Abstract
Purpose
Osteonecrosis of the jaw (ONJ) is an established side effect of intravenous bisphosphonates and other anti-resorptive medications. Although bisphosphonates are frequently prescribed for patients with the skeletal disorder fibrous dysplasia (FD), there are no reports of ONJ in this population. This has led some to conclude that FD patients are at low risk for the development of bisphosphonate-related ONJ.
Patients and Methods
Patients were evaluated as part of a longstanding FD natural history study at the NIH.
Results
Out of 76 FD patients who were treated with bisphosphonates, four developed ONJ (5.4%). Three patients developed ONJ in areas of FD bone, and one in an area of normal bone. All four patients had features known to be associated with ONJ in the general population, including long-term, high dose, intravenous bisphosphonate treatment, periodontal and endodontic infections, and dentoalveolar surgical procedures.
Conclusions
These cases establish ONJ as a potential complication of bisphosphonate treatment in patients with FD. The presence of established risk factors for ONJ in this group of FD patients suggests that high risk individuals may be identified prior to the development of ONJ. Clinicians should use caution in prescribing bisphosphonates to FD patients, and should do so only for established indications.
Introduction
Fibrous dysplasia/McCune-Albright syndrome (FD/MAS, OMIM 174800) is a genetic disorder arising from somatic activating mutations in GNAS, which codes for the signaling protein, Gsα [1]. This mutation leads to constitutive receptor activation, resulting in increased Gsα signaling and dysregulated cyclic AMP production [2]. In the skeleton, GNAS mutations lead to increased proliferation and impaired differentiation of skeletal progenitor cells, resulting in formation of FD lesions that are highly vascular and prone to expansion, deformity, fracture, and pain [3, 4]. FD may affect one bone (monostotic) or multiple (polyostotic), and may occur in isolation or in combination with café-au-lait skin macules and hyperfunctioning endocrinopathies, including precocious puberty, hyperthyroidism, growth hormone excess, hypercortisolism, and renal phosphate wasting. The combination of FD and one or more extraskeletal features is termed McCune-Albright syndrome (MAS) [2]. FD may affect any area of the skeleton, and occurs frequently in the craniofacial area [5]. Morbidity in the skull relates primarily to FD expansion, resulting in facial asymmetry and, less commonly, functional deficits including malocclusion, dental anomalies, and vision and hearing impairment [6, 7].
The mainstay of treatment for FD is surgical, and there are no medical therapies capable of altering the disease course. Anti-resorptive therapy with bisphosphonates has been advocated as a potential treatment due to the high levels of osteoclastogenesis present in FD tissue, and the established role of bisphosphonates in inhibiting osteoclast function [8]. Bisphosphonates inhibit bone resorption by incorporating into the hydroxyapatite crystal and inhibiting osteoclast function when they are taken up by active osteoclasts. This group of drugs is used to treat conditions of excessive bone resorption, such as osteoporosis [9], Paget's disease [10], malignancies with skeletal metastases [11], and other skeletal disorders such as osteogenesis imperfecta [12]. The role of these medications in the management of FD has not been fully elucidated. Early case reports and small series described subjective improvements in pain and variable effects on the radiographic appearance of FD lesions [13-15]; however, a placebo-controlled trial of the oral bisphosphonate alendronate showed no effects on pain or FD lesion appearance [16]. At present, there is little evidence to support an effect of bisphosphonates on FD quality or lesion expansion; however, intravenous formulations are generally considered beneficial for FD-related bone pain, and are frequently prescribed for this indication [2, 17, 18].
The first cases of bisphosphonate-related osteonecrosis of the jaws (BRONJ) were reported in 2003 and 2004 by Marx and Ruggiero [19, 20]. Since that time, other antiresorptive (denosumab) and anti-cancer medications (i.e. sirolimus, bevacizumab) have been implicated in jaw necrosis; as of 2015, the AAOMS Special Committee has relabeled this diagnosis as “Medication-related Osteonecrosis of the Jaws” [21]. Despite the frequent use of bisphosphonates in FD patients, there are no reports in the literature describing ONJ. This has led to postulation that patients with FD may be at lower risk for ONJ in comparison to the general population of patients treated with bisphosphonates [18]. Here we describe three patients in the NIH FD/MAS natural history study who developed ONJ subsequent to bisphosphonate treatment.
Methods
The patients in this report were evaluated at the NIH Clinical Center as part of a longstanding FD/MAS natural history study. All patients gave informed consent, and the protocol was approved by the Institutional Review Board of the National Institute of Dental and Craniofacial Research, NIH. This study followed the Declaration of Helsinki on medical protocol and ethics.
Results
Out of 146 subjects with FD seen at the NIH, 76 (46%) had received treatment with bisphosphonates for management of bone pain. Four patients developed ONJ subsequent to bisphosphonate treatment, described in detail below.
Case 1
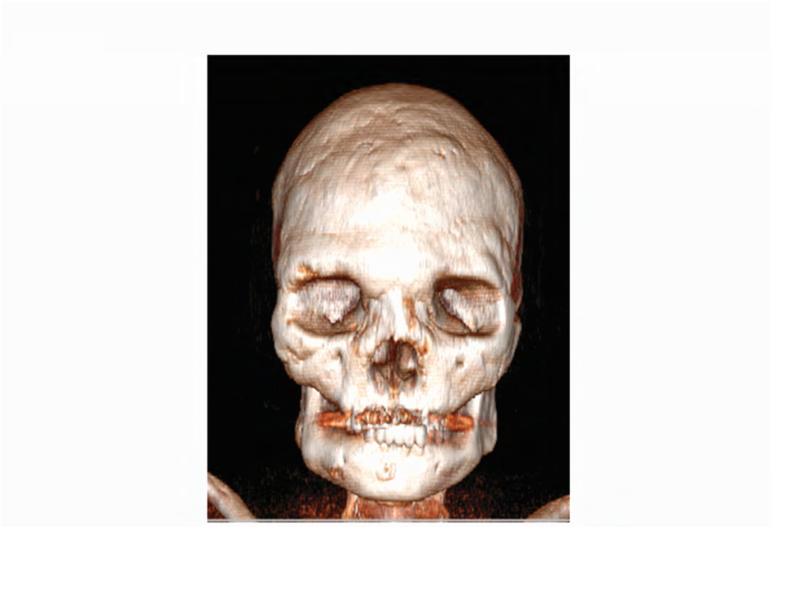
A 47-year-old woman presented with pain and cold sensitivity three months following extraction of the left maxillary second molar. The patient has a history of FD/MAS manifested by severe bony involvement in the craniofacial region, thorax, and femur; café-au-lait macules; and MAS-associated growth hormone excess (Figure 1). Her history is significant for numerous craniofacial surgeries, including bilateral optic nerve decompressions, and multiple maxillary and mandibular recontouring procedures. Her clinical course was complicated by severe FD-related bone pain, unresponsive to over-the-counter analgesics and amitriptyline. At age 36 years, eleven years prior to her current presentation, the patient was placed on bisphosphonates for pain management, including a 3-year course of pamidronate 180 mg every 3 months from ages 36 to 39 years, followed by a 6-year course of zoledronic acid 4 mg every 3 months from ages 39 to 45 years.
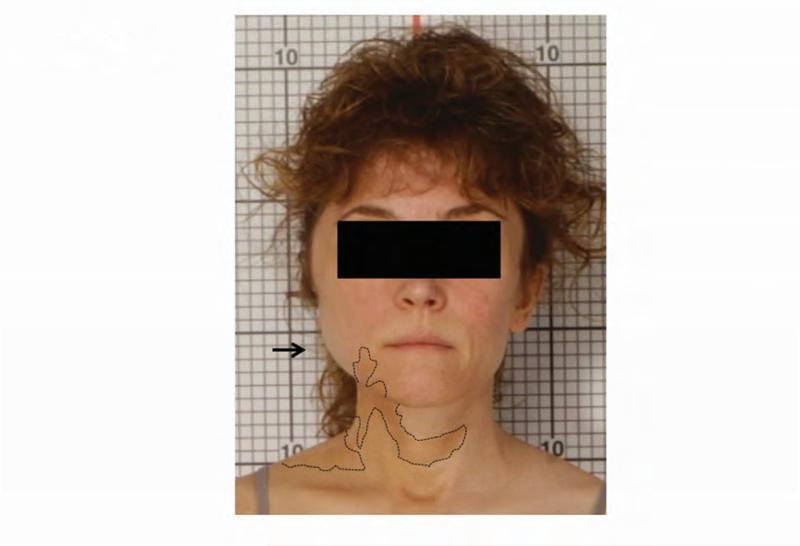
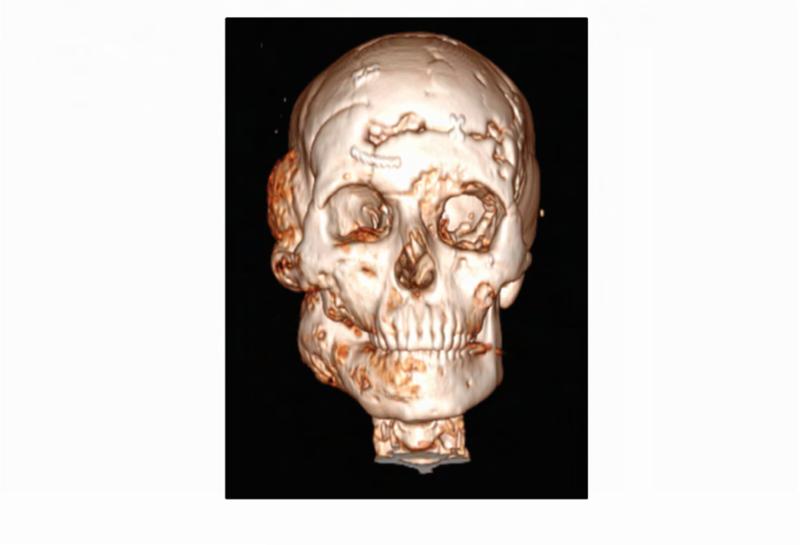
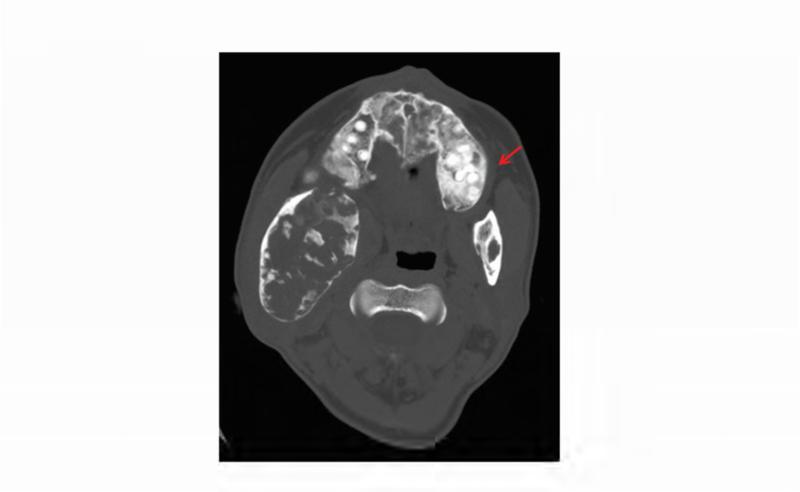
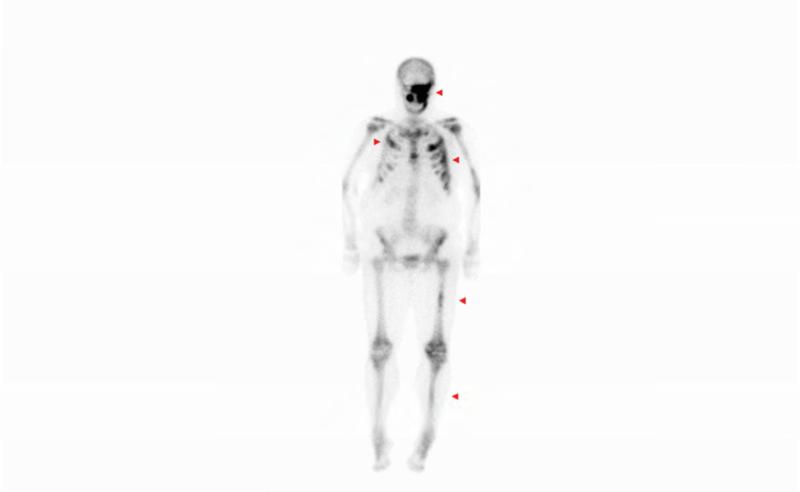
Figure 1. Case 1 clinical images.
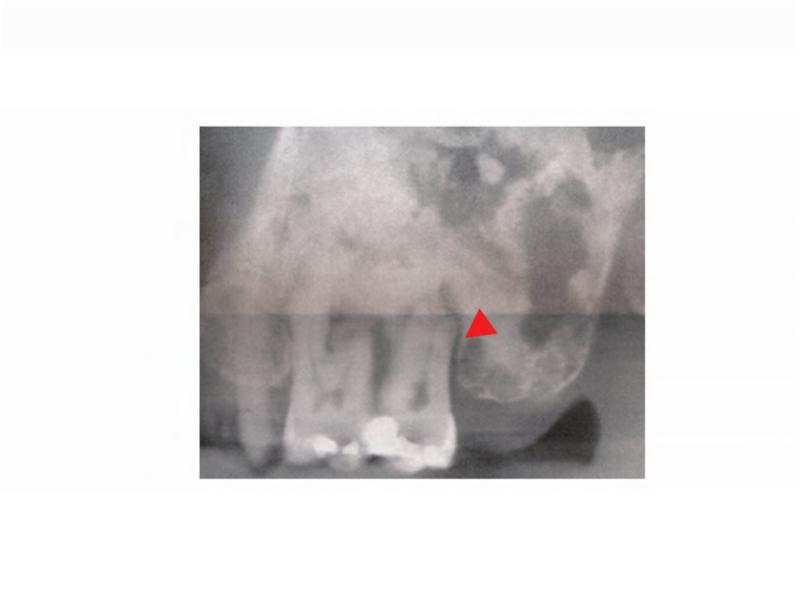
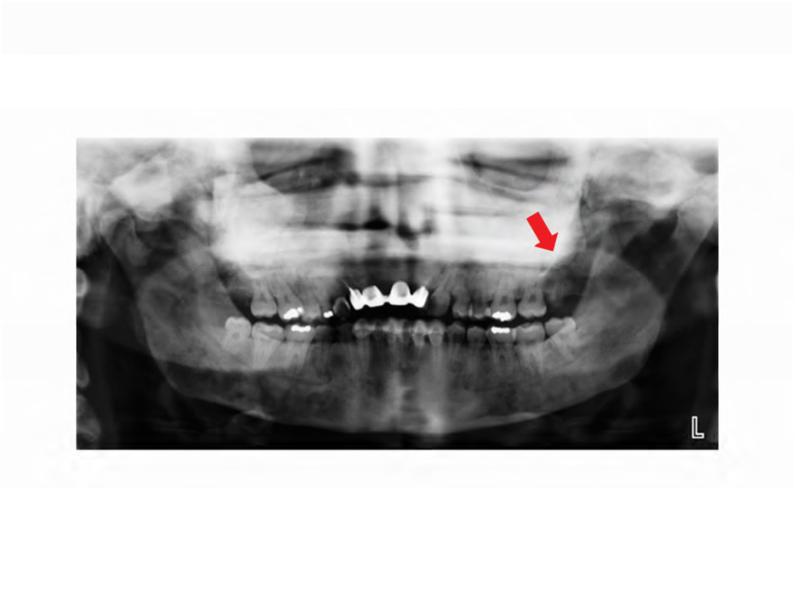
A. 99Technetium bone scintigraphy shows multiple areas of increased uptake consistent with fibrous dysplasia involving the skull, ribs, pelvis, and lower extremities (red arrowheads). B. Photograph demonstrating asymmetric expansion of the right mandible (black arrow). Note the café-au-lait macule involving the jaw, shoulder and neck with characteristic features, including jagged, “coast of Maine” borders and location respecting the midline of the body (dashed lines). C. Three-dimensional computed tomography demonstrates facial asymmetry with multiple expansile lesions particularly affecting the right side of the skull. D. Axial computed tomography images show extensive craniofacial fibrous dysplasia demonstrating the characteristic “ground glass” appearance with multiple areas of radiolucency. Note the left maxilla and alveolar ridge with extensive fibrous dysplasia involvement encasing the left maxillary first and second molars (ADA #14 and 15)(red arrow).
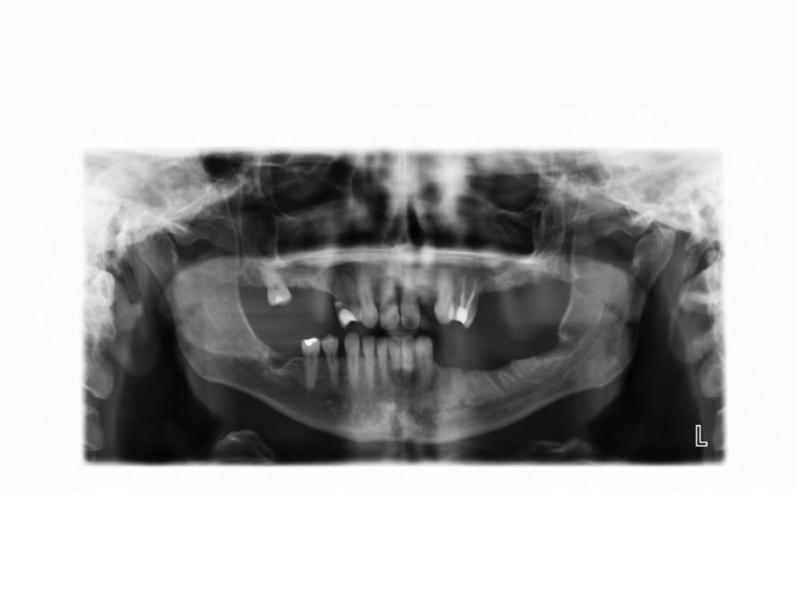
The patient has an extensive dental history with numerous restorative, endodontic, and periodontal procedures. One year prior to presentation, the patient's general dentist observed gingival swelling around the left maxillary first and second molars (ADA #14 and 15, respectively), and diagnosed a distal endodontic-periodontal lesion with probing depth of 12 mm; she was noted to have generalized mild, chronic periodontitis with localized aggressive periodontitis at the left maxillary molars, and purulent exudate from the site. Of note, the left maxilla and alveolar ridge had extensive FD involvement. The patient was referred to a periodontist and was treated with scaling and root planing, gingivectomy, and oral antibiotics. One year later, the patient reported lingering pain in the left maxillary second molar; her general dentist observed a periapical radiolucency and prescribed a one week course of Penicillin V. The patient was evaluated by an endodontist who determined that the left maxillary second molar was nonrestorable secondary to the persistent endodontic-periodontal lesion (Figure 2B), and extraction was recommended. The patient was referred to an oral and maxillofacial surgeon for extraction of the left maxillary second molar. Three months post-extraction, the patient returned to her oral-maxillofacial surgeon with complaints of cold sensitivity, severe pain, and a non-healing extraction site. Given her history of bisphosphonate treatment, in combination with symptomatic, exposed bone which had been present for greater than 8 weeks, she was diagnosed with Stage 2 osteonecrosis (ONJ) of the left posterior maxilla. Radiographically, the left maxillary alveolar ridge was noted to have bone resorption and osteosclerotic areas within the FD lesion (Figure 2A). Of note, the patient had discontinued zoledronic acid 2 years prior to undergoing extraction, and received one additional dose of zoledronic acid 2 weeks prior to her ONJ diagnosis. Following her ONJ diagnosis local debridement was performed three times over the next year. She was treated with oral cephalexin. Cultures were obtained on two occasions, but did not reveal a predominant species. Ten months after her ONJ diagnosis, the maxillary left first molar (ADA #14) was found to be mobile and periodontally compromised (Figure 2C). This tooth was surgically extracted, and the area was debrided. Post-operatively, oral hygiene was stressed, and the patient was prescribed fluoridated toothpaste and triannual dental prophylaxis. Two months following extraction of the left maxillary first molar, the patient reported improved healing and some soft tissue coverage. One year following extraction of the left maxillary first molar, the patient presented with pain and discomfort; a panoramic radiograph showed a persistent fistula (Figure 2D). The patient received cefalexin and reported pain relief. Two years later, the patient again presented with pain and swelling. An intraoral examination revealed moderate erythema of the mucosa around the lesion with no discharge. Two days later the patient underwent an incision and drainage. At that time, she was placed on a long-term course of oral antibiotics; however, compliance has been intermittent, and 5 years after her initial diagnosis she continues to have active ONJ.
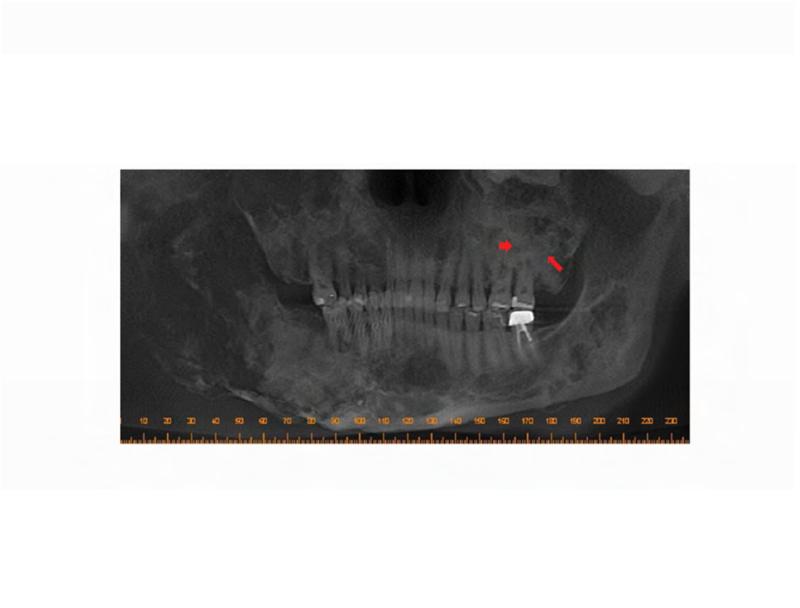
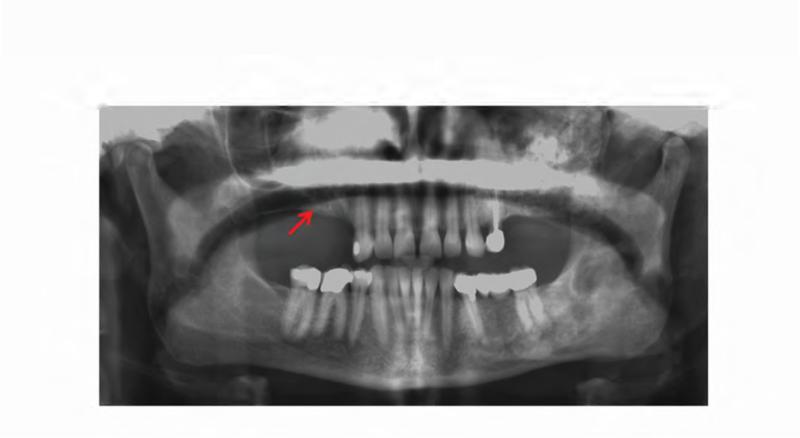
Figure 2. Case 1 dental images.
A. Pre-operative panoramic radiograph taken prior to extraction of the left maxillary second molar and three months prior to initial presentation of osteonecrosis. Both the left maxillary first and second molars are located within fibrous dysplasia bone (red arrows). B. Periapical radiograph taken prior to extraction of the left maxillary second molar (ADA #15). Note the perio-endo lesion associated with the right maxillary second molar (red arrowhead). C. A panoramic radiograph taken four months post-extraction of the left maxillary second molar shows bone resorption and osteosclerosis of the lesion (red arrow). D. Gutta percha points within the persistent fistula.
Case 2
A 23-year-old male with intellectual disability and FD/MAS presented for routine dental consultation. His disease burden included extensive polyostotic FD involving the skull, proximal humerus, bilateral ribs and femurs (Figure 3). Additional features of MAS included neonatal hypercortisolism treated with bilateral adrenalectomy, hyperthyroidism treated with total thyroidectomy, and pancreatic intraductal papillary mucinous neoplasms treated with pancreatoduodenectomy. His clinical course was complicated by post-operative right-sided vision loss after a prophylactic right optic nerve decompression at age 5. He has a history of severe FD-related bone pain treated with zoledronic acid 4 mg every three months from ages 18 to 23 years.
Figure 3. Case 2 clinical and dental images.
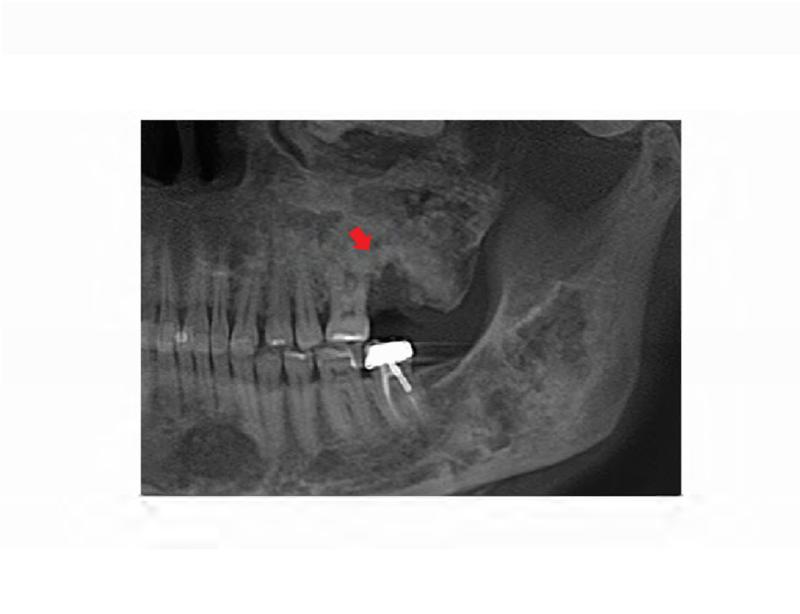
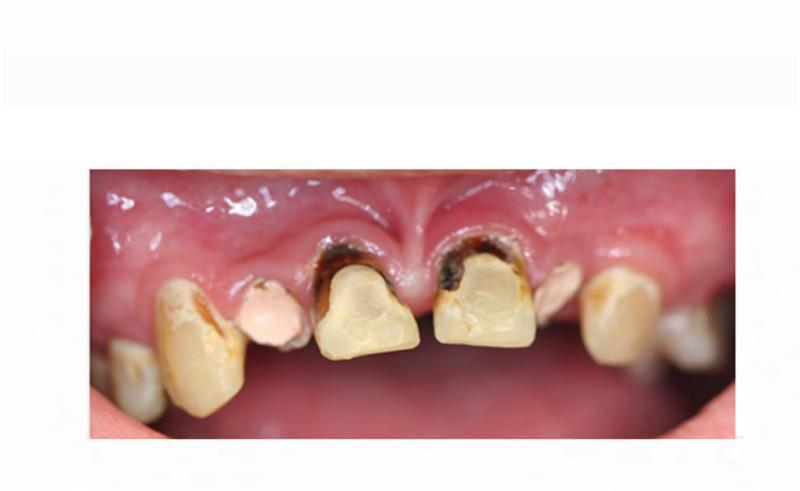
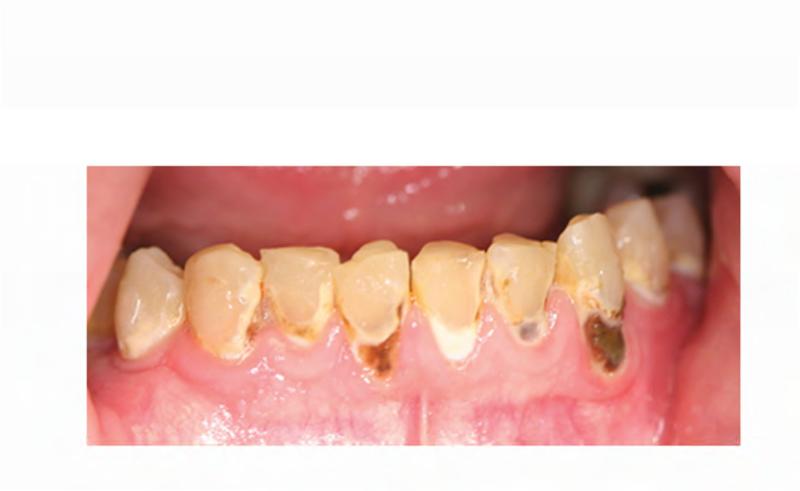
A. Technetium-99 bone scan highlighting areas of increased radiotracer uptake in bone (red arrowheads) affected by fibrous dysplasia in the skull and bilateral femora, humeri, and fibulae. B. Clinical photo depicting facial dysmorphism resulting from mild vertical dystopia, and diffuse expansion of maxillary and mandibular fibrous dysplasia. C. Three-dimensional computed tomography reconstruction reveals diffuse craniofacial involvement with fibrous dysplasia. D. Panoramic radiograph demonstrating the region of significantly decayed left maxillary third molar (red arrow).
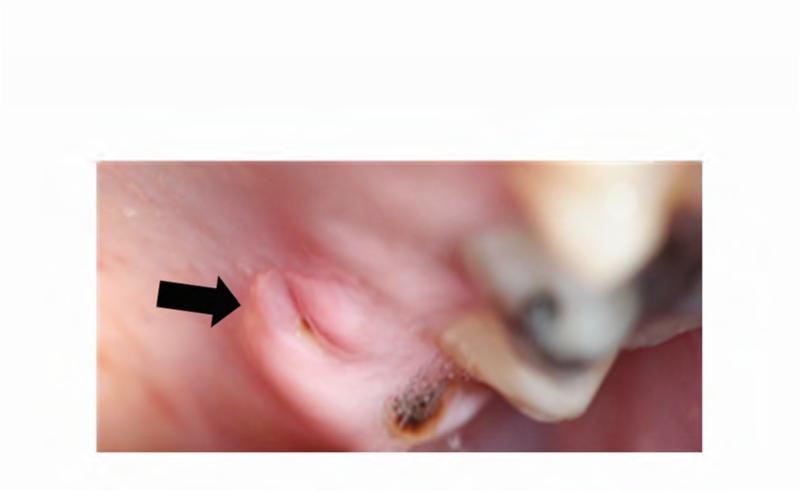
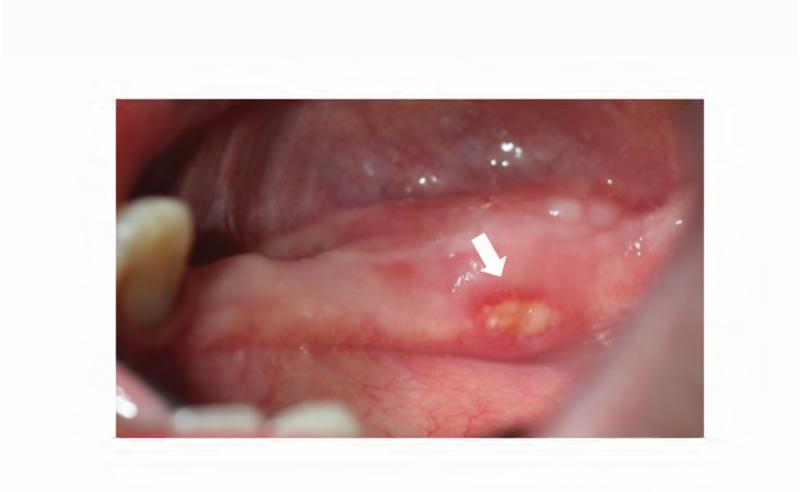
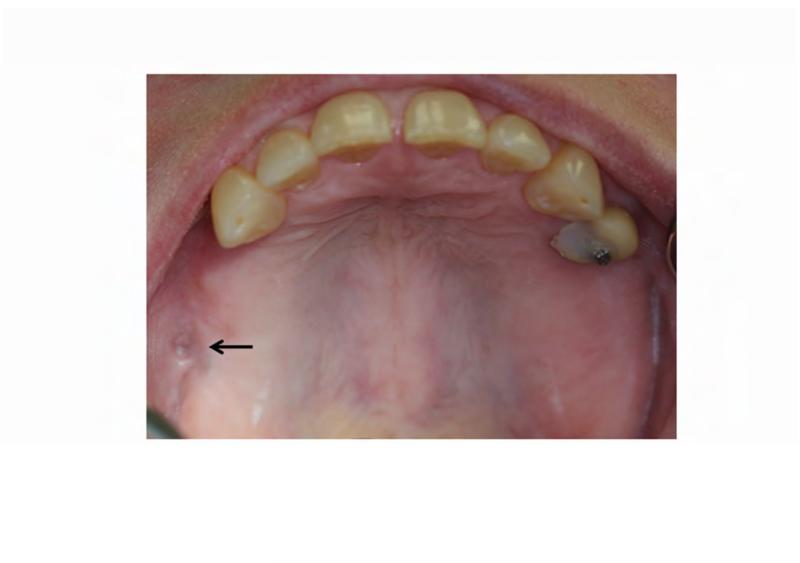
The patient's complex dental history included orthodontic treatment to correct his malocclusion, starting with a palatal expander one year prior to his current presentation. During orthodontic treatment, the patient sustained a traumatic fracture of the maxillary left lateral incisor. The family requested that the orthodontic appliance be removed due to difficulty in maintaining oral hygiene. When the appliance was removed, extensive decay was noted on all of the maxillary and mandibular anterior teeth (Figure 4A and B). During a routine dental consult at the NIH, a 3-4 mm, circular area of exposed bone was noted on the left palate adjacent to the left maxillary second and third molars (ADA #15 and 16) (Figure 4C). Remarkably, the area of exposed bone was in an area of FD, but did not occur in an area that had been covered by the palatal expander. Additionally, the left maxillary third molar was decayed to the gingival margin, and numerous caries were noted throughout the dentition. Neither the patient nor the family was aware of the lesion or the carious tooth, and the patient denied pain or sensitivity. No erythema or purulence was associated with the exposed bone. Based on his history of bisphosphonate treatment and the duration of exposed bone, the patient was diagnosed with Stage 1 ONJ. As a result of more pressing medical issues, the patient did not return for follow-up until three years later. At that time, necrotic bone was still noted to be present (Figure 4D), along with the carious left maxillary third molar (Figure 3D). The patient was referred to a tertiary care center near his home for treatment, and is being closely monitored.
Figure 4. Case 2 intraoral photographs.
A & B. Photographs of the anterior maxillary and mandibular teeth, respectively, illustrate rampant dental caries and stagnant plaque after the removal of the patient's braces, and the fractured maxillary left lateral incisor. C. An area of exposed bone is visible on the left palate adjacent to the left maxillary second and third molars (black arrow). D. Photo of the site of osteonecrosis covered by granulation tissue at the patient's follow-up appointment three years after its initial documentation.
Case 3
A 57-year-old man with FD/MAS presented for dental clearance prior to orthopedic surgery. The patient has severe FD involving the lower extremities and pelvis, café-au-lait macules, and renal phosphate wasting. He has a history of multiple pathologic fractures requiring orthopedic surgeries. Eleven years prior to his current presentation, the patient received a 2-year course of bisphosphonates from ages 46-48 years for treatment of FD-related bone pain. He was initially treated with zoledronic acid 6 mg every 6 months for the first year, and transitioned to 4 mg every three months for the subsequent year.
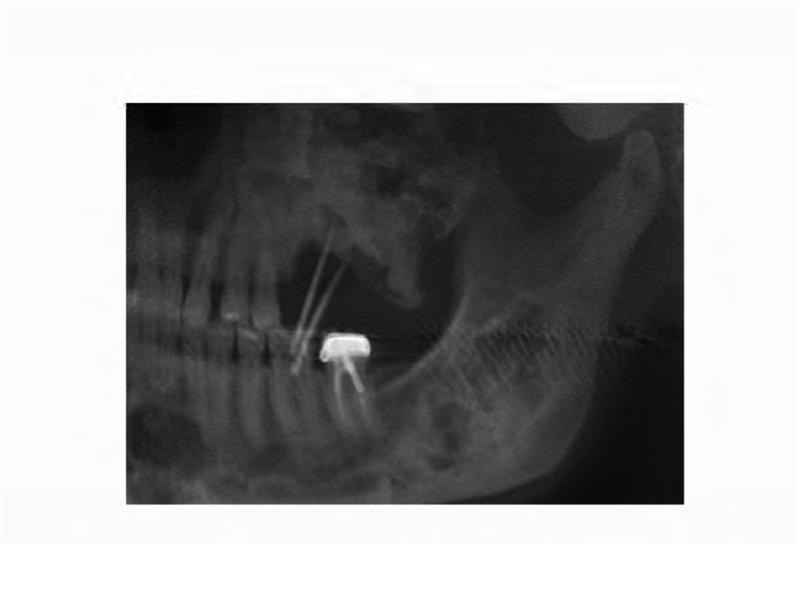
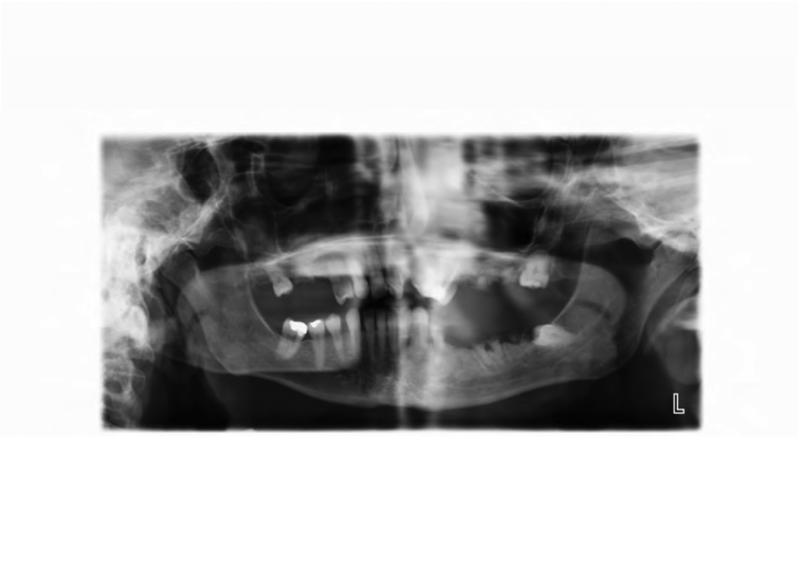
The patient's dental care has been sporadic due to financial issues and dental phobia. Seven months prior to his current presentation, he underwent a pre-operative dental evaluation in preparation for a planned total femur replacement. The general dentist observed carious root tips, generalized caries, and a horizontal partial-bony impacted left mandibular first molar (Figure 5A). Ten teeth were recommended for extraction, but the patient elected to limit extraction to the left mandibular first and second molars (ADA #18-19), and left mandibular first premolar (ADA #21). Post-extraction, the patient was prescribed a daily chlorhexidine rinse. At a follow-up evaluation two months later, the patient elected to have the right maxillary second molar (ADA #2) and left maxillary first, second, and third molars (ADA #14-16) extracted.
Figure 5. Case 3 dental images.
A. A panoramic radiograph depicting the poor state of the dentition before dental treatment. B. Panoramic radiograph taken six months after extractions and restorative treatment.
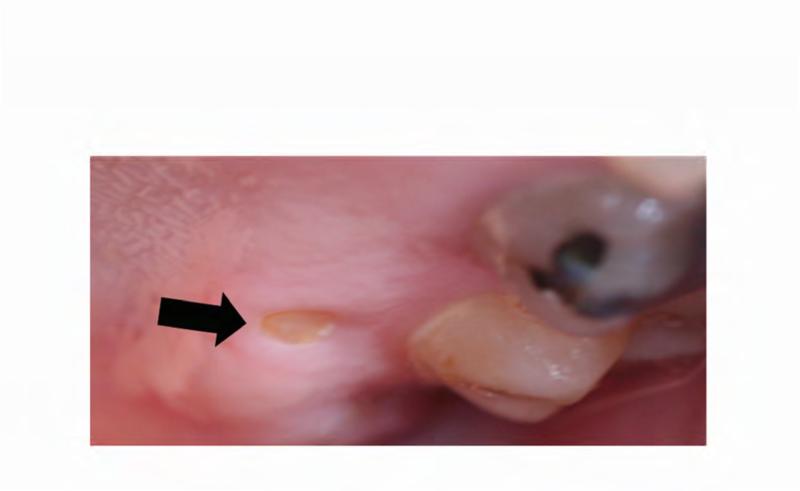
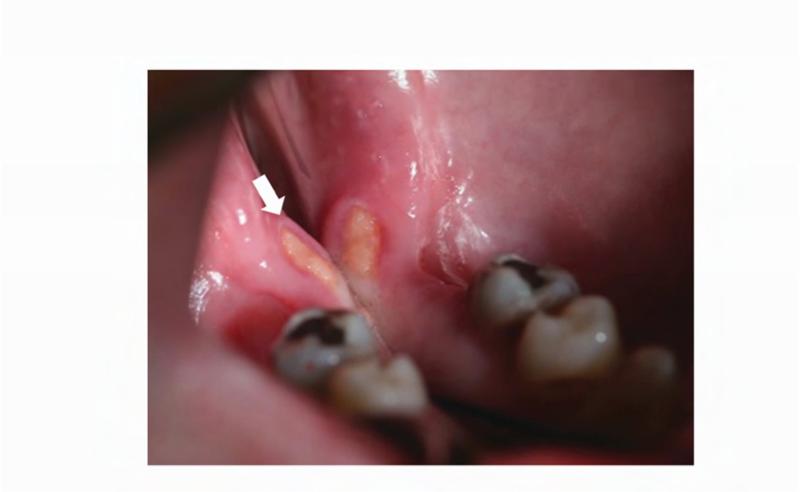
At a follow-up evaluation two months later, planned restorative work on the right maxillary first molar and right maxillary first premolar was performed. During the restorative appointment, the dentist noted a cariogenic pulp exposure on the right mandibular first molar (ADA #30), and this tooth was subsequently extracted, along with the left maxillary lateral incisor (ADA #10), left mandibular first molar (ADA #17) (Figure 5B). Two weeks post extractions, the patient presented to the dental clinic with pain and discomfort as a result of impacted food in the bilateral extraction sites; the sites were irrigated and the patient was instructed to continue home irrigation. At a follow-up evaluation three months later, the intraoral examination revealed exposed bone in the lingual mandible, bilaterally: 3×3 mm on the left and 3×2mm on the right side (Figure 6A and B). The patient was asymptomatic and subsequently diagnosed with Stage 1 ONJ. The patient was placed on chlorhexidine rinses with close observation.
Figure 6. Case 3 intraoral photographs.
Photographs were taken 2 weeks after initial ONJ presentation. A. Right posterior mandible with exposed bone along the lingual alveolar ridge (image is reflected in mirror). B. Patient's left mandibular lingual alveolus, reflected in mirror. Area of exposed bone is present along the posterior mylohyoid ridge.
Case 4
A 57-year-old female with FD/MAS presented for routine dental consultation. The patient has polyostotic FD affecting the craniofacial region, ribs and femurs and tibiae (Fig 7A). MAS-related endocrinopathies include precocious puberty and hyperthyroidism status-post thyroidectomy for poorly-differentiated clear cell carcinoma. As a result of chronic FD-related bone pain, the patient was prescribed a course of bisphosphonates, which included pamidronate 60 mg every 3 months from ages 36-39 years, followed by zoledronic acid 4 mg every 6 months from ages 39-49 years.
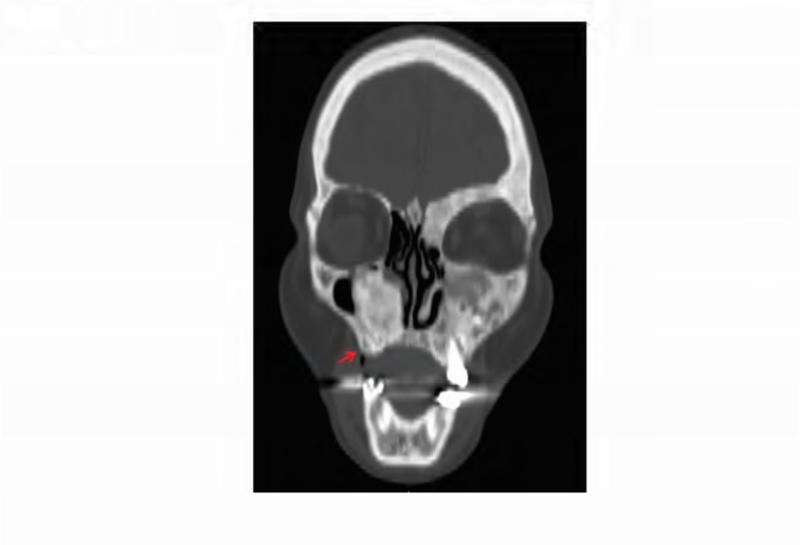
Figure 7. Case 4 clinical, dental and intraoral images.
A. Technetium-99 bone scan highlighting areas of increased radiotracer uptake in bone (red arrowheads) affected by fibrous dysplasia in the skull, ribs, femur, and tibia. B. Panoramic radiograph taken at initial presentation, with the arrow pointing to the region of extraction 7 months prior. C. Coronal computed tomography showing characteristic homogenous ground glass appearance of FD in the area of extraction (arrow). D. Clinical photo showing 2 mm bony exposure of the right maxillary alveolar ridge in the area of extraction (arrow).
The patient's dental history is defined by sporadic dental care, including multiple restorative procedures and extractions. Seven months prior to presentation, the right maxillary first molar and second premolar (ADA #3 and 4, respectively) were deemed non-restorable and were extracted by the patient's home providers. Upon examination at the NIH, a 2 mm non-healing bony defect of the right maxillary alveolar ridge and potential radiographic fistula were noted (Fig 7B and D). Of note, FD was present in the bilateral maxilla, including in the region of the extractions (Fig 7C). The patient was asymptomatic and denied pain or sensitivity, and was subsequently diagnosed with Stage 1 ONJ. The patient reported that her home dentist never mentioned the exposed bone, and 1.5 years later reports that the right maxillary wound has healed without complication. Approximately a year after her visit to the NIH, the patient also recounted that the left maxillary canine and first premolar (ADA #11 and 12) were extracted due to mobility and significant decay. Three months following this extraction the patient denied complications, stating that her wounds had completely healed, and that a removable partial denture was fabricated.
Discussion
ONJ is an established complication of bisphosphonate use, and appears to occur uncommonly in association with FD/MAS. To our knowledge, these cases are the only reports of bisphosphonate-associated ONJ in patients treated for FD, and cases 1, 2 and 4 are the only reported instances of ONJ occurring in FD bone. In the NIH cohort of 76 subjects treated with bisphosphonates, this represents a 5.4% prevalence of bisphosphonate-related ONJ. While this cohort is not sufficiently large to draw conclusions regarding prevalence in the general FD population, these cases establish that contrary to previous thinking, patients with FD are at risk for the development of bisphosphonate-related ONJ, and should be counseled and monitored accordingly.
The pathophysiology of bisphosphonate-related ONJ in FD and other disorders is not well-understood. One proposed mechanism includes inhibition of angiogenesis resulting in interruption of vascular supply to the jaws [21, 22]. This is supported by studies demonstrating antiangiogenic properties of bisphosphonates [23, 24], and increasing evidence of a potential association between ONJ and other antiangiogenic agents such as tyrosine kinase inhibitors and an anti-VEGF monoclonal antibody [25, 26]. FD bone is highly vascular [7], and it is unknown whether inhibition of angiogenesis is a contributory factor in the development of ONJ in FD patients.
Another commonly cited hypothesis links the pathophysiology of bisphosphonate-related ONJ with suppression of osteoclastic bone resorption and remodeling [21, 22]. This may explain the predominant localization of ONJ to the jaws, where intracortical remodeling rates are thought be increased in comparison to other skeletal sites [27, 28], although it should be noted that more recent studies have challenged this paradigm [29, 30]. High bone turnover and increased osteoclastogenesis are characteristic of FD lesions [3]. The effects of bisphosphonates on osteoclast activity within FD lesions have not been fully elucidated; however one study reported no detectable effect on histomorphometric indices, including resorption parameters, in biopsy specimens of patients treated with pamidronate [15]. This is consistent with long-term studies which did not find a consistent effect of bisphosphonate treatment on FD lesion size or radiographic appearance [16, 31]. The potential role of suppression of bone remodeling and/or resorption in the development of these patients’ ONJ is therefore unclear.
Age-related changes in craniofacial FD may also potentially impact on the development of ONJ. The natural history of FD lesions is to become established during early childhood, expand during linear growth, and remain relatively quiescent in adulthood [5]. Craniofacial lesions in older individuals typically become less homogeneous radiographically, developing discrete radiolucent, “cystic” appearing areas over time [2]. These findings are mirrored histologically, with lesions in older patients showing fewer features characteristic of FD, and in some cases demonstrating normal bone and marrow histology, complete with restoration of hematopoiesis [32]. It is possible that decreased vascularity and bone turnover over time may predispose older individuals with FD to the development of ONJ. However, the occurrence of ONJ in FD bone in Case 2, who presented at the relatively young age of 23 years, argues against this possibility.
The patients presented in this report had multiple features known to be associated with bisphosphonate-related ONJ in the general population. ONJ occurs more commonly in conjunction with nitrogen containing bisphosphonates, in particular zoledronic acid, as opposed to lower potency non-nitrogen containing and oral formulations [33, 34]. Higher doses, intravenous formulations, duration of treatment, and more frequent dosing intervals are also correlated with increased risk [35, 36]. All patients in this report had frequent treatment with zoledronic acid at high doses and over a long-term period. In addition all had concomitant periodontal and endodontic infections, and underwent dentoalveolar surgical procedures, which are also established risk factors for ONJ [37]. The development of ONJ in these patients was likely the result of a combination of these multiple risk factors.
Current ONJ treatment guidelines stress the need for disease prevention with regular dental examinations and professional prophylaxis before beginning bisphosphonate/non-bisphosphonate antiresorptives or antiangiogenic medication regimens [38]. Periodontal surgical procedures are treated in the same manner as oral and maxillofacial surgical procedures [39]. Evaluation and treatment for MAS-associated endocrinopathies is an essential element of care in FD patients, and should be performed prior to initiating medical or dental treatments. In active ONJ, treatments can range from debridement of the necrotic bone to aggressive resection of the affected area [38]. Post-operative regrowth is common following conservative debulking and recontouring surgeries in craniofacial FD; therefore, if ONJ is treated surgically, the FD bone should be monitored to assess response to surgery [40]. As more FD/MAS patients are treated with bisphosphonates and anti-osteoclastic drugs, it is important to investigate ONJ and other long-term effects of these drugs in order to develop appropriate treatment and monitoring guidelines.
These cases establish ONJ as a potential complication of bisphosphonate treatment in patients with FD/MAS. The presence of established risk factors for ONJ in this group of FD patients suggests that high risk individuals may be identified prior to the development of ONJ. Population studies are needed to identify additional, FD-specific risk factors, and to determine the prevalence of bisphosphonate-related ONJ in FD/MAS patients. Future studies investigating the effect of bisphosphonates on histology, turnover, and other morphometric indices may provide insights into the pathophysiology of medication-related ONJ in FD and other conditions. The identification of this potential side effect also highlights the need to be judicious in administering bisphosphonates to patients with FD without a clearly established indication. The current literature does not support a beneficial effect of bisphosphonate treatment on FD beyond the treatment of FD-related bone pain. Similar to non-FD patients, the development of ONJ may potentially be mitigated by 1) obtaining pre-treatment dental screening and initiation of appropriate dental care, 2) using the lowest dose and interval necessary to maintain a therapeutic effect, and 3) utilizing clinical judgment regarding use of alternate dosing schedules or drug holidays in patients who require invasive dental procedures [21, 41, 42].
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, NIDCR. This research was also funded by the NIH Medical Research Scholars Program, a public-private partnership supported jointly by the NIH and generous contributions to the Foundation for the NIH from the Doris Duke Charitable Foundation, American Association for Dental Research, Howard Hughes Medical Institute, and the Colgate-Palmolive Company, as well as alumni of student research programs and other individual supporters. For a complete list, please visit the Foundation website at: http://www.fnih.org.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Weinstein LS, Shenker A, Gejman PV, Merino MJ, Friedman E, Spiegel AM. Activating mutations. of the stimulatory G protein in the McCune-Albright syndrome. N Engl J Med. 1991;325:1688. doi: 10.1056/NEJM199112123252403. [DOI] [PubMed] [Google Scholar]
- 2.Boyce AM, Collins MT. Fibrous Dysplasia/McCune-Albright Syndrome. In: Pagon RA, Adam MP, Ardinger HH, Wallace SE, Amemiya A, Bean LJH, Bird TD, Dolan CR, Fong CT, Smith RJH, Stephens K, editors. GeneReviews(R) University of Washington, Seattle; Seattle WA: 1993. [PubMed] [Google Scholar]
- 3.Collins MT, Riminucci M, Bianco P. Fibrous dysplasia. In: Rosen C, editor. Primer on the metabolic . bone diseases and disorders of mineral metabolism 8th ed. American Society of Bone and Mineral Research; Washington, D.C: 2013. p. 786. [Google Scholar]
- 4.Ito H, Waga S, Sakakura M. Fibrous dysplasia of the skull with increased vascularity in the . angiogram. Surg Neurol. 1985;23:408. doi: 10.1016/0090-3019(85)90218-6. [DOI] [PubMed] [Google Scholar]
- 5.Hart ES, Kelly MH, Brillante B, Chen CC, Ziran N, Lee JS, Feuillan P, Leet AI, Kushner H, Robey PG, Collins MT. Onset, progression, and plateau of skeletal lesions in fibrous dysplasia and the relationship to functional outcome. J Bone Miner Res. 2007;22:1468. doi: 10.1359/jbmr.070511. [DOI] [PubMed] [Google Scholar]
- 6.Akintoye SO, Boyce AM, Collins MT. Dental perspectives in fibrous dysplasia and McCune-Albright syndrome. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;116:e149. doi: 10.1016/j.oooo.2013.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee J FE, Chen Y, Kim H, Lustig L, Akintoye S, Collins M, Kaban L. Clinical guidelines for the management of craniofacial fibrous dysplasia. 2012;24S2(Suppl 1)(Suppl 1):S2. doi: 10.1186/1750-1172-7-S1-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Riminucci M, Liu B, Corsi A, Shenker A, Spiegel AM, Robey PG, Bianco P. The histopathology of fibrous dysplasia of bone in patients with activating mutations of the Gs alpha gene: site-specific patterns and recurrent histological hallmarks. J Pathol. 1999;187:249. doi: 10.1002/(SICI)1096-9896(199901)187:2<249::AID-PATH222>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 9.Papapoulos S. Bisphosphonates for postmenopausal osteoporosis. In: Rosen C, editor. Primer on the metabolic bone diseases and disorders of mineral metabolism 8th ed. American Society of Bone and Mineral Research; Washington, D.C: 2013. p. 412. [Google Scholar]
- 10.Wat WZ. Current perspectives on bisphosphonate treatment in Paget's disease of bone. Ther Clin Risk Manag. 2014;10:977. doi: 10.2147/TCRM.S58367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mhaskar R, Redzepovic J, Wheatley K, Clark OA, Miladinovic B, Glasmacher A, Kumar A, Djulbegovic B. Bisphosphonates in multiple myeloma: a network meta-analysis. Cochrane Database Syst Rev. 2012;5:CD003188. doi: 10.1002/14651858.CD003188.pub3. [DOI] [PubMed] [Google Scholar]
- 12.Biggin A, Munns CF. Osteogenesis imperfecta: diagnosis and treatment. Curr Osteoporos Rep. 2014;12:279. doi: 10.1007/s11914-014-0225-0. [DOI] [PubMed] [Google Scholar]
- 13.Liens D, Delmas PD, Meunier PJ. Long-term effects of intravenous pamidronate in fibrous dysplasia of bone. Lancet. 1994;343:953. doi: 10.1016/s0140-6736(94)90069-8. [DOI] [PubMed] [Google Scholar]
- 14.Chapurlat RD, Hugueny P, Delmas PD, Meunier PJ. Treatment of fibrous dysplasia of bone with intravenous pamidronate: long-term effectiveness and evaluation of predictors of response to treatment. Bone. 2004;35:235. doi: 10.1016/j.bone.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 15.Plotkin H, Rauch F, Zeitlin L, Munns C, Travers R, Glorieux FH. Effect of pamidronate treatment in children with polyostotic fibrous dysplasia of bone. J Clin Endocrinol Metab. 2003;88:4569. doi: 10.1210/jc.2003-030050. [DOI] [PubMed] [Google Scholar]
- 16.Boyce AM, Kelly MH, Brillante BA, Kushner H, Wientroub S, Riminucci M, Bianco P, Robey PG, Collins MT. A randomized, double blind, placebo-controlled trial of alendronate treatment for fibrous dysplasia of bone. J Clin Endocrinol Metab. 2014:jc20141371. doi: 10.1210/jc.2014-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chapurlat RD. Medical therapy in adults with fibrous dysplasia of bone. J Bone Miner Res. 2006;21(Suppl 2):P114. doi: 10.1359/jbmr.06s222. [DOI] [PubMed] [Google Scholar]
- 18.Tessaris D, Matarazzo P, Lala R, Defabianis P. Odontoiatric perspectives and osteonecrosis of the jaw as a possible adverse effect of bisphosphonates therapy in fibrous dysplasia and McCune-Albright syndrome. J Pediatr Endocrinol Metab. 2015 doi: 10.1515/jpem-2015-0300. [DOI] [PubMed] [Google Scholar]
- 19.Marx RE. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: a growing epidemic. J Oral Maxillofac Surg. 2003;61:1115. doi: 10.1016/s0278-2391(03)00720-1. [DOI] [PubMed] [Google Scholar]
- 20.Ruggiero SL, Mehrotra B, Rosenberg TJ, Engroff SL. Osteonecrosis of the jaws associated with the use of bisphosphonates: a review of 63 cases. J Oral Maxillofac Surg. 2004;62:527. doi: 10.1016/j.joms.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 21.Ruggiero SL, Dodson TB, Fantasia J, Goodday R, Aghaloo T, Mehrotra B, O'Ryan F. American Association of Oral and Maxillofacial Surgeons position paper on medication-related osteonecrosis of the jaw--2014 update. J Oral Maxillofac Surg. 2014;72:1938. doi: 10.1016/j.joms.2014.04.031. [DOI] [PubMed] [Google Scholar]
- 22.Allen MR, Burr DB. The pathogenesis of bisphosphonate-related osteonecrosis of the jaw: so many hypotheses, so few data. J Oral Maxillofac Surg. 2009;67:61. doi: 10.1016/j.joms.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 23.Santini D, Vincenzi B, Galluzzo S, Battistoni F, Rocci L, Venditti O, Schiavon G, Angeletti S, Uzzalli F, Caraglia M, Dicuonzo G, Tonini G. Repeated intermittent low-dose therapy with zoledronic acid induces an early, sustained, and long-lasting decrease of peripheral vascular endothelial growth factor levels in cancer patients. Clin Cancer Res. 2007;13:4482. doi: 10.1158/1078-0432.CCR-07-0551. [DOI] [PubMed] [Google Scholar]
- 24.Vincenzi B, Santini D, Dicuonzo G, Battistoni F, Gavasci M, La Cesa A, Grilli C, Virzi V, Gasparro S, Rocci L, Tonini G. Zoledronic acid-related angiogenesis modifications and survival in advanced breast cancer patients. J Interferon Cytokine Res. 2005;25:144. doi: 10.1089/jir.2005.25.144. [DOI] [PubMed] [Google Scholar]
- 25.Lescaille G, Coudert AE, Baaroun V, Ostertag A, Charpentier E, Javelot MJ, Toledo R, Goudot P, Azerad J, Berdal A, Spano JP, Ruhin B, Descroix V. Clinical study evaluating the effect of bevacizumab on the severity of zoledronic acid-related osteonecrosis of the jaw in cancer patients. Bone. 2014;58:103. doi: 10.1016/j.bone.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 26.Sivolella S, Lumachi F, Stellini E, Favero L. Denosumab and anti-angiogenetic drug-related osteonecrosis of the jaw: an uncommon but potentially severe disease. Anticancer Res. 2013;33:1793. [PubMed] [Google Scholar]
- 27.Garetto LP, Chen J, Parr JA, Roberts WE. Remodeling dynamics of bone supporting rigidly fixed titanium implants: a histomorphometric comparison in four species including humans. Implant Dent. 1995;4:235. doi: 10.1097/00008505-199500440-00002. [DOI] [PubMed] [Google Scholar]
- 28.Han ZH, Palnitkar S, Rao DS, Nelson D, Parfitt AM. Effects of ethnicity and age or menopause on the remodeling and turnover of iliac bone: implications for mechanisms of bone loss. J Bone Miner Res. 1997;12:498. doi: 10.1359/jbmr.1997.12.4.498. [DOI] [PubMed] [Google Scholar]
- 29.Cheong S, Sun S, Kang B, Bezouglaia O, Elashoff D, McKenna CE, Aghaloo TL, Tetradis S. Bisphosphonate uptake in areas of tooth extraction or periapical disease. J Oral Maxillofac Surg. 2014;72:2461. doi: 10.1016/j.joms.2014.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ristow O, Gerngross C, Schwaiger M, Hohlweg-Majert B, Kehl V, Jansen H, Hahnefeld L, Otto S, Pautke C. Is bone turnover of jawbone and its possible over suppression by bisphosphonates of etiologic importance in pathogenesis of bisphosphonate-related osteonecrosis? J Oral Maxillofac Surg. 2014;72:903. doi: 10.1016/j.joms.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 31.Chapurlat RD, Delmas PD, Liens D, Meunier PJ. Long-term effects of intravenous pamidronate in fibrous dysplasia of bone. J Bone Miner Res. 1997;12:1746. doi: 10.1359/jbmr.1997.12.10.1746. [DOI] [PubMed] [Google Scholar]
- 32.Kuznetsov SA, Cherman N, Riminucci M, Collins MT, Robey PG, Bianco P. Age-dependent demise of GNAS-mutated skeletal stem cells and “normalization” of fibrous dysplasia of bone. J Bone Miner Res. 2008;23:1731. doi: 10.1359/jbmr.080609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.King AE, Umland EM. Osteonecrosis of the jaw in patients receiving intravenous or oral bisphosphonates. Pharmacotherapy. 2008;28:667. doi: 10.1592/phco.28.5.667. [DOI] [PubMed] [Google Scholar]
- 34.Durie BG, Katz M, Crowley J. Osteonecrosis of the jaw and bisphosphonates. N Engl J Med. 2005;353:99. doi: 10.1056/NEJM200507073530120. [DOI] [PubMed] [Google Scholar]
- 35.Hoff AO, Toth BB, Altundag K, Johnson MM, Warneke CL, Hu M, Nooka A, Sayegh G, Guarneri V, Desrouleaux K, Cui J, Adamus A, Gagel RF, Hortobagyi GN. Frequency and risk factors associated with osteonecrosis of the jaw in cancer patients treated with intravenous bisphosphonates. J Bone Miner Res. 2008;23:826. doi: 10.1359/JBMR.080205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bamias A, Kastritis E, Bamia C, Moulopoulos LA, Melakopoulos I, Bozas G, Koutsoukou V, Gika D, Anagnostopoulos A, Papadimitriou C, Terpos E, Dimopoulos MA. Osteonecrosis of the jaw in cancer after treatment with bisphosphonates: incidence and risk factors. J Clin Oncol. 2005;23:8580. doi: 10.1200/JCO.2005.02.8670. [DOI] [PubMed] [Google Scholar]
- 37.Marx RE, Sawatari Y, Fortin M, Broumand V. Bisphosphonate-induced exposed bone (osteonecrosis/osteopetrosis) of the jaws: risk factors, recognition, prevention, and treatment. J Oral Maxillofac Surg. 2005;63:1567. doi: 10.1016/j.joms.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 38.Aghaloo T, Hazboun R, Tetradis S. Pathophysiology of Osteonecrosis of the Jaws. Oral Maxillofac Surg Clin North Am. 2015;27:489. doi: 10.1016/j.coms.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goodday RH. Preventive Strategies for Patients at Risk of Medication-related Osteonecrosis of the Jaw. Oral Maxillofac Surg Clin North Am. 2015;27:527. doi: 10.1016/j.coms.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 40.Denadai R, Raposo-Amaral CA, Marques FF, Ghizoni E, Buzzo CL, Raposo-Amaral CE. Strategies for the Optimal Individualized Surgical Management of Craniofacial Fibrous Dysplasia. Ann Plast Surg. 2015 doi: 10.1097/SAP.0000000000000640. [DOI] [PubMed] [Google Scholar]
- 41.Hellstein JW, Adler RA, Edwards B, Jacobsen PL, Kalmar JR, Koka S, Migliorati CA, Ristic H. Managing the care of patients receiving antiresorptive therapy for prevention and treatment of osteoporosis: executive summary of recommendations from the American Dental Association Council on Scientific Affairs. J Am Dent Assoc. 2011;142:1243. doi: 10.14219/jada.archive.2011.0108. [DOI] [PubMed] [Google Scholar]
- 42.Khan AA, Morrison A, Hanley DA, Felsenberg D, McCauley LK, O'Ryan F, Reid IR, Ruggiero SL, Taguchi A, Tetradis S, Watts NB, Brandi ML, Peters E, Guise T, Eastell R, Cheung AM, Morin SN, Masri B, Cooper C, Morgan SL, Obermayer-Pietsch B, Langdahl BL, Al Dabagh R, Davison KS, Kendler DL, Sandor GK, Josse RG, Bhandari M, El Rabbany M, Pierroz DD, Sulimani R, Saunders DP, Brown JP, Compston J. Diagnosis and management of osteonecrosis of the jaw: a systematic review and international consensus. J Bone Miner Res. 2015;30:3. doi: 10.1002/jbmr.2405. [DOI] [PubMed] [Google Scholar]