Abstract
Purpose
Over the last decade, few novel antibiotics have been approved by the Food and Drug Administration (FDA) for pediatric use. For most anti-infective agents, including antibiotics, extrapolation of efficacy from adults to children is possible if the disease and therapeutic exposures are similar between the 2 populations. This approach reduces the number of studies required in children, but relies heavily on exposure matching between children and adults. Failures in exposure matching can lead to delays in pediatric approvals of new anti-infective agents. We sought to determine the extent of exposure matching, defined by a comparison of area under the concentration-time curve, between children and adults, for anti-infective drug products submitted to the FDA for approval.
Methods
We reviewed anti-infective submissions to the FDA (2002–2014) for pediatric indication. We included drug products administered via oral, intravenous, or intramuscular administration routes, and those with AUC estimates for children in available FDA reports. Our main outcome of interest was the proportion of drugs with median (or mean) pediatric AUC within 20% of the median (or mean) reported adult value.
Findings
We identified 29 drug products that met inclusion criteria, 14 (48%) of which had mean (or median) AUCs of all submitted age groups within 20% of that in adults. Only route of administration and drug class were associated with pediatric AUC within 20% of adult AUC.
Implications
Future research is needed to define criteria for and predictors of successful exposure matching of anti-infectives between children and adults.
Keywords: anti-infectives, pediatrics, exposure matching, pharmacokinetics/pharmacodynamics
INTRODUCTION
In the United States, recent legislation has aimed to address deficiencies in pediatric drug product labeling. Through the Best Pharmaceuticals for Children Act (BPCA), sponsors are provided incentives to submit pediatric pharmacokinetic (PK) and pharmacodynamic (PD) studies of drugs in children.1 These incentives have resulted in an expansion of pediatric clinical research, submission of pediatric data to the Food and Drug Administration (FDA), and pediatric labeling changes, including 466 changes after the initial BPCA renewal in 2002.2
Despite the large number of label changes, a substantial number of pediatric clinical trials have failed to meet their proposed objectives. In one analysis of 158 studies conducted to obtain pediatric drug labels, 18% of drug products were not labeled for pediatric use, and 6% were labeled for pediatric use but not for the studied indication.3 Potential reasons for these failures include lack of appropriate dosing range, lack of efficacy, lack of pediatric formulation, trial design failure, insufficient sample size, and poor dose selection.3–5
To improve the success of pediatric submissions and to ensure appropriate allocation of limited resources, the FDA developed a pediatric study planning and extrapolation algorithm that provides guidance for industry about the necessary studies required for drug approval for a pediatric indication.6 According to this algorithm, if the disease progression and response to intervention are expected to be similar between children and adults, required clinical trials are limited to those that evaluate the PK and safety of the drugs. This is true for most anti-infective agents. Therefore, the goal of PK studies for anti-infectives is to identify doses in children that result in drug exposures shown to be efficacious in adults. Achieving similar drug exposures between children and adults (i.e., exposure matching) is paramount to extrapolation of efficacy from adult studies.
Given the need to achieve exposure matching between children and adults, one of the most important reasons for failure to obtain pediatric approval for anti-infectives is poor dose selection that occurs early in the drug development process.4 Therefore, pediatric drug development may have improved efficiency and success as well as decreased costs if doses for early phase trials are more appropriately selected.
Available methods for dose selection using adult data include population PK models, which incorporate allometric scaling, maturational and organ functions, and physiologically based PK modeling, among others. It is unclear in public reviews, which of these methods, if any, sponsors are using for pediatric drug development. Further, although several FDA guidance documents discuss approaches for matching systemic exposure between two populations,7–11 there is no well accepted approach for evaluating matching between adult and pediatric populations. We sought to describe the extent to which area under the concentration versus time curve (AUC), a well-accepted measure of average drug concentration over a time period,12 was comparable between children and adults using publicly available data.
METHODS
Data Sources
Since 2002, medical, clinical pharmacology, and statistical review documents are regularly published on the FDA website for each pediatric submission.13 The medical and clinical pharmacology review documents describe or summarize studies that were conducted to obtain pediatric approval.
We identified all anti-infective drug products submitted to the FDA for a pediatric indication and available online in the FDA websites from January 1, 2002 through August 28, 2014. We reviewed clinical pharmacology and medical review reports for each anti-infective submission. If available documentation on the FDA website was incomplete, we retrieved publications from PubMed related to the referenced studies in the FDA reports.
We extracted anti-infective characteristics including known pathways of elimination from the FDA product label or drug reference applications (i.e., Micromedex®, Lexicomp®, DailyMed [http://dailymed.nlm.nih.gov]).
Inclusion/Exclusion Criteria
The unit of observation to identify exposure matching between children and adults was the drug submitted for pediatric approval. We included an anti-infective in our analysis if it met the following criteria: 1) oral, intravenous, or intramuscular administration; and 2) inclusion of AUC estimates in available reports for pediatric age groups of interest. Submissions for a new physical design of a previously approved drug product (e.g., new scored tablet, previously unscored) were not eligible for inclusion.
The unit of observation to identify anti-infective trial characteristics associated with exposure matching was a trial submitted to support an application. We included an anti-infective trial in our analysis if the trial was the first to describe a drug’s PK/PD properties in a pediatric age group.
Definitions and Outcomes
We used area under the curve from time zero to infinity (AUC0-∞) or zero to a given time point (AUC0-t) available in drug submissions to determine exposure matching between children and adults. We preferentially extracted median values if available for both children and adults, followed by geometric mean, and then arithmetic mean.
For individual drugs, we described exposure matching between children and adults by the following criteria: 1 median (or mean) pediatric AUC within 20% of the reported adult value; 2) median (or mean) pediatric AUC within 50% of the reported adult value; or 3) ≥75% of pediatric AUC ranges within the reported adult range. Pediatric exposure was defined as “within 20% of the median (or mean) adult exposure” if the ratio of the pediatric to adult AUC was 0.8 to 1.2 for all age groups in which sponsors were seeking approval. For example, the sponsor for caspofungin sought approval for ages 3 months to 17 years. We extracted median values to calculate the AUCchild/AUCadult ratio for each of the following age groups: 3–24 months, 2–11 years, and 12–17 years. If a single value of AUCchild/AUCadult ratio in any of these age groups did not meet our predefined criteria (0.8–1.2), we classified the drug as not being within 20% of the adult median exposure. Similarly, we calculated exposure matching within 50% using an AUCchild/AUCadult ratio of 0.5 to 1.5.
Pediatric AUC ranges were 1) defined as the median and observed range of values if available for both children and adults for a given drug, or 2) calculated as the mean AUC ± 2 standard deviations for a given age group if median and range were not available. The default adult AUC was obtained from the reported value in healthy volunteers unless medical and PK reports specifically noted that exposure from patients was used as the target for exposure matching. If adult exposure was not reported in the FDA documents, pediatric exposures were compared to the most liberal (values closes to pediatric values) data in healthy adults identified in the FDA product label. We determined the percentage of the AUC range for each age group (e.g., 3–24 months, 2–11 years, and 12–17 years for caspofungin) that was within the calculated AUC range for adults. We summarized drug exposure matching according to whether ≥75% of the AUC ranges for the entire pediatric age range was within the calculated adult range. Each age group was weighted according to the number of years it contributed to the overall drug submission. For example, the caspofungin 2–11 years age group accounted for ~60% of the total age submission (10 years in this range/17 total years in the submission = 0.60). The AUC range for this age group was 0.70 of the adult range, and the weighted value was 0.42 (0.70 × 0.60). The sum of weighted values for all age groups submitted (3–24 months, 2–11 years, and 12–17 years) determined whether caspofungin was classified as having ≥75% of the pediatric AUC range within the adult range.
We also directly compared exposure matching by AUCchild/AUCadult ratio and range of AUCchild/AUCadult ratios for each anti-infective trial that determined drug exposure in the following FDA-defined pediatric age groups: neonates (<1 month), infants (1 month to <2 years), children (2 to <12 years), and adolescents (12 to 16 years). We presented the data in graphical form for each age group.
Data Collection
We collected specific application, drug product, and trial details for each anti-infective. In addition to exposure-matching data, we extracted the following details from the FDA reports: 1) sponsor requests and FDA approval of new or expanded anti-infective indications for all age groups of interest; 2) routes of administration and elimination; 3) anti-infective class (antiviral, antibacterial, antifungal); 4) year of pediatric anti-infective approval; 5) drug development phase of clinical trial in which sponsors collected qualifying PK/PD data; 6) PK data analysis methods; 7) extrapolation of efficacy from studies in adults; and 7) method the sponsors used to determine dose in the PK/PD clinical trial. For the method used to determine dose, we specifically noted whether prior to trial initiation, a sponsor explicitly mentioned or described use of the following methods: 1) scaled the adult dose to children using a body size measure (e.g., linear or allometric) or applied a maturational function; 2) developed a strategy to evaluate multiple different doses/dose combinations within one study to determine the correct pediatric dose; or 3) used data from a previous pilot study of PK/PD properties in children.
Statistical Analyses
We used standard summary statistics, including percentages, means, and ranges, to describe the study variables. Fisher’s exact tests were used to compare exposure matching by anti-infective and trial characteristics. We used linear regression and Cochran–Armitage tests for trend to identify directionality of significant results. We used STATA 13.1 (StataCorp LP, College Station, TX, USA) for all statistical analyses and considered a P-value < .05 statistically significant.
RESULTS
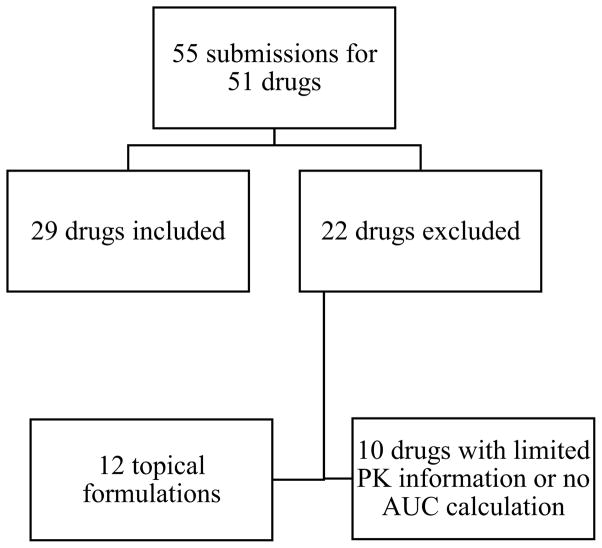
We reviewed 55 applications for 51 anti-infectives submitted to the FDA for approval (Figure 1). For 4 of the submitted anti-infectives (8%), we retrieved publications from PubMed regarding trials conducted and submitted to the FDA. Of the 51 evaluated anti-infectives, 12 (24%) were topical formulations and 10 had limited AUC information specifically available for the population of interest. Of the 10 drugs excluded for limited AUC information, 5 (50%) were antivirals. The remaining 29 anti-infectives (54%) met our criteria for analysis. Sponsors submitted applications to solely obtain a new pediatric indication for 20/29 anti-infectives and to solely expand the pediatric indication for 6/29 anti-infectives. Darunavir and tenofovir each had one submission for a new pediatric indication and a second to expand the pediatric indication, while atazanavir had 2 submissions, each to expand the pediatric indication. The majority of anti-infectives were antivirals (21/29, 72%), indicated for HIV (14/29, 48%), and administered orally (23/29, 79%) (Table I).
Figure 1.
Flow chart of drug inclusion in the final analysis.
Table I.
Included Drugs and Drug/Study Characteristics
| Drug | Drug Class | Route of Administration | Predominant Route of Elimination | Pediatric Trial Phase | Ages Studied (years) | Ages Approved (years) |
|---|---|---|---|---|---|---|
| Adefovir | Antiviral | Oral | Renal unchangeda | 1/2 | 12–17 | 12–17 |
| Amoxicillin Clavulanate XR | Antibacterial | Oral | Renal unchanged | 1 | 7–15 | 7–15 |
| Atazanavirb,c | Antiviral | Oral | Hepatic then fecalg | 1/2; 3b | 6–17; 0.25–5 | 6–17; 0.25–5 |
| Azithromycin | Antibacterial | Oral | Other | 1 | 0.5–16 | 0.5–16 |
| Caspofungind | Antifungal | Intravenous | Hepatic then renal | 1 | 0.25–11 | 0.25–17 |
| Darunavire | Antiviral | Oral | Hepatic then fecalg | 1,2 | 3–17 | 3–17 |
| Dolutegravir | Antiviral | Oral | Feces unchanged | 1/2 | 12–17 | 12–17 |
| Efavirenzf | Antiviral | Oral | Hepatic then fecalg | 1/2 | 0.25–2 | 0.25–2 |
| Emtricitabinef | Antiviral | Oral | Renal unchangeda | 1 | 0–0.25 | 0–0.25 |
| Entecavir | Antiviral | Oral | Renal unchanged | 2 | 2–15 | 2–15 |
| Ertapenem | Antibacterial | Intravenous | Hepatic then renal | 1 | 0.25–17 | 0.25–17 |
| Etravirine | Antiviral | Oral | Hepatic then fecalg | 1 | 6–17 | 6–17 |
| Famciclovir | Antiviral | Oral | Hepatic then renal | 1/2 | 0.083–12 | None |
| Fosamprenavirf | Antiviral | Oral | Hepatic then fecalg | 2 | 0.08–5 | 0.08–5 |
| Levofloxacin | Antibacterial | Oral/Intravenous | Renal unchanged | 1 | 0.5–17 | 0.5–17 |
| Linezolid | Antibacterial | Intravenous | Hepatic then renal | 1 | 0–17 | 0–17 |
| Lopinavir/Ritonavirf | Antiviral | Oral | Hepatic then fecalg | 1/2 | 0.04–0.5; 13–17 | 0.04–0.5; 13–17 |
| Micafungin | Antifungal | Intravenous | Hepatic then renal | 1 | 0.33–16 | 0.33–16 |
| Nelfinavird,f | Antiviral | Oral | Hepatic then fecalg | 1 | 0–1 | None |
| Oseltamivir | Antiviral | Oral | Hepatic then renal | 1 | 1–17 | 1–17 |
| PEG-Interferon alfa-2b | Antiviral | Subcutaneous | Hepatic then renal | 1/3 | 3–17 | 3–17 |
| Raltegravir | Antiviral | Oral | Hepatic then fecalg | 1/2 | 2–17 | 2–17 |
| Ritonavir | Antiviral | Oral | Hepatic then fecalg | 1/2 | 0.08–1 | 0.08–1 |
| Tenofovire | Antiviral | Oral | Renal unchangeda | 3 | 2–17 | 2–17 |
| Terbinafine | Antifungal | Oral | Hepatic then renal | 1/2 | 4–17 | 4–17 |
| Tipranavir | Antiviral | Oral | Hepatic then fecalg,h | 1/2 | 2–17 | 2–17 |
| Valacyclovir | Antiviral | Oral | Renal unchangeda | 1 | 1–17 | 1–17 |
| Valgancyclovir | Antiviral | Oral | Hepatic then renal | 1 | 0.33–16 | 0.33–16 |
Administered as prodrug and active metabolite eliminated in the urine, largely unchanged
Two applications submitted to expand the pediatric indication.
Atazanavir without ritonavir evaluated separately from atazanavir with ritonavir.
Ages studied and ages approved do not match.
One new application submitted; a second application to expand the pediatric indication.
Purpose of the application was to expand the pediatric indication.
Some hepatic metabolism with primary excretion into feces; also unchanged drug eliminated in feces.
When administered with ritonavir, there is minimal metabolism.
Among the 29 anti-infectives, exposure matching by median (or mean) AUC was variable. Considering initial PK trials for anti-infectives included in this study, 14 anti-infectives (48%) had a median (or mean) AUC of all submitted age groups within 20% of that in adults, while 22 (76%) had a median (or mean) AUC within 50% of that in adults (Table II). Route of administration and anti-infective class were the only statistically significant differences between matched and unmatched drugs within 20% (Table III). All anti-infectives that had a median (or mean) AUC within 20% were orally administered, and 13/14 (93%) were antivirals. All anti-infectives that sought approval for only adolescents (4 drugs) matched adult and pediatric median (or mean) exposures within 20%.
Table II.
Comparison of Median (or Mean) AUC and AUC Ranges for Anti-infectives in Children and Adults
| Drug | Within 20% Adult Mediana | Within 50% Adult Mediana | % Pediatric Range Within Adult Rangeb |
|---|---|---|---|
| Adefovir | Yes | Yes | 78–100 |
| Amoxicillin Clavulanate XR | No | Yes | 40–81 |
| Atazanavir w/o ritonavir | No | No | 71–100 |
| Atazanavir w/ ritonavir | Yes | Yes | 100 |
| Azithromycin | No | No | 42–100 |
| Caspofungin | No | Yes | 60–73 |
| Darunavir | Yes | Yes | n/a |
| Dolutegravir | Yes | Yes | 93 |
| Efavirenz | Yes | Yes | 94 |
| Emtricitabine | Yes | Yes | 43–79 |
| Entecavir | Yes | Yes | 81–100 |
| Ertapenem | No | Yes | 3–8 |
| Etravirine | No | Yes | 97–100 |
| Famciclovir | No | No | 0–100 |
| Fosamprenavir | No | Yes | 31–45 |
| Levofloxacin | No | No | 0–100 |
| Linezolid | No | No | 0–44 |
| Lopinavir/ritonavir | No | No | 53–100 |
| Micafungin | No | No | 28–49 |
| Nelfinavir | Yes | Yes | 66–90 |
| Oseltamivir | Yes | Yes | 85–100 |
| PEG-Interferon alfa-2b + ribavirin | No | Yes | 52–56 |
| Raltegravir | Yes | Yes | 23–61 |
| Ritonavir | Yes | Yes | 44–67 |
| Tenofovir | Yes | Yes | 73–100 |
| Terbinafine | Yes | Yes | 88–100 |
| Tipranavir | No | Yes | 59–93 |
| Valacyclovir | Yes | Yes | 71–100 |
| Valgancyclovir | No | Yes | 55–100 |
Abbreviation: AUC, area under the concentration-time curve.
Mean values used if medians were unavailable.
Presented as a range of percentages (e.g., 78–100) to account for variability in each age groups of interest for a specific drug. For example, 0 to 4 year olds for drug A may have a pediatric range entirely within the adult range (i.e., 100%), but only 78% of the range for 5 to 8 year olds is within the adult range. This is listed as 78–100.
Table III.
Drug Characteristics Associated With Median Pediatric AUC Within 20% of Median Adult AUCa
| AUCchild/AUCadult ratio 0.8–1.2 N = 14 |
AUCchild/AUCadult ratio < 0.8 or > 1.2 N = 15 |
|
|---|---|---|
| Route of administration, No. (%) | ||
| Oral | 14 (100) | 9 (60) |
| Intravenous | 0 | 3 (20) |
| Both | 0 | 2 (13) |
| Other | 0 | 1 (7) |
| Route of elimination, No. (%) | ||
| Renal unchanged | 5 (36) | 2 (13) |
| Hepatic then renal | 2 (14) | 7 (47) |
| Hepatic then fecal | 7 (50) | 5 (33) |
| Other | 0 | 1 (7) |
| Indication, No. (%) | ||
| HIV | 9 (64) | 5 (33) |
| Intra-abdominal | 1 (7) | 1 (7) |
| Systemic | 3(21) | 5 (33) |
| Candidiasis | 0 | 2 (13) |
| Other | 1 (7) | 2 (13) |
| Drug class, No. (%) | ||
| Antiviral | 13 (93) | 8 (53) |
| Antibacterial | 0 | 5 (33) |
| Antifungal | 1 (7) | 2 (13) |
| Age range, No. (%) | ||
| Only 12–17 years | 4 (29) | 0 |
| > 2 years | 5 (36) | 5 (33) |
| > 28 days | 3 (21) | 8 (53) |
| > birth | 2 (14) | 2 (13) |
| Year approved, No. (%) | ||
| > 2008 | 8 (57) | 13 (87) |
| < 2008 | 6 (43) | 2 (13) |
| Approved indication, No. (%) | 13 (93) | 13 (87) |
Abbreviation: AUC, area under the concentration-time curve.
Mean values used if medians were unavailable
Exposure matching by AUC range criteria was also variable. Range data were available or able to be calculated for all anti-infectives except darunavir. Fourteen (48%) drugs had ≥1 submitted age group in which the pediatric AUC range was completely within the published adult range. Fifteen anti-infectives (52%) had ≥75% of the pediatric AUC ranges within the adult range. Compared to antibacterial drug class and intravenous route of administration, antiviral drug class and oral route of administration were indicative of exposure matching by the 75% range criteria. Eleven anti-infectives (44%) were within 20% of the adult mean (or median) and met the 75% range criteria.
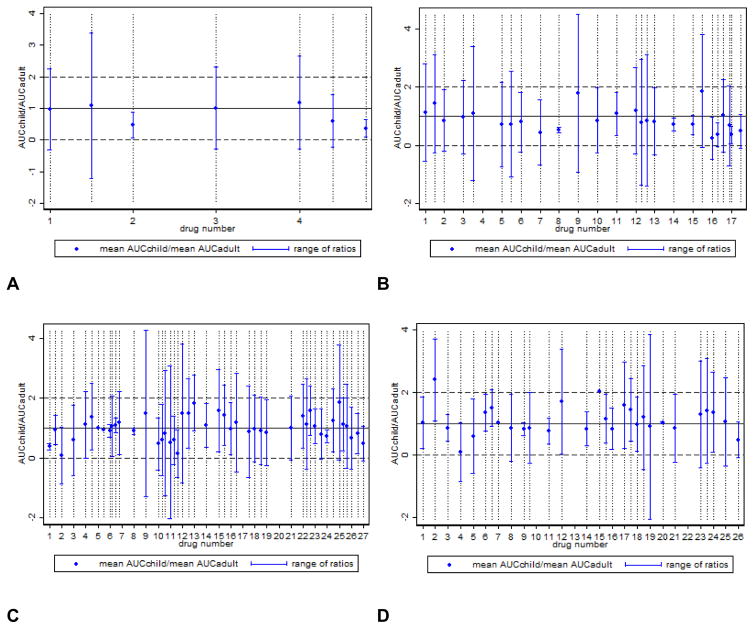
The variability in exposure matching by our criteria is magnified when the drugs are classified by age, according to the following groups: neonates (< 30 days), infants (1 month to <2 years), children (2 to 11 years), and adolescents (12 to 16 years) (Figure 2).
Figure 2.
Comparison of pediatric to adult area under the concentration-time curve (AUC) without outliers: age (A) <30 days, (B) 1 month–<2 years, (C) 2–11 years, and (D) 12–16 years. Drug products were considered outliers and excluded from the figure if the range of ratios for the drug product was > 2.5 SD of the range of ratios for the age group of interest. Multiple studies for a single drug will have the same identifying number (e.g., 3 studies supporting the 2–11 age group for a drug will be located on the x-axis at 7.0, 7.4, 7.8). Blue dots represent the AUCchild/AUCadult median (or mean) ratio. The solid black line represents the equivalence median (or mean) AUC between children and adults (ratio=1). Horizontal dashed lines represent the upper and lower bounds of a normalized adult AUC range. Ratios and ranges that fall within the dashed lines indicate pediatric AUC values within the adult range.
Of the 29 anti-infectives included in our analysis, all but 3 (atazanavir without ritonavir from 2007 submission, famciclovir, and nelfinavir) received FDA approval for the entire age range for which sponsors were seeking approval. Among the anti-infectives that were approved, mean (or median) AUCs for children were within 20% of adult AUCs for 46% of anti-infectives. Similarly, ≥75% of the child AUC range was within the adult range for 54% of approved anti-infectives. Of the anti-infectives that were approved, 12/26 (46%) relied on some extrapolation of efficacy from adult studies. Of these, 5 (42%) anti-infectives matched exposure by mean AUC 20% or range criteria.
Finally, we examined trial characteristics that might influence the similarity between pediatric and adult exposures. In total, we analyzed 39 trials. Non-compartmental analysis was the predominant method of PK analysis (23/39 trials, 59%). To determine the dose studied in the pediatric trial, sponsors commonly scaled by body size or applied an age-dependent organ function (e.g., creatinine clearance) to the adult dose (20/39, 51%). Of these 20 studies, 6 studies (30%) scaled the adult dose using body weight, 9 (45%) used body surface area, 4 (25%) used the equivalent adult dose in adolescent populations, and 1 (5%) used an age-dependent maturational factor to determine the dose. Phase of trial, method of PK analysis, and overall method of initial dose determination (e.g., application of a body size measure or applied maturational function, strategy to evaluate doses within one study, or use of pilot data) were not associated with exposure matching. However, all trials that used body weight-based dosing met mean AUC 20% criteria, and 78% of trials that used body surface area did not meet this criteria (P = .007).
DISCUSSION
According to our results, differences in exposure matching by AUC occurred in the initial pediatric PK studies of ≥50% of the anti-infectives evaluated in this study. Because this study focuses on the early stages of pediatric drug submission, we cannot comment on exposure matching for the FDA-approved dose. However, FDA approval occurred for most drugs in our study. The observed discrepancy between exposure matching in initial PK studies and drug approval could be because 1) anti-infective approval does not rely on information obtained from exposure matching in early PK studies alone or on information included in publicly available reports; 2) anti-infective approval does not rely on our definition of exposure matching; 3) exposure measures other than AUC were used for exposure matching; 4) anti-infective approval relies on exposure matching information in some pediatric sub-populations to grant approval for the whole pediatric population; or 5) pediatric exposures should be different from those in adults given discrepant therapeutic indices or possibilities for drug resistance, between the two populations.3,5–11,14,15
It is likely that exposure matching does not rely on our definition; to date, criteria for exposure matching between children and adults for pediatric drug approvals have not been defined in the United States.7–11 Instead, sponsors define their own targets for exposure matching for each drug product. The result of this method is variability in the exposure measure of interest and variability in acceptable parameters for exposure matching. For example, this variability is evident in the submissions for adefovir and fosempranavir. For adefovir exposure in children, sponsors targeted the adult maximum concentration (Cmax, 18.4 ng/mL) and AUC0-∞ (230.3 ng*hr/mL); however, sponsors did not explicitly define the minimum and maximum acceptable values of Cmax or AUC0-∞. In contrast, sponsors more clearly defined the targets for fosemprenavir, ranging from the 25th percentile for concentration at a defined time-point (Ctau) to the 95th percentile for AUC observed in healthy adults.
In contrast to the variable definitions for exposure matching observed in submissions to the FDA, we defined specific criteria that we applied to all anti-infective submissions. In defining these criteria, we considered PK and drug exposure variability. Exposure matching using summary data alone (e.g., median or mean AUC for a given population) is risky in children because it oversimplifies the population under study. Most pediatric early phase studies are small, are conducted in patients with the disease, and may include a relatively wide age range. These factors all increase the variability in observed exposures. Therefore, it is possible for a drug in children to have, on average, very similar exposures to adults, but in reality have only toxic or sub-therapeutic exposure ranges. This discrepancy between average exposures and ranges of exposures may be even more pronounced if you examine exposure matching by age, as depicted in Figure 2.
Possible discrepancy between average values and ranges as well as variability in the extent of exposure matching by our criteria suggest the need to consider several key factors in the development of formal exposure-matching criteria for children and adults. These factors include 1) the ideal primary exposure measure for exposure-matching analysis (this will likely vary by drug), 2) use of individual compared to summary data to calculate exposure matching, 3) the need for varying criteria by pediatric age group based on underlying variability in drug disposition, and 4) consideration of a drug’s therapeutic index.
Although it is clear that the development of defined criteria for exposure matching between children and adults is necessary, it is less clear what drug or trial characteristics influence exposure matching according to the defined criteria. We anticipated that age of the population of interest (e.g., only adolescents or only children and adolescents compared to neonates) and method of dose determination would greatly influence the extent of exposure matching. In a recent analysis, 87/92 (95%) of drug products submitted to the FDA had recommendations for equivalent dosing of adolescent and adult patients.16 However, age was not a statistically significant determinant of exposure matching by our criteria.
Method of dose determination was not associated with exposure matching by our criteria despite known developmental changes in body composition and maturation in organ function that occur with age and should influence method of dose determination.17,18 For example, scaling of adult dosing to pediatric patients using allometric principles can account for differences in developmental PK for children >2 years of age; however, empirical models using maturational functions or physiological models based on in vitro data are typically needed to describe drug disposition in younger children.19,20 Reasons for lack of association between age or method of dose determination and exposure matching may include: 1) limited number of anti-infectives included in our analysis (29/51 met inclusion and exclusion criteria); 2) skewed distribution of age groups of interest among anti-infectives (e.g., 4 studies including neonates, and 4 studies that included only adolescents); and 3) sponsors’ use of maturational factors or physiological principles in appropriate populations.
The limitations of our study include 1) a limited number of anti-infectives that met inclusion and exclusion criteria, 2) availability of predominantly HIV drugs for analysis, where concerns about drug toxicity may disproportionately influence dose selection in early phase trials, 3) limited ability to capture additional factors (e.g., study sample size and variation in ages) that potentially influence successful exposure matching, 4) ability to extract only information that was publicly available, which may have varied by drug, 5) lack of consistency in the content and structure of documents submitted to the FDA, 6) access to summary-level data and not patient-level data, 7) inclusion of AUC as the only measure of successful exposure matching, and 8) inability to determine clinical relevance of our defined criteria for exposure matching.
Access to only summary-level data and inclusion of only AUC data are likely the most important of these limitations. Use of summary data mandated calculations to obtain AUC ranges for children and adults; however, this method may not have reflected actual observed minimum and maximum AUC values. Further, although AUC is one of the most widely available PK parameters for anti-infectives and other drug classes, it is unknown whether AUC is the best PK parameter to determine exposure matching.14 For many anti-infectives, PK/PD indices that have been predictive of antimicrobial efficacy include Cmax/minimum inhibitory concentration (MIC), AUC/MIC, time above MIC, or trough concentration/half maximal inhibitory concentration.15 Given the specific interest in extrapolation of efficacy from adult to pediatric populations, such parameters with proven association to efficacy may be more relevant than AUC alone.
CONCLUSIONS
Despite certain limitations, our study highlights several areas for improvement in exposure matching of adult to pediatric anti-infectives. These areas for improvement include the need to 1) define criteria for successful exposure matching that accounts for PK variability in pediatric populations; 2) design trials, accounting for developmental changes that affect drug disposition; 3) improve granularity and standardization of sponsor submissions to the FDA; and 4) concentrate resources on exposure matching and reduce studies of pediatric efficacy for drugs that may extrapolate efficacy from adult data. The ability to address these areas in future studies may increase the likelihood of success in obtaining a pediatric indication, improve the breadth of pediatric labeling, and help to conserve limited financial resources that are currently available for research in pediatric therapeutics.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Field MJ, Boat TF, editors. Committee on Pediatric Studies Conducted Under the Best Pharmaceuticals for Children Act (BPCA) and the Pediatric Research Equity Act (PREA), Board on Health Sciences Policy, Institute of Medicine, Policy Framework for BPCA and PREA. Safe and effective medicines for children: pediatric studies conducted under the Best Pharmaceuticals for Children Act and the Pediatric Research Equity Act. Washington, DC: National Academies Press; 2012. pp. 63–88. Available from: http://www.ncbi.nlm.nih.gov/books/NBK202032/ [PubMed] [Google Scholar]
- 2.Food and Drug Administration. New pediatric labeling information database. [Accessed November 2014];FDA website. www.accessdata.fda.gov/scripts/sda/sdNavigation.cfm?sd=labelingdatabase.
- 3.Burckart GJ, Momper JD. Pediatric clinical pharmacology in regulatory and drug development sciences: lessons learned and the path forward. In: Mulberg AE, Murphy D, Dunne J, Mathis LL, editors. Pediatric drug development: concepts and applications. 2. chap 23 Hoboken, NJ: John Wiley & Sons; 2013. [Google Scholar]
- 4.Benjamin DK, Jr, Smith PB, Jadhav P, Gobburu JV, Murphy MD, Hasselblad V, et al. Pediatric antihypertensive trial failures: analysis of end points and dose range. Hypertension. 2008;51(4):834–40. doi: 10.1161/HYPERTENSIONAHA.107.108886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Momper JD, Mulugeta Y, Burckart GJ. Failed pediatric drug development trials. Clin Pharmacol Ther. 2015;98(3):245–51. doi: 10.1002/cpt.142. [DOI] [PubMed] [Google Scholar]
- 6.Food and Drug Administration. General clinical pharmacology considerations for pediatric studies for drugs and biological products: guidance for industry. [Accessed 26 December 2014];FDA website. www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm425885.pdf.
- 7.Food and Drug Administration. Draft guidance for industry. Bioavailability and bioequivalence studies submitted in NDAs or INDs--general consideration 2003. [Accessed 16 March 2015];FDA website. www.fda.gov/downloads/Drugs/.../Guidances/ucm070124.pdf.
- 8.Food and Drug Administration. Draft guidance for industry. Drug interaction studies: study design, data analysis, implications for dosing, and labeling recommendations 2012. [Accessed 16 March 2015];FDA website. www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm292362.pdf.
- 9.Food and Drug Administration. Draft guidance for industry. Pharmacokinetics in patients with impaired renal function—study design, data analysis, and impact on dosing and labeling 2010. [Accessed 16 March 2015];FDA website. www.fda.gov/downloads/Drugs/Guidances/UCM204959.pdf.
- 10.Food and Drug Administration. Draft guidance for industry. Pharmacokinetics in patients with impaired hepatic function: study design, data analysis, and impact on dosing and labeling, 2003. [Accessed 16 March 2015];FDA website. www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm072123.pdf.
- 11.Food and Drug Administration. Draft guidance for industry. Clinical pharmacology data to support a demonstration of biosimilarity to a reference product. [Accessed 16 March 2015];FDA website. www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm397017.pdf.
- 12.Food and Drug Administration. Guidance for industry. Exposure-response relationships—study design, data analysis, and regulatory applications. [Accessed 16 March 2015];FDA website. www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm072109.pdf.
- 13.Food and Drug Administration. Medical, statistical, and clinical pharmacology reviews of pediatric studies conducted under Section 505A and 505B of the Federal Food, Drug, and Cosmetic Act (the Act), as amended by the FDA Amendments Act of 2007 (FDAAA) [Accessed 15 November 2014];FDA website. www.fda.gov/Drugs/DevelopmentApprovalProcess/DevelopmentResources/ucm049872.htm.
- 14.Panel on Antiretroviral Guidelines for Adults and Adolescents. [Accessed 26 December 2014];Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. http://aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf.
- 15.Mueller M, de la Pena A, Derendorf H. Issues in pharmacokinetics and pharmacodynamics of anti-infective agents: kill curves versus MIC. Antimicrob Agents Chemother. 2004;48(2):369–77. doi: 10.1128/AAC.48.2.369-377.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Momper JD, Mulugeta Y, Green DJ, Karesh A, Krudys KM, Sachs HC, et al. Adolescent dosing and labeling since the Food and Drug Administration Amendments Act of 2007. JAMA Pediatr. 2013;167(10):926–32. doi: 10.1001/jamapediatrics.2013.465. [DOI] [PubMed] [Google Scholar]
- 17.Kearns GL, Abdel-Rahman SM, Alander SW, Blowey DL, Leeder JS, Kauffman RE. Developmental pharmacology—drug disposition, action, and therapy in infants and children. N Engl J Med. 2003;349(12):1157–67. doi: 10.1056/NEJMra035092. [DOI] [PubMed] [Google Scholar]
- 18.van den Anker JN, Schwab M, Kearns GL. Developmental pharmacokinetics. Handb Exp Pharmacol. 2011;205:51–75. doi: 10.1007/978-3-642-20195-0_2. [DOI] [PubMed] [Google Scholar]
- 19.Holford N, Heo YA, Anderson B. A pharmacokinetic standard for babies and adults. J Pharm Sci. 2013;102(9):2941–52. doi: 10.1002/jps.23574. [DOI] [PubMed] [Google Scholar]
- 20.Barrett JS, Della Casa Alberighi O, Laer S, Meibohm B. Physiologically based pharmacokinetic (PBPK) modeling in children. Clin Pharmacol Ther. 2012;92(1):40–9. doi: 10.1038/clpt.2012.64. [DOI] [PubMed] [Google Scholar]