Abstract
Background
Heart failure is the leading cause for 30-day all-cause readmission, the reduction of which is a goal of the Affordable Care Act. There is a growing interest in understanding the impact of evidence-based heart failure therapy on 30-day all-cause readmission. In the current study, we examined the impact of angiotensin-converting enzyme inhibitors or angiotensin receptor blockers (ACEI-ARBs) on 30-day all-cause readmission in heart failure.
Methods
Of the 1384 hospitalized Medicare beneficiaries with heart failure and left ventricular ejection fraction <45% discharged alive from 106 Alabama hospitals (1998–2001) without prior ACEI-ARB use and without known contraindications to ACEI-ARB use, 734 received new predischarge prescriptions for these drugs. Using propensity scores for ACEI-ARB initiation, we assembled a matched cohort of 477 pairs of patients balanced on 32 baseline characteristics (mean age 75 years, 46% women, 26% African American).
Results
30-day all-cause readmissions occurred in 18% and 24% of matched patients receiving and not receiving ACEI-ARBs, respectively (hazard ratio {HR}, 0.74; 95% confidence interval {CI}, 0.56–0.97; p=0.030). ACEI-ARB use was also associated with lower risk of 30-day all-cause mortality (HR, 0.56; 95% CI, 0.33–0.98; p=0.041) and of the combined endpoint of 30-day all-cause readmission or 30-day all-cause mortality (HR, 0.73; 95% CI, 0.56–0.94; p=0.017). All associations remained significant at 1-year post-discharge.
Conclusions
Among hospitalized patients with heart failure and reduced ejection fraction, the use of ACEI-ARBs was associated with a significantly lower risk of 30-day all-cause readmission and 30-day all-cause mortality; both beneficial associations persisted during long-term follow-up.
Keywords: ACEI or ARB, heart failure, hospital readmission
Heart failure is the leading cause of hospital admission and readmission for Medicare beneficiaries aged 65 years and older in the United States.1 The 2010 Patient Protection and Affordable Care Act has made provisions for financial penalties for hospitals with above-average 30-day all-cause readmissions. In 2014, when the law was first implemented, 66% of hospitals paid $227 million, a 2% loss in total Medicare inpatient payments for the year.2 According to the Congressional Budget Office, over the next 10 years, hospitals may collectively lose about 7 billion dollars in Medicare payments for failing the cost-driven metric of 30-day all-cause readmission.3 As the 30-day all-cause readmission rate in heart failure remains high,1 and few intervention appears to be effective,4 there is a growing interest in understanding the role of evidence-based therapy on 30-day all-cause readmission in patients with heart failure.
National heart failure guidelines recommends the use of angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin receptor blockers (ARBs) in patients with heart failure and reduced ejection fraction.5 These drugs have favorable hemodynamic and neuroendocrine effects and have been shown to improve clinical outcomes in patients with heart failure and reduced ejection fraction.6 Heart failure medications with a favorable hemodynamic effect may alleviate symptoms and may also reduce hospital admission and readmission.7–9 in the current study, we tested the hypothesis that discharge prescription of ACEIs or ARBs will be associated with lower 30-day all-cause hospital readmission in patients with heart failure and reduced ejection fraction.
MATERIALS AND METHODS
Data Source and Study Patients
The current study is based on the Alabama Heart Failure Project, the details of which have been described previously.7,10,11 Briefly, medical records of 8555 unique fee-for-service Medicare beneficiaries discharged with a principal discharge diagnosis of with heart failure from 106 Alabama hospitals between July 1, 1998 and October 31, 2001 were abstracted by trained technicians.10 A diagnosis of heart failure was based on the International Classification of Diseases, 9th Revision, Clinical Modification codes for heart failure. Of the 8555 patients, 8049 were discharged alive, of which 5479 (68%) had data on left ventricular ejection fraction and 3067 (55%) had ejection fraction <45%.
Assembly of an Inception Cohort
To minimize bias associated with prevalent use of drugs, we created an inception cohort by excluding 1591 (52% of the 3067) patients who were receiving ACEIs or ARBs prior to hospital admission.12 We also excluded 92 patients who had prior documented records of contraindications or intolerance to these drugs or had baseline serum potassium greater than 5.5 mEq/ml. Because of emerging evidence of benefit of ACEIs or ARBs in heart failure patients with chronic kidney disease, these patients were not excluded.13,14 Of the 1384 patients with heart failure and reduced ejection fraction and without any contraindication for use of ACEIs or ARBs, 734 (53%) received discharge prescriptions for these drugs. Extensive data on other baseline characteristics including demographics, medical history, medications at discharge, hospital course, and discharge disposition were also collected by chart abstraction. 7,10
Propensity Matching: Assembly of a Balanced Cohort
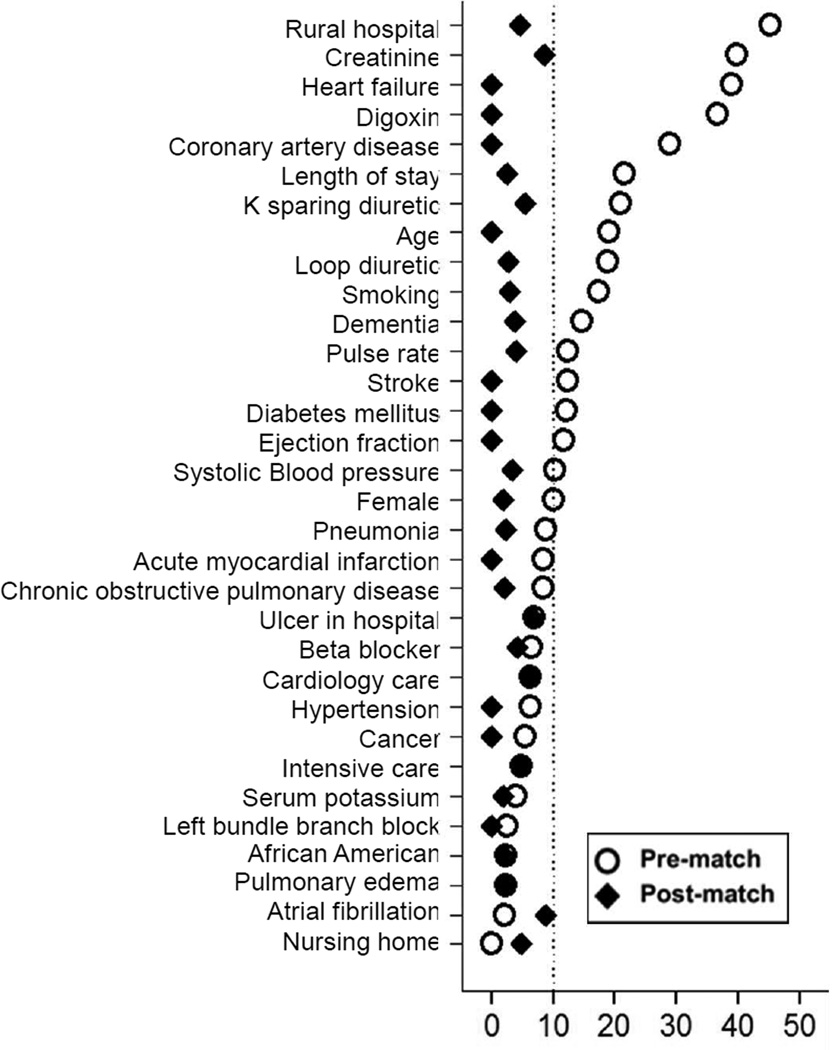
Due to imbalances in the baseline characteristics between patients receiving and not receiving ACEIs or ARBs (Table 1 and Figure 1), propensity score matching approach was used to assemble a balanced cohort of patients receiving and not receiving these drugs.15,16 Propensity scores or predicted probability of receiving a discharge prescription for ACEIs or ARBs were estimated for each of the 1384 patients using a non-parsimonious multivariable logistic regression model using 32 baseline characteristics as covariates.7,13,17,18 Using a greedy matching protocol described elsewhere,17,18 we were able to match 477 patients receiving ACEIs or ARBs with 477 patients not receiving these drugs who had similar propensity scores. Absolute standardized differences before and after matching were estimated for all 32 baseline characteristics to examine between-group covariate balance and were presented as a Love plot.19 An absolute standardized difference of 0% indicates no residual bias, and differences <10% are considered inconsequential.
Table 1.
Baseline characteristics of hospitalized Medicare beneficiaries with heart failure and left ventricular ejection fraction <45%, by the receipt of a new discharge prescription for angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin receptor blocker (ARBs), before and after propensity score matching
| n (%) or mean (±SD) | Pre-match (N=1384) |
Post-match (N=954) |
||||
|---|---|---|---|---|---|---|
| ACEIs or ARBs |
P value | ACEIs or ARBs |
P value | |||
| No (n=650) | Yes (n=734) | No (n=477) | Yes (n=477) | |||
| Age (years) | 76 (±10) | 74 (±11) | 0.004 | 75.0 (±10) | 75.4 (±10) | 0.544 |
| Female | 285 (44) | 359 (49) | 0.059 | 224 (47) | 217 (46) | 0.696 |
| African American | 167 (26) | 199 (27) | 0.894 | 120 (25) | 123 (26) | 0.881 |
| Nursing home residents | 32 (5) | 35 (5) | 0.003 | 21 (4) | 25 (5) | 0.644 |
| Current smoker | 73 (11) | 123 (17) | 0.003 | 62 (13) | 59 (12) | 0.848 |
| Left ventricular ejection fraction (%) | 29 (±9) | 28 (±8) | 0.002 | 29 (±9) | 29 (±8) | 0.761 |
| Past medical history | ||||||
| Prior heart failure | 494 (76) | 429 (58) | 0.000 | 336 (70) | 332 (70) | 0.809 |
| Coronary artery disease | 434 (67) | 390 (53) | 0.001 | 290 (50) | 291 (50) | 1.000 |
| Hypertension | 428 (66) | 465 (63) | 0.333 | 314 (66) | 313 (66) | 1.000 |
| Atrial fibrillation | 178 (27) | 205 (28) | 0.821 | 123 (26) | 140 (30) | 0.229 |
| Left bundle branch block | 133 (21) | 149 (20) | 0.941 | 95 (20) | 95 (20) | 1.000 |
| Diabetes mellitus | 284 (44) | 276 (38) | 0.021 | 197 (41) | 194 (41) | 0.898 |
| Stroke | 151 (23) | 131 (18) | 0.013 | 99 (21) | 100 (21) | 1.000 |
| Chronic obstructive pulmonary disease | 246 (38) | 252 (34) | 0.174 | 173 (36) | 176 (37) | 0.893 |
| Dementia | 64 (10) | 45 (6) | 0.010 | 32 (7) | 36 (8) | 0.694 |
| Cancer | 17 (3) | 28 (4) | 0.209 | 13 (3) | 14 (3) | 1.000 |
| Clinical / laboratory findings | ||||||
| Pulse (beats per minute) | 93 (±24) | 96 (±24) | 0.011 | 94 (±25) | 95 (±24) | 0. 624 |
| Systolic blood pressure (mmHg) | 146 (±30) | 149 (±28) | 0.073 | 148 (±31) | 147 (±28) | 0.767 |
| Pulmonary edema by chest x-ray | 473 (73) | 543 (74) | 0.611 | 350 (73) | 341 (72) | 0.557 |
| Serum creatinine (mEq/L) | 1.9 (±1.4) | 1.4 (±1.1) | 0.001 | 1.6 (±1.1) | 1.5 (±1.2) | 0.199 |
| eGFR (ml/min/1.73 m2) | 54 (± 24) | 57 (± 25) | 0.047 | 56 (± 27) | 57 (± 25) | 0.798 |
| In-hospital events | ||||||
| Pneumonia | 193 (30) | 193 (26) | 0.159 | 129 (27) | 122 (26) | 0.671 |
| Acute myocardial infarction | 44 (7) | 35 (5) | 0.109 | 22 (5) | 23 (5) | 1.000 |
| Pressure ulcer | 65 (10) | 61 (8) | 0.276 | 45 (9) | 51 (11) | 0.586 |
| Hospital and care characteristics | ||||||
| Rural hospital | 144 (45) | 179 (24) | 0.327 | 107 (22) | 115 (24) | 0.604 |
| Cardiology consult | 426 (66) | 503 (69) | 0.237 | 313 (66) | 300 (63) | 0.422 |
| Intensive care unit | 35 (5) | 28 (4) | 0.162 | 23 (5) | 20 (4) | 0.761 |
| Length of stay (days) | 7.6 (±6) | 6.5 (±4) | 0.000 | 6.8 (±4) | 6.9 (±4) | 0.810 |
| Discharge medications | ||||||
| Beta-blockers | 190 (29) | 238 (32) | 0.199 | 154 (32) | 145 (30) | 0.578 |
| Digoxin | 299 (41) | 431 (59) | <0.001 | 252 (51) | 242 (49) | 0.544 |
| Loop diuretics | 522 (80) | 635 (87) | 0.002 | 317 (83) | 319 (84) | 0.930 |
| Potassium-sparing diuretics | 92 (14) | 158 (22) | 0.000 | 82 (17) | 71 (15) | 0.367 |
| Potassium supplements | 280 (43) | 328 (45) | 0.547 | 212 (44) | 207 (43) | 0.794 |
Figure 1.
Love plot displaying absolute standardized differences for 32 baseline characteristics of hospitalized Medicare beneficiaries with heart failure and left ventricular ejection fraction <45% receiving and not receiving a new discharge prescription for angiotensin-converting enzyme inhibitors or angiotensin receptor blocker, before and after propensity score matching
Outcomes
The primary outcome of the current analysis was 30-day all-cause readmission. Secondary outcomes included 30-day readmissions due to heart failure, 30-day all-cause mortality, and 30-day composite end point of 30-day all-cause mortality or all-cause readmissions. In addition, we also examined these outcomes at one year after index hospital discharge. Data on all outcomes and time to first occurrence of each outcome were obtained from the Centers for Medicare and Medicaid Services Medicare data files.7,10
Statistical Analysis
Baseline characteristics were compared using Pearson’s Chi-square and Wilcoxon rank-sum tests for pre-match, and McNemar’s test and paired sample t-test for post-match comparisons, as appropriate. Cox regression models were used to examine associations of ACEI or ARB use with all outcomes among matched patients.7,11 In addition, we used Kaplan-Meier survival analysis to generates plots for 30-day all-cause readmissions by ACEI or ARB use in the matched cohort. All statistical tests were two-tailed with a p-value <0.05 considered significant. IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp. was used for data analyses.
RESULTS
Baseline Characteristics
Matched patients had a mean age (±SD) of 75 (±10) years, 46% were women, and 26% were African American. Pre-match imbalances in the distribution of age, race, history of prior heart failure, comorbidities and treatment between cohort receiving and not receiving ACEIs or ARBs were well balanced after matching (Table 1 and Figure 1).
Associations with 30-Day Outcomes
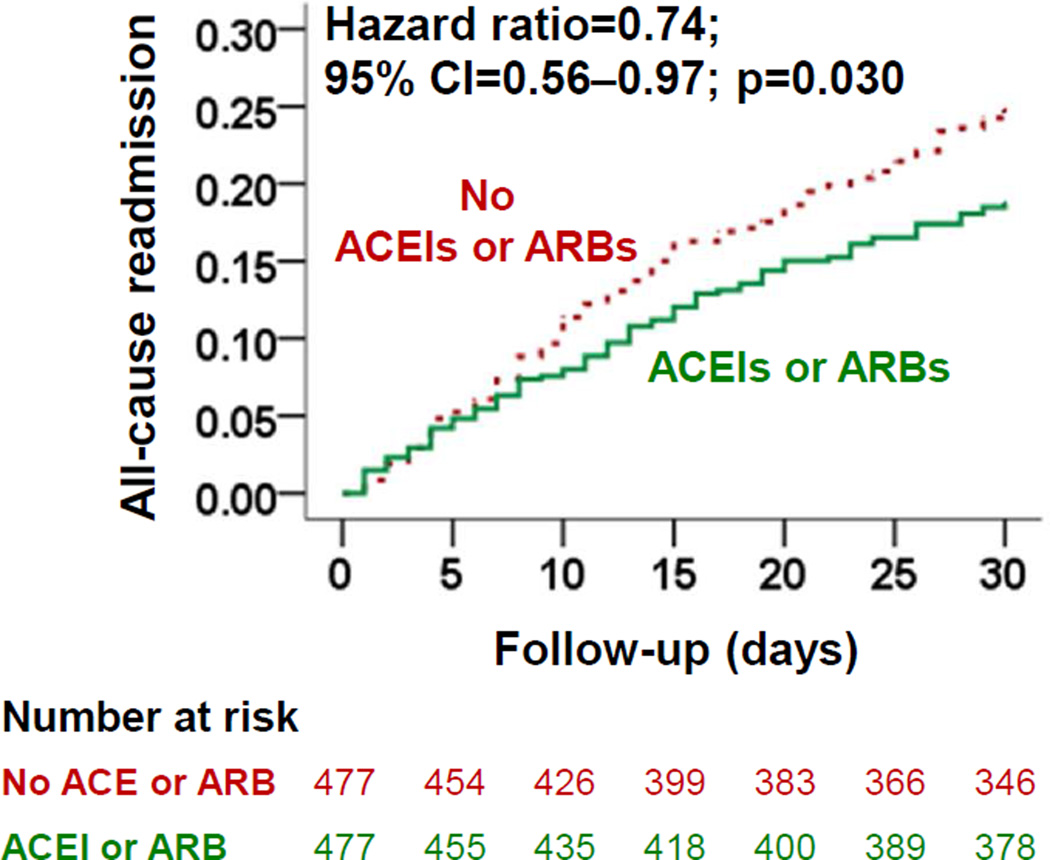
30-day all-cause readmission occurred in 18% (88/477) and 24% (116/477) of matched patients receiving and not receiving a discharge prescription for ACEIs or ARBs, respectively (hazard ratio {HR} associated with ACEI or ARB use, 0.74; 95% confidence interval {CI}: 0.56–0.97; Table 2 and Figure 2). Findings from our sensitivity analysis demonstrate that of the 477 matched pairs, 180 patients clearly outlived their matched counterparts, 102 of which were receiving ACEIs or ARBs. In the absence of hidden bias, a sign-score test for matched data with censoring suggest that beneficial association of ACEI or ARB use with 30-day all-cause readmission maybe sensitive to a potential unmeasured confounder (p=0.0736).
Table 2.
Association between pre-discharge initiation of angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin receptor blocker (ARBs) and 30-day post-discharge outcomes in a propensity-matched cohort of hospitalized Medicare beneficiaries with heart failure and left ventricular ejection fraction <45%
| 30-day outcomes | % (events) |
Absolute risk diff.* |
Hazard ratio† (95% confidence interval) |
|
|---|---|---|---|---|
| ACEIs or ARBs | ||||
| No (n=477) | Yes (n=477) | |||
| All-cause readmission | 24% (116) | 18% (88) | −6% | 0.74 (0.56–0.97); p=0.030 |
| Heart failure readmission | 14% (65) | 7% (35) | −7% | 0.52 (0.35–0.79); p=0.002 |
| All-cause mortality | 7% (35) | 4% (20) | −3% | 0.56 (0.33–0.98); p=0.041 |
| All-cause mortality or all-cause readmission | 28% (133) | 21% (100) | −7% | 0.73 (0.56–0.94); p=0.017 |
Absolute risk differences were calculated by subtracting percent events in patients receiving no ACEIs or ARBs from those receiving those drugs
The hazard ratios compared patients receiving ACEIs or ARBs versus those not receiving these drugs. These hazard ratios were calculated by treating patients without events during the first 30 days as censored.
Figure 2.
Kaplan-Meier plots displaying association between pre-discharge initiation of angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin receptor blocker (ARBs) and 30-day all-cause readmission in a propensity-matched cohort of hospitalized Medicare beneficiaries with heart failure and left ventricular ejection fraction <45%. (CI=confidence interval)
There was no evidence of heterogeneity in the association of ACEI or ARB use with 30-day all-cause readmission in subgroups of patients based on an estimated glomerular filtration rate cutoff of 45 mL/min/1.73 m2. ACEI or ARB use was associated with significantly lower risk of 30-day heart failure readmission (7% vs. 14% for those not receiving these drugs; HR, 0.52; 95% CI, 0.35–0.79; Table 2). This association was insensitive to a potential unmeasured confounder (58 of the 92 patients who clearly outlived their matched counterparts were receiving ACEIs or ARBs; sign-score test p=0.0123).
30-day all-cause mortality occurred in 4% and 7% of patients receiving and not receiving ACEIs or ARBs, respectively (HR, 0.56; 95% CI, 0.33–0.98; Table 2). For this outcome, of the 53 patients that clearly outlived their matched counterparts, 34 received ACEIs or ARBs (sign-score test p=0.0394). The use of ACEIs or ARBs was also associated with a significantly lower risk for 30-day all–cause mortality or all-cause readmission (HR, 0.73; 95% CI, 0.56–0.94; Table 2). This association was also insensitive to a potential unmeasured confounder (sign-score test p = 0.0312).
Associations with 12-Month Outcomes
During the first year after hospital discharge, 64% and 69% of patients receiving and receiving ACEIs or ARBs had all-cause readmissions, respectively (HR, 0.81; 95% CI, 0.69–0.95; Table 3). A discharge prescription of ACEI or ARB was associated with a significant lower risk for other one-year outcomes (Table 3).
Table 3.
Association between pre-discharge initiation of angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin receptor blocker (ARBs) and 1-year post-discharge outcomes in a propensity-matched cohort of hospitalized Medicare beneficiaries with heart failure and left ventricular ejection fraction <45%
| 1-year outcomes | % (events) |
Absolute risk diff.* |
Hazard ratio† (95% confidence interval) |
|
|---|---|---|---|---|
| ACEIs or ARBs | ||||
| No (n=477) | Yes (n=477) | |||
| All-cause readmission | 69% (329) | 64% (304) | −5% | 0.81 (0.69–0.95); p=0.008 |
| Heart failure readmission | 38% (179) | 29% (136) | −9% | 0.68 (0.54–0.84); p=0.001 |
| All-cause mortality | 37% (178) | 31% (148) | −6% | 0.77 (0.62–0.96); p=0.020 |
| All-cause mortality or all-cause readmission | 78% (374) | 73% (347) | –5% | 0.82 (0.70–0.94); p=0.006 |
Absolute risk differences were calculated by subtracting percent events in patients receiving no ACEIs or ARBs from those receiving those drugs
The hazard ratios compared patients receiving ACEIs or ARBs versus those not receiving ACEIs or ARBs. These hazard ratios were calculated by treating patients without events during the first 12 months as censored.
DISCUSSION
Findings from the current study demonstrate that among a well-balanced cohort of older Medicare beneficiaries with heart failure and reduced ejection fraction who were hospitalized for acute decompensation a new discharge prescription for ACEIs or ARBs was associated with a significantly lower risk of 30-day all-cause and heart failure readmissions. The use of these drugs was also associated with lower risk of 30-day all-cause mortality, as well as the combined outcome of 30-day all-cause mortality or all-cause readmission. These beneficial associations persisted at one year after index hospitalization. These findings based on propensity-matched cohort of real world patients with heart failure provide new insights about the role of ACEIs or ARBs in lowering the risk of 30-day all-cause readmission in these patients, an important contemporary outcome of financial implication.
The lower 30 day all-cause readmissions associated with discharge initiation of ACEIs or ARBs observed in our study, is almost entirely explained by lower rate of heart failure readmissions. In our study among 477 pairs of matched patients receiving vs. not receiving ACEIs or ARBs, there were 116 events of 30-day all-cause readmission in patients not receiving these drugs, of which 65 were due to worsening heart failure and 51 were due to non-heart failure related causes. On the other hand, among those receiving these drugs, there were 88 events of 30-day all-cause readmission of which 35 were heart failure related and 53 were non-heart failure related. Therefore, the 28 (116 – 88) fewer events of 30-day all-cause readmission among those receiving ACEIs or ARBs can be almost entirely explained by the 30 (65 – 35) fewer events of 30-day heart failure readmission in that group. Findings from the Studies of Left Ventricular Dysfunction (SOLVD) – Treatment trial and the Candesartan in Heart failure - Assessment of moRtality and Morbidity (CHARM) – Alternative trial suggest that the long-term clinical benefits of ACEIs or ARBs in patients with heart failure and reduced ejection fraction are mediated by its favorable effects on ventricular remodeling and disease progression.20,21 Findings from those trials also demonstrated that the clinical benefits of these drugs start early and are likely mediated by their favorable hemodynamic effects, which also likely explain the beneficial associations observed in our study. To the best of our knowledge this is the first study to demonstrate a beneficial association of ACEIs or ARBs with 30-day all-cause readmission in real world patients with heart failure and reduced ejection fraction.
Despite limitations of the cost-driven metric of 30-day all-cause readmission, heart failure remains the leading cause for this outcome, which has gained new importance due to provision of financial penalties in the Affordable Care Act for hospitals with higher than average readmission rates. ACEIs or ARBs are under-prescribed despite evidence of a favorable survival and hospitalization benefits.22,23 Importantly, these drugs are often temporarily discontinued in patients with renal insufficiency despite emerging evidence of their beneficial effects in these patients.13,14 Further, these drugs are often not restarted in the outpatient setting.24 Findings from our study suggest that initiation of ACEIs or ARBs prior to hospital discharge may provide additional benefit of lowered 30-day all-cause readmissions in patients with heart failure and reduced ejection fraction. This is important as hospitals are under pressure to identify means to lower 30-day all-cause readmission, and many are adopting expensive transition of care interventions with little proven benefit.4
Several potential limitations of our study merit discussion. Patients were restricted to fee-for-service Medicare beneficiaries from a single state during 1999–2001. Of this cohort, only 30% were receiving beta-blockers, very few receiving aldosterone antagonists, and presumably, none receiving device-based interventions. However, neither of these drugs have any proven benefit for 30-day all-cause readmission and despite other modern advances in device-based therapy, the 30-day readmissions rates remains high.1 Another limitation of study was lack of data on dosage of ACEIs or ARBs at discharge as well as post-discharge adherence data.
Conclusions
Among Medicare beneficiaries with heart failure and reduced ejection fraction hospitalized for acute decompensation, compared to patients not receiving ACEIs or ARBs, those receiving these drugs had lower risk of 30-day all-cause readmission and 30-day all-cause mortality, and these beneficial associations persisted up to one year post-discharge. Our findings of clinical effectiveness of ACEIs or ARBs in reducing 30-day all-cause hospital readmission in the real-world patients with heart failure and reduced ejection fraction suggest that these drugs may play an important role in reducing readmission, a growing public health problem and a target for financial penalties under the Affordable Care Act.
Clinical significance.
Angiotensin-converting enzyme inhibitor and angiotensin receptor blocker use is associated with lower risk of 30-day all-cause readmissions and 30-day all-cause mortality in older hospitalized Medicare beneficiaries with heart failure and reduced ejection fraction.
These beneficial associations persisted for one year after discharge.
Acknowledgments
Source of Funding: Dr. Ahmed was in part supported by the National Institutes of Health through grants (R01-HL085561, R01-HL085561-S and R01-HL097047) from the National Heart, Lung, and Blood Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: None
Authors’ roles: All authors had access to the data and a role in writing the manuscript
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Veterans Health Administration.
References
- 1.Jencks SF, Williams MV, Coleman EA. Rehospitalizations among Patients in the Medicare Fee-for-Service Program. N Engl J Med. 2009;360:1418–1428. doi: 10.1056/NEJMsa0803563. [DOI] [PubMed] [Google Scholar]
- 2.Boccuti C, Casillas G. Aiming for fewer hospital u-turns: The medicare hospital readmission reduction program. [Access date: April 18, 2016];Kaiser family foundation. http://kff.org/medicare/issue-brief/aiming-for-fewer-hospital-u-turns-the-medicare-hospital-readmission-reduction-program/ [Google Scholar]
- 3.Davis K, Guterman S, Bandeali F. [Access date: April 18, 2016];The affordable care act and medicare: How the law is changing the program and the challenges that remain. The commonwealth fund. http://www.commonwealthfund.org/~/media/files/publications/fundreport/2015/jun/1821_davis_aca_and_medicare_v2.Pdf. [Google Scholar]
- 4.Feltner C, Jones CD, Cene CW, et al. Transitional care interventions to prevent readmissions for persons with heart failure: a systematic review and meta-analysis. Ann Intern Med. 2014;160:774–784. doi: 10.7326/M14-0083. [DOI] [PubMed] [Google Scholar]
- 5.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–e239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 6.The SOLVD Investigators. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991;325:293–302. doi: 10.1056/NEJM199108013250501. [DOI] [PubMed] [Google Scholar]
- 7.Ahmed A, Bourge RC, Fonarow GC, et al. Digoxin use and lower 30-day all-cause readmission for Medicare beneficiaries hospitalized for heart failure. Am J Med. 2014;127:61–70. doi: 10.1016/j.amjmed.2013.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bourge RC, Fleg JL, Fonarow GC, et al. Digoxin reduces 30-day all-cause hospital admission in older patients with chronic systolic heart failure. Am J Med. 2013;126:701–708. doi: 10.1016/j.amjmed.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.The Digitalis Investigation Group. The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med. 1997;336:525–533. doi: 10.1056/NEJM199702203360801. [DOI] [PubMed] [Google Scholar]
- 10.Feller MA, Mujib M, Zhang Y, et al. Baseline characteristics, quality of care, and outcomes of younger and older Medicare beneficiaries hospitalized with heart failure: findings from the Alabama Heart Failure Project. Int J Cardiol. 2012;162:39–44. doi: 10.1016/j.ijcard.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhatia V, Bajaj NS, Sanam K, et al. Beta-blocker Use and 30-day All-cause Readmission in Medicare Beneficiaries with Systolic Heart Failure. Am J Med. 2015;128:715–721. doi: 10.1016/j.amjmed.2014.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Danaei G, Tavakkoli M, Hernan MA. Bias in observational studies of prevalent users: lessons for comparative effectiveness research from a meta-analysis of statins. Am J Epidemiol. 175:250–262. doi: 10.1093/aje/kwr301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahmed A, Fonarow GC, Zhang Y, et al. Renin-angiotensin inhibition in systolic heart failure and chronic kidney disease. Am J Med. 2012;125:399–410. doi: 10.1016/j.amjmed.2011.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edner M, Benson L, Dahlstrom U, Lund LH. Association between renin-angiotensin system antagonist use and mortality in heart failure with severe renal insufficiency: a prospective propensity score-matched cohort study. Eur Heart J. 2015;11 doi: 10.1093/eurheartj/ehv268. [DOI] [PubMed] [Google Scholar]
- 15.Rosenbaum PRRD. The central role of propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 16.Rubin DB. The design versus the analysis of observational studies for causal effects: parallels with the design of randomized trials. Stat Med. 2007;26:20–36. doi: 10.1002/sim.2739. [DOI] [PubMed] [Google Scholar]
- 17.Ahmed A, Husain A, Love TE, et al. Heart failure, chronic diuretic use, and increase in mortality and hospitalization: an observational study using propensity score methods. Eur Heart J. 2006;27:1431–1439. doi: 10.1093/eurheartj/ehi890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahmed MI, White M, Ekundayo OJ, et al. A history of atrial fibrillation and outcomes in chronic advanced systolic heart failure: a propensity-matched study. Eur Heart J. 2009;30:2029–2037. doi: 10.1093/eurheartj/ehp222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wahle C, Adamopoulos C, Ekundayo OJ, Mujib M, Aronow WS, Ahmed A. A propensity-matched study of outcomes of chronic heart failure in younger and older adults. Arch Gerontol Geriatr. 2009;49:165–171. doi: 10.1016/j.archger.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.The SOLVD Investigators. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991;325:293–302. doi: 10.1056/NEJM199108013250501. [DOI] [PubMed] [Google Scholar]
- 21.Granger CB, McMurray JJ, Yusuf S, et al. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting-enzyme inhibitors: the CHARM-Alternative trial. Lancet. 2003;362:772–776. doi: 10.1016/S0140-6736(03)14284-5. [DOI] [PubMed] [Google Scholar]
- 22.Fonarow GC, Abraham WT, Albert NM, et al. Association between performance measures and clinical outcomes for patients hospitalized with heart failure. JAMA. 2007;297:61–70. doi: 10.1001/jama.297.1.61. [DOI] [PubMed] [Google Scholar]
- 23.Hernandez AF, Hammill BG, O'Connor CM, Schulman KA, Curtis LH, Fonarow GC. Clinical effectiveness of beta-blockers in heart failure: findings from the OPTIMIZE-HF (Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure) Registry. J Am Coll Cardiol. 2009;53:184–192. doi: 10.1016/j.jacc.2008.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Butler J, Arbogast PG, Daugherty J, Jain MK, Ray WA, Griffin MR. Outpatient utilization of angiotensin-converting enzyme inhibitors among heart failure patients after hospital discharge. J Am Coll Cardiol. 2004;43:2036–2043. doi: 10.1016/j.jacc.2004.01.041. [DOI] [PubMed] [Google Scholar]