Abstract
Previously, we found an anxiolytic effect of ziprasidone augmentation to escitalopram (compared to placebo augmentation) in patients with depression in an 8-week, randomized, double-blind, parallel-group, placebo-controlled trial. Here, we conducted a post-hoc analysis, comparing changes in the Hamilton Depression (HDRS) and Anxiety (HAM-A) rating scales between patients with anxious depression versus nonanxious depression, using a moderator analysis. HDRS total change scores from baseline and endpoint were not significantly different (interaction term p=0.91) in patients with anxious depression on ziprasidone augmentation (n=19; −9.1 ± 4.9) or placebo (n=19; −6.1 ± 8.9) versus patients without anxious depression on ziprasidone (n=52; −5.5 ± 6.7) or placebo (n=49; −2.3 ± 4.5). There was a trend towards statistical significance (interaction term p=0.1) in favor of patients without anxious depression for a difference in HAM-A total change scores from baseline to endpoint (patients with anxious depression on ziprasidone augmentation (n=19; −2.7 ± 5.3) or placebo (n=19; −3.3 ± 5.8) versus patients without anxious depression on ziprasidone (n=51; −3.9 ± 6.6) or placebo (n=44; −0.9 ± 4.7)). Ziprasidone augmentation was equally efficacious in treating depression in patients with versus without anxious depression. However, the observed anxiolytic effect for patients with higher anxiety was not clinically significant.
Keywords: anxious depression, ziprasidone augmentation, treatment-resistant depression, psychopharmacology, anxiolytic
Introduction
Anxious depression, often defined as major depressive disorder with significant anxiety burden, is a common clinical depression subtype.(Fava et al., 2004, Ionescu et al., 2013) Up to 50% of patients with depression may meet criteria for “anxious” depression, representing a large portion of depressed patients. Anxious depression subtype is also over-represented among patients with treatment-resistant depression (TRD)—representing a large treatment challenge for clinicians.(Ionescu et al., 2014) Although treatment with serotonergic antidepressants (e.g., selective serotonin reuptake inhibitors (SSRIs), serotonin-norepinephrine reuptake inhibitors (SNRIs), and tricyclic antidepressants (TCAs)) can result in initial treatment success in some cases, patients with anxious depression can often require second- and third-line therapy in order to achieve sustained response or remission.(Ionescu et al., 2014, Wiethoff et al., 2010, Wu et al., 2013a) Furthermore, anxious depression puts patients at a higher risk than those without anxious depression for suicidal thinking and attempts (Fava et al., 2008, Seo et al., 2011) and side-effects,(Ionescu et al., 2014, Chan et al., 2012, Thase et al., 2012, Farabaugh et al., 2012, Fava et al., 2008, Wu et al., 2013b) which contribute to overall poorer treatment outcomes.(Ionescu et al., 2014, Farabaugh et al., 2012, Fava et al., 2008, Wiethoff et al., 2010, Wu et al., 2013b, Thase et al., 2012)
In conjunction with serotonergic antidepressants, atypical antipsychotic medications—specifically, quetiapine and aripiprazole—have been FDA approved as augmentation agents for the treatment of unipolar depression. Augmentation with atypical antipsychotics offers approximately two-fold higher odds of achieving remission compared to augmentation with placebo in patients with TRD.(Spielmans et al., 2013, Nelson and Papakostas, 2009, Papakostas et al., 2007) As a consequence, atypical antipsychotics are often called upon clinically as adjunctive therapy to relieve symptoms among TRD patients with anxious major depressive disorder (MDD), while several studies have examined data on the use of atypical antipsychotics aripiprazole and quetiapine for anxious depression (14, 15).
We recently published the results of a double-blind, placebo-controlled trial of ziprasidone augmentation of escitalopram for MDD patients with persistent depressive symptoms despite receiving treatment with open-label escitalopram in which the antidepressant and anxiolytic efficacy of ziprasidone was demonstrated in this patient population.(Papakostas et al., 2015) In fact, the number needed to treat (NNT) for response appeared to be more favorable with respect to anxiolytic than antidepressant effects in that study (NNT of 4 vs 7, respectively). Therefore, in light of this finding, and the need to expand our treatment options for patients with anxious MDD, in the present work we conducted a post-hoc analysis of mood and anxiety outcomes for patients with and without anxious MDD enrolled in that trial.
Materials and Methods
Patient Selection
In accordance with the Declaration of Helsinki and after approval by the institutional review board (IRB), patients for this study were recruited from three separate academic medical centers in the United States: Massachusetts General Hospital, Vanderbilt University, and the University of Alabama at Birmingham between July 2008 and October 2013. All patients signed written informed consent prior to study participation.
A qualified study doctor screened all patients. All were deemed to be in good physical health after basic laboratory testing, urine toxicology/pregnancy screening, and a physical exam by a study physician. Eligible patients were men and women between the ages of 18–65 years old, with a primary psychiatric diagnosis of major depressive disorder (in a current depressive episode), according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria and confirmed by the Structured Clinical Interview for DSM-IV (SCID-I/P).(First et al., 1997) In addition, eligible patients had a total score > 10 at screening on the 16-item Quick Inventory of Depressive Symptomatology-Self Rated scale (QIDS-SR).(Rush et al., 2003) Patients were permitted to take the following psychotropic concomitant medications, insofar as the daily doses remained stable throughout the entire study and had been stable for at least two weeks prior to screening: benzodiazepines (or benzodiazepine-like agents), anticonvulsants, lithium, and buspirone.
Patients were ineligible if any of the following criteria were met at screening or during the study: 1) high risk for suicide or homicide (as deemed by the study investigator); 2) unstable medical illness (including uncontrolled seizure disorder); 3) history of multiple adverse drug reactions or allergies (including allergy to the study drug); 4) substance use disorder, according to DSM-IV evaluation, within the last six months; 5) bipolar disorder (current or past); 6) psychotic disorder or psychotic symptoms (current or past); 7) women of childbearing potential who were not using an effective form of contraception, or who were pregnant, breastfeeding, or lactating; 8) failure of >3 antidepressant treatment trials of adequate dose and duration during the current episode of depression, as assessed by the Massachusetts General Hospital Antidepressant Treatment History Questionnaire;(Chandler et al., 2010) 9) current antipsychotic use, including current or past trials of ziprasidone; 10) current antidepressant use; and 11) participation in an experimental psychotropic drug study within 3 months of screening.
Study Design
This study was an 8-week, randomized, double-blind, parallel-group, placebo controlled trial of ziprasidone augmentation of escitalopram. The study was carried out in two phases: Phase 1 consisted of an 8-week, single-arm, open-label trial of escitalopram; Phase 2 consisted of 8 additional weeks of a randomized, double-blind, placebo-controlled trial of adjunctive ziprasidone in escitalopram nonresponders.
Phase 1: Open-labeled trial of escitalopram
After screening, all eligible patients were enrolled in an 8-week open-label trial of escitalopram. Patients were seen in the clinic weekly for a total of 8 visits. Escitalopram dosing started at 10mg by mouth daily (the minimum acceptable dose for the study). For the first four weeks, this dose could be increased by 10mg per week (up to a maximum of 30mg daily), as deemed clinically necessary and safe by the study doctor and with the consent of the patient. After week four, further dose adjustments were prohibited—unless reductions were deemed clinically necessary—during the remainder of the open-label (Phase 1) and subsequent double-blind (Phase 2) trials.
Phase 2: Double-blind, placebo-controlled ziprasidone augmentation
At the conclusion of Phase 1, patients were enrolled into Phase 2 if the following criteria were met: 1) continued depressive episode, according to DSM-IV criteria; 2) QIDS-SR score ≥10; and 3) remained in good physical health. The following criteria were exclusionary for Phase 2 enrollment: 1) abnormal serum potassium or magnesium levels; 2) evidence of untreated hypothyroidism; 3) a positive urine drug screen; and/or 4) significant cardiac conduction problems (e.g., atrial fibrillation, atrial flutter, atrio-ventricular block, of disqualifying electrocardiogram changes (i.e., prolonged QTc or QRS intervals). In addition to the HDRS17, the 14-item (HAM-A) (Hamilton, 1959) was administered during all visits.
In Phase 2, patients were randomized to receive either ziprasidone augmentation or placebo in a 1:1 fashion. A central randomization center allocated treatments via a computer-generated list of random numbers. This list was blinded to all investigators, study clinicians, clinical raters, and patients. Both placebo and ziprasidone (20mg) were administered in identical capsule form. Independent pharmacists were responsible for preparing the capsules; the study drug was pre-packaged in bottles and consecutively numbered according to the randomization schedule. Patients were instructed to take one capsule twice daily with a full meal, in addition to continuing on the same dosage of escitalopram that they were on at the end of Phase 1.
Similar to the schedule in Phase 1, patients in Phase 2 were seen once a week for 8 weeks. After the first visit in Phase 2, study clinicians could choose to increase the dose of the study drug in 1-capsule, twice-per-day, weekly increments; this resulted in a dose range for ziprasidone of 20–80mg twice daily (i.e., 40–160mg total per day). Dosage could also be lowered, when deemed appropriate by study clinicians, due to intolerable or uncomfortable side effects. Inability to tolerate the minimum doses of ziprasidone or escitalopram resulted in termination from the study. At each study visit, adherence was determined by pill count. Those with <80% adherence were withdrawn from the study.
Anxious Depression Definition
Criteria for anxious depression were as follows: 1) presence of major depressive disorder, as determined by the Structured Clinical Interview for DSM-IV Disorders (SCID); and 2) a score of ≥7 on the Hamilton Depression Rating Scale (HDRS17) anxiety-somatization factor score (Cleary and Guy, 1977) at baseline. From these criteria, patients were designated as either having or not having anxious depression. The anxiety-somatization factor score consists of the following six items: anxiety (general), anxiety (somatic), somatic symptoms (general), somatic symptoms (gastrointestinal), hypochondriasis, and insight. This scale (derived almost four decades ago from a factor analysis of the HDRS,(Cleary and Guy, 1977)) has been shown to be a useful measure for assessing anxious depression status in both clinical and research populations,(McClintock et al., 2011) and has been used to study dimensionally-defined anxious depression in large-scale research trials.(Fava et al., 2008, Wiethoff et al., 2010, Wu et al., 2013a)
Main Outcome Measures
The HDRS was defined a priori to test antidepressant efficacy of ziprasidone augmentation. The HAM-A was defined a priori as the outcome measure for testing the anxiolytic efficacy of ziprasidone augmentation.
Statistical Analyses
Data from all randomized patients were used in the analyses via the intention-to-treat (ITT) method. All patients were assigned to either the anxious or nonanxious depressed group. Chi-square tests were done to compare clinical characteristics between anxious versus nonanxious depressed patients. An intention-to-treat (ITT) ANCOVA analysis was done comparing treatment outcome (ziprasidone versus placebo) using the HDRS17 and HAM-A between patients with anxious versus patients without anxious depression (moderator analysis). Analyses were conducted controlling for baseline scores. All tests were conducted with a significance level of 0.05 (2-tailed), using STATA SE Version 12 statistical software. Statistical power for these tests was >0.8 to detect medium effect sizes.
Results
Thirty-eight (27.3%) of the 139 study patients met criteria for anxious depression at the beginning of Phase 2. Of the 71 patients randomly assigned to ziprasidone augmentation, 19 (26.8%) met criteria for anxious depression; 19 (27.9%) of the 68 patients allocated to placebo met criteria for anxious depression.
In the moderator analysis, HDRS total change scores from baseline and endpoint were not significantly different (interaction term p=0.91) in patients with anxious depression on ziprasidone augmentation (n=19; change scores −9.1 ± 4.9) or placebo (n=19; change scores −6.1 ± 8.9) versus patients without anxious depression on ziprasidone (n=52; change scores −5.5 ± 6.7) or placebo (n=49; change scores −2.3 ± 4.5); Figure 1.
Figure 1. Improvements in HDRS Scores on Ziprasidone Augmentation vs. Placebo Between Anxious Depression and Depression Without Anxiety.
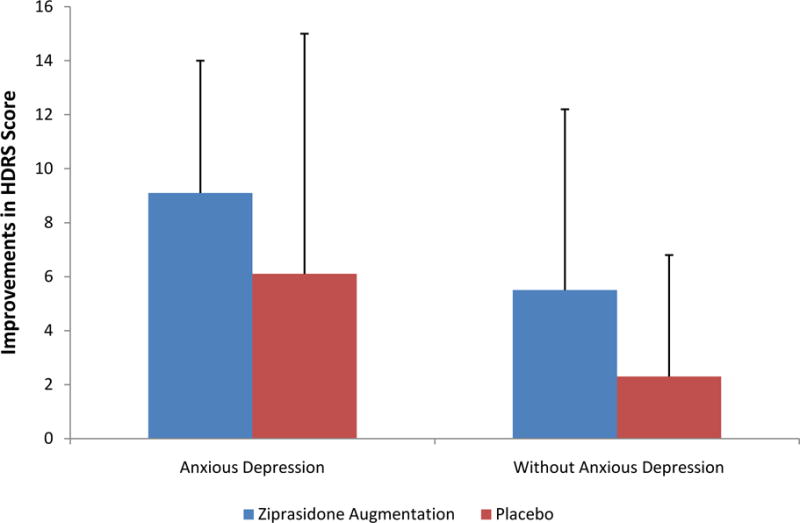
HDRS=Hamilton Depression Rating Scale
133 patients completed at least one HAM-A rating after baseline. In the second moderator analysis, there was a trend towards statistical significance (interaction term p=0.1) for a difference in HAM-A total change scores from baseline to endpoint in patients with anxious depression on ziprasidone augmentation (n=19; change scores −2.7 ± 5.3) or placebo (n=19; change scores −3.3 ± 5.8) versus patients without anxious depression on ziprasidone (n=51; change scores −3.9 ± 6.6) or placebo (n=44; change scores −0.9 ± 4.7); Figure 2. In other words, though there was no statistically significant difference in HAM-A changes in patients on ziprasidone vs. placebo augmentation in the depressed groups (regardless of anxiety status), there was a trend towards ziprasidone being superior to placebo in the depressed group without anxiety.
Figure 2. Improvements in HAM-A Scores on Ziprasidone Augmentation vs. Placebo Between Anxious Depression and Depression Without Anxiety.
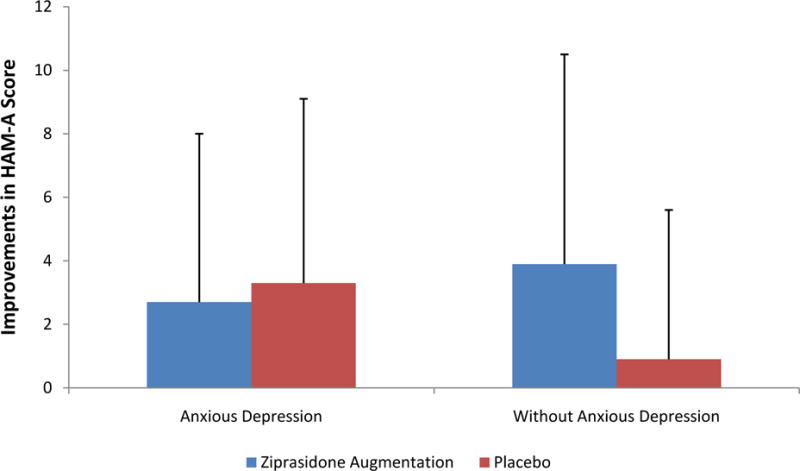
HAM-A=Hamilton Anxiety Rating Scale
Discussion
In this study, ziprasidone augmentation was equally efficacious for treating both depression and anxiety in patients with versus without anxious depression. Specifically, there was an approximately three-point difference in reduction of HDRS scores in favor of adjunctive ziprasidone versus placebo for the two patient groups. However, there was a trend towards statistical significance for differential anxiolytic efficacy between the two patient groups, with a greater difference in HAM-A scores for ziprasidone versus placebo in favor of patients with lower levels of anxiety (compared to those with anxious depression). Therefore, while ziprasidone augmentation seems a reasonable antidepressant approach for patients with and without anxious depression, further care will likely be required for residual anxiety symptoms in the anxious depression subtype.
It is interesting to contrast findings from our study with those available for the other atypical antipsychotic agents. Previously, pooled data from two adjunctive quetiapine trials did find significant antidepressant efficacy for quetiapine versus placebo in patients with anxious depression (with a mean difference in reduction in Montgomery-Asberg Depression Rating Scale (MADRS) scores in patients treated with 150mg or 300mg of adjunctive quetiapine versus placebo of 2.6 and 3.3, respectively).(Bandelow et al., 2014) Interestingly enough, however, equivalent differences in efficacy for patients with low levels of anxiety in those two trials were not in the clinically significant range (1.7 versus 1.2 for 150mg and 300mg, respectively). Equivalent differences in changes in HAM-A scores for these two populations were 1.65 and 2.03 (in favor of quetiapine 150mg and 300mg, respectively, p<0.05) versus 1 and 0.8 (in favor of quetiapine 150mg and 300mg, respectively, p>0.05). Therefore, in contrast to our findings, it appears as though quetiapine, perhaps due its sedative properties, would be a better choice for treating mood symptoms in anxious depressed patients, than those without. Drug placebo differences in HAM-A scores in either of the two groups were not impressive with the exception of the 300mg dose with respect to anxiolytic efficacy in patients with anxious MDD.
In a separate report, Trivedi and colleagues (Trivedi et al., 2008) found that the difference in reduction in MADRS scores between adjunctive aripiprazole versus placebo was 44% greater for patients with nonanxious (versus anxious) depression (2.5 versus 3.6, both being statistically significant). Furthermore, it look longer for adjunctive aripiprazole to separate out from placebo in the anxious depression group compared to the nonanxious depression group (separation occurred at week 2 vs. week 1, respectively). Therefore, in contrast to our findings, it appears as though the antidepressant effect is greater among patients without anxious depression during treatment with adjunctive aripiprazole versus placebo than patients with anxious depression, which may be related to high rates of akathisia reported among patients with anxious depression treated with aripiprazole (25.5%). Unfortunately, HAM-A changes in anxious versus nonanxious depression are not noted in the manuscript for the purposes of comparison. Therefore, in contrast to quetiapine, the antidepressant effects of aripiprazole augmentation appear to be more pronounced among patients with lower levels of anxiety.
Our study had several notable strengths, as it was designed as a randomized, tri-site, double-blind, placebo-controlled trial with an escitalopram open-label treatment lead-in. However, several limitations must be addressed. For one, using the HDRS anxiety-somatization factor score to define “anxious depression” is only one of many definitional methods.(Ionescu et al., 2013) Nevertheless, this method has been employed by many large-scale treatment trials, lending evidence towards its overall usefulness.(Ionescu et al., 2013) In addition, the total percentage of patients with anxious depression in our sample was only 27.3%—much lower than prior estimates of around 50% for other study samples.(Ionescu et al., 2013, Ionescu et al., 2014) Perhaps due to the escitalopram-lead in, many patients originally meeting criteria for “anxious” depression may not have met criteria for the ziprasidone augmentation portion of the study. Indeed, SSRIs, such as escitalopram, have been shown to be efficacious in treating anxious depression (though anxious patients often experience more side effects and relapse sooner than their nonanxious counterparts with depression).(Ionescu et al., 2014) Furthermore, the patients that were randomized in Phase 2 likely represented a group with a more “treatment-resistant” type of anxious depression; therefore, superior efficacy with ziprasidone may have been more difficult to demonstrate in this particular cohort. This may also explain the trend toward significance for ziprasidone augmentation being superior in treating anxiety in nonanxious patients compared to those with anxious depression.
In conclusion, ziprasidone augmentation of escitalopram appears equally efficacious with respect to mood symptoms in the treatment of both anxious and nonanxious depression. However, the observed anxiolytic effect size for patients with higher levels of anxiety was not found to be in the clinically significant range. These results, along with those published for adjunctive aripiprazole and quetiapine, suggest that residual anxiety symptoms among TRD patients with anxious depression represent a major unmet need.
Acknowledgments
The authors would like to acknowledge the National Institute for Mental Health (NIMH) for funding through an R01 mechanism (R01MH081235 awarded to G.I.P.), Pfizer Inc. (for providing free blinded ziprasidone/placebo pills), and Forest Laboratories, Inc. (for providing free escitalopram)
Footnotes
Clinical Trial Registration: Ziprasidone Augmentation of SSRIs for Patients With Major Depressive Disorder (MDD) That do Not Sufficiently Respond to Treatment With SSRIs; Number: NCT00633399; URL: http://clinicaltrials.gov/show/NCT00633399.
Conflicts and Disclosures:
George I. Papakostas: Consultant: Abbott Laboratories, AstraZeneca PLC, Avanir Pharmaceuticals, Brainsway Ltd, Bristol-Myers Squibb Company, Cephalon Inc., Dey Pharma, L.P., Eli Lilly Co., GlaxoSmithKline, Evotec AG, H. Lundbeck A/S, Inflabloc Pharmaceuticals, Jazz Pharmaceuticals, Novartis Pharma AG, Otsuka Pharmaceuticals, PAMLAB LLC, Pfizer Inc., Pierre Fabre Laboratories, Ridge Diagnostics (formerly known as Precision Human Biolaboratories), Shire Pharmaceuticals, Sunovion Pharmaceuticals, Takeda Pharmaceutical Company LTD, Theracos, Inc., and Wyeth, Inc.; Grant/Research Support: AstraZeneca PLC, Bristol-Myers Squibb Company, Forest Pharmaceuticals, the National Institute of Mental Health, PAMLAB LLC, Pfizer Inc., Ridge Diagnostics (formerly known as Precision Human Biolaboratories), Sunovion Pharmaceuticals, and Theracos, Inc.; Honoraria (for consulting or educational activities): Abbott Laboratories, Astra Zeneca PLC, Avanir Pharmaceuticals, Bristol-Myers Squibb Company, Brainsway Ltd, Cephalon Inc., Dey Pharma, L.P., Eli Lilly Co., Evotec AG, GlaxoSmithKline, Inflabloc Pharmaceuticals, Jazz Pharmaceuticals, H. Lundbeck A/S, Novartis Pharma AG, Otsuka Pharmaceuticals, Pamlab LLC, Pfizer, Pierre Fabre Laboratories, Ridge Diagnostics, Shire Pharmaceuticals, Sunovion Pharmaceuticals, Takeda Pharmaceutical Company LTD, Theracos, Inc., Titan Pharmaceuticals, and Wyeth Inc.; Speaker or Advisory Boards: BristolMyersSquibb Co and Pfizer, Inc. (Previous). This study was supported by the National Institute for Mental Health (NIMH R01MH081235), Pfizer Inc. (providing free blinded ziprasidone/placebo pills), and Forest Laboratories, Inc. (providing free escitalopram).
Richard C. Shelton: Consultant: Cerecor, Inc., Clintara, LLC, Janssen Pharmaceutica, MSI Methylation Sciences, Inc., Naurex, Inc., Nestle’ Health-Pamlab, Inc., Pfizer, Inc., Ridge Diagnostics. Grant/research support: Alkermes, Inc., Assurex Health, Avanir Pharmaceuticals, Forest Pharmaceuticals (Allergan), Genomind, Janssen Pharmaceutica, Johnson & Johnson, Naurex Inc., Novartis Inc., Otsuka Pharmaceuticals, Nestle’ Health-Pamlab, Inc., Takeda Pharmaceuticals.
Dawn F. Ionescu: Received research funding from a Young Investigator Award through the Brain and Behavior Research Foundation, KL2/CMeRIT Award from the Harvard Catalyst, K23 award from the National Institute for Mental Health (1K23MH107776-01), and from the Executive Committee on Research at Massachusetts General Hospital.
Contributor Information
Dawn F. Ionescu, Email: dionescu@mgh.harvard.edu.
Richard C. Shelton, Email: rshelton@uab.edu.
Lee Baer, Email: baer.lee@mgh.harvard.edu.
Kathryn H. Meade, Email: khmeade@mgh.harvard.edu.
Michaela B. Swee, Email: mbswee@gmail.com.
Maurizio Fava, Email: mfava@mgh.harvard.edu.
George I. Papakostas, Email: gpapakostas@mgh.harvard.edu.
References
- BANDELOW B, BAUER M, VIETA E, EL-KHALILI N, GUSTAFSSON U, EARLEY WR, ERIKSSON H. Extended release quetiapine fumarate as adjunct to antidepressant therapy in patients with major depressive disorder: pooled analyses of data in patients with anxious depression versus low levels of anxiety at baseline. World J Biol Psychiatry. 2014;15:155–66. doi: 10.3109/15622975.2013.842654. [DOI] [PubMed] [Google Scholar]
- CHAN HN, RUSH AJ, NIERENBERG AA, TRIVEDI M, WISNIEWSKI SR, BALASUBRAMANI GK, FRIEDMAN ES, GAYNES BN, DAVIS L, MORRIS D, FAVA M. Correlates and outcomes of depressed out-patients with greater and fewer anxious symptoms: a CO-MED report. Int J Neuropsychopharmacol. 2012;15:1387–99. doi: 10.1017/S1461145711001660. [DOI] [PubMed] [Google Scholar]
- CHANDLER GM, IOSIFESCU DV, POLLACK MH, TARGUM SD, FAVA M. RESEARCH: Validation of the Massachusetts General Hospital Antidepressant Treatment History Questionnaire (ATRQ) CNS Neurosci Ther. 2010;16:322–5. doi: 10.1111/j.1755-5949.2009.00102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLEARY P, GUY W. Factor analysis of Hamilton depression scale. Drugs Exp Clin Res. 1977:115–120. [Google Scholar]
- FARABAUGH A, ALPERT J, WISNIEWSKI SR, OTTO MW, FAVA M, BAER L, PERLIS R, FRIEDMAN E, NYER M, BITRAN S, BALASUBRAMANI GK, INAMORI A, TRIVEDI M, THASE ME. Cognitive therapy for anxious depression in STAR(*) D: what have we learned? J Affect Disord. 2012;142:213–8. doi: 10.1016/j.jad.2012.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAVA M, ALPERT JE, CARMIN CN, WISNIEWSKI SR, TRIVEDI MH, BIGGS MM, SHORES-WILSON K, MORGAN D, SCHWARTZ T, BALASUBRAMANI GK, RUSH AJ. Clinical correlates and symptom patterns of anxious depression among patients with major depressive disorder in STAR*D. Psychol Med. 2004;34:1299–308. doi: 10.1017/s0033291704002612. [DOI] [PubMed] [Google Scholar]
- FAVA M, RUSH AJ, ALPERT JE, BALASUBRAMANI GK, WISNIEWSKI SR, CARMIN CN, BIGGS MM, ZISOOK S, LEUCHTER A, HOWLAND R, WARDEN D, TRIVEDI MH. Difference in treatment outcome in outpatients with anxious versus nonanxious depression: a STAR*D report. Am J Psychiatry. 2008;165:342–51. doi: 10.1176/appi.ajp.2007.06111868. [DOI] [PubMed] [Google Scholar]
- FIRST MB, SPITZER RL, GIBBON M, WILLIAMS JBW. Structured Clinical Interview for DSM-IV Axis I Disorders. Washington, DC: American Psychiatric Press; 1997. [Google Scholar]
- HAMILTON M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32:50–5. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- IONESCU DF, NICIU MJ, HENTER ID, ZARATE CA. Defining anxious depression: a review of the literature. CNS Spectr. 2013;18:252–60. doi: 10.1017/S1092852913000114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IONESCU DF, NICIU MJ, RICHARDS EM, ZARATE CA., JR Pharmacologic treatment of dimensional anxious depression: a review. Prim Care Companion CNS Disord. 2014;16 doi: 10.4088/PCC.13r01621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCCLINTOCK SM, HUSAIN MM, BERNSTEIN IH, WISNIEWSKI SR, TRIVEDI MH, MORRIS D, ALPERT J, WARDEN D, LUTHER JF, KORNSTEIN SG, BIGGS MM, FAVA M, RUSH AJ. Assessing anxious features in depressed outpatients. Int J Methods Psychiatr Res. 2011;20:e69–82. doi: 10.1002/mpr.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NELSON JC, PAPAKOSTAS GI. Atypical antipsychotic augmentation in major depressive disorder: a meta-analysis of placebo-controlled randomized trials. Am J Psychiatry. 2009;166:980–91. doi: 10.1176/appi.ajp.2009.09030312. [DOI] [PubMed] [Google Scholar]
- PAPAKOSTAS GI, FAVA M, BAER L, SWEE MB, JAEGER A, BOBO WV, SHELTON RC. Ziprasidone Augmentation of Escitalopram for Major Depressive Disorder: Efficacy Results From a Randomized, Double-Blind, Placebo-Controlled Study. Am J Psychiatry. 2015 doi: 10.1176/appi.ajp.2015.14101251. appiajp201514101251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAPAKOSTAS GI, SHELTON RC, SMITH J, FAVA M. Augmentation of antidepressants with atypical antipsychotic medications for treatment-resistant major depressive disorder: a meta-analysis. J Clin Psychiatry. 2007;68:826–31. doi: 10.4088/jcp.v68n0602. [DOI] [PubMed] [Google Scholar]
- RUSH AJ, TRIVEDI MH, IBRAHIM HM, CARMODY TJ, ARNOW B, KLEIN DN, MARKOWITZ JC, NINAN PT, KORNSTEIN S, MANBER R, THASE ME, KOCSIS JH, KELLER MB. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry. 2003;54:573–83. doi: 10.1016/s0006-3223(02)01866-8. [DOI] [PubMed] [Google Scholar]
- SEO HJ, JUNG YE, KIM TS, KIM JB, LEE MS, KIM JM, LIM HW, JUN TY. Distinctive clinical characteristics and suicidal tendencies of patients with anxious depression. J Nerv Ment Dis. 2011;199:42–8. doi: 10.1097/NMD.0b013e3182043b60. [DOI] [PubMed] [Google Scholar]
- SPIELMANS GI, BERMAN MI, LINARDATOS E, ROSENLICHT NZ, PERRY A, TSAI AC. Adjunctive atypical antipsychotic treatment for major depressive disorder: a meta-analysis of depression, quality of life, and safety outcomes. PLoS Med. 2013;10:e1001403. doi: 10.1371/journal.pmed.1001403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THASE ME, DEMYTTENAERE K, EARLEY WR, GUSTAFSSON U, UDD M, ERIKSSON H. Extended release quetiapine fumarate in major depressive disorder: analysis in patients with anxious depression. Depress Anxiety. 2012;29:574–86. doi: 10.1002/da.21970. [DOI] [PubMed] [Google Scholar]
- TRIVEDI MH, THASE ME, FAVA M, NELSON CJ, YANG H, QI Y, TRAN QV, PIKALOV A, CARLSON BX, MARCUS RN, BERMAN RM. Adjunctive aripiprazole in major depressive disorder: analysis of efficacy and safety in patients with anxious and atypical features. J Clin Psychiatry. 2008;69:1928–36. [PubMed] [Google Scholar]
- WIETHOFF K, BAUER M, BAGHAI TC, MOLLER HJ, FISHER R, HOLLINDE D, KIERMEIR J, HAUTH I, LAUX G, CORDES J, BRIEGER P, KRONMULLER KT, ZEILER J, ADLI M. Prevalence and treatment outcome in anxious versus nonanxious depression: results from the German Algorithm Project. J Clin Psychiatry. 2010;71:1047–54. doi: 10.4088/JCP.09m05650blu. [DOI] [PubMed] [Google Scholar]
- WU Z, CHEN J, YUAN C, HONG W, PENG D, ZHANG C, CAO L, FANG Y. Difference in remission in a Chinese population with anxious versus nonanxious treatment-resistant depression: a report of OPERATION study. J Affect Disord. 2013a;150:834–9. doi: 10.1016/j.jad.2013.03.012. [DOI] [PubMed] [Google Scholar]
- WU Z, CHEN J, YUAN C, HONG W, PENG D, ZHANG C, CAO L, FANG Y. Difference in remission in a Chinese population with anxious versus nonanxious treatment-resistant depression: A report of OPERATION study. J Affect Disord. 2013b doi: 10.1016/j.jad.2013.03.012. [DOI] [PubMed] [Google Scholar]


