Abstract
Background
The risk of cardiovascular problems due to diabetes mellitus is highest among older Mexicans, and yet what remains to be determined is the association between muscle weakness and diabetes in this population. Therefore, the purpose of this study was to determine the association between muscle strength and diabetes among Mexican adults greater than 50 years old.
Design
Cross-sectional.
Setting
National sample of households in both urban and rural areas.
Participants
A sub-sample of 1,841 individuals, aged 50 years and older, was included from the 2012 Mexican Health and Aging Study (MHAS).
Measurements
Strength was assessed using a hand-held dynamometer, and the single largest reading from either hand was normalized to body mass (NGS). Conditional inference tree analyses were used to identify sex-specific NGS weakness thresholds. Linear regression was used to examine the association between NGS and HbA1c, and logistic regression was used to assess the association between weakness and risk of diabetes (HbA1c ≥6.5% [≥48 mmol/mol]), after controlling for age, sex and waist circumference.
Results
Normalized grip strength was inversely associated with HbA1c (β=−1.56; p<0.001). Optimal sex-specific NGS weakness thresholds to detect diabetes were ≤0.46 and ≤0.30 for men and women respectively. Weakness was associated with significantly increased odds of diabetes (OR: 1.69, 95%CI: 1.37-2.10), even after adjusting for age, sex, and waist circumference.
Conclusions
NGS was robustly associated with diabetes and other cardiometabolic risk factors in older Mexicans. This simple screen may serve as a valuable tool to identify adults that are at risk for negative health consequences or early mortality, and that might benefit from lifestyle interventions to reduce risk.
Keywords: insulin resistance, diabetes, muscle weakness, aging, epidemiology
Introduction
Diabetes is a leading cause of mortality that is estimated to affect over 400 million adults globally,1 particularly in low- and middle-income countries where more than 80% of diabetes deaths occur 2. For example, in Mexico the lifetime risk of diagnosed diabetes is projected to reach 50% by 2050,3 and there is also a high prevalence of impaired glucose tolerance,4 and undiagnosed or uncontrolled diabetes.5 From 1970 to 2010, Mexican mortality fell by two-fold for individuals aged >50 years (from 33% to 17%), and this has been attributed to expanded health-care infrastructure and access in poorer states.6 However, older adults are at increased risk for chronic cardiometabolic diseases such as diabetes, a known driver of both years lived with a disability and disability-adjusted life years in Mexico.7 An age-related decline in physical function and deterioration in muscle morphology contribute to exaggerated risk of diabetes at the individual level; and yet, increases in the incidence of diagnosed diabetes, combined with declining mortality levels, have led to an acceleration of lifetime diabetes risk and more years spent with diabetes at the population level. Therefore, the aging Mexican population poses a substantial burden to the country's future healthcare system.
Early screening and promotion efforts for healthy aging among higher-risk populations are vital to mitigate the incidence of diabetes and other preventable comorbidities; thereby curtailing the escalating healthcare costs associated with chronic conditions. There has been an increase in the amount of evidence that highlights the role of muscular strength preservation as a protective factor for cardiometabolic health across populations. Recent investigations8-10 have shown that low muscular strength is independently associated with increased odds of the metabolic syndrome and diabetes in adults, and that cut points or centiles11 for low normalized strength may be used to predict increased risk. Moreover, longitudinal data have demonstrated that chronic hyperglycemia12 and greater fat mass13 (i.e., two hallmark risk factors for diabetes) are associated with diminished muscle quality and weakness. There is also mounting evidence that indicates a robust inverse association between low strength and cardiometabolic risk clustering among children and adolescents,14-16 thus reinforcing the need for early and improved clinical screening strategies across populations. Therefore, the purposes of this study were to examine the independent association between handgrip strength capacity and diabetes in a large sample of aging adults in Mexico, and to identify potential sex-specific weakness thresholds for detection of diabetes.
Research Design and Methods
Study Population
The Mexican Health and Aging Study (MHAS) was designed to prospectively evaluate the impact of disease on the health, function, and mortality of adults over the age of 50 years in a national sample of households in both urban and rural areas of Mexico. The overall goal of the study is to examine the aging process, including the impact of disease and disability in a large representative panel of older Mexicans, as previously described in detail.17,18 The MHAS study protocols and instruments were approved by the Institutional Review Board or Ethics Committee of the University of Texas Medical Branch, the Instituto Nacional de Estadistica y Geografia (INEGI) in Mexico, and the Instituto Nacional de Salud Publica (INSP) in Mexico.
Of the 15,723 participants who were interviewed in the 2012 MHAS (survey wave 3), a sub-sample of 2,086 was selected in order to collect anthropometric measures, blood pressure readings, performance tests, and blood biomarkers. Of these, 1,841 participants had (1) complete demographic and anthropometric data; (2) valid strength data from a handgrip dynamometer; and (3) the necessary blood samples obtained for non-fasting glycohemoglobin determination.
Anthropometric Factors
Each participant wore light clothing and no shoes while being weighed on a digital Toledo scale (Mettler-Toledo International, Inc., Columbus, OH). Height was measured using a fixed stadiometer. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared (kg/m2). Standard categories were applied to determine if each participant was normal weight (18.5-24.9 kg/m2), overweight (25.0-29.9 kg/m2), or obese (≥ 30.0 kg/m2).19 Individuals with BMI <18.5 kg/m2 were excluded (n=15), due to the known association between underweight status and diabetes risk in older adults.20 Waist circumference was measured to the nearest 0.1 cm at the level of the iliac crest, and used in the analyses as a continuous variable.
Cardiometabolic Parameters
Participants were tested on routine cardiometabolic parameters. Resting systolic and diastolic blood pressures were measured twice with a mercury sphygmomanometer by trained staff. Non-fasting measures of total cholesterol, HDL-cholesterol, thyroid-stimulating hormone, serum 25-Hydroxyvitamin D, and C-reactive protein concentrations were also measured. Non-fasting serum measures of glycohemoglobin (%) were included as a diagnostic test for diabetes, which reflects average plasma glucose for the previous ~3 months. HbA1c was measured using A1cNow assay, a method that is National Glycohemoglobin Standardization Program certified. Participant diabetes status was based on elevated non-fasting HbA1c (≥6.5% [≥48 mmol/mol]), which reflects uncontrolled diabetes 21.
Exposure Variable
Grip Strength
Strength was assessed using a hydraulic handgrip dynamometer (Jamar Hydraulic Dynamometer, model 5030J1; JA Preston Corp., Clifton, NJ). A trained examiner explained and demonstrated the protocol to the participant, then adjusted the grip size of the dynamometer to the participant's dominant hand size, and asked the participant to squeeze the dynamometer for a practice trial. Thereafter, the participant was instructed to start the test with his/her dominant hand, and was asked to squeeze the dynamometer with maximal effort, exhaling while squeezing. The test was then repeated with the opposite hand. Each hand was tested two times, alternating hands between trials with a 60-second rest between measurements on the same hand. The grip test was performed in the standing position unless the participant was physically limited. Participants were excluded from this component if they were unable to hold the dynamometer and perform strength testing with both hands. Participants who had surgery on either hand or wrist in the last three months were not tested on that particular hand. Since the link between muscle strength and both physical function and chronic health is mediated by the proportion of strength relative to body mass, grip strength was normalized (NGS) as strength per body mass (i.e., ).
Statistical Analysis
All statistical analyses were conducted using SAS 9.3 (SAS Institute, Cary, NC) and R version 3.1.2 (R Foundation for Statistical Computing, Vienna, Austria). Descriptive characteristics and cardiometabolic profiles are provided as means, standard errors, and percentages. Differences in these characteristics across strength categories were tested using linear and logistic regression for continuous and categorical variables respectively, after creating appropriate categories and dummy coding for each. A similar strategy was used to test differences for outcomes between men and women.
Threshold Analyses
Conditional inference tree analyses were used to determine risk thresholds of NGS in differentiating the risk for diabetes among all participants. Unlike a recent study in older adults that used classification and regression tree (CART) analysis to identify cut points for weakness,22 we chose not to incorporate this method because it tends to overfit. The conditional inference tree method recursively partitions participants into mutually exclusive groups defined by predictor cut points, grouping together participants with similar outcome probabilities.23 However, in contrast with CART, it utilizes a formal statistical framework to evaluate the recursive partitioning, taking into account both the distributional properties of the measures and multiple comparison between groups. This technique is also free of modeling assumptions, which allows for optimal concurrent validity, by identifying those cut points with the strongest association with diabetes. Moreover, it does not require an a priori specified number of cut points, and thus it can provide more than a single threshold to predict the outcome. The analysis was conducted using R software with the party package.23
To assess the odds of diabetes in the entire sample, we utilized the multivariate logistic regression modeling approach. Known risk factors including gender, age, waist circumference, and NGS (both continuous [per 0.10 variation in strength relative to body mass] and dichotomous for “weakness”) were adjusted in the model. The logistic regression model with the highest Akaike information criterion (AIC) was retained as the final model.
Results
Descriptive and cardiometabolic characteristics are presented as means, standard errors, and percentages across sexes in Table 1. Men were stronger than women in both absolute and normalized grip strength capacity (p<0.001). Prevalence of obesity, abdominal obesity, diabetes status, high CRP, and vitamin D deficiency were all significantly greater in Mexican women as compared to men; whereas, prevalence of low HDL and hypertension were higher among Mexican men.
Table 1.
Descriptive and cardiometabolic characteristics of the study population by sex.
| All (n=1,841) | Men (n=743) | Women (n=1,098) | |
|---|---|---|---|
| Age, years | 63.55 (9.52) | 64.35 (9.51)* | 62.99 (9.49) |
| Body Mass, kg | 69.65 (14.98) | 74.38 (14.67)* | 66.49 (14.34) |
| Body Mass Index (BMI), kg/m2 | 29.06 (9.55) | 27.88 (4.65) | 29.86 (11.68)* |
| Obesity (BMI>30), % | 37.10 | 28.01 | 43.20* |
| Waist Circumference (WC), cm | 98.09 (12.53) | 99.92 (12.38) | 96.86 (12.49) |
| Abdominal Obesity (Sex-Specific WC), % | 62.30 | 40.70 | 76.70* |
| Grip Strength, kg | 26.28 (8.94) | 32.40 (8.81)* | 22.13 (6.24) |
| Normalized Grip Strength (Relative to Body Mass) | 0.38 (0.12) | 0.44 (0.12)* | 0.34 (0.10) |
| Glycohemoglobin, % | 6.82 (1.87) | 6.67 (1.74) | 6.93 (1.96)* |
| Total Cholesterol, mg/dL | 200.43 (46.31) | 197.40 (47.93) | 202.46 (45.10)* |
| HDL-Cholesterol, mg/dL | 41.16 (10.37) | 39.11 (9.87) | 42.54 (10.46)* |
| Low HDL (<40 mg/dL), % | 47.10 | 56.10* | 41.10 |
| Thyroid-Stimulating Hormone, μIU/mL | 2.88 (5.66) | 2.63 (3.18) | 3.05 (6.70) |
| Serum 25-Hydroxyvitamin D, ng/mL | 24.22 (8.63) | 26.20 (9.30)* | 22.90 (7.87) |
| Vitamin D Deficiency (<20 ng/dL), % | 32.20 | 24.50* | 37.30 |
| C-Reactive Protein, mg/dL | 4.26 (7.22) | 3.33 (4.31)* | 4.89 (8.59) |
| High CVD Risk (≥3.0 mg/dL), % | 73.30 | 33.30 | 46.90* |
| Systolic Blood Pressure (SBP), mmHg | 138.46 (20.96) | 140.45 (20.67)* | 137.13 (21.06) |
| Diastolic Blood Pressure (DBP), mmHg | 78.89 (11.10) | 80.18 (11.63)* | 78.02 (10.65) |
| Hypertensive (SBP>140 mmHg or DBP>90 mmHg), % | 44.30 | 47.40* | 42.30 |
Significant differences between sexes (p<0.05): Denotes as sex with higher risk.
Abbreviations: HDL-High Density Lipoprotein; CVD-Cardiovascular Disease.
Samples sizes varied according to the specific variable.
Threshold Analysis
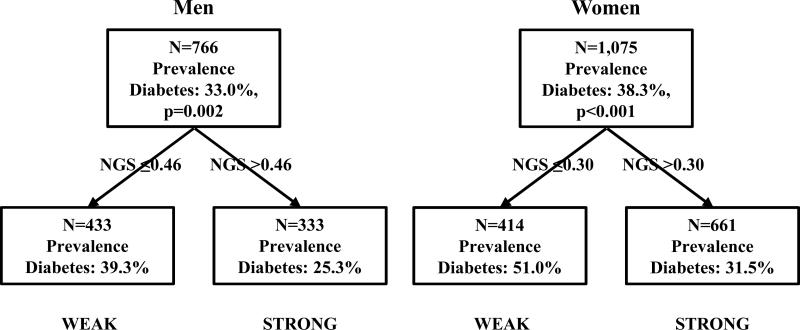
Conditional inference trees predicting diabetes confirmed different low strength thresholds for men and women. Figure 1 provides results for the primary definitions. The cutoff identified in men was based on having normalized grip strength less than or equal to 0.46 (i.e., grip strength in kg ≤ [0. 46 × body mass in kg]) versus greater than 0.46. Among women the identified NGS cutoff was based on having normalized grip strength less than or equal to 0.30 versus greater than 0.30.
Figure 1.
Conditional inference trees for baseline strength as predictors of diabetes (HbA1c levels ≥6.5% [≥48 mmol/mol]) in men and women.
In both men and women, prevalence of obesity and abdominal obesity were significantly higher among individuals in the weak NGS category (Table 2). Weak individuals also had significantly higher prevalences of vitamin D deficiency (women only), elevated CRP (both men and women), and hypertension (women only) (all p<0.01). Diabetes prevalence, according to elevated HbA1c (≥6.5% [≥48 mmol/mol]), was significantly higher among weak versus strong individuals and was 39.3% vs. 25.3% for men, and 51.0% vs, 31.5% for women, for weak and strong NGS respectively.
Table 2.
Demographic and cardiometabolic characteristics stratified by sex and normalized grip strength.
| Men | Women | |||
|---|---|---|---|---|
| NGS≤0.46 (n=433) | NGS>0.46 (n=333) | NGS≤0.30 (n=414) | NGS>0.30 (n=661) | |
| Age, years | 66.14 (9.56)* | 61.76 (8.54) | 64.57 (9.34)* | 61.79 (9.22) |
| Body Mass, kg | 77.74 (15.68)* | 70.22 (12.12) | 72.00 (15.99)* | 63.68 (12.40) |
| Body Mass Index (BMI), kg/m2 | 29.23 (4.93)* | 26.21 (3.63) | 32.32 (6.06)* | 28.13 (4.66) |
| Obesity (BMI>30), % | 39.70* | 13.60 | 62.90* | 32.80 |
| Waist Circumference (WC), cm | 103.41 (12.69)* | 95.62 (10.52) | 103.00 (12.46)* | 93.62 (11.25) |
| Abdominal Obesity (Sex-Specific WC), % | 54.30* | 24.10 | 88.90* | 70.20 |
| Grip Strength, kg | 27.97 (7.39)* | 38.16 (6.86) | 17.06 (4.86)* | 24.97 (4.95) |
| Normalized Grip Strength (Relative to Body Mass) | 0.36 (0.07)* | 0.55 (0.08) | 0.24 (0.05)* | 0.40 (0.07) |
| Glycohemoglobin, % | 6.72 (1.64) | 6.57 (1.77) | 7.16 (1.93)* | 6.79 (1.95) |
| Total Cholesterol, mg/dL | 194.34 (45.59) | 201.99 (50.75)* | 201.84 (50.42) | 201.64 (42.23) |
| HDL-Cholesterol, mg/dL | 38.47 (9.45)* | 39.90 (10.37) | 41.24 (9.96)* | 43.38 (10.68) |
| Low HDL (<40 mg/dL), % | 52.80 | 58.60 | 39.40 | 44.20 |
| Thyroid-Stimulating Hormone, μIU/mL | 2.62 (2.90) | 2.65 (3.54) | 3.37 (7.62) | 2.89 (6.24) |
| Serum 25-Hydroxyvitamin D, ng/mL | 25.42 (8.67) | 27.53 (9.81)* | 21.47 (7.45)* | 23.77 (7.95) |
| Vitamin D Deficiency (<20 ng/dL), % | 26.60 | 21.9 | 43.20* | 33.90 |
| C-Reactive Protein, mg/dL | 3.42 (3.67) | 3.13 (4.91) | 5.90 (7.50)* | 4.14 (8.67) |
| High CVD Risk (≥3.0 mg/dL), % | 38.00* | 27.4 | 58.70* | 40.10 |
| Systolic Blood Pressure (SBP), mmHg | 141.43 (21.11) | 139.99 (20.10) | 141.08 (21.32)* | 134.83 (20.48) |
| Diastolic Blood Pressure (DBP), mmHg | 79.59 (11.82) | 80.99 (11.23) | 78.28 (10.64) | 77.89 (10.64) |
| Hypertensive (SBP>140 mmHg or DBP>90 mmHg), % | 48.40 | 46.10 | 47.00* | 39.60 |
Significant difference within sexes, between weak (NGS ≤0.46 for men and ≤0.30 for women) and strong (NGS>0.46 for men and >0.30 for women) participants (p<0.05): Denoted as group with higher risk.
Abbreviations: NGS-Normalized Grip Strength; HDL-High Density Lipoprotein; CVD-Cardiovascular Disease
Samples sizes varied according to the specific variable.
In the adjusted models (Table 3), women were at higher odds of having diabetes than men (<0.001), and waist circumference was positively associated with diabetes. Moreover, for every 0.10 decrement in normalized strength, there was a 1.22 times increased odds for diabetes (p<0.001). Weakness was associated with significantly increased odds of diabetes (OR: 1.69, 95% CI: 1.37-2.10), even after adjusting for age, sex, and waist circumference.
Table 3.
Multiple logistic regression models for independent predictors of diabetes in Mexican adults, with NGS as a dichotomous variable denoting sex-specific weakness.
| Model Predictor(s) | Odds Ratio | 95% CIs | p-value | |
|---|---|---|---|---|
| Diabetes (HbA1c ≥ 6.5%) | ||||
| Age (years) | 0.99 | 0.99-1.01 | 0.38 | |
| Sex (Reference: Male) | 1.59 | 1.29-1.94 | <0.001 | |
| Waist circumference | 1.03 | 1.02-1.03 | <0.001 | |
| Weak Normalized Grip Strength | 1.69* | 1.37-2.10** | <0.001 | |
OR for weakness (NGS ≤0.46 and ≥0.30 for men and women respectively).
95% CIs for weakness.
Discussion
The principal finding of this study was that normalized grip strength is significantly associated with diabetes in older Mexican men and women. Specifically, for every 0.1 decrement in strength-to-body mass-ratio, there was a 22% increased adjusted odds of diabetes. Furthermore, when using sex-specific NGS thresholds, weak men and women both had significantly greater diabetes prevelance and higher prevalences of certain cardiometabolic abnormalities (e.g., abdominal obesity, vitamin D deficiency, elevated CRP, and hypertension) compared to their strong counterparts. These findings indicate that hand grip strength is a simple and inexpensive technique that identifies risk for diabetes and other cardiometabolic determinates of health in older Mexican adults.
Global life expectancy has increased in recent years; and yet, obesity is also expected to increase by 12% among older adults by the year 2030.24. Obesity has remained highly prevelant in older Mexican men and women, putting them at elevated risk for chronic cardiometabolic diseases such as diabetes -- a leading cause of early mortality in Mexico.2,25,26 The present investigation identified 28.0% of men and 43.2% of women as obese, and 33.0% of men and 38.3% of women as diabetic. These results differ slightly from an investigation by Rodríguez-Saldaña et al.,27 who showed non-diabetics were more obese than persons with diabetes, and that diabetes was more common in men than women. Comparisons across studies are difficult however, as different criteria were used to diagnose diabetes, participants were aged at least 65 years, and participants were only residing in retirement housing in Mexico City. Older Mexican adults are known to have less muscle mass and greater visceral adiposity than non-Hispanic Whites and Blacks in the U.S., suggesting that behavioral factors associated with these age-related changes may mediate the association between functional declines and risk for metabolic disease.28 Indeed, we found that diabetes prevalence was 14.0% and 19.5% greater in weak men and women compared to their stronger counterparts, respectively. Moreover, a recent study by Kumar et al.29 demonstrated that older obese Mexicans are at a greater risk for physical disability than their non-obese counterparts. Both obesity and diabetes are predictors of functional declines in older adults;30 however, the simultaneus loss of muscle and increase in adiposity with age also contributes to reduced functional performance and increased risk of diabetes. Therefore, these are highly interrelated factors that contribute to diabetes prevalence and weakness, making it difficult to fully understand the direction of causation. Considering that persons with diminished functional performance are at greater risk for developing diabetes, improved strategies to reduce diabetes risk in older Mexican populations should be emphasized.31 These findings underscore the consequences of sarcopenic obesity, particularly as a primary contributer to diabetes risk in older adults.32,33
Sarcopenic obesity is associated with many negative chronic cardiometabolic determinates of health, including abdominal obesity, elevated inflammatory markers, and hypertension.34 Older adults are at particularly elevated risk for sarcopenic obesity, as age-related decrements in muscle size and physical activity become accelerated.34,35 Our results demonstrate that weak men and women were more likely to be obese, had higher levels of central obesity, and were at higher risk for cardiovascular disease from elevated CRP levels. Greater vitamin D deficiency and hypertension were more prevalent only in weak women. These results align with other studies suggesting that central obesity, inflammatory markers, vitamin D deficiency, and hypertension are greater in older adults that are weaker.36,37 These findings confirm that older Mexicans are at similar risk for developing chronic cardiometabolic diseases associated with muscle weakness.
Hand grip strength has been recognized as a valid technique to predict risk of disability, which is significantly associated with diabetes in older Mexican Americans in the U.S..38 Another recent study that used age- and sex-specific NGS cuttoffs from 4,066 U.S. adults, revealed that for every 0.5 decrement in NGS, there was a 26% increased odds of diabetes.9 By comparison, the present investigation included 1,841 Mexican participants aged at least 50 years and found for every 0.1 decrement in NGS, there was a 22% increased odds of diabetes. A 5-year prospective cohort study by Al Snih et al. 39 determined 2,488 older Mexican adults were more at risk for mortality when hand grip strength was low. Considering that diabetes is a leading cause of mortality in Mexico, the association between hand grip strength as a determinent of muscle weakness, sarcopenic obesity, diabetes, and mortality should be further studied.2 This investigation also demonstrated that waist circumference was positively associated with diabetes and women had higher odds of having diabetes than men. These results concur with previous studies that have identified central obesity as a risk factor for diabetes and that women have more fat mass and lower absolute and relative strength than men, putting older Mexican women at greater risk for diabetes.34,40
This study has a number of strengths that extend the current body of literature on diabetes prevention. Analyses were conducted on a large number of participants who have been followed several years. To our knowledge, this is the first study to investigate the role that muscle weakness plays on diabetes prevalence in older Mexicans. The threshold modeling technique used in this investigation identified two strength cutpoints and two respective risk categories, which may be incorporated into a clinical setting for screening older Mexicans that are at risk for diabetes. Also, hand grip strength was normalized to body mass, making comparisons across body sizes possible. Despite these strengths, some limitations of the present study should be noted. Physical activitiy and nutritional data were not examined, and thus future efforts should seek to determine how these variables mediate the association between hand grip strength and diabetes. Further, as with all cross-sectional studies, a limitation of this investigation is the inability to unravel the direction of causation. Whether lower NGS “cause” an elevated risk for diabetes in older Mexicans, or if diabetes-related musculoskeletal conditions (e.g., neuropathy, diabetic cheiroarthropathy, flexor tenosynovitis, etc.) themselves, are a cause of diminished muscle function, is an interesting and complex topic. Lastly, other tests, such as quadriceps strength were unavailable, making comparisons between hand grip strength and other tests of muscle weakness unknown. Future investigations should explore the association between hand grip strength on chronic cardiometabolic diseases in other ethnicities and to examine the attributable risks of physical disability status and hand grip strength on incident diabetes in older adults.
Conclusion
Individuals with low NGS had a greater prevalence of diabetes and other cardiometabolic risk factors as compared to their strong counterparts. The odds of prevalent diabetes increased as NGS decreased. Health professionals should encourage older Mexican adults, and especially women, to engage in physical activities that help preserve or improve muscle strength in an effort to prevent chronic cardiometabolic diseases. NGS should also be used to monitor and assess muscle weakness in older Mexicans and other older populations.
Acknowledgments
Funding Sources
This work was supported by the National Institutes of Health (R01AG018016, Dr. Rebeca Wong, PI) and by the Instituto Nacional de Estadística y Geografía (INEGI) in Mexico. Dr. Peterson is funded by the National Institutes of Health (KO1 HD074706).
Role of the Sponsors: The funders had no role in the design and conduct of the study; the collection, analysis, and interpretation of the data; or the preparation, review, or approval of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions:
R.W. acquired research data. M.P. and P.Z. performed all analyses. M.P. R.W. and R.M. wrote the manuscript. R.W., K.M., and S.A.S. reviewed/edited manuscript, and contributed to the interpretation of the results. All authors reviewed the final submitted manuscript.
Conflict of Interest Statement:
The author report no conflicts of interest.
References
- 1.Grp IDA . IDF Diabetes Atlas. 7th Edition International Diabetes Federation; 2015. [Google Scholar]
- 2.WHO . Global Health Estimates: Deaths by Cause, Age, Sex and Country, 2000-2012. World Health Organization; Geneva: 2014. [Google Scholar]
- 3.Meza R, Barrientos-Gutierrez T, Rojas-Martinez R, et al. Burden of type 2 diabetes in Mexico: past, current and future prevalence and incidence rates. Prev Med. 2015;81:445–50. doi: 10.1016/j.ypmed.2015.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Urena-Bogarin EL, Martinez-Ramirez HR, Torres-Sanchez JR, Hernandez-Herrera A, Cortes-Sanabria L, Cueto-Manzano AM. Prevalence of pre-diabetes in young Mexican adults in primary health care. Fam Pract. 2015;32:159–64. doi: 10.1093/fampra/cmu047. [DOI] [PubMed] [Google Scholar]
- 5.Kumar A, Wong R, Ottenbacher KJ, Al Snih S. Prediabetes, undiagnosed diabetes, and diabetes among Mexican adults: findings from the Mexican Health and Aging Study. Ann Epid. 2016 doi: 10.1016/j.annepidem.2015.12.006. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Norheim OF, Jha P, Admasu K, et al. Avoiding 40% of the premature deaths in each country, 2010-30: review of national mortality trends to help quantify the UN Sustainable Development Goal for health. Lancet. 2015;385:239–52. doi: 10.1016/S0140-6736(14)61591-9. [DOI] [PubMed] [Google Scholar]
- 7.Murray CJ, Vos T, Lozano R, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2197–223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- 8.Senechal M, McGavock JM, Church TS, et al. Cut points of muscle strength associated with metabolic syndrome in men. Med Sci Sports Exerc. 2014;46:1475–81. doi: 10.1249/MSS.0000000000000266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peterson MD, Zhang P, Choksi P, Markides KS, Al Snih S. Muscle Weakness Thresholds for Prediction of Diabetes in Adults. Sports Med. 2016 doi: 10.1007/s40279-015-0463-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mainous AG, Tanner RJ, Anton SD, Jo A. Grip Strength as a Marker of Hypertension and Diabetes in Healthy Weight Adults. American journal of preventive medicine. 2015;49:850–8. doi: 10.1016/j.amepre.2015.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peterson MD, Krishnan C. Growth Charts for Muscular Strength Capacity With Quantile Regression. American journal of preventive medicine. 2015;49:935–8. doi: 10.1016/j.amepre.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kalyani RR, Metter EJ, Egan J, Golden SH, Ferrucci L. Hyperglycemia predicts persistently lower muscle strength with aging. Diabetes Care. 2015;38:82–90. doi: 10.2337/dc14-1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moore AZ, Caturegli G, Metter EJ, et al. Difference in muscle quality over the adult life span and biological correlates in the Baltimore Longitudinal Study of Aging. Journal of the American Geriatrics Society. 2014;62:230–6. doi: 10.1111/jgs.12653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen DD, Gomez-Arbelaez D, Camacho PA, et al. Low muscle strength is associated with metabolic risk factors in Colombian children: the ACFIES study. PloS one. 2014;9:e93150. doi: 10.1371/journal.pone.0093150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Artero EG, Ruiz JR, Ortega FB, et al. Muscular and cardiorespiratory fitness are independently associated with metabolic risk in adolescents: the HELENA study. Pediatr Diabetes. 2011;12:704–12. doi: 10.1111/j.1399-5448.2011.00769.x. [DOI] [PubMed] [Google Scholar]
- 16.Peterson MD, Zhang P, Saltarelli WA, Visich PS, Gordon PM. Low Muscle Strength Thresholds for the Detection of Cardiometabolic Risk in Adolescents. American journal of preventive medicine. 2015 doi: 10.1016/j.amepre.2015.09.019. [DOI] [PubMed] [Google Scholar]
- 17.Wong R, Michaels-Obregon A, Palloni A. Cohort Profile: The Mexican Health and Aging Study (MHAS). Int J Epidemiol. 2015 doi: 10.1093/ije/dyu263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wong R, Michaels-Obregon A, Palloni A, et al. Progression of aging in Mexico: the Mexican Health and Aging Study (MHAS) 2012. Salud Publica Mex. 2015;57(Suppl 1):S79–89. doi: 10.21149/spm.v57s1.7593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ogden CL, Carroll MD, Flegal KM. Prevalence of obesity in the United States. Jama. 2014;312:189–90. doi: 10.1001/jama.2014.6228. [DOI] [PubMed] [Google Scholar]
- 20.Sairenchi T, Iso H, Irie F, Fukasawa N, Ota H, Muto T. Underweight as a predictor of diabetes in older adults: a large cohort study. Diabetes care. 2008;31:583–4. doi: 10.2337/dc07-1390. [DOI] [PubMed] [Google Scholar]
- 21.International Expert C. Nathan DM, Balkau B, et al. International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes care. 2009;32:1327–34. doi: 10.2337/dc09-9033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alley DE, Shardell MD, Peters KW, et al. Grip strength cutpoints for the identification of clinically relevant weakness. J Gerontol A Biol Sci Med Sci. 2014;69:559–66. doi: 10.1093/gerona/glu011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hothorn T, Hornik K, Zeileis A. Unbiased recursive partitioning: A conditional inference framework. J Comput Graph Stat. 2006;15:651–74. [Google Scholar]
- 24.Salomon JA, Wang H, Freeman MK. Healthy life expectancy for 187 countries, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010 (vol 380, pg 2144, 2012). Lancet. 2013;381:628. doi: 10.1016/S0140-6736(12)61690-0. [DOI] [PubMed] [Google Scholar]
- 25.Aguilar-Salinas CA, Monroy OV, Gomez-Perez FJ, et al. Characteristics of patients with type 2 diabetes in Mexico - Results from a large population-based nationwide survey. Diabetes care. 2003;26:2021–6. doi: 10.2337/diacare.26.7.2021. [DOI] [PubMed] [Google Scholar]
- 26.Barquera S, Campos-Nonato I, Hernández-Barrera L, et al. Obesity and central adiposity in Mexican adults: results from the Mexican National Health and Nutrition Survey 2006. Salud publica de Mexico. 2009;51:S595–S603. doi: 10.1590/s0036-36342009001000014. [DOI] [PubMed] [Google Scholar]
- 27.Rodriguez-Saldana J, Morley JE, Reynoso MT, et al. Diabetes mellitus in a subgroup of older Mexicans: prevalence, association with cardiovascular risk factors, functional and cognitive impairment, and mortality. Journal of the American Geriatrics Society. 2002;50:111–6. doi: 10.1046/j.1532-5415.2002.50016.x. [DOI] [PubMed] [Google Scholar]
- 28.Aleman Mateo H, Lee SY, Javed F, et al. Elderly Mexicans have less muscle and greater total and truncal fat compared to African-Americans and Caucasians with the same BMI. The journal of nutrition, health & aging. 2009;13:919–23. doi: 10.1007/s12603-009-0252-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumar A, Karmarkar AM, Tan A, et al. The effect of obesity on incidence of disability and mortality in Mexicans aged 50 years and older. Salud publica de Mexico. 2015;57(Suppl 1):S31–8. doi: 10.21149/spm.v57s1.7587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anton SD, Karabetian C, Naugle K, Buford TW. Obesity and diabetes as accelerators of functional decline: can lifestyle interventions maintain functional status in high risk older adults? Experimental gerontology. 2013;48:888–97. doi: 10.1016/j.exger.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morrato EH, Hill JO, Wyatt HR, Ghushchyan V, Sullivan PW. Physical activity in U.S. adults with diabetes and at risk for developing diabetes, 2003. Diabetes care. 2007;30:203–9. doi: 10.2337/dc06-1128. [DOI] [PubMed] [Google Scholar]
- 32.Fielding RA, Vellas B, Evans WJ, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. Journal of the American Medical Directors Association. 2011;12:249–56. doi: 10.1016/j.jamda.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Villareal DT, Apovian CM, Kushner RF, Klein S, American Society for N, Naaso TOS. Obesity in older adults: technical review and position statement of the American Society for Nutrition and NAASO, The Obesity Society. Obesity research. 2005;13:1849–63. doi: 10.1038/oby.2005.228. [DOI] [PubMed] [Google Scholar]
- 34.Zamboni M, Mazzali G, Fantin F, Rossi A, Di Francesco V. Sarcopenic obesity: a new category of obesity in the elderly. Nutrition, metabolism, and cardiovascular diseases : NMCD. 2008;18:388–95. doi: 10.1016/j.numecd.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 35.Stenholm S, Harris TB, Rantanen T, Visser M, Kritchevsky SB, Ferrucci L. Sarcopenic obesity: definition, cause and consequences. Curr Opin Clin Nutr Metab Care. 2008;11:693–700. doi: 10.1097/MCO.0b013e328312c37d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Janssen HC, Samson MM, Verhaar HJ. Vitamin D deficiency, muscle function, and falls in elderly people. The American journal of clinical nutrition. 2002;75:611–5. doi: 10.1093/ajcn/75.4.611. [DOI] [PubMed] [Google Scholar]
- 37.Schaap LA, Pluijm SM, Deeg DJ, Visser M. Inflammatory markers and loss of muscle mass (sarcopenia) and strength. The American journal of medicine. 2006;119:526, e9–17. doi: 10.1016/j.amjmed.2005.10.049. [DOI] [PubMed] [Google Scholar]
- 38.Al Snih S, Markides KS, Ottenbacher KJ, Raji MA. Hand grip strength and incident ADL disability in elderly Mexican Americans over a seven-year period. Aging clinical and experimental research. 2004;16:481–6. doi: 10.1007/BF03327406. [DOI] [PubMed] [Google Scholar]
- 39.Al Snih S, Markides KS, Ray L, Ostir GV, Goodwin JS. Handgrip strength and mortality in older Mexican Americans. Journal of the American Geriatrics Society. 2002;50:1250–6. doi: 10.1046/j.1532-5415.2002.50312.x. [DOI] [PubMed] [Google Scholar]
- 40.Visser M, Kritchevsky SB, Goodpaster BH, et al. Leg muscle mass and composition in relation to lower extremity performance in men and women aged 70 to 79: the health, aging and body composition study. Journal of the American Geriatrics Society. 2002;50:897–904. doi: 10.1046/j.1532-5415.2002.50217.x. [DOI] [PubMed] [Google Scholar]