Abstract
Background
No studies have examined whether use of sedation during a Tc-99m dimercaptosuccinic acid (DMSA) renal scan reduces patient discomfort.
Objective
To compare discomfort level during a DMSA scan to the discomfort level during other frequently performed uroradiologic tests, and to determine whether use of sedation during a DMSA scan modifies the level of discomfort.
Materials and methods
We examined the discomfort level in 798 children enrolled in the Randomized Intervention for children with Vesicoureteral Reflux (RIVUR) and Careful Urinary Tract Infection Evaluation (CUTIE) studies by asking parents to rate their child's discomfort level with each procedure on a scale from 0 to 10. We compared discomfort during the DMSA scan and the DMSA image quality between centers in which sedation was used >90% of the time (sedation centers), centers in which sedation was used <10% of the time (non-sedation centers), and centers in which sedation was used on a case-by-case basis (selective centers).
Results
Mean discomfort level was highest for voiding cystourethrogram (6.4), followed by DMSA (4.0), followed by ultrasound (2.4; P<0.0001). Mean discomfort level during the DMSA scan was significantly higher at non-sedation centers than at selective centers (P<0.001). No difference was apparent in discomfort level during the DMSA scan between sedation centers and selective centers (P=0.12), or between the sedation centers and non-sedation centers (P=0.80). There were no differences in the proportion with uninterpretable DMSA scans according to sedation use.
Conclusion
Selective use of sedation in children 12–36 months of age can reduce the discomfort level experienced during a DMSA scan.
Keywords: Anxiolytic, Children, Dimercaptosuccinic acid, Renal scintigraphy, Sedation, Ultrasound, Voiding cystourethrogram
Introduction
Use of sedation during the Tc-99m dimercaptosuccinic acid (DMSA) renal scan, a procedure performed to evaluate the presence or absence and extent of pyelonephritis and renal scarring in children with urinary tract infections (UTIs), remains contentious. Some centers routinely use sedation for young children undergoing a DMSA scan, whereas others use sedation infrequently. Opponents of routine sedation maintain that a DMSA scan is unlikely to cause significant discomfort and that the majority of children can comfortably undergo the procedure with adequate preparation and distraction. In contrast, proponents of sedation argue that immobilization of uncooperative children, which is often necessary for obtaining acceptable images, is best accomplished using sedation.
In contrast to the plethora of studies on the utility of sedation during a voiding cystourethrogram (VCUG) [1–13], to our knowledge only two studies have examined the discomfort levels during a DMSA scan. Nelson et al. [1] found that parents consider their child's discomfort level during a VCUG to be significantly higher than discomfort during DMSA scan, and higher than discomfort during a renal sonogram. Train et al. [6] reported that appropriate patient preparation and distraction can successfully reduce the proportion of children undergoing sedation for DMSA. No studies have examined whether the use of sedation during the imaging portion of the DMSA scan is associated with less discomfort.
Our objectives were (1) to compare the level of discomfort during a DMSA scan with the level of discomfort experienced during other frequently performed uroradiologic tests (sonography and voiding cystourethrography), (2) to compare the level of discomfort experienced by young children during a DMSA scan at centers that do and do not routinely use sedation, and (3) to compare the quality of the DMSA scans obtained at centers that do and do not routinely use sedation.
Materials and methods
We examined the parent-perceived degree of discomfort during uroradiologic imaging tests performed in 607 children enrolled in the Randomized Intervention for children with Vesicoureteral Reflux (RIVUR) trial and 195 children enrolled in the Careful Urinary Tract Infection Evaluation (CUTIE) study. The methods of the RIVUR trial and CUTIE study have been described [14–16]. Briefly, we enrolled children ages 2–72 months who presented with a first or second UTI from both primary and subspecialty care settings at clinical trial centers throughout North America. Children with grades I–IV vesicoureteral reflux (VUR) were enrolled in the RIVUR study whereas those without VUR were enrolled in the parallel CUTIE study. Not all RIVUR sites participated in the CUTIE study. Each child in these studies had sonography, a VCUG, and a DMSA scan soon after the index UTI. In both studies, the DMSA scan was repeated 2 years after the index UTI. In the RIVUR study, a DMSA scan was performed 1 year and a VCUG performed 2 years after the index UTI. Both studies were approved by institutional review boards of the participating institutions. Collection of data regarding discomfort during imaging tests was planned before patient enrollment started for the RIVUR and CUTIE studies.
Following each imaging test, we asked parents to rate how much discomfort their child had with each of these procedures on a rating scale (Fig. 1). Study coordinators recorded whether sedation was used and, if so, they ascertained the type of sedation by reviewing the child's medical records. We included only imaging tests for which the discomfort questionnaire was obtained within 2 weeks of the imaging test. The only exception to this rule was the baseline VCUG; some discomfort questionnaires were completed 2 weeks or more after the baseline VCUG (maximum of 112 days). We included all imaging tests conducted at any time during the 2-year study period except for four cases with missing data regarding either the sedative used or the level of discomfort.
Fig. 1. Instrument used to assess discomfort with imaging tests.
The DMSA scan has three phases. In the first phase (injection), DMSA is injected intravenously. The second phase (distribution), during which DMSA is distributed to the tissues, takes 1.5–3 h. During this time the child usually leaves the radiology suite. In the third phase (imaging), which takes 30–60 min, uncooperative children often need to be immobilized by their parents or by using a papoose board. If used, sedation is administered right before the third phase. Sites that use sedation provided additional information regarding sedation to patients. Otherwise, because there was a common standard operating procedure for performing the DMSA across all sites, as well as a standardized description of the procedure in the common consent for the study, we do not expect large differences in the preparation for the test or in the procedure among centers. The time required for the imaging portion of the test was likely relatively constant across institutions because the counts required for each image were specified in the procedure manual. Information regarding child life specialist participation during the DMSA scan was not collected on a case-by-case basis. However, after the study was completed, we queried the principal investigators and research coordinators at each site regarding child-life involvement at their respective sites.
We defined “sedation centers” as centers that used sedation for >90% of DMSA scans. Similarly, we defined “non-sedation centers” as those that used sedation in <10% of DMSA scans. The remaining centers, in which the use sedation was more selective, were categorized as “selective centers.” All six selective centers had policies for sedation that were based on the child's age: two sites routinely sedated children 1–3 years of age, two sedated children <4 years of age, one sedated children <5 years of age and one sedated children 6 months to 5 years of age.
DMSA quality was categorized as adequate or inadequate by two independent reference radiologists who were unaware whether sedation was used. Disagreements were resolved by discussion. Inadequate scans were those that were uninterpretable because of poor quality (most often because of excessive movement during the procedure).
Discomfort levels were compared using linear models adjusted for age, race, gender, site, insurance type, primary caregiver's education level, and fever. We used generalized estimated equations (with exchangeable correlation structure) to account for correlation of multiple observations in the same child. Repeated measures analysis of variance (ANOVA) was used to compare discomfort levels among centers. Univariable and multivariable logistic regression was used to investigate the relationship between use of sedation and adequacy of DMSA scans.
Results
Table 1 describes the clinical and demographic characteristics of 798 children in the analytic sample. Overall, 725 (91%) were girls and 612 (77%) were white; median age was 12 months. A total of 1,215 DMSA scans, 1,159 VCUGs and 768 renal US exams were performed during the study period. A total of 27% of DMSA scans were conducted on the same day as a VCUG. Sedation was used in 390 (32%) of the 1,215 DMSA scans, including 77 (92%) of the 84 DMSA scans performed at the 3 sedation centers, 5 (1%) of the 631 DMSA scans performed at the 10 non-sedation centers, and 308 (62%) of the 500 DMSA scans performed at the 6 selective centers. Sedation was not used for any of the US examinations. Similarly, 95%) (1,104/1,159) VCUGs were performed without sedation.
Table 1. Demographic and clinical characteristics of the children included.
| Characteristic | Total (n=798) n (%) |
|---|---|
| Age | |
| 2–24 months | 534 (67) |
| 24–72 months | 264 (33) |
| Gender | |
| Female | 725 (91) |
| Male | 73 (9) |
| Race | |
| White | 612 (77) |
| Non-white | 175 (22) |
| Missing | 11 (1) |
| Ethnicity | |
| Hispanic | 118 (15) |
| Not Hispanic | 677 (85) |
| Missing | 3 (<1) |
| Primary caregiver education | |
| High school graduate or less | 226 (28) |
| >High school | 567 (71) |
| Missing | 5 (1) |
| Index UTI | |
| Febrile | 667 (84) |
| Afebrile | 131 (16) |
UTI urinary tract infection
Data regarding child-life participation were available for 18 of the 19 participating sites; fewer than 1% of DMSA scans were performed at the site with missing data. Of the responding centers, child life was present routinely at 33% (1 of 3) of sedation centers, 22% (2 of 9) of non-sedation centers and 33% of selective centers (2 of 6).
Discomfort level comparison among the three imaging tests
The mean discomfort level (confidence interval) on a scale of 0 to 10 was highest for VCUG (6.4, confidence interval [CI] 6.2–6.5), followed by DMSA (4.0, CI 3.8–4.1) and US exam (2.4, CI 2.1–2.6), with P<0.001 for all three pairwise comparisons (Fig. 2).
Fig. 2.

Graph shows perceived discomfort level for three frequently performed imaging tests of the urinary tract. DMSA dimercaptosuccinic acid renal scan, VCUG voiding cystourethrogram
Effect of sedation on discomfort during DMSA
Mean discomfort level (confidence interval) reported by children's parents was 4.1 (3.5–4.7) at sedation centers, 4.2 (4.0–4.5) at non-sedation centers and 3.6 (3.3–3.8) at selective centers; the mean discomfort level at non-sedation centers was significantly higher than that observed at selective centers (P<0.001). No significant difference in discomfort level was apparent between sedation centers and selective centers (P=0.12) or between sedation centers and non-sedation centers (P=0.80). Sedation, non-sedation and selective centers differed systematically according to patient characteristics (age, gender, race, ethnicity, maternal education, insurance type, and presence of fever at the time of the index UTI). Nevertheless adjusting for these variables in the analysis did not alter our findings; adjusted P values for non-sedation vs. selective, sedation vs. selective, and sedation vs. non-sedation centers were <0.0001, 0.17, and 0.55, respectively.
Factors associated with use of sedation at selective centers
Sedation was significantly more likely to be used in younger children. At the selective centers, mean age of sedated children ranged from 18 months to 31 months, whereas mean age of non-sedated children ranged from 43 months to 48 months. Race, gender, ethnicity, maternal insurance, maternal education level, and fever at the time of the index UTI were not associated with the use of sedation. Specifically, children 12–36 months of age were significantly more likely to be sedated than children outside this age range (odds ratio 2.32; 95% CI 1.83, 2.94).
Efficacy of sedatives used
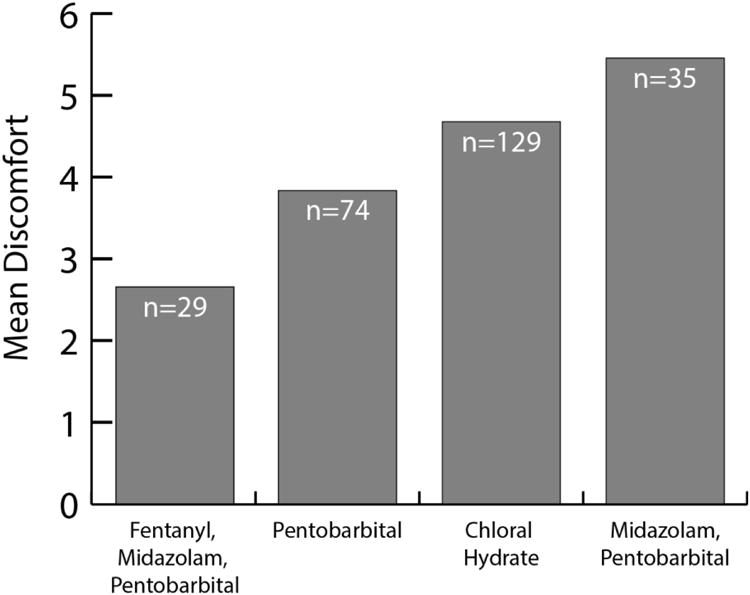
At selective centers (Fig. 3), children who received a combination of fentanyl-pentobarbital-midazolam had significantly lower discomfort levels than those who received pentobarbital alone (2.7 vs. 3.8), choral hydrate (2.7 vs. 4.7) or midazolam-pentobarbital (2.7 vs. 5.5). Adjusted P-values were 0.03, <0.001 and <0.001, respectively. Adjustment for site was not performed because most sites used only one or two sedative types. We were not able to compare various sedatives because 96% of sedated children received propofol.
Fig. 3. Graph shows efficacy of the sedatives used in selective centers.
Effect of sedation on DMSA quality
Radiologists categorized 22 of 1,215 DMSA scans (2%) as being uninterpretable. There were 0/84 (0%) uninterpretable DMSA scans at sedation centers, 12/631 (2%) at non-sedation centers and 10/500 (2%) at selective centers (P=0.58). Use of sedation was not associated with interpretability of the scan: 1.5% of scans in which sedation was used versus 1.9% of scans in which sedation was not used were judged to be uninterpretable (P=0.89). Similarly, at selective centers there was no difference in the proportion of uninterpretable scans performed with and without sedation (P=0.41).
Factors associated with DMSA quality
The only variable associated with DMSA quality was age; mean age of children with uninterpretable scans was significantly lower than the mean age of children with interpretable scans (20 months vs. 34 months, P=0.003).
Discussion
We found no difference in the perceived discomfort level or scan quality at centers that did or did not routinely use sedation for the imaging portion of the DMSA scan. Accordingly, routine use of sedation does not appear to be particularly beneficial. We also found that children scanned at centers where the use of sedation was more selective (determined mostly by age) had less discomfort than children at centers that routinely used sedation or centers that seldom used sedation. This suggests that restricting sedation to individual children 12–36 months of age who are unlikely to hold still during the study may be the best overall strategy to reduce discomfort. Developmentally, children in the 12- to 36-month age range have more difficulty remaining motionless for a prolonged period compared with infants, who often fall asleep after a period of crying (provided that they are well-fed during the distribution phase of the study), or compared with older children who can be distracted more easily (e.g., by a movie or game). The lower discomfort scores from centers that use sedation selectively was likely because these centers maximized the benefits of sedation while minimizing its risks; the perceived discomfort levels during the DMSA scan at such centers were much closer to the discomfort levels of a US scan than that of a VCUG.
At selective centers there was significant variation in the perceived discomfort level according to the sedative used. Children who received an agent containing an analgesic (e.g., fentanyl) had lower levels of perceived discomfort than children receiving agents containing only sedatives or anxiolytics. Because allocation of agents used for sedation was not random, we cannot make definitive conclusions regarding the efficacy of various sedatives. Nevertheless our data suggest that the combination of fentanyl, midazolam and pentobarbital might be more effective than other sedative combinations.
The perceived discomfort level of the DMSA scan fell between that of the US and the VCUG tests, which is consistent with findings from Nelson et al. [1]. This is not entirely surprising. During a DMSA scan an intravenous line is placed and some children are restrained. During a VCUG, urinary catheterization is performed and all children are restrained. During a US examination, no invasive procedures are performed and children are generally not restrained.
Because these data are observational, our conclusions are limited. Sedation centers used propofol almost exclusively, limiting the generalizability of our findings. Unmeasured confounders such as the degree of preparation before the procedure and the use of distraction during the procedure could have differed between centers. However there was little apparent difference among centers regarding the preparation for the test, the actual procedures, or the use of child life specialists. The rating scale we used to measure discomfort has not been validated; however, the use of rating scales for pain is widely accepted. We did not examine the effect of sedation on the time required to perform the DMSA scan; doing so in future studies might provide helpful information. The relative efficacy of various sedatives also merits further study. Finally, we did not ask about adverse events related to the use of sedation.
Conclusion
It appears that selective use of sedation during a DMSA scan might reduce the perceived discomfort level experienced by children during this procedure. The use of sedation did not reduce the proportion of DMSA scans that were uninterpretable.
Footnotes
Compliance with ethical standards: Conflicts of interest: None
References
- 1.Nelson CP, Chow JS, Rosoklija I, et al. Patient and family impact of pediatric genitourinary diagnostic imaging tests. J Urol. 2012;188:1601–1607. doi: 10.1016/j.juro.2012.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Azarfar A, Esmaeeili M, Farrokh A, et al. Oral midazolam for voiding dysfunction in children undergoing voiding cystourethrography: a controlled randomized clinical trial. Nephrourol Mon. 2014;6:e17168. doi: 10.5812/numonthly.17168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mandell GA, Cooper JA, Majd M, et al. Procedure guideline for pediatric sedation in nuclear medicine. J Nucl Med. 1997;38:1640–1643. [PubMed] [Google Scholar]
- 4.Zier JL, Kvam KA, Kurachek SC, Finkelstein M. Sedation with nitrous oxide compared with no sedation during catheterization for urologic imaging in children. Pediatr Radiol. 2007;37:678–684. doi: 10.1007/s00247-007-0508-z. [DOI] [PubMed] [Google Scholar]
- 5.Akil I, Ozkol M, Ikizoglu OY, et al. Premedication during micturating cystourethrogram to achieve sedation and anxiolysis. Pediatr Nephrol. 2005;20:1106–1110. doi: 10.1007/s00467-005-1874-0. [DOI] [PubMed] [Google Scholar]
- 6.Train H, Colville G, Allan R, Thurlbeck S. Paediatric 99mTc-DMSA imaging: reducing distress and rate of sedation using a psychological approach. Clin Radiol. 2006;61:868–874. doi: 10.1016/j.crad.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 7.Elder JS, Longenecker R. Premedication with oral midazolam for voiding cystourethrography in children: safety and efficacy. AJR Am J Roentgenol. 1995;164:1229–1232. doi: 10.2214/ajr.164.5.7717236. [DOI] [PubMed] [Google Scholar]
- 8.Herd DW, McAnulty KA, Keene NA, Sommerville DE. Conscious sedation reduces distress in children undergoing voiding cystourethrography and does not interfere with the diagnosis of vesicoureteric reflux: a randomized controlled study. AJR Am J Roentgenol. 2006;187:1621–1626. doi: 10.2214/AJR.05.1216. [DOI] [PubMed] [Google Scholar]
- 9.Stokland E, Andreasson S, Jacobsson B, et al. Sedation with midazolam for voiding cystourethrography in children: a randomised double-blind study. Pediatr Radiol. 2003;33:247–249. doi: 10.1007/s00247-003-0874-0. [DOI] [PubMed] [Google Scholar]
- 10.Rao J, Kennedy SE, Cohen S, Rosenberg AR. A systematic review of interventions for reducing pain and distress in children undergoing voiding cystourethrography. Acta Paediatr. 2012;101:224–229. doi: 10.1111/j.1651-2227.2011.02482.x. [DOI] [PubMed] [Google Scholar]
- 11.Sandy NS, Nguyen HT, Ziniel SI, et al. Assessment of parental satisfaction in children undergoing voiding cystourethrography without sedation. J Urol. 2011;185:658–662. doi: 10.1016/j.juro.2010.09.120. [DOI] [PubMed] [Google Scholar]
- 12.Keidan I, Zaslansky R, Weinberg M, et al. Sedation during voiding cystourethrography: comparison of the efficacy and safety of using oral midazolam and continuous flow nitrous oxide. J Urol. 2005;174:1598–1600. doi: 10.1097/01.ju.0000176595.49213.13. [DOI] [PubMed] [Google Scholar]
- 13.Ferguson GG, Chen C, Yan Y, et al. The efficacy of oral midazolam for decreasing anxiety in children undergoing voiding cystourethrogram: a randomized, double-blind, placebo controlled study. J Urol. 2011;185:2542–2546. doi: 10.1016/j.juro.2011.01.031. [DOI] [PubMed] [Google Scholar]
- 14.Keren R, Carpenter MA, Hoberman A, et al. Rationale and design issues of the Randomized Intervention for Children with Vesicoureteral Reflux (RIVUR) study. Pediatrics. 2008;122:S240–250. doi: 10.1542/peds.2008-1285d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keren R, Shaikh N, Pohl H, et al. Risk factors for recurrent urinary tract infection and renal scarring. Pediatrics. 2015;136:e13–21. doi: 10.1542/peds.2015-0409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoberman A, Greenfield SP, Mattoo TK, et al. Antimicrobial prophylaxis for children with vesicoureteral reflux. N Eng J Med. 2014;370:2367–2376. doi: 10.1056/NEJMoa1401811. [DOI] [PMC free article] [PubMed] [Google Scholar]