Abstract
Increased production of amyloid-β (Aβ)42 peptide, derived from the amyloid-β protein precursor, and its subsequent aggregation into oligomers and plaques constitutes a hallmark of Alzheimer’s disease (AD). We here report on a family of low molecular weight molecules, the Aftins (Amyloid-β Forty-Two Inducers), which, in cultured cells, dramatically affect the production of extracellular/secreted amyloid peptides. Aftins trigger β-secretase inhibitor and γ-secretase inhibitors (GSIs) sensitive, robust upregulation of Aβ42, and parallel down-regulation of Aβ38, while Aβ40 levels remain stable. In contrast, intracellular levels of these amyloids appear to remain stable. In terms of their effects on Aβ38/Aβ40/Aβ42 relative abundance, Aftins act opposite to γ-secretase modulators (GSMs). Aβ42 upregulation induced by Aftin-5 is unlikely to originate from reduced proteolytic degradation or diminished autophagy. Aftin-5 has little effects on mitochondrial functional parameters (swelling, transmembrane potential loss, cytochrome c release, oxygen consumption) but reversibly alters the ultrastructure of mitochondria. Aftins thus alter the Aβ levels in a fashion similar to that described in the brain of AD patients. Aftins therefore constitute new pharmacological tools to investigate this essential aspect of AD, in cell cultures, allowing (1) the detection of inhibitors of Aftin induced action (potential ‘anti-AD compounds’, including GSIs and GSMs) but also (2) the identification, in the human chemical exposome, of compounds that, like Aftins, might trigger sustained Aβ42 production and Aβ38 down-regulation (potential ‘pro-AD compounds’).
Keywords: Aftins, Alzheimer’s disease, amyloid-β, Aβ38, Aβ40, Aβ42, γ-secretase, γ-secretase modulators, mitochondria
INTRODUCTION
Following its description by Aloïs Alzheimer at the beginning of the 20th century, the neurodegenerative disease bearing the name of the German neuropathologist has been the object of numerous studies. Despite extensive investigation, the causes and mechanisms underlying the initiation and development of this disease still remain poorly understood (reviewed in [1, 2]). There is only a relatively mild consensus on the respective roles and importance of the two best described events associated with Alzheimer’s disease (AD), namely (i) the release of amyloid-β (Aβ) peptides by proteolytic processing of the amyloid-β protein precursor (AβPP) and their extracellular aggregation in oligomers and ultimately in plaques [3–5] and (ii) hyperphosphorylation of the microtubule-binding protein tau by various kinases and subsequent intracellular aggregation as paired helical filaments [reviewed in 6–8].
Aβ peptides are the endproducts of two successive proteolytic actions catalyzed first by β-secretase (BACE 1, β-site AβPP cleaving enzyme 1) and then by γ-secretase, a complex of four proteins: presenilin-1/2 (PSEN1/PSEN2) (the catalytic subunit, an aspartyl protease), nicastrin, anterior pharynx-defective 1, and presenilin enhancer 2 (PEN-2) (reviewed in [9]). The action of β-secretase on AβPP leads to a soluble extracellular fragment (sAβPPβ) and a membrane bound fragment (C99 or βCTF, β-carboxyl-terminal fragment). γ-Secretase then acts on this C-terminal fragment of AβPP, leading to the possibly stepwise [10] generation of Aβ peptides of various lengths, such as Aβ38, Aβ40, Aβ42, Aβ43, and the AβPP intracellular domain (AICD). Aβ40 is the main Aβ peptide. However, there is considerable data showing that generation of Aβ42 is strongly correlated with the onset and development of AD. In autosomal dominant forms of early onset AD, which represents less than 1% of all AD cases, mutations affect AβPP, PSEN1, and PSEN2 (reviewed in [11]) and all lead to enhanced production of Aβ42 or increase in the Aβ42/Aβ40 ratio, a critical factor in the onset of AD [12]. Aβ42 is more toxic than Aβ40 and this appears to be linked to its higher stability and ability to oligomerize and aggregate in plaques [13].
In a previous article, we reported on the properties of a family of tri-substituted purines, the Aftins (Amyloid-β Forty-Two Inducers) [14]. These low molecular weight compounds induce robust production of Aβ42 in cultured cells while leaving Aβ40 levels unaffected. They bind specific mitochondrial proteins and reversibly alter mitochondrial structure. They constitute new pharmacological tools to investigate the molecular mechanism underlying the modified Aβ42/Aβ40 ratio associated with AD.
We have synthesized and tested a small library of 52 Aftins and related purine analogues. In this article, we report on an optimized Aftin (Aftin-5) which lacks cellular toxicity at high doses, while dramatically modifying extracellular Aβ38/Aβ40/Aβ42 production in cultured N2a-AβPP695 and other cells (primary neuronal cells, HEK293 cells expressing AβPP, normal N2a cells). Aftin-5 triggers massive upregulation of Aβ42 and parallel down-regulation of Aβ38, while Aβ40 levels remain stable. Intracellular levels of these amyloids remain stable. These effects are sensitive to pharmacological inhibitors of β-secretase or γ-secretase (GSIs) and to a γ-secretase modulator (GSM). We show that upregulation of Aβ42 by Aftin-5 is unlikely to be due to reduced degradation by proteases or diminished autophagy. Aftin-5 has no significant effects on mitochondrial functional parameters such as swelling, transmembrane potential loss, cytochrome c release, and oxygen consumption (complex I or II activation). Mitochondria ultrastructure is modestly, but reversibly, affected. Aftin-5 thus appears to alter Aβ relative abundance in cells in a fashion similar to that described in the brain of AD patients [15–17]. Aftin-5 therefore constitutes a new pharmacological tool that could be used to investigate this essential aspect of AD in cell cultures. The established cellular system allows (i) the detection of inhibitors of this Aftin-5 induced action (potential ‘anti-AD compounds’) but also (ii) the identification of other compounds like Aftins that might trigger massive Aβ42 production and Aβ38 down-regulation (potential ‘pro-AD compounds’). Aftin-5 may also be a first step toward the generation of a chemically-induced animal model of AD.
MATERIAL AND METHODS
Synthesis of Aftins and γ-secretase modulator
N6-dimethylaminopurine (N6DA) (1) was obtained from Sigma Aldrich (Lyon, France). Aftins of Fig. 1 were synthesized as follows. Compound (2) was obtained upon heating 2-amino-6-chloropurine with N-methylbenzylamine in 2-propanol. Aftin-1 (3) and Aftin-2 (4) were prepared from compound (2). Aftin-3 (5) was also obtained in a two steps procedure starting from 2-amino-6-chloropurine. Aftin-4 (6) and Aftin-5 (7) were obtained in a three steps procedure starting from 2,6-dichloropurine. (R)-Roscovitine (8) was synthesized as previously described [18]. The ‘Torrey Pines’ compound (9) was synthesized following a published procedure [19]. Detailed preparation procedures and characterization of these products are provided in the Supplementary data (available online: http://www.j-alz.com/issues/35/vol35-1.html#supplementarydata01). All compounds were solubilized as 100 mM stock solutions in 100% dimethylsulfoxide (DMSO) and diluted just prior to use. Aftin-5 is available from ManRos Therapeutics, 29680-Roscoff, France (E-mail: meijer@manros-therapeutics.com).
Fig. 1.
Structure of the compounds used in this study. N6-diamino-purine (N6DA) (1), compound (2), Aftin-1 (3), Aftin-2 (4), Aftin-3 (5), Aftin-4 (6), Aftin-5 (7), (R)-roscovitine (8) and “Torrey Pines” compound (9).
Other reagents
DMSO, Nonidet P-40, Tween-20, bovine serum albumin (BSA), Na2CO3, NaHCO3, citric acid monohydrate, Na2HPO4.2H2O, H2O2, thiorphan, captopril, quinaprilat, phosphoramidon, digitonin, and rapamycin were purchased from Sigma Aldrich. Protease inhibitors mix (Complete) was obtained from Roche (Boulogne-Billancourt, France). Streptavidin-horseradish peroxidase (HRP) conjugate was purchased from Thermo Scientific Pierce (Brebières, France). o-Phenylenediamine dihydrochloride (OPD) tablets were from Invitrogen (St. Aubin, France). DAPT (N-[(3,5-difluorophenyl)acetyl]-L-alanyl-2-phenyl]glycine-1,1-dimethylethyl ester), BMS299897, 3-methyladenine and bafilomycin A1 were obtained from Tocris Bioscience (Lille, France). Paraformaldehyde (16% stock) was purchased from Electron Microscopy Sciences (EMS, Hatfield, USA). β-secretase inhibitor IV was from Calbiochem (Molsheim, France).
Cell culture
N2a-AβPP695 cells (Rockefeller University) were cultured in Dulbecco’s minimal essential medium (DMEM)/OptiMEM (1 : 1 v/v; Gibco, InVitrogen, St. Aubin, France) containing 0.2 mg/mL Geniticin (Geneticin Selective Antibiotic, Gibco) and 5% fetal bovine serum (FBS, Gibco, EU approved origin) in a humidified, 5% CO2 incubator. Cells were split routinely every 4/5 days. They were first rinsed with PBS (Gibco) and detached from the plate bottom using 4 mL Versene (Gibco) at room temperature (RT) for 3–4 min. Eight mL of fresh medium were added to the cell suspension, and the mix was centrifuged for 3 min at 1,000 rpm. The cell pellet was resuspended in fresh medium before seeding (1/10 dilution) in new flasks.
Sample preparation (extracellular amyloids)
N2a-AβPP695 cells were seeded at 10,000 cells/well in a 96 well plate with modified media (0.5% FBS) and incubated overnight. Cells were treated with fresh media and different compounds (equal quantity of DMSO), then incubated for 18 h in a humidified, 5% CO2 incubator. The plate was finally centrifuged to remove cell fragments before collecting supernatant samples for amyloids levels determination (see below).
Sample preparation (intracellular amyloids)
N2a-AβPP695 cells were seeded at 2 × 106 cells in a T25 flask with modified media (0.5% FBS) and incubated overnight. Cells were treated with fresh media and different compounds (equal quantity of DMSO), then incubated for 18 h in a humidified, 5% CO2 incubator. The supernatants were collected and centrifuged before testing in an ELISA capture assay. Cells were washed with PBS (Gibco) and then scraped in 1.5 mL of PBS + protease inhibitors. Cells were centrifuged (1,000 rpm for 3 min at 4°C) and cell pellets were resuspended in PBS + protease inhibitors + 1% Nonidet P-40 and sonicated. After centrifugation (14,000 rpm for 15 min at 4°C), amyloid concentrations were determined in the supernatants by an ELISA capture assay (see below).
ELISA capture assay
Aβ38, Aβ40, and Aβ42 levels were measured in a double antibody sandwich ELISA using a combination of monoclonal antibody (mAb) 6E10 (SIG-39320, Covance, Eurogentec, Seraing, Belgium) and biotinylated polyclonal Aβ38 [20], Aβ40, or Aβ42 [21] antibodies (provided by Dr. P.D. Mehta, Institute for Basic Research in Developmental Disabilities, Staten Island, USA). Briefly, 100 μl mAb 6E10 diluted in carbonate-bicarbonate buffer (0.015 M Na2CO3 + 0.035 M NaHCO3, pH 9.6), was coated in the wells of microtiter plates (Maxisorp, NuncTher-moFisher Scientific, Illkirch, France) and incubated overnight at 4°C. The plates were washed with PBST (PBS containing 0.05% Tween-20) and blocked for 1 h with 1% BSA in PBST to avoid non-specific binding. Each of the Aβ38, Aβ40, or Aβ42 antibodies did not cross-react with the other amyloid peptides (data not shown). Standard curves were prepared with synthetic amyloids and each of the three antibodies. Fitting was performed using a 4 parameters sigmoid equation (SigmaPlot, Systat).
Following a washing step, experimental samples were added into the wells and incubation was carried out for 2 h at RT and overnight at 4°C. Plates were washed before incubation with biotinylated polyclonal antibody diluted in PBST + 0.5% BSA at RT for 75 min. After a washing step, streptavidin-HRP conjugate, diluted in PBS + 1% BSA, was added and incubation was carried out for 45 min at RT. After washing, 100 μl OPD in citrate buffer (0.049M citric acid monohydrate + 0.1M Na2HPO4.2H2O + 1 mL H2O2 30%/L) pH 5.0 were added. The reaction was stopped after 15 min with 100 μL 1 N sulfuric acid. The optical density was measured at 490 nm in a microELISA reader (BioTek Instrument, El 800, Gen 5 software).
Cell viability
To measure cell viability, the MTS (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2 -(4-sulfophenyl)-2H-tetrazolium) assay (CellTiter 96 AQueous One Solution Cell Proliferation Assay, Promega, Charbonnières-les-bains, France) was used in the same wells as the capture ELISA assay. Briefly, 20 μL of MTS reagent was added in each well containing 100 μL of media and incubation then proceeded for 3 h (37°C, 5% CO2, and 95% humidity). Measurements were made at OD 490Δ630 nm using a microELISA reader.
Autophagy assessment
Autophagy was assessed by counting of LC3 foci by immunofluorescence. Briefly, cells were grown on glass coverslips, fixed with 4% paraformaldehyde, permeabilized using 50 μM digitonin and stained with a monoclonal anti-LC3 antibody (Enzo Life Sciences, Lausen, Switzerland). Images were acquired with a DFC310FX camera on a Leica AF6000 microscope using a 40× NA 0.85 objective (Leica Microsystems). Automated counting of LC3 foci was performed using Image J (NIH).
Mitochondria studies
Swelling, transmembrane potential loss, cytochrome c release, oxygen consumption (through complex I or complex II activation), and electron microscopy were carried out as described in details in the Supplementary data (see also http://www.mitologics.com).
RESULTS
Aftins, a family of roscovitine-related purines, dramatically increase production of Aβ42 in N2a cells, but not Aβ40
In a search for compounds inhibiting the CK1 kinase [22, 23] and possibly modifying Aβ production in cultured N2a-AβPP695 cells (N2a cells stably expressing human AβPP695), we identified a sub-family of purines, related to the kinase inhibitor roscovitine (Fig. 1, compound 8) [24] and able to decrease the Aβ40/Aβ42 ratio. This family of compounds was named Aftins, which stands for Amyloid Forty Two Inducers [14]. Here we have prepared and tested a small library of 52 purines and related compounds. A selection of these molecules is shown in Fig. 1. N6-substituted purines were synthesized and added at various concentrations to N2a-AβPP695 cells. After 18 h incubation, levels of Aβ40 and Aβ42 in cell supernatants were determined by an ELISA assay and cell viability was estimated by the MTS assay (see Material & Methods section). A subset of compounds massively increased the production of Aβ42 (Fig. 2, upper panel) while Aβ40 level remained unaffected (Fig. 2, lower panel). This screen allowed to select the most active Aftins (in terms of Aβ42 induction) displaying the lowest cytotoxicity (MTS assay). The Aβ42-inducing compounds have all lost the H-bonding potential at N6 (indicated by an asterisk on roscovitine), which is key to the binding of roscovitine (8) to its kinase targets [25], either through methylation [Aftins-1 (3), -2 (4), -4 (6), -5 (7)] or replacement of the nitrogen atom by an oxygen [Aftin-3 (5)]. This is necessary but not sufficient as Aftins also require a hydrophobic substituent at position 9 [compare inactive compounds N6DA (1) and compound (2) to Aftin-1 (3) and -4 (6)]. Kinase assays showed that Aftins are kinase inactive (data not shown), while the kinase inhibiting roscovitine shows little effect on Aβ42 levels (Fig. 2A). As for Aftin-4 [14], running Aftin-5 (10 μM) on the KinomeScan panel of 402/442 kinases revealed no interaction with any of the kinases (Supplementary Table 1). Kinase inhibition activity therefore does not seem to be involved in the biological activity of Aftins.
Fig. 2.
Dose-dependent effect of various purines on extracellular Aβ42 and Aβ40 production in N2a-AβPP695 cells. Cells were exposed to various concentrations of compounds 1–6, 8 for 18 h. Extracellular Aβ42 (upper panel) and Aβ40 (lower panel) levels were measured by an ELISA assay and are expressed as fold change over the level of control, vehicle-treated cells. Representative of two independent experiments, errors bars represent standard deviation of triplicate values.
Non-cytotoxic Aftin-5 upregulates Aβ42 and down-regulates Aβ38
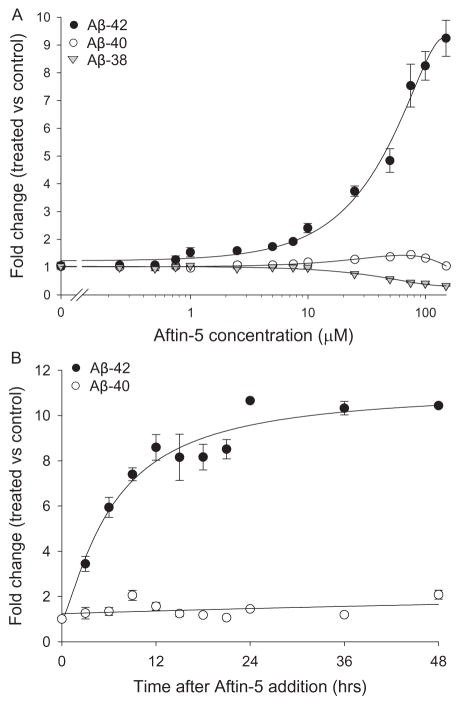
Among all the compounds tested, Aftin-5 (7), the purvalanol equivalent of Aftin-4 (N6-methyl-roscovitine), did not show any cytotoxic effects on cells even at the highest concentration tested (100 μM) or at the longest exposure (6 days) (data not shown). Comparison of the effects of Aftin-4 and Aftin-5 on the survival of various cell lines illustrated the lower cell toxicity of Aftin-5 (Table 1). Dose-response curves showed that Aftin-5 gradually induced increasing levels of Aβ42 production (up to >900% increase), while the levels of Aβ40 remained essentially stable (maximum 40% increase) (Fig. 3A). In contrast, the levels of Aβ38 were found to decrease (>70%) in parallel to the increase of Aβ42. Time-course experiments showed that Aβ42 production reached a plateau about 12 h after Aftin-5 addition and remained stable even up to 48 h (Fig. 3B). These results were confirmed with non-transfected N2a cells, with HEK293 stably expressing AβPPsw (data not shown) and with primary neuronal cell cultures (Mabondzo et al., in preparation). Aβ42 production induced by Aftin-4 was bell-shaped, indicative of some cell toxicity at high doses. In contrast, Aβ42 production induced by Aftin-5 was linear up to the highest dose tested (150 μM).
Table 1.
Compared cytotoxicity of Aftin-4 and Aftin-5. The different cell lines were exposed to increasing concentrations of each compound and their viability was assessed 24 h later by the MTS assay as described in the Material & Methods section. IC50 values were determined from the dose-response curves and are presented in μM
| Cell line | Aftin-4 | Aftin-5 |
|---|---|---|
| SH-SY5Y | 106 | 180 |
| HT22 | 74 | 194 |
| N2a | 100 | 178 |
| N2a-AβPP695 | 90 | 150 |
Fig. 3.
Effect of Aftin-5 on extracellular Aβ production in N2a-AβPP695 cells. A) Aftin-5 dose-dependent effect on extracellular amyloids levels. Cells were exposed to various concentrations of Aftin-5 for 18 h. Extracellular Aβ38, Aβ40, and Aβ42 levels were measured by an ELISA assay and are expressed as fold change over the level of control, vehicle-treated cells. B) Aftin-5 time-dependent effect on Aβ40 and Aβ42 levels. Cells were exposed to 100 μM Aftin-5 for various periods of time and extracellular Aβ40 and Aβ42 levels were measured by an ELISA assay. Representative of three independent experiments, errors bars represent standard deviation of triplicate values.
Aftin induced upregulation of Aβ42 requires active β- and γ-secretases
To evaluate the importance of β-secretase in the effects of Aftin-5, we used a pharmacological inhibitor of β-secretase, β-secretase inhibitor IV. The drug inhibited production of both Aβ40 and Aβ42 in a dose-dependent manner (data not shown). To investigate the importance of γ-secretases in the effects of Aftin-5, we made use of two potent pharmacological inhibitors of γ-secretases (GSIs), DAPT [26] and BMS299897 [27]. Both drugs dose-dependently inhibited Aftin-5 induced production of Aβ42 (Fig. 4). Both drugs also reduced the level of basal Aβ40 and Aβ38. IC50 values (Aβ38/Aβ40/Aβ42: 112/62/43 nM and 88/56/29 nM, for DAPT and BMS 299897, respectively) were in agreement with reported IC50 values for these GSIs (25–56 nM and 8–300 nM, respectively). A similar sensitivity of Aftin-4’s effects to γ-secretase inhibitors has been observed (data not shown).
Fig. 4.
Extracellular Aβ42 production induced by Aftin-5 and basal level of Aβ40 are inhibited by γ-secretase inhibitors. Cells were exposed to increasing concentrations of DAPT or BMS 299897 and 1 h later to 100 μM Aftin-5. Extracellular Aβ40 and Aβ42 levels were measured after 18 h and are expressed relative to their levels produced by Aftin-5 treated cells in the absence of γ-secretase inhibitor. Representative of two independent experiments, errors bars represent standard deviation of triplicate values.
Upregulation of Aβ42 and down-regulation of Aβ38 by Aftin-5 are counteracted by γ-secretase modulator
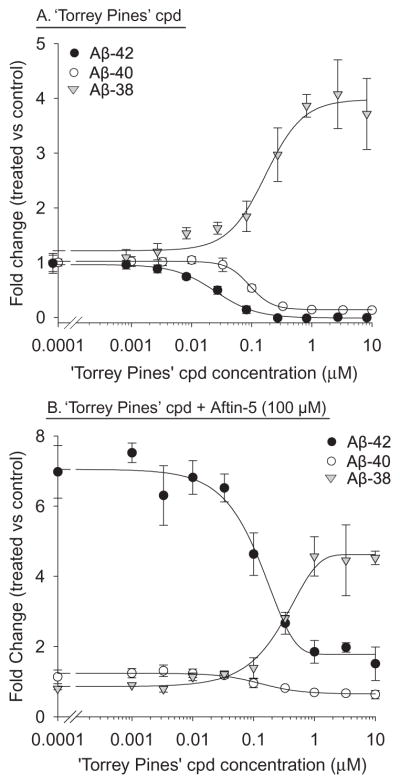
To evaluate the mechanism of action of Aftin-5 we next made use of a recently reported γ-secretase modulator (GSM), the ‘Torrey Pines’ compound [28, 29]. This compound down-regulated Aβ42 and Aβ40 production, in a dose-dependent manner, while it triggers a parallel upregulation of Aβ38 level (Fig. 5A) in N2a-AβPP695 cells as previously reported in HEK293/sw cells (stably expressing Swedish mutant AβPP) [29, 30]. When increasing concentrations of the ‘Torrey Pines’ compound were added to N2a-AβPP695 cells 1 h prior to 100 μM Aftin-5, a dose-dependent inhibition of Aftin-5 induced Aβ42 upregulation and Aβ38 down-regulation was observed (Fig. 5B). This shows that Aftin-5 and the GSM ‘Torrey Pines’ have opposite effects on the production of Aβ38 and Aβ42 peptides in N2a-AβPP695 cells.
Fig. 5.
γ-Secretase modulator ‘Torrey Pines’ counter-balances Aftin-5 effects. A) GSM ‘Torrey Pines’ dose-dependent effect on extracellular Aβ38, Aβ40, and Aβ42 levels in N2a-AβPP695 cells. Cells were exposed to various concentrations of ‘Torrey Pines’ compound for 18 h. Extracellular Aβ levels were measured by an ELISA assay and are expressed as fold change over the level of control, vehicle-treated cells. B) GSM ‘Torrey Pines’ dose-dependently inhibits Aftin-5 upregulation of Aβ42 and down-regulation of Aβ38. Cells were exposed to various concentrations of ‘Torrey Pines’ compound and 1 h later to 100 μM Aftin-5. Incubation was carried out for 18 h. Extracellular Aβ levels were measured by an ELISA assay and are expressed as fold change over the level of control, Aftin-5 treated cells. Representative of three independent experiments, errors bars represent standard deviation of triplicate values.
Aftin-5-induced upregulation of Aβ42 and down-regulation of Aβ38 concerns extracellular excreted amyloids, but not intracellular amyloids
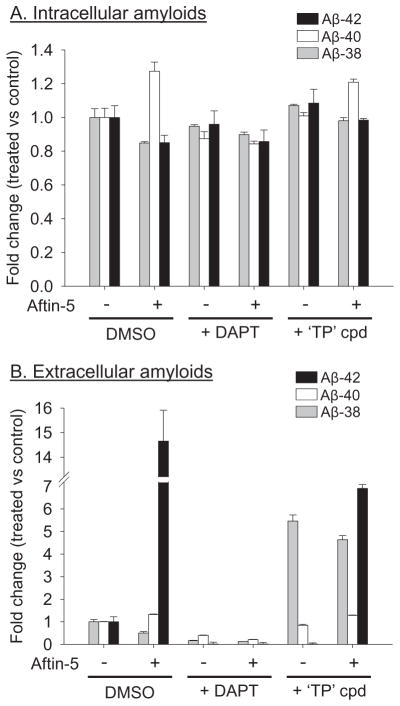
We next measured both extracellular and intracellular levels of the three amyloids in N2a-AβPP695 cells following exposure to Aftin-5. Cells were exposed for 18 h to 100 μM Aftin-5, in the absence or presence of 1 μM DAPT or ‘Torrey Pines’ compound. Both extra-cellular and intracellular amyloids were detected and quantified by the ELISA assay. The validity of the intracellular amyloid assay was confirmed by spiking (and recovering) some samples with a known amount of Aβ42 (data not shown). Results show that Aftin-5 did not modify the levels of intracellular Aβ38, Aβ40, and Aβ42 (Fig. 6A), while the levels of the extracellular amyloids (Fig. 6B) were modified as described above. DAPT down-regulated the levels of all three extracellular amyloids but not that of intracellular amyloids. The ‘Torrey Pines’ compound had the expected effects on extracellular amyloids, and no effects on intracellular amyloids. These results suggest that Aftins affect the production of extracellular amyloids only. However intracellular amyloids are more difficult to detect and these results might be invalidated by other extraction and detection methods.
Fig. 6.
Aftin-5 modifies extracellular but not intracellular Aβ38/Aβ40/Aβ42 ratios. Cells were exposed to 100 μM Aftin-5 for 18 h, in the absence or presence of 1 μM DAPT or 1 μM ‘Torrey Pines’ compound. Cell supernatant (A) and cell pellets (B) were recovered and Aβ38, Aβ40, and Aβ42 amyloid levels were measured by an ELISA assay. Amyloid levels are expressed as fold change over their concentrations in control, non-treated cells. Basal extracellular (A) and intracellular (B) levels of amyloids were, respectively, 61.9 and 83.5 pg/mL (Aβ38), 471 and 48.9 pg/mL (Aβ40), 28.9 and 33.6 pg/mL (Aβ42). Representative of two independent experiments, errors bars represent standard deviation of triplicate values.
Aftin-5-induced upregulation of Aβ42 is not due to down-regulation of Aβ proteolytic degradation or autophagy
Increased accumulation of Aβ42 peptides could be due to an increased production (i.e., release from AβPP) or to a decreased disposal (proteolytic degradation or autophagy), mimicking the reported reduced clearance of amyloids in AD brain [31]. Although it is unlikely that these elimination mechanisms would specifically remove Aβ42, leave Aβ40 untouched, and allow an increase in Aβ38 level, we tested their potential implications in the levels of all three amyloid peptides.
There are a number of reported amyloid-degrading enzymes (neprilysin, endothelin-converting enzyme (ECE-1, ECE-2), angiotensin converting enzyme (ACE), insulin-degrading enzyme (IDE), cathepsin B, matrix metalloproteinase) (reviewed in [32]). We therefore tested the effects of various inhibitors of these enzymes, alone or in combination with Aftin-5, on the levels of amyloid peptides after incubation of N2a-AβPP695. An 18 h exposure to 0.1–100 μM thiorphan (neprilysin inhibitor), captopril and quinaprilat (ACE inhibitors), or phosphoramidon (IDE inhibitor) had no effects on extracellular levels of amyloid Aβ38, Aβ40, or Aβ42 (data not shown). These results suggest that these amyloid elimination mechanisms are probably not significant in our cellular systems and that inhibition of amyloid peptide degradation is unlikely to be the driving mechanism of action of Aftin-5. However, other amyloid degradation pathways, not challenged by the above-mentioned inhibitors, may be involved.
An alternative explanation for Aftin-5 triggered increase in Aβ42 level could be a reduced autophagy. It has indeed been reported that autophagy is one of the mechanisms that contribute to the elimination of amyloid peptides [33–36], that deterioration in the autophagylysosomal system participates to Aβ42 neurotoxicity [35, 37], and that autophagy is linked to AD [38–40]. Therefore an inhibition of autophagy might increase amyloid peptides levels. Exposure of N2a-AβPP695 cells for 18 h to a classical inhibitor of autophagy (0.033–10 mM 3-methyladenine) did not interfere with Aftin-5 induced extracellular Aβ42 production (data not shown). Furthermore, 0.01–20 μM rapamycin, a well characterized autophagy inducer, did not prevent the increase in extracellular Aβ42 level induced by 100 μM Aftin-5 (data not shown), although it triggered a classical hallmark of autophagy, namely the accumulation of LC3 foci (Supplementary Figure 1). Neither Aftin-5 nor its inactive analog compound (2) impaired rapamycin-induced autophagy in N2a-AβPP695 cells (Supplementary Figure 1). Neither drugs alone had significant effects on autophagy (Supplementary Figure 1).
We conclude that the change in Aβ38/Aβ40/Aβ42 relative abundance induced by Aftin-5 is unlikely to be dominantly due to a reduction in proteolytic or autophagic activity. This might be different in primary neurons or in an animal situation.
Mitochondrial effects
In a previous article, we reported that Aftin-4 had significant effects on the morphology of mitochondria and that several mitochondrial proteins were specifically binding to agarose immobilized Aftin-4 [14]. We therefore investigated the effects of the much less toxic Aftin-5 on mitochondria. We first tested the effects of Aftin-5 treatment (1.6 to 200 μM) on several biological parameters of mitochondria isolated from N2a-AβPP695 (Supplementary data section on mitochondria). The following parameters were measured in these isolated mitochondria: transmembrane potential loss (Δψm loss), cytochrome c release, oxygen consumption (through complex I or complex II activation).
Aftin-5 had no important effects when added directly to isolated mitochondria prepared from untreated N2a-AβPP695 cells (Supplementary data), only a slight stimulation of oxygen consumption was measured under conditions of complex I activation. We also investigated the mitochondrial effects of Aftin-5 on cultured N2a-AβPP695 cells. At the cellular level, the mitochondrial parameters were essentially undistinguishable between control and Aftin-5 treated cells (Supplementary data), except for global respiration functions where a slight inhibition was measured at 24 h.
We next analyzed the ultrastructure of mitochondria following Aftin-5 treatment of isolated mitochondria or intact cells. Treatment of mitochondria isolated from N2a-AβPP695 cells with 100 μM Aftin-5 did not induce any swelling or cristae remodeling, suggesting that the compound has no direct effect on mitochondrial structure (Fig. 7A). This is in accordance with the spectrofluorimetry results. As a control, calcium induced a strong swelling reversed by cyclosporin A (CsA), an inhibitor of mitochondrial permeability transition pore. N2a-AβPP695 cells were next treated for 6 h or 24 h with 100 μM Aftin-5. Electron microscopy analysis of cells revealed little detectable effects at 6 h, but a partial mitochondrial swelling without disruption of cristae at 24 h (Fig. 7B), without cell death induction. These effects were reversible upon washing Aftin-5 away from cells. As a positive control, doxorubicin altered the structure of cristae and induced cell death. Altogether these results suggest that Aftin-5 has some structural effects on mitochondria which, like for Aftin-4, are reversible.
Fig. 7.
Aftin-5 has modest effects on mitochondrial structure. A) Ultrastructure of isolated mitochondria exposed to 100 μM Aftin-5, 50 μM Calcium, or 50 μM Calcium + Cyclosporin A (CsA). B) Ultrastructure of mitochondria within cells exposed to 100 μM Aftin-5 for 6 or 24 h, or to 5 μM doxorubicin. Representative of two independent experiments.
DISCUSSION
In this article we confirm and extend the results described in a previous article [14]. In cell cultures (immortalized cell lines and primary neuronal cultures) Aftins robustly increase the production and extracellular release of Aβ42 while Aβ40 levels remain stable. In contrast, Aftins trigger important parallel down-regulation of Aβ38. This effect on the Aβ38/Aβ40/Aβ42 peptides relative abundance triggered in cells by Aftins is similar to that previously observed, yet to a much lower extent, with fenofibrate, celecoxib, and indomethacin [35]. Among all the purines derivatives and analogues tested, we have selected Aftin-5 as one of the most potent Aftins. In addition, it displays little cell toxicity (up to 100 μM) or animal toxicity (per os doses up to 150 mg/kg daily for 20 days) (data not shown). Aftin-5 is thus the pharmacological tool we recommend.
Molecular mechanism of action of Aftins
The action of Aftins on cells requires rather high concentrations (50–150 μM). Despite extensive chemical synthesis work, we were able to improve only slightly the potency of the initial Aftin-4 [14]. Furthermore we have not been able to establish a clear-cut structure activity relationship (SAR) in our Aftins library. The structural requirements for Aftins’ biological activity can be summarized as follows: (i) the methyl (or other aryl) substituent on position N6 is a key requirement, (ii) the N9 position can accommodate larger substituents than the corresponding kinase inhibitors, (iii) substitution at C2 is necessary, but no specific modifications could be identified. The lack of unambiguous SAR probably comes from the fact that the biological assay is a cellular assay (change in the ratio of extracellular Aβ38/Aβ40/Aβ42 peptides) rather than a molecular assay, implying that multiple parameters might contribute to the measured signal (cell permeability, action on different molecular targets, off-target binding, intracellular distribution, saturation effect, intracellular degradation, etc., all of which may vary from one Aftin to the other). Affinity chromatography on immobilized Aftin-4 has already been allowed the identification of mitochondrial proteins (mitofilin, voltage-dependent anion channel (VDAC) and prohibitin) as well as PSEN and AβPP as interacting ligants. The use of photo-affinity labeled compounds would further help us uncover direct targets of Aftins, some of which might be one of these 5 candidates. The targets directly interacting with Aftins (rather than associated protein complexes) remain to be identified. At this stage, we feel that protein kinases are quite unlikely targets of Aftins. KinomeScan analysis of Aftin-4 [14] and Aftin-5 (Supplementary Table 1) revealed no significant interaction between these Aftins with any of the 402 or 442 kinases, respectively.
The change in Aβ38/Aβ40/Aβ42 peptides relative abundance induced by Aftin-5 requires active γ-secretase, as demonstrated by the complete inhibition of Aftins by γ-secretase inhibitors [14] (Fig. 4). This suggests the possibility that Aftin-5 directly binds to γ-secretase, modifying its substrate specificity toward the production of Aβ42, at the expense of Aβ38, while the production of Aβ40 remains unaffected. Aftins might then also modify the cleavage sites on other substrates of γ-secretase [41]. Another possibility is that Aftins directly bind to the substrate, AβPP, and thereby alters the interaction with γ-secretase, leading to a shift of the cleavage site on AβPP. Aftins would then act on the AβPP transmembrane domain to drive γ-secretase’s cleaving activity toward longer Aβ peptides at the expense of shorter versions [10]. An alternative explanation would be an interaction with mitochondrial proteins as demonstrated for Aftin-4 [14]. In this respect, the association of γ-secretase with VDAC [42, 43] and the specific binding of VDAC to immobilized Aftin-4 [14] are particularly interesting.
The effects of Aftins and the other Aβ42 increasing agents on the levels of different Aβ38 and Aβ42 peptides (Fig. 3A) [14, 44] are opposite to those induced by the ‘Torrey Pines’ GSM (Fig. 5) [28–30, 45–47], suggesting a mutually exclusive action of these two families of γ-secretase modulating agents. GSMs action also depends on presenilin mutations [24, 45, 48]. The mechanisms of action of GSMs falls in several categories (reviewed in [49–51]: direct binding to Aβ and/or AβPP [52–55], to PEN-2 [22], and PSEN-1 [30, 46, 47, 56]. Structurally different GSMs may thus target different components of the AβPP/ γ-secretase complex. Besides the two families of γ-secretase modulating agents (GSMs and Aftins) which modify the Aβ38/Aβ40/Aβ42 peptides ratio in opposite ways, mutations in the AβPP transmembrane domain also appear to modulate the proportions of amyloid peptides species [57–59]. In particular Lysine 624 (Lys28 in the Aβ peptides) appears to be a critical determinant for the length of the Aβ peptide released by presenilins as mutation to an Alanine or Glutamine leads to a decrease in the production of Aβ40 and Aβ42 peptides in favor of the production of shorter Aβ peptides (Aβ33, Aβ34, Aβ37, Aβ38, Aβ39) [55]. These mutations partially mimic the effects of GSMs. In fact the effects of GSMs also depend on the sequence of the AβPP transmembrane segment [60].
Cellular mechanism of action of Aftins
Comparison of intracellular and extracellular levels of Aβ38, Aβ40, and Aβ42 following exposure to Aftin-5 suggests that, at least under the extraction and assays conditions used here, Aftin-5 modifies the ratio of extracellular Aβ38/Aβ40/Aβ42 (Fig. 6A), but not that of intracellular amyloid peptides (Fig. 6B). This extracellular production/release of Aβ and Aβ42 in particular is clearly relevant to what happens in AD [61].
Besides a direct interaction with γ-secretase or its substrate AβPP, we also investigated the possibility that increased Aβ42 peptide level induced by Aftins could be due to globally reduced degradation or disposal of amyloid peptides. Although we cannot formally exclude a decreased proteolytic degradation by one or several of the numerous proteases that target amyloid peptides [26], we feel this is an unlikely explanation for the action of Aftins. Indeed, if this was the case we would expect an upregulation of Aβ38 and Aβ40 which, respectively, partially disappears or remains stable. Furthermore, addition of inhibitors of such degradation systems did not lead to massive increases of Aβ42 (data not shown). Similarly, down-regulation of autophagy is unlikely to provide an explanation for the increase in Aβ42, and even less so for the down-regulation of Aβ38.
Although Aftin-4 has some clear yet reversible effects on mitochondria structure, Aftin-5 also affects the mitochondria though to a lesser extent. Aftin-5 has no detectable functional effects on isolated mitochondria and mitochondria isolated from Aftin-5 treated cells also display normal physiological characteristics. In contrast when the ultrastructure of cells exposed to Aftin-5 is analyzed, some alterations are seen, which are less obvious than those seen with Aftin-4 (Fig. 7). These results suggest that Aftin-5, like Aftin-4, also interacts with mitochondria in a reversible way. When Aftin is washed away from treated cells or when mitochondria are isolated from treated cells (a procedure that includes numerous washes), mitochondria regain a normal structure. We believe that these modest morphological changes are not dependent on Aβ42 upregulation or Aβ38 down-regulation.
Aftins, new pharmacological tools to investigate amyloid peptides production: Toward a chemical animal model of AD?
Aftins, and particularly Aftin-5, constitute new pharmacological tools to investigate the generation of amyloid peptides from AβPP and particularly the regulation of the balance between Aβ38, Aβ40, and Aβ42 peptides. Aftin-5 allows a shift in the Aβ peptides relative abundance which is reminiscent of that observed in AD. Aftins’ effects on amyloid peptide levels have been observed in several cell lines whether or not they overexpress AβPP, as well as in primary neuronal cultures [14] (Mabondzo et al., in preparation). We are currently investigating the molecular mechanism of action of Aftins. We are also investigating the effects of Aftin-5 in animals, both wild-type mice and AD models such as AβPP/PS1 mice. The overall goal is to establish a chemically induced AD model, similar to the MPTP-induced Parkinsonism model [62, 63].
Altogether we believe that Aftin-5 will contribute to the understanding of the pathological mechanisms that favor the γ-secretase dependent production of Aβ42 and subsequent neurological disorders.
Cellular screens for ‘anti-AD’ and ‘pro-AD’ compounds
The ELISA assay we have set up allows simple, cheap, and fast detection of Aβ38, Aβ40, and Aβ42 peptides released extracellularly from cultured cells exposed to Aftin-5. This simple set-up can be turned to a large scale, cell based assay to screen for inhibitors of Aβ42 production. This would allow the easy detection of β-secretase and γ-secretase inhibitors (Fig. 4) or GSMs (Fig. 5). It might also allow the detection of compounds acting through other pathways (interaction between presenilin and other partners of the γ-secretase complex, interaction with AβPP, interaction with the elements composing the lipid rafts where γ-secretase is enclosed, interaction with intra-cellular proteins involved in the trafficking of AβPP, γ-secretase, and the amyloid peptides, interaction with protein involved in the degradation or withdrawal of amyloids, etc.). All these compounds have a potential as possible ‘anti-AD’ drugs that would deserve further investigation.
The very same cell culture and amyloid detection system allows the detection of Aftin-related compounds (in terms of biological activity rather than structure), namely compounds that upregulate Aβ42 and down-regulate Aβ38. These compounds represent potential hazards as possible ‘pro-AD’ compounds that deserve careful handling, and controlled/regulated exposure. We envisage the testing of a set of natural products but also of anthropic molecules humans are exposed to (the so-called ‘human chemical exposome’). We believe our simple screening test, using Aftin-5 as a positive, reference compound, should be included in safety screens designed to detect potentially hazardous molecules among the large number of compounds that are already on or being brought to the market. These molecules may constitute environmental factors contributing to the onset, development, and acceleration of AD. Their detection and identification would constitute a first step in a preventive action to reduce or even eradicate late onset AD which accounts for more than 99% of all AD cases.
Acknowledgments
This work was supported by the ‘Fonds Unique Interministériel” (FUI) PHARMASEA project (LM and HG), the “Association France-Alzheimer (Finistère)” (LM), CRITT-Santé Bretagne (LM) and by NIH grant AG09464 (M.F.). AH is recipient of a CIFRE PhD fellowship.
Authors’ disclosures available online (http://www.j-alz.com/disclosures/view.php?id=1632).
Footnotes
Supplementary data available online: http://www.j-alz.com/issues/35/vol35-1.html#supplementarydata01
References
- 1.Huang Y, Mucke L. Alzheimer mechanisms and therapeutics strategies. Cell. 2012;148:1204–1222. doi: 10.1016/j.cell.2012.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Selkoe DJ, Mandelkow E, Holtzman DM. The Biology of Alzheimer Disease. Cold Spring Harbor Press; Cold Spring Harbor, New York: 2012. p. 511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. Erratum in: Science 297, 2209. [DOI] [PubMed] [Google Scholar]
- 4.Benilova I, Karran E, De Strooper B. The toxic Aβ oligomer and Alzheimer’s disease: An emperor in need of clothes. Nat Neurosci. 2012;15:349–357. doi: 10.1038/nn.3028. [DOI] [PubMed] [Google Scholar]
- 5.Karran E, Mercken M, De Strooper B. The amyloid cascade hypothesis for Alzheimer’s disease: An appraisal for the development of therapeutics. Nat Rev Drug Discov. 2011;10:698–712. doi: 10.1038/nrd3505. [DOI] [PubMed] [Google Scholar]
- 6.Iqbal K, Liu F, Gong CX, Grundke-Iqbal I. Tau in Alzheimer disease and related tauopathies. Curr Alzheimer Res. 2010;7:656–664. doi: 10.2174/156720510793611592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee VM, Brunden KR, Hutton M, Trojanowski JQ. Developing therapeutic approaches to tau, selected kinases, and related neuronal protein targets. Cold Spring Harb Perspect Med. 2011;1:a006437. doi: 10.1101/cshperspect.a006437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Obulesu M, Venu R, Somashekhar R. Tau mediated neurodegeneration: An insight into Alzheimer’s disease pathology. Neurochem Res. 2011;36:1329–1335. doi: 10.1007/s11064-011-0475-5. [DOI] [PubMed] [Google Scholar]
- 9.De Strooper B, Vassar R, Golde T. The secretases: Enzymes with therapeutic potential in Alzheimer disease. Nat Rev Neurol. 2010;6:99–107. doi: 10.1038/nrneurol.2009.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takami M, Nagashima Y, Sano Y, Ishihara S, Morishima-Kawashima M, Funamoto S, Ihara Y. gamma-Secretase: Successive tripeptide and tetrapeptide release from the transmembrane domain of beta-carboxyl terminal fragment. J Neurosci. 2009;29:13042–13052. doi: 10.1523/JNEUROSCI.2362-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bateman RJ, Aisen PS, De Strooper B, Fox NC, Lemere CA, Ringman JM, Salloway S, Sperling RA, Windisch M, Xiong C. Autosomal-dominant Alzheimer’s disease: A review and proposal for the prevention of Alzheimer’s disease. Alzheimers Res Ther. 2011;3:1. doi: 10.1186/alzrt59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuperstein I, Broersen K, Benilova I, Rozenski J, Jonckheere W, Debulpaep M, Vandersteen A, Segers-Nolten I, Van Der Werf K, Subramaniam V, Braeken D, Callewaert G, Bartic C, D’Hooge R, Martins IC, Rousseau F, Schymkowitz J, De Strooper B. Neurotoxicity of Alzheimer’s disease Aβ peptides is induced by small changes in the Aβ42 to Aβ40 ratio. EMBO J. 2010;29:3408–3420. doi: 10.1038/emboj.2010.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McGowan E, Pickford F, Kim J, Onstead L, Eriksen J, Yu C, Skipper L, Murphy MP, Beard J, Das P, Jansen K, Delucia M, Lin WL, Dolios G, Wang R, Eckman CB, Dickson DW, Hutton M, Hardy J, Golde T. Abeta42 is essential for parenchymal and vascular amyloid deposition in mice. Neuron. 2005;47:191–199. doi: 10.1016/j.neuron.2005.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bettayeb K, Oumata N, Zhang Y, Luo W, Bustos V, Galons H, Greengard P, Meijer L, Flajolet M. A chemical route towards Alzheimer’s disease? FASEB J. 2012;26:5115–5123. doi: 10.1096/fj.12-212985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Findeis MA. The role of amyloid beta peptide 42 in Alzheimer’s disease. Pharmacol Ther. 2007;116:266–286. doi: 10.1016/j.pharmthera.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 16.Pannee J, Portelius E, Oppermann M, Atkins A, Hornshaw M, Zegers I, Höjrup P, Minthon L, Hansson O, Zetterberg H, Blennow K, Gobom J. A selected reaction monitoring (SRM)-based method for absolute quantification of Aβ38, Aβ40, and Aβ42 in cerebrospinal fluid of alzheimer’s disease patients and healthy controls. J Alzheimers Dis. 2013;33:1021–1032. doi: 10.3233/JAD-2012-121471. [DOI] [PubMed] [Google Scholar]
- 17.Blennow K, Zetterberg H, Fagan AM. Fluid biomarkers in Alzheimer disease. Cold Spring Harb Perspect Med. 2012;2:a006221. doi: 10.1101/cshperspect.a006221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oumata N, Ferandin Y, Meijer L, Galons H. Practical synthesis of roscovitine and CR8. Org Process Res Dev. 2009;13:641–644. [Google Scholar]
- 19.Cheng S, Comer DD, Mao L, Balow GP, Pleynet D. World Intellectual Property Organization. 2004. compounds and uses thereof in modulating amyloid beta. Pub. No. WO/2004-110350. [Google Scholar]
- 20.Mehta PD, Pirttila T. Increased cerebrospinal fluid A beta38/A beta42 ratio in Alzheimer disease. Neurodegener Dis. 2005;2:242–245. doi: 10.1159/000090363. [DOI] [PubMed] [Google Scholar]
- 21.Potempska A, Mack K, Mehta P, Kim KS, Miller DL. Quantification of sub-femtomole amounts of Alzheimer amyloid beta peptides. Amyloid. 1999;6:14–21. doi: 10.3109/13506129908993283. [DOI] [PubMed] [Google Scholar]
- 22.Flajolet M, He G, Heiman M, Lin A, Nairn AC, Greengard P. Regulation of Alzheimer’s disease amyloid-beta formation by casein kinase I. Proc Natl Acad Sci U S A. 2007;104:4159–4164. doi: 10.1073/pnas.0611236104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oumata N, Bettayeb K, Ferandin Y, Demange L, Lopez-Giral A, Goddard M-L, Myrianthopoulos V, Mikros E, Flajolet M, Greengard P, Meijer L, Galons H. Roscovitine-derived, dual-specificity inhibitors of cyclin-dependent kinases (CDKs) and casein kinase 1 (CK1) J Med Chem. 2008;51:5229–5242. doi: 10.1021/jm800109e. [DOI] [PubMed] [Google Scholar]
- 24.Meijer L, Raymond E. Roscovitine and other purines as kinase inhibitors. From starfish oocytes to clinical trials. Accounts Chem Res. 2003;36:417–425. doi: 10.1021/ar0201198. [DOI] [PubMed] [Google Scholar]
- 25.Bettayeb K, Oumata N, Echalier A, Ferandin Y, Endicott JA, Galons H, Meijer L. CR8, a potent and selective, roscovitine-derived inhibitor of cyclin-dependent kinases. Oncogene. 2008;27:5797–5807. doi: 10.1038/onc.2008.191. [DOI] [PubMed] [Google Scholar]
- 26.Dovey HF, John V, Anderson JP, Chen LZ, de Saint Andrieu P, Fang LY, Freedman SB, Folmer B, Goldbach E, Holsztynska EJ, Hu KL, Johnson-Wood KL, Kennedy SL, Kholodenko D, Knops JE, Latimer LH, Lee M, Liao Z, Lieberburg IM, Motter RN, Mutter LC, Nietz J, Quinn KP, Sacchi KL, Seubert PA, Shopp GM, Thorsett ED, Tung JS, Wu J, Yang S, Yin CT, Schenk DB, May PC, Altstiel LD, Bender MH, Boggs LN, Britton TC, Clemens JC, Czilli DL, Dieckman-McGinty DK, Droste JJ, Fuson KS, Gitter BD, Hyslop PA, Johnstone EM, Li WY, Little SP, Mabry TE, Miller FD, Audia JE. Functional gamma-secretase inhibitors reduce beta-amyloid peptide levels in brain. J Neurochem. 2001;76:173–181. doi: 10.1046/j.1471-4159.2001.00012.x. [DOI] [PubMed] [Google Scholar]
- 27.Zhao B, Yu M, Neitzel M, Marugg J, Jagodzinski J, Lee M, Hu K, Schenk D, Yednock T, Basi G. Identification of gamma-secretase inhibitor potency determinants on presenilin. J Biol Chem. 2008;283:2927–2938. doi: 10.1074/jbc.M708870200. [DOI] [PubMed] [Google Scholar]
- 28.Kounnas MZ, Danks AM, Cheng S, Tyree C, Ackerman E, Zhang X, Ahn K, Nguyen P, Comer D, Mao L, Yu C, Pleynet D, Digregorio PJ, Velicelebi G, Stauderman KA, Comer WT, Mobley WC, Li YM, Sisodia SS, Tanzi RE, Wagner SL. Modulation of gamma-secretase reduces beta-amyloid deposition in a transgenic mouse model of Alzheimer’s disease. Neuron. 2010;67:769–780. doi: 10.1016/j.neuron.2010.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kretner B, Fukumori A, Gutsmiedl A, Page RM, Luebbers T, Galley G, Baumann K, Haass C, Steiner H. Attenuated Abeta42 responses to low potency gamma-secretase modulators can be overcome for many pathogenic presenilin mutants by second-generation compounds. J Biol Chem. 2011;286:15240–15251. doi: 10.1074/jbc.M110.213587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ebke A, Luebbers T, Fukumori A, Shirotani K, Haass C, Baumann K, Steiner H. Novel γ-secretase enzyme modulators directly target presenilin protein. J Biol Chem. 2011;286:37181–37186. doi: 10.1074/jbc.C111.276972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mawuenyega KG, Sigurdson W, Ovod V, Munsell L, Kasten T, Morris JC, Yarasheski KE, Bateman RJ. Decreased clearance of CNS beta-amyloid in Alzheimer’s disease. Science. 2010;330:1774. doi: 10.1126/science.1197623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miners JS, Barua N, Kehoe PG, Gill S, Love S. Aβ-degrading enzymes: Potential for treatment of Alzheimer disease. J Neuropathol Exp Neurol. 2011;70:944–959. doi: 10.1097/NEN.0b013e3182345e46. [DOI] [PubMed] [Google Scholar]
- 33.Pickford F, Masliah E, Britschgi M, Lucin K, Narasimhan R, Jaeger PA, Small S, Spencer B, Rockenstein E, Levine B, Wyss-Coray T. The autophagy-related protein beclin 1 shows reduced expression in early Alzheimer disease and regulates amyloid beta accumulation in mice. J Clin Invest. 2008;118:2190–2199. doi: 10.1172/JCI33585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ling D, Song HJ, Garza D, Neufeld TP, Salvaterra PM. Abeta42-induced neurodegeneration via an age-dependent autophagic-lysosomal injury in Drosophila. PLoS One. 2009;4:e4201. doi: 10.1371/journal.pone.0004201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jaeger PA, Pickford F, Sun CH, Lucin KM, Masliah E, Wyss-Coray T. Regulation of amyloid precursor protein processing by the Beclin 1 complex. PLoS One. 2010;5:e11102. doi: 10.1371/journal.pone.0011102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tian Y, Bustos V, Flajolet M, Greengard P. A small-molecule enhancer of autophagy decreases levels of Abeta and APP-CTF via Atg5-dependent autophagy pathway. FASEB J. 2011;25:1934–1942. doi: 10.1096/fj.10-175158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ling D, Salvaterra PM. Brain aging and Aβ1-42 neurotoxicity converge via deterioration in autophagylysosomal system: A conditional Drosophila model linking Alzheimer’s neurodegeneration with aging. Acta Neuropathol. 2011;121:183–191. doi: 10.1007/s00401-010-0772-0. [DOI] [PubMed] [Google Scholar]
- 38.Lee JH, Yu WH, Kumar A, Lee S, Mohan PS, Peterhoff CM, Wolfe DM, Martinez-Vicente M, Massey AC, Sovak G, Uchiyama Y, Westaway D, Cuervo AM, Nixon RA. Lysosomal proteolysis and autophagy require presenilin 1 and are disrupted by Alzheimer-related PS1 mutations. Cell. 2010;141:1146–1158. doi: 10.1016/j.cell.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nixon RA, Yang DS. Autophagy failure in Alzheimer’s disease-locating the primary defect. Neurobiol Dis. 2011;43:38–45. doi: 10.1016/j.nbd.2011.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang DS, Stavrides P, Mohan PS, Kaushik S, Kumar A, Ohno M, Schmidt SD, Wesson DW, Bandyopadhyay U, Jiang Y, Pawlik M, Peterhoff CM, Yang AJ, Wilson DA, St George-Hyslop P, Westaway D, Mathews PM, Levy E, Cuervo AM, Nixon RA. Therapeutic effects of remediating autophagy failure in a mouse model of Alzheimer disease by enhancing lysosomal proteolysis. Autophagy. 2011;7:788–789. doi: 10.4161/auto.7.7.15596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beel AJ, Sanders CR. Substrate specificity of gamma-secretase and other intramembrane proteases. Cell Mol Life Sci. 2008;65:1311–1334. doi: 10.1007/s00018-008-7462-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wakabayashi T, Craessaerts K, Bammens L, Bentahir M, Borgions F, Herdewijn P, Staes A, Timmerman E, Vandeker-ckhove J, Rubinstein E, Boucheix C, Gevaert K, De Strooper B. Analysis of the gamma-secretase interactome and validation of its association with tetraspanin-enriched microdomains. Nat Cell Biol. 2009;11:1340–1346. doi: 10.1038/ncb1978. [DOI] [PubMed] [Google Scholar]
- 43.Hur JY, Teranishi Y, Kihara T, Yamamoto NG, Inoue M, Hosia W, Hashimoto M, Winblad B, Frykman S, Tjernberg LO. Identification of novel γ-secretase-associated proteins in detergent-resistant membranes from brain. J Biol Chem. 2012;287:11991–12005. doi: 10.1074/jbc.M111.246074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kukar T, Murphy MP, Eriksen JL, Sagi SA, Weggen S, Smith TE, Ladd T, Khan MA, Kache R, Beard J, Dodson M, Merit S, Ozols VV, Anastasiadis PZ, Das P, Fauq A, Koo EH, Golde TE. Diverse compounds mimic Alzheimer disease-causing mutations by augmenting Abeta42 production. Nat Med. 2005;11:545–550. doi: 10.1038/nm1235. [DOI] [PubMed] [Google Scholar]
- 45.Page RM, Baumann K, Tomioka M, Pérez-Revuelta BI, Fukumori A, Jacobsen H, Flohr A, Luebbers T, Ozmen L, Steiner H, Haass C. Generation of Abeta38 and Abeta42 is independently and differentially affected by familial Alzheimer disease-associated presenilin mutations and gamma-secretase modulation. J Biol Chem. 2008;283:677–683. doi: 10.1074/jbc.M708754200. [DOI] [PubMed] [Google Scholar]
- 46.Ohki Y, Higo T, Uemura K, Shimada N, Osawa S, Berezovska O, Yokoshima S, Fukuyama T, Tomita T, Iwatsubo T. Phenylpiperidine-type γ-secretase modulators target the transmembrane domain 1 of presenilin 1. EMBO J. 2011;30:4815–4824. doi: 10.1038/emboj.2011.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Crump CJ, Fish BA, Castro SV, Chau DM, Gertsik N, Ahn K, Stiff C, Pozdnyakov N, Bales KR, Johnson DS, Li YM. Piperidine acetic acid based γ-secretase modulators directly bind to Presenilin-1. ACS Chem Neurosci. 2011;2:705–710. doi: 10.1021/cn200098p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Czirr E, Cottrell BA, Leuchtenberger S, Kukar T, Ladd TB, Esselmann H, Paul S, Schubenel R, Torpey JW, Pietrzik CU, Golde TE, Wiltfang J, Baumann K, Koo EH, Weggen S. Independent generation of Abeta42 and Abeta38 peptide species by gamma-secretase. J Biol Chem. 2008;283:17049–17054. doi: 10.1074/jbc.M802912200. [DOI] [PubMed] [Google Scholar]
- 49.Wolfe MS. γ-Secretase inhibitors and modulators for Alzheimer’s disease. J Neurochem. 2012;120(Suppl 1):89–98. doi: 10.1111/j.1471-4159.2011.07501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oehlrich D, Berthelot DJ, Gijsen HJ. γ-Secretase modulators as potential disease modifying anti-Alzheimer’s drugs. J Med Chem. 2011;54:669–698. doi: 10.1021/jm101168r. [DOI] [PubMed] [Google Scholar]
- 51.Borgegard T, Juréus A, Olsson F, Rosqvist S, Sabirsh A, Rotticci D, Paulsen K, Klintenberg R, Yan H, Waldman M, Stromberg K, Nord J, Johansson J, Regner A, Parpal S, Malinowsky D, Radesater AC, Li T, Singh R, Eriksson H, Lundkvist J. First and second generation γ-secretase modulators (GSMs) modulate amyloid-β (Aβ) peptide production through different mechanisms. J Biol Chem. 2012;87:11810–11819. doi: 10.1074/jbc.M111.305227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Richter L, Munter LM, Ness J, Hildebrand PW, Dasari M, Unterreitmeier S, Bulic B, Beyermann M, Gust R, Reif B, Weggen S, Langosch D, Multhaup G. Amyloid beta 42 peptide (Abeta42)-lowering compounds directly bind to Abeta and interfere with amyloid precursor protein (APP) transmembrane dimerization. Proc Natl Acad Sci U S A. 2010;107:14597–14602. doi: 10.1073/pnas.1003026107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Botev A, Munter LM, Wenzel R, Richter L, Althoff V, Ismer J, Gerling U, Weise C, Koksch B, Hildebrand PW, Bittl R, Multhaup G. The amyloid precursor protein C-terminal fragment C100 occurs in monomeric and dimeric stable conformations and binds γ-secretase modulators. Biochemistry. 2011;50:828–835. doi: 10.1021/bi1014002. [DOI] [PubMed] [Google Scholar]
- 54.Kukar TL, Ladd TB, Bann MA, Fraering PC, Narlawar R, Maharvi GM, Healy B, Chapman R, Welzel AT, Price RW, Moore B, Rangachari V, Cusack B, Eriksen J, Jansen-West K, Verbeeck C, Yager D, Eckman C, Ye W, Sagi S, Cottrell BA, Torpey J, Rosenberry TL, Fauq A, Wolfe MS, Schmidt B, Walsh DM, Koo EH, Golde TE. Substrate-targeting gamma-secretase modulators. Nature. 2008;453:925–929. doi: 10.1038/nature07055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kukar TL, Ladd TB, Robertson P, Pintchovski SA, Moore B, Bann MA, Ren Z, Jansen-West K, Malphrus K, Eggert S, Maruyama H, Cottrell BA, Das P, Basi GS, Koo EH, Golde TE. Lysine 624 of the amyloid precursor protein (APP) is a critical determinant of amyloid β peptide length: Support for a sequential model of γ-secretase intramembrane proteolysis and regulation by the amyloid β precursor protein (APP) juxtamembrane region. J Biol Chem. 2011;286:39804–39812. doi: 10.1074/jbc.M111.274696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lleó A, Berezovska O, Herl L, Raju S, Deng A, Bacskai BJ, Frosch MP, Irizarry M, Hyman BT. Nonsteroidal anti-inflammatory drugs lower Abeta42 and change presenilin 1 conformation. Nat Med. 2004;10:1065–1066. doi: 10.1038/nm1112. [DOI] [PubMed] [Google Scholar]
- 57.Munter LM, Voigt P, Harmeier A, Kaden D, Gottschalk KE, Weise C, Pipkorn R, Schaefer M, Langosch D, Multhaup G. GxxxG motifs within the amyloid precursor protein transmembrane sequence are critical for the etiology of Abeta42. EMBO J. 2007;26:1702–1712. doi: 10.1038/sj.emboj.7601616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kienlen-Campard P, Tasiaux B, Van Hees J, Li M, Huysseune S, Sato T, Fei JZ, Aimoto S, Courtoy PJ, Smith SO, Constantinescu SN, Octave JN. Amyloidogenic processing but not amyloid precursor protein (APP) intracellular C-terminal domain production requires a precisely oriented APP dimer assembled by transmembrane GXXXG motifs. J Biol Chem. 2008;283:7733–7744. doi: 10.1074/jbc.M707142200. Erratum in: J Biol Chem 283, 12680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sagi SA, Lessard CB, Winden KD, Maruyama H, Koo JC, Weggen S, Kukar TL, Golde TE, Koo EH. Substrate sequence influences γ-secretase modulator activity, role of the transmembrane domain of the amyloid precursor protein. J Biol Chem. 2011;286:39794–39803. doi: 10.1074/jbc.M111.277228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gong P, Vetrivel KS, Nguyen PD, Meckler X, Cheng H, Kounnas MZ, Wagner SL, Parent AT, Thinakaran G. Mutation analysis of the presenilin 1 N-terminal domain reveals a broad spectrum of gamma-secretase activity toward amyloid precursor protein and other substrates. J Biol Chem. 2010;285:38042–38052. doi: 10.1074/jbc.M110.132613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jonsson T, Atwal JK, Steinberg S, Snaedal J, Jonsson PV, Bjornsson S, Stefansson H, Sulem P, Gudbjartsson D, Maloney J, Hoyte K, Gustafson A, Liu Y, Lu Y, Bhangale T, Graham RR, Huttenlocher J, Bjornsdottir G, Andreassen OA, Jönsson EG, Palotie A, Behrens TW, Magnusson OT, Kong A, Thorsteinsdottir U, Watts RJ, Stefansson K. A mutation in APP protects against Alzheimer’s disease and age-related cognitive decline. Nature. 2012;488:96–99. doi: 10.1038/nature11283. [DOI] [PubMed] [Google Scholar]
- 62.Prediger RD, Aguiar AS, Jr, Moreira EL, Matheus FC, Castro AA, Walz R, De Bem AF, Latini A, Tasca CI, Farina M, Raisman-Vozari R. The intranasal administration of 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine (MPTP): A new rodent model to test palliative and neuroprotective agents for Parkinson’s disease. Curr Pharm Des. 2011;17:489–507. doi: 10.2174/138161211795164095. [DOI] [PubMed] [Google Scholar]
- 63.Fox SH, Brotchie JM. The MPTP-lesioned non-human primate models of Parkinson’s disease. Past, present, and future. Prog Brain Res. 2010;184:133–157. doi: 10.1016/S0079-6123(10)84007-5. [DOI] [PubMed] [Google Scholar]