <0.0001 |
| Left-axis deviation‡ | 1.36 (1.02–1.80) | 0.03 |
| Right-axis deviation‡ | 1.48 (0.92–2.39) | 0.11 |
| Extreme QRS axis‡ | 4.22 (1.54–11.6) | 0.005 |
| Abnormal T-wave axis | 1.10 (0.79–1.53) | 0.577 |
QRS-T angle analyzed as a continuous variable;
Using QRS-T angle of >97° as a cut-off compared to the rest of the subjects as reference.
Compared to normal QRS axis of −30° to 90° inclusive.
Abnormal QRS axis defined as <−30° or >90°; Left-axis deviation, −31° to −90°; Right-axis deviation, 91° to 180°; Extreme QRS axis, 181° to 269°; Abnormal T-wave, <−15° or >105°.
Figure 3.
Bar chart showing the proportion of case versus control subjects in each QRS-T angle category.
Table 3.
Sensitivity, specificity and the Youden index at different QRS-T angle cut-offs
| Cases n=666) |
Controls (n=863) |
OR (95% CI) | P value | Sensitivity (%) |
Specificity (%) |
Youden index |
|
|---|---|---|---|---|---|---|---|
| QRS-T Angle >70° | 298 | 231 | 2.22 (1.79–2.75) | <0.0001 | 45.3 | 72.5 | 0.178 |
| QRS-T Angle >80° | 274 | 196 | 2.34 (1.91–2.97) | <0.0001 | 41.4 | 76.7 | 0.181 |
| QRS-T Angle >90° | 246 | 170 | 2.39 (1.90–3.01) | <0.0001 | 37.2 | 80.0 | 0.172 |
| QRS-T Angle >100° | 224 | 145 | 2.51 (1.97–3.19) | <0.0001 | 34.1 | 83.0 | 0.171 |
| QRS-T Angle >110° | 194 | 120 | 2.55 (1.97–3.29) | <0.0001 | 29.9 | 85.6 | 0.155 |
Multivariate analysis
On multivariate analysis, a QRS-T angle cut-off of 90° was used and two models were adopted to adjust for potential confounders. These results are presented in Table 4. We included all variables that showed a significant difference between the cases and controls on univariate analysis as well as variables that were previously found to have an association with QRS-T angle. These include age, gender, heart rate, hypertension, diabetes mellitus, prior myocardial infarction, prolonged intraventricular conduction, ECG LVH and left ventricular dysfunction.6,7,26,27 A QRS-T angle of >90° remained significantly associated with SCA after adjusting for these variables.
Table 4.
Multivariate models of variables associated with sudden cardiac arrest
| Model 1 (n=1529) | Model 2 (n=880)* | |||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| QRS-T angle >90° | 1.8 (1.34–2.30) | <0.0001 | 2.2 (1.59–3.10) | <0.0001 |
| Age | 1.0 (0.99–1.01) | 0.47 | 1.0 (1.00–1.02) | 0.12 |
| Male gender | 1.1 (0.86–1.38) | 0.47 | 1.0 (0.71–1.32) | 0.83 |
| Heart rate | 1.0 (1.03–1.04) | <0.0001 | 1.0 (1.01–1.03) | <0.0001 |
| Prolonged intraventricular conduction | 1.0 (0.78–1.40) | 0.78 | 1.1 (0.74–1.56) | 0.69 |
| Hypertension | 1.2 (0.94–1.54) | 0.14 | 1.1 (0.78–1.60) | 0.54 |
| Diabetes mellitus | 1.6 (1.25–2.00) | 0.0001 | 1.8 (1.30–2.38) | 0.0002 |
| Previous MI | 1.1 (0.86–1.36) | 0.49 | 1.2 (0.90–1.62) | 0.22 |
| ECG LVH | 1.2 (0.86–1.66) | 0.28 | 1.0 (0.67–1.50) | 0.99 |
| Severely impaired LVEF† | - | - | 1.6 (1.09–2.46) | 0.02 |
LVEF denotes left ventricular ejection fraction; MI, myocardial infarction; LVH by ECG, left ventricular hypertrophy by electrocardiographic criteria
Prolonged intraventricular conduction defined as QRS duration (QRSD) >110ms; Severely impaired LVEF, ≤35%.
For Model 2, results were calculated using the non-missing data as the denominator.
Information on LVEF was available for 330 cases and 402 controls.
Discussion
In this community-based study of SCA, a frontal QRS-T angle of greater than 90° on the resting 12-lead ECG was significantly associated with SCA independent of age, gender, heart rate, baseline comorbidities, prolonged intraventricular conduction, ECG LVH and most importantly LVEF. Our findings serve to consolidate the prognostic significance of the QRS-T angle and establish the potential utility of the abnormally wide frontal QRS- T angle as a risk marker of SCA independent of the LVEF.
The QRS-T angle is a representation of axial differences between ventricular depolarization and repolarization. In healthy individuals with structurally normal hearts, despite marginal gender differences, the QRS-T angle has been shown to be narrow.26 Hence, a wide QRS-T angle reflects an imbalance of electrical heterogeneity or discordance of depolarization and repolarization,28 thus representing a vulnerable arrhythmic substrate.
The abnormally wide spatial QRS-T angle was associated with cardiovascular mortality in several populations8,9,11,29 and, in a cohort of patients with ICD for primary prevention, a wide frontal QRS-T angle was predictive of overall mortality and appropriate device therapy.30 However, the calculation of the spatial QRS-T angle requires additional software involving vectocardiograms that many physicians are not familiar with, and this hinders widespread clinical use. Since Zhang et al showed the frontal QRS-T angle as a suitable clinical substitute for the spatial QRS-T angle,11 several authors have described its prognostic value in cardiovascular morbidity and mortality30,31 and sudden cardiac death.12 These studies, however, excluded subjects with intraventricular conduction disturbance (IVCD) or were limited by the lack of echocardiographic data.
The main strength of our study is the inclusion of all potential confounders namely age, gender, heart rate, hypertension, prior myocardial infarction, prolonged intraventricular conduction, ECG LVH and LV dysfunction;6,7,26,27 and even when adjusting for these variables, QRS-T angle was independently associated with SCA. It is also notable that the subjects with prolonged intraventricular conduction in our study accounted for 26.4% of cases and 18.9% of controls; and even after correcting for these variables in a multivariate model, QRS-T angle remained predictive of SCA. This finding suggests that despite the deviation in axes as a result of electro-anatomical changes from conduction delays, a large difference in the depolarization and repolarization axes remains prognostic for SCA.
When we examine the subjects with abnormal QRS axis in greater detail, it is not surprising that the subjects with an extreme QRS axis had the highest risk of SCA compared to those with a normal QRS axis. It is interesting, however, that abnormal left-axis deviation also conferred an increased risk of SCA (n=230; OR 1.36; 95% CI, 1.02–1.80; p=0.03). Two hundred and six (64.1%) of these subjects had a QRS-T angle of >90°. A QRS-T angle cut-off of >90° in this group remained associated with SCA independent of the presence of prolonged intraventricular conduction (OR 2.14; 95% CI, 1.21–3.80; p=0.009). These findings suggest that the QRS-T angle has incremental value over an abnormal QRS axis.
Unlike previous reports, an abnormal T-wave axis was not associated with SCD in our study.12,21,32 This goes against the contention that QRS-T angle does not add further value to T-wave axis.33 There could be a few reasons for this. Aro et al defined the normal T-wave axis as 0° to 90° and, since the T-wave axis was determined manually at 10° intervals, an abnormal T-axis was regarded as <−10° and >100° that is marginally different from our definition. We included patients with bundle branch blocks (excluded by Aro et al), and this could also have affected the overall proportions with abnormal T-wave axis. However, we performed a sensitivity analysis excluding patients with prolonged intraventricular conduction and the results were unchanged (n=1190; OR 1.15; 95% CI, 0.77 to 1.72; p=0.508). Among other studies, Rautaharju et al studied the spatial T-axis in relation to coronary heart disease while Kors et al studied the frontal T-wave axis in an elderly cohort with a higher proportion of comorbidities and structural heart disease.21,29 Beyond the specifics of differences in methodology, our data suggest the QRS-T angle could be a more robust tool than just using the T-wave axis alone.
There are inherent challenges in determining an optimal cut-off value for QRS-T angle in terms of the trade-off between sensitivity and specificity values. Aro et al chose 100° as a cut-off for predicting SCD while other studies have used 90° as a cut-off for patients with non-ischemic cardiomyopathy and post myocardial infarction, looking at adverse outcomes and left ventricular dysfunction, respectively.27,30 Importantly, an ROC analysis was performed in two of these studies providing support for the use of 90° as a cut-off value. In our ROC analysis, the different cut-off values for QRS-T angle yielded only marginal differences in Youden index as shown in Table 3. Hence, it would not be appropriate to determine a definitive cut-off value from our results. Based on past literature and the positive findings in our multivariate analysis, we propose a cut-off value of 90°, recognizing that this is a specific marker with at least modest sensitivity for increased SCA risk in the community.
Limitations
We recognize several limitations to our study, especially those inherent to community-based studies, and these should be considered when interpreting our findings. Clinical data was not universally available among all our study subjects. This is because approximately 40% of subjects who suffer SCA have this condition as the first manifestation of cardiac disease and hence may not have had any prior medical evaluations. LVEF was available in about 58% of our study population; hence, the inclusion of LVEF in our multivariate analysis resulted in decreased power.
Conclusion
A wide frontal QRS-T angle greater than 90° was associated with an increased risk of SCA independent of demographic characteristics, baseline comorbidities, prolonged intraventricular conduction, ECG LVH and LVEF in this population. This simple ECG tool should be evaluated as a measurement that could potentially enhance clinical risk stratification for SCA.
Acknowledgments
Funded in part by National Heart, Lung and Blood Institute Grants R01HL122492 and R01HL126938 to Dr Chugh. Dr Chugh holds the Pauline and Harold Price Professor of Cardiac Electrophysiology at Cedars-Sinai Medical Center, Los Angeles, California.
Footnotes
Disclosures: None
References
- 1.Wellens HJ, Schwartz PJ, Lindemans FW, Buxton AE, Goldberger JJ, Hohnloser SH, Huikuri HV, Kääb S, La Rovere MT, Malik M, Myerburg RJ, Simoons ML, Swedberg K, Tijssen J, Voors AA, Wilde AA. Risk stratification for sudden cardiac death: current status and challenges for the future. Eur Heart J. 2014;35:1642–1651. doi: 10.1093/eurheartj/ehu176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stecker EC, Vickers C, Waltz J, Socoteanu C, John BT, Mariani R, McAnulty JH, Gunson K, Jui J, Chugh SS. Population-based analysis of sudden cardiac death with and without left ventricular systolic dysfunction: two-year findings from the Oregon Sudden Unexpected Death Study. J Am Coll Cardiol. 2006;47:1161–1166. doi: 10.1016/j.jacc.2005.11.045. [DOI] [PubMed] [Google Scholar]
- 3.Narayanan K, Reinier K, Uy-Evanado A, Teodorescu C, Chugh H, Marijon E, Gunson K, Jui J, Chugh SS. Frequency and determinants of implantable cardioverter defibrillator deployment among primary prevention candidates with subsequent sudden cardiac arrest in the community. Circulation. 2013;128:1733–1738. doi: 10.1161/CIRCULATIONAHA.113.002539. [DOI] [PubMed] [Google Scholar]
- 4.Fishman GI, Chugh SS, Dimarco JP, Albert CM, Anderson ME, Bonow RO, Buxton AE, Chen P-S, Estes M, Jouven X, Kwong R, Lathrop DA, Mascette AM, Nerbonne JM, O’Rourke B, Page RL, Roden DM, Rosenbaum DS, Sotoodehnia N, Trayanova NA, Zheng Z-J. Sudden cardiac death prediction and prevention: report from a National Heart, Lung, and Blood Institute and Heart Rhythm Society workshop. Circulation. 2010;122:2335–2348. doi: 10.1161/CIRCULATIONAHA.110.976092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Narayanan K, Chugh SS. The 12-lead electrocardiogram and risk of sudden death: current utility and future prospects. Europace. 2015 Oct 1;17(suppl 2):ii7–ii13. doi: 10.1093/europace/euv121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dilaveris P, Gialafos E, Pantazis A, Synetos A, Triposkiadis F, Gialafos J. The spatial QRS-T angle as a marker of ventricular repolarisation in hypertension. J Hum Hypertens. 2001;15:63–70. doi: 10.1038/sj.jhh.1001129. [DOI] [PubMed] [Google Scholar]
- 7.Dilaveris P, Gialafos E, Pantazis A, Synetos A, Triposkiadis F, Stamatelopoulos S, Gialafos J. Spatial aspects of ventricular repolarization in postinfarction patients. Pacing Clin Electrophysiol. 2001;24:157–165. doi: 10.1046/j.1460-9592.2001.00157.x. [DOI] [PubMed] [Google Scholar]
- 8.Kardys I, Kors JA, van der Meer IM, Hofman A, van der Kuip DA, Witteman JC. Spatial QRS-T angle predicts cardiac death in a general population. Eur Heart J. 2003;24:1357–1364. doi: 10.1016/s0195-668x(03)00203-3. [DOI] [PubMed] [Google Scholar]
- 9.Yamazaki T, Froelicher VF, Myers J, Chun S, Wang P. Spatial QRS-T angle predicts cardiac death in a clinical population. Heart Rhythm. 2005;2:73–78. doi: 10.1016/j.hrthm.2004.10.040. [DOI] [PubMed] [Google Scholar]
- 10.Walsh JA, Soliman EZ, Ilkhanoff L, Ning H, Liu K, Nazarian S, Lloyd-Jones DM. Prognostic value of frontal QRS-T angle in patients without clinical evidence of cardiovascular disease (from the Multi-Ethnic Study of Atherosclerosis) Am J Cardiol. 2013;112:1880–1884. doi: 10.1016/j.amjcard.2013.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Z-M, Prineas RJ, Case D, Soliman EZ, Rautaharju PM ARIC Research Group. Comparison of the prognostic significance of the electrocardiographic QRS/T angles in predicting incident coronary heart disease and total mortality (from the atherosclerosis risk in communities study) Am J Cardiol. 2007;100:844–849. doi: 10.1016/j.amjcard.2007.03.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aro AL, Huikuri HV, Tikkanen JT, Junttila MJ, Rissanen HA, Reunanen A, Anttonen O. QRS-T angle as a predictor of sudden cardiac death in a middle-aged general population. Europace. 2012;14:872–876. doi: 10.1093/europace/eur393. [DOI] [PubMed] [Google Scholar]
- 13.Chugh SS, Jui J, Gunson K, Stecker EC, John BT, Thompson B, Ilias N, Vickers C, Dogra V, Daya M, Kron J, Zheng Z-J, Mensah G, McAnulty J. Current burden of sudden cardiac death: multiple source surveillance versus retrospective death certificate-based review in a large U.S. community. J Am Coll Cardiol. 2004;44:1268–1275. doi: 10.1016/j.jacc.2004.06.029. [DOI] [PubMed] [Google Scholar]
- 14.Reinier K, Stecker EC, Vickers C, Gunson K, Jui J, Chugh SS. Incidence of sudden cardiac arrest is higher in areas of low socioeconomic status: a prospective two year study in a large United States community. Resuscitation. 2006;70:186–192. doi: 10.1016/j.resuscitation.2005.11.018. [DOI] [PubMed] [Google Scholar]
- 15.Chugh SS, Reinier K, Singh T, Uy-Evanado A, Socoteanu C, Peters D, Mariani R, Gunson K, Jui J. Determinants of prolonged QT interval and their contribution to sudden death risk in coronary artery disease: the Oregon Sudden Unexpected Death Study. Circulation. 2009;119:663–670. doi: 10.1161/CIRCULATIONAHA.108.797035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chugh SS, Uy-Evanado A, Teodorescu C, Reinier K, Mariani R, Gunson K, Jui J. Women have a lower prevalence of structural heart disease as a precursor to sudden cardiac arrest: the ore-SUDS (Oregon Sudden Unexpected Death Study) J Am Coll Cardiol. 2009;54:2006–2011. doi: 10.1016/j.jacc.2009.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reddy PR, Reinier K, Singh T, Mariani R, Gunson K, Jui J, Chugh SS. Physical activity as a trigger of sudden cardiac arrest: the Oregon Sudden Unexpected Death Study. Int J Cardiol. 2009;131:345–349. doi: 10.1016/j.ijcard.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teodorescu C, Reinier K, Dervan C, Uy-Evanado A, Samara M, Mariani R, Gunson K, Jui J, Chugh SS. Factors associated with pulseless electric activity versus ventricular fibrillation: the Oregon sudden unexpected death study. Circulation. 2010;122:2116–2122. doi: 10.1161/CIRCULATIONAHA.110.966333. [DOI] [PubMed] [Google Scholar]
- 19.Adabag AS, Peterson G, Apple FS, Titus J, King R, Luepker RV. Etiology of sudden death in the community: results of anatomical, metabolic, and genetic evaluation. Am Heart J. 2010;159:33–39. doi: 10.1016/j.ahj.2009.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Surawicz B, Childers R, Deal BJ, Gettes LS, Bailey JJ, Gorgels A, Hancock EW, Josephson M, Kligfield P, Kors JA, Macfarlane P, Mason JW, Mirvis DM, Okin P, Pahlm O, Rautaharju PM, van Herpen G, Wagner GS, Wellens H American Heart Association; Electrocardiography and Arrhythmias Committee; Council on Clinical Cardiology; American College of Cardiology Foundation; Heart Rhythm Society. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part III: intraventricular conduction disturbances: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; the Heart Rhythm Society: endorsed by the International Society for Computerized Electrocardiology. Circulation. 2009;17(119):e235–e240. doi: 10.1161/CIRCULATIONAHA.108.191095. [DOI] [PubMed] [Google Scholar]
- 21.Kors JA, de Bruyne MC, Hoes AW, van Herpen G, Hofman A, van Bemmel JH, Grobbee DE. T axis as an indicator of risk of cardiac events in elderly people. Lancet. 1998;352:601–605. doi: 10.1016/S0140-6736(97)10190-8. [DOI] [PubMed] [Google Scholar]
- 22.Assanelli D, Rago L, Salvetti M, Di Castelnuovo A, Badilini F, Vaglio M, Zito F, Donati MB, de Gaetano G, Iacoviello L. Moli-sani Project Investigators: T-wave axis deviation, metabolic syndrome and cardiovascular risk: results from the MOLI-SANI study. J Electrocardiol. 2012;45:546–550. doi: 10.1016/j.jelectrocard.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 23.Sokolow M, Lyon TP. The ventricular complex in right ventricular hypertrophy as obtained by unipolar precordial and limb leads. American heart journal. 1949 Aug 31;38(2):273–294. doi: 10.1016/0002-8703(49)91335-6. [DOI] [PubMed] [Google Scholar]
- 24.Casale PN, Devereux RB, Kligfield P, Eisenberg RR, Miller DH, Chaudhary BS, Phillips MC. Electrocardiographic detection of left ventricular hypertrophy: development and prospective validation of improved criteria. Journal of the American College of Cardiology. 1985 Sep 1;6(3):572–580. doi: 10.1016/s0735-1097(85)80115-7. [DOI] [PubMed] [Google Scholar]
- 25.Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3:32–35. doi: 10.1002/1097-0142(1950)3:1<32::aid-cncr2820030106>3.0.co;2-3. doi: 10.1002/1097-0142(1950)3: 1<32::AID-CNCR2820030106>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 26.Scherptong RW, Henkens IR, Man SC, Le Cessie S, Vliegen HW, Draisma HH, Maan AC, Schalij MJ, Swenne CA. Normal limits of the spatial QRS-T angle and ventricular gradient in 12-lead electrocardiograms of young adults: dependence on sex and heart rate. J Electrocardiol. 2008;41:648–655. doi: 10.1016/j.jelectrocard.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 27.Li Y-H, Ren X-J, Han Z-H, Wang Y-L, Wang Y, Zhang J-R, Chen F. Value of the frontal planar QRS-T angle on cardiac dysfunction in patients with old myocardial infarction. Int J Clin Exp Med. 2013;6:688–692. [PMC free article] [PubMed] [Google Scholar]
- 28.Draisma HH, Schalij MJ, van der Wall EE, Swenne CA. Elucidation of the spatial ventricular gradient and its link with dispersion of repolarization. Heart Rhythm. 2006;3:1092–1099. doi: 10.1016/j.hrthm.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 29.Rautaharju PM, Kooperberg C, Larson JC, LaCroix A. Electrocardiographic abnormalities that predict coronary heart disease events and mortality in postmenopausal women: the Women’s Health Initiative. Circulation. 2006;113:473–480. doi: 10.1161/CIRCULATIONAHA.104.496091. [DOI] [PubMed] [Google Scholar]
- 30.Pavri BB, Hillis MB, Subačius H, Brumberg GE, Schaechter A, Levine JH, Kadish A. Defibrillators in Nonischemic Cardiomyopathy Treatment Evaluation (DEFINITE) Investigators: Prognostic value and temporal behavior of the planar QRS-T angle in patients with nonischemic cardiomyopathy. Circulation. 2008;117:3181–3186. doi: 10.1161/CIRCULATIONAHA.107.733451. [DOI] [PubMed] [Google Scholar]
- 31.Walsh JA, Soliman EZ, Ilkhanoff L, Ning H, Liu K, Nazarian S, Lloyd-Jones DM. Prognostic value of frontal QRS-T angle in patients without clinical evidence of cardiovascular disease (from the Multi-ethnic study of atherosclerosis) Am J Cardiol. 2013;112:1880–1884. doi: 10.1016/j.amjcard.2013.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rautaharju PM, Nelson JC, Kronmal RA, Zhang ZM, Robbins J, Gottdiener JS, Furberg CD, Manolio T, Fried L. Usefulness of T-axis deviation as an independent risk indicator for incident cardiac events in older men and women free from coronary heart disease (the Cardiovascular Health Study) Am J Cardiol. 2001;88:118–123. doi: 10.1016/s0002-9149(01)01604-6. [DOI] [PubMed] [Google Scholar]
- 33.Macfarlane PW. The frontal plane QRS-T angle. Europace. 2012;14:773–775. doi: 10.1093/europace/eus057. [DOI] [PubMed] [Google Scholar]