Abstract
Graphene based nanomaterials possess remarkable physiochemical properties suitable for diverse applications in electronics, telecommunications, energy and healthcare. The human and environmental exposure to graphene-based nanomaterials is increasing due to advancements in the synthesis, characterization and large-scale production of graphene and the subsequent development of graphene based biomedical and consumer products. A large number of in vitro and in vivo toxicological studies have evaluated the interactions of graphene-based nanomaterials with various living systems such as microbes, mammalian cells, and animal models. A significant number of studies have examined the short- and long-term in vivo toxicity and biodistribution of graphene synthesized by variety of methods and starting materials. A key focus of these examinations is to properly associate the biological responses with chemical and morphological properties of graphene. Several studies also report the environmental and genotoxicity response of pristine and functionalized graphene. This review summarizes these in vitro and in vivo studies and critically examines the methodologies used to perform these evaluations. Our overarching goal is to provide a comprehensive overview of the complex interplay of biological responses of graphene as a function of their physio-chemical properties.
Keywords: Graphene, toxicity, in vitro, in vivo, antimicrobial, environmental, biodistribution
Graphical abstract
2. Introduction
Carbon nanomaterials such as fullerenes, carbon nanotubes and graphene are the most widely researched class of materials and hold immense potential to impact several scientific disciplines [1–3]. Their transformative potential has been recognized with multiple honors including the Kavli and Nobel Prize [4, 5]. Owing to the distinct arrangement of sp2 bonded carbon atoms, each carbon nanomaterial can exhibit significantly different physical, morphological and chemical properties.
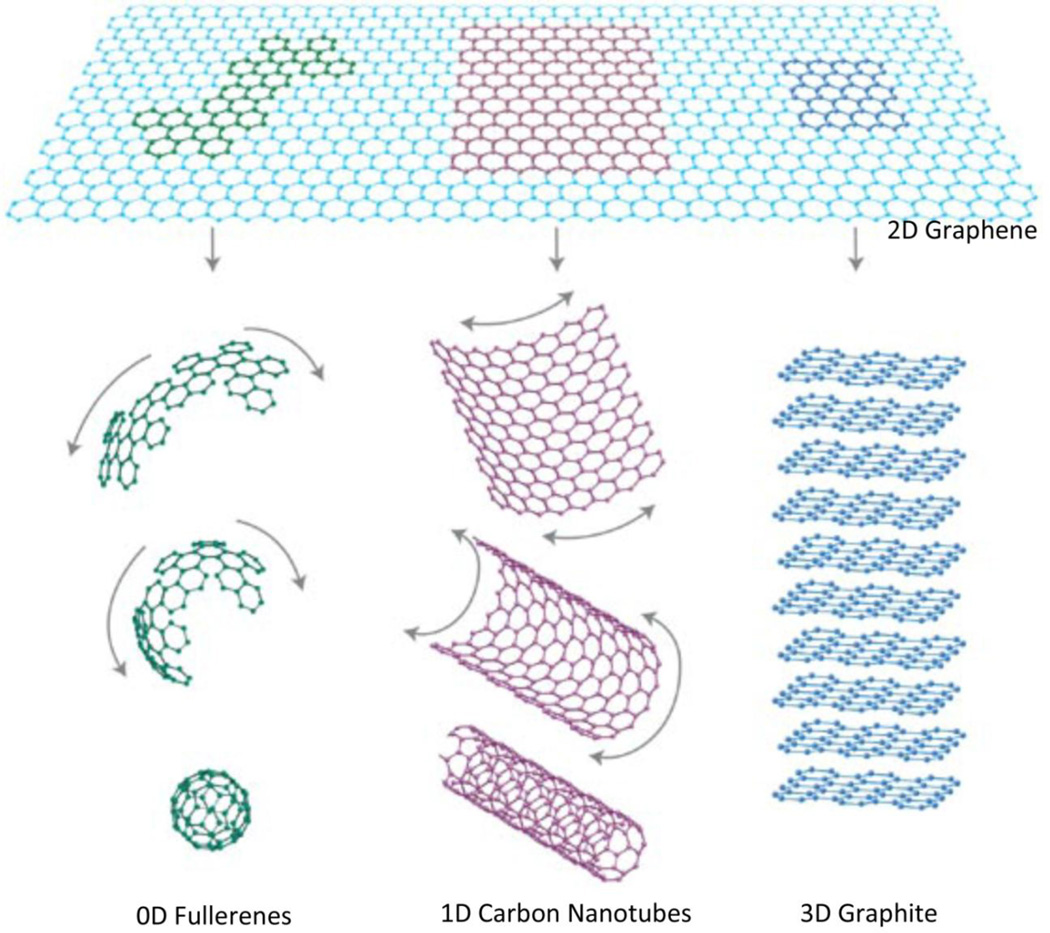
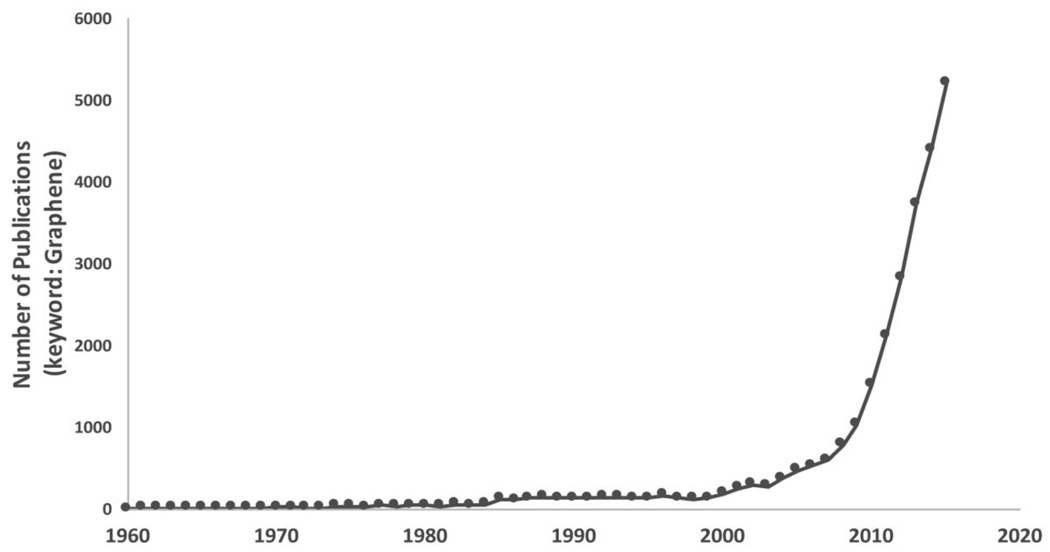
Graphene, a two-dimensional (2D) sheet of carbon atoms packed in a honeycomb lattice is widely regarded as a basic building block of graphitic allotropes (Figure 1)[6]. The theoretical existence of graphene was discussed over 55 years ago by Slonczewski and Weiss [7]. Landau, Peierls and Mermin reported that existence of atomically thin 2D crystals (such as graphene) was practically impossible due to thermodynamic instabilities, a theory that was supported by several independent experimental observations [8–11]. However, in 2004, Novoselov and Geim isolated single sheets of graphene by micromechanical cleavage of graphite or the “scotch-tape method” [12] and characterized their quantum electrodynamics [13, 14]. Since then research on graphene has exploded. The number of research papers published on graphene has been increasing exponentially (Figure 2) attracting scientists from all areas of science and technology towards the graphene “gold-rush”. In 2013, the European Union announced the graphene flagship project – a $1.3 billion 10 year investment in graphene research and development to translate graphene-based technologies from academic labs to the marketplace [15]. The Korean Graphene Project, also announced in 2013, is a $44 million 5 year investment for graphene research [16]. In 2011, United Kingdom committed £50 million investment for graphene research [17]. Recently, in October 2015, Chinese company Huawei Technologies has announced a $1 billion 5 year investment towards the development of information and communications technologies focused on graphene [18].
Figure 1.
Graphene is the building material for 0D fullerenes, 1D carbon nanotubes and 3D graphite. Schematic adapted from Reference [6] with permission, copyright © Macmillan Publishers Limited, 2007.
Figure 2.
Number of publications with the keyword ‘graphene’ from 1960–2015. Data retrieved from PubMed (www.ncbi.nlm.nih.gov).
Graphene has interesting optical, thermal, mechanical and electrical properties. The sp2 hybridization of 2D graphene plane results in delocalized out of plane π bonds that provide an exceptionally high carrier mobility (~ 200,000 cm2 V−1 s−1 for suspended graphene [19, 20] and ~500,000 cm2 V−1 s−1 for graphene-based field effect transistors) [21, 22]. Graphene exhibits room temperature quantum hall effect for electrons and holes [13, 23]. Graphene sheets also exhibit high surface area (2630 m2 g−1) [21], thermal conductivity (~5000 Wm K−1) [24], mechanical property (Young’s modulus of ~ 1 TPa) [25] and optical transparency (single layer graphene absorbs ~2.3% of visible light) [26].
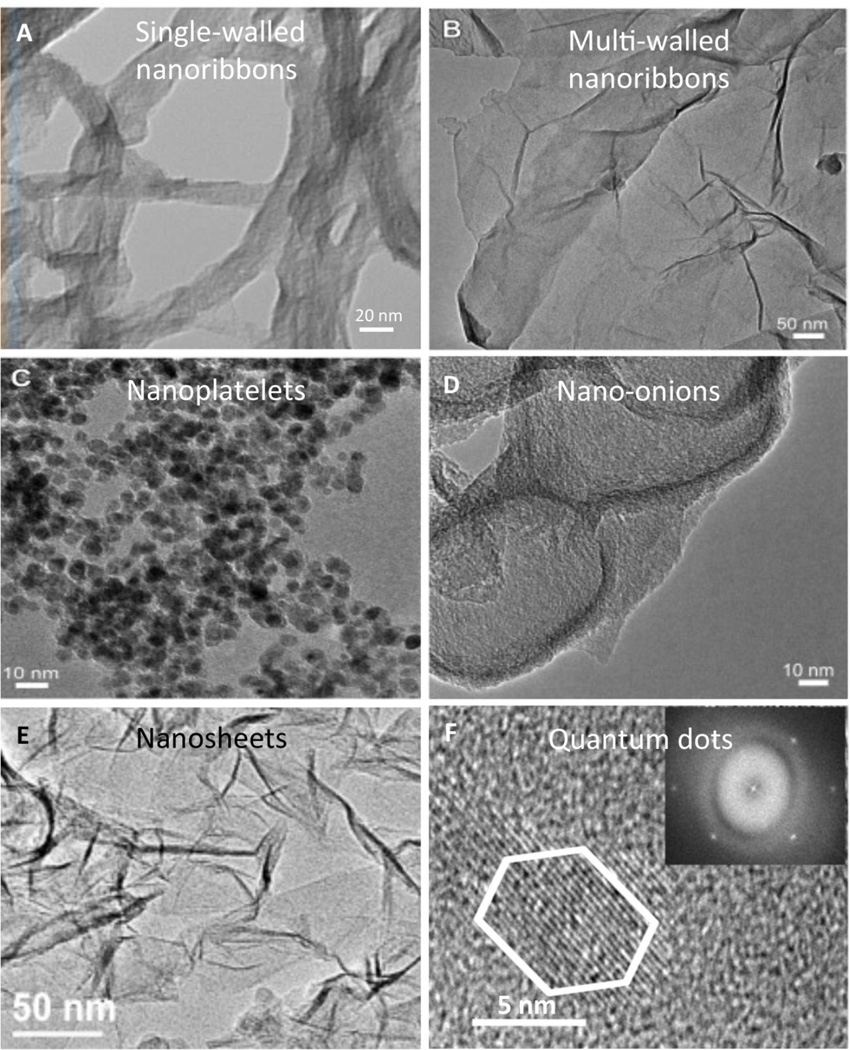
Graphene can be synthesized using various physical (such as mechanical cleavage (“scotch tape method”) [27] or arc discharge [28]) and chemical methods (chemical vapor deposition [29], Hummer’s method (chemical oxidation of graphite followed by mechanical exfoliation) [30] or longitudinal unzipping of carbon nanotubes [31]). Depending on the method of synthesis, graphene can exist in various morphologies such as sheets, platelets, ribbons, onions and quantum dots (Figure 3). Pristine graphene is apolar and very hydrophobic. It needs to be oxidized to improve its dispersibility in aqueous media.
Figure 3.
Representative transmission electron microscopy images of (A and B) graphene nanoribbons, (C) graphene nanoplatelets, (D) graphene nanoonions, (E) graphene nanosheets and (F) graphene quantum dots. Image (A) adapted from Reference [41], (B–D) adapted from Reference [44], (E) adapted from Reference [96] and (F) adapted from Reference [146], with permissions. (A) copyright © American Chemical Society 2013, (B–D) copyright © Elsevier 2014, (E) copyright © Elsevier 2015, and (F) copyright © American Chemical Society, 2013.
Oxidized graphene is typically synthesized via chemical oxidation. Depending on the synthesis or morphology of the graphene, oxidized graphene are referred by various terminologies. For example, oxidized graphene prepared by Hummer’s method is typically referred as graphene oxide (GO) or graphene nanoplatelet. Oxidized graphene prepared by longitudinal unzipping are referred as graphene oxide nanoribbons. The pristine sp2 characteristic of graphene can to large extend (but not completely) be restored by treating oxidized graphene nanoparticles with reducing agents such as hydrazine, hydrogen iodide, etc. [32]. Although the presence of hydrogen bonds between the polar oxidative functional groups (such as oxide, acid, alcohol, epoxide etc.) of oxidized graphene imparts colloidal stability, the dispersibility of oxidized graphene in aqueous and biological media is inadequate for several biomedical applications. Functionalization strategies have been employed to further improve graphene’s aqueous dispersibility. Graphene can be covalently or non-covalently functionalized with several chemical moieties (for instance amine) or biological molecules (such as nucleic acids and proteins). Oxidized graphene nanoparticle-based formulations has been extensively explored for several biomedical applications such as bioimaging [33–35], drug and gene delivery [36–38], photothermal therapy [39, 40], tissue engineering [41–43], and stem cell technology [44, 45]. Pristine or nearly pristine (oxidized graphene treated with reducing agents) graphene have also been investigated for several biomedical applications [27, 35, 46, 47].
The evaluation of in vitro cytotoxicity and in vivo biocompatibility is critical to develop nanoparticle-based formulations for biomedical applications. The potential widespread use of graphene-based nanomaterials for commercial materials science applications will increase their interactions with biological and environmental constituents. Furthermore, a thorough analysis of the biocompatibility of graphene is an essential prerequisite before their use for in-vivo biomedical applications. Consequently, several studies have been performed to assess the in vitro and in vivo cyto- and bio- compatibility of graphene-based nanomaterials [48–56]. These studies indicate that the toxicity of graphene is dependent on the complex interplay of several physiochemical properties such as shape, size, oxidative state, functional groups, dispersion state, synthesis methods, route and dose of administration, and exposure times [48–57]. Post synthesis processing steps could lead to disruption of graphene structure and production of smaller carbonaceous debris or methods to synthesize graphene could lead to the incorporation of several metallic impurities in the final product. These confounding factors may elicit variable toxicity responses [58–60].
In this article, we provide a comprehensive review of recent in vitro and in vivo toxicity studies using graphene-based nanomaterials and examine the methodologies used to perform these evaluations. We also review studies investigating the effects of graphene on antimicrobial biota (eg. bacteria and fungi) and environmental constituents (e.g. crops, waste water, etc.). Finally we summarize the current understanding of the toxicity mechanisms of graphene-based nanomaterials. The goal of this article is to provide the readers with an overview on graphene toxicity and its dependence on the various physiochemical properties of graphene. Such an understanding could lead to development of strategies to mitigate potential adverse effects for successful development of graphene–based consumer and healthcare products.
3. In vitro toxicity
The assessment of in vitro cytotoxicity is the initial first step towards significantly expensive and elaborate in vivo studies. Table 1 summarizes the in vitro cytotoxicity of graphene and graphene oxide (GO) assessed using several representative cell lines at various treatment concentrations.
Table 1.
In vitro cytotoxicity of graphene
| Material | Characte rization |
Properties | Treatment Concentrati on |
Cell line |
Assays | Conclusions | Referenc es |
|---|---|---|---|---|---|---|---|
| Graphene, SWCNT |
TEM, SEM, AFM, microscop y, spectrosco py, X-ray diffraction |
G: Thickness = 3– 5nm |
0.01–100 µg/ml |
PC12 cells |
MTT, LDH, ROS, Caspase 3/7 |
Dose and shape dependent cytotoxicity was observed for graphene and SWCNT. |
Zhang et al. [61] |
| GO, ox- SWCNT |
AFM, FTIR, EDS |
GO: Length = 100nm; Height = 1nm |
1 µg/ml | HepG 2 cells |
MTT, ROS, FITC, DFDA Fluorescence analysis, 2D LC-MS Proteome Analysis |
GO shows less mitochondrial damage, ROS generation, apoptotic cell population compared to cells treated with ox- SWCNTs. |
Yuan et. al. [63] |
| GO | AFM, DLS, FTIR, Uv- Vis |
GO: Thickness = 1nm; Size = 100– 600nm |
10–100 µg/ml |
SH- SY5Y cells |
MTT, Caspase- 3 |
Viability of cells are dose and time dependent. No apoptosis induced by GO. MAP2 expression and neurite length improved. |
Lv et. al. [64] |
| GNO, GONP, GONR (PEG- DSPE dispersed) |
TEM, Raman, TGA, Zeta potential, Hydrodyn amic diameter |
GNO: Diameter = 50–300 nm; ID/IG = 0.92; ζ- potential = −32.3 ±1.35; Hydrodynamic radius = 460.76 ±53.58 |
5–300 µg/ml | Huma n adMS Cs and bmMS Cs |
Alamar blue, Calcein AM, Adipogenic differentiation (oil red O) and Osteogenic differentiation (alizarin red, ALP, calcium deposition) |
Dose dependent cytotoxicity (not time dependent; Graphene does not affect differentiation potential of human stem cells |
Talukdar et. al. [44] |
| GONR: Width = 60–90 nm, Length = 500–1500 nm; ID/IG = 1.28; ζ- potential = −26.3 ±0.75; Hydrodynamic radius = 457.5 ± 35.70 |
|||||||
| GONP: Diameter = 20–40 nm, Thickness = 3–5 nm; ID/IG = 1.09; ζ-potential = − 12.47 ± 0.12; Hydrodynamic radius = 296.4 ± 20.32 |
|||||||
| GONR and GONP |
Raman, XPS |
GONR: ID/IG = 1.09; Lattice Size = 22.2 nm; C/O ratio = 1.9; Percentage of C=O groups = 28.22 |
3–400 µg/ml | A549 cells |
MTT, WST-8 | Size and functional group dependent toxicity; GONR exhibit greater toxicity than GONP due to presence of greater number of carbonyl groups and greater length |
Chng et. al. [66] |
| GONP: ID/IG = 0.88; Lattice size = 19.1 nm; C/O ratio = 1.9; Percentage of C=O groups = 11.06 |
|||||||
| GONR, rGONR, GOS and rGOS |
TEM, SEM, AFM, XPS, Raman spectrosco py |
GONR and rGONR: Length = 10µm, width = 50–200 nm; Thickness = 1 nm; O/C ratio = 54% for GONR and 19% for rGONR; Increase in ID/IG ratio upon reduction |
0.01–100 µg/ml |
Huma n MSCs |
ROS assay, RNA efflux, cell viability (FDA) assay, Comet assay, Giemsa staining |
Dose and shape dependent cytotoxicity with GONRs more cytotoxic than GOS. GONRs and rGONRs induce DNA fragmentation and chromosomal aberrations at 1 µg/ml. |
Akhavan et. al. [67] |
| GOS and rGOS: Thickness = 1.2 nm; Lateral size = 2µm; Reduction in oxygen content and increase in ID/IG ratio upon reduction |
|||||||
| GP | TEM, z- potential |
GP: Diameter = 450nm−1.5µm; z- potential = −9.61 |
5–100µg/ml | U87, U118 |
Trypan blue, XXT-based proliferation, LDH, apoptosis kit |
Activated apoptosis and necrosis in U87 cells whereas only apoptosis was activated in U118 cells. |
Jaworski et. al. [68] |
| Pristine-G, COOH- GO |
TEM, AFM, Raman, XPS |
Thickness = 0.5µm |
0–300µg/ml | Vero cells |
Alamar Blue, LDH, Apoptosis, ROS, |
Surface functionalization of graphene is critical for pacifying strong hydrophobic interaction associated with toxicity effects. |
Sasidhara n et. al. [69] |
| GQD- NH2, GQD- COOH, GQD-CO- N(CH3)2 |
UV-Vis, TEM, FTIR |
GQD-NH2: Diameter = 7.5 nm; UV-Vis peak = 230 nm; Ex/Em = 420/500 nm; FTIR peaks at 1627 cm−1 (C=O), 1417 cm−1 (N-H), 1328 cm−1 (C-N), |
0–200µg/ml | A549 and C6 glioma cells |
MTT, Trypan blue, Fluorescence imaging, |
No cytotoxic effects upto 200µg/ml treatment for all GQDs. Intracellular accumulation of GQDs was observed, nuclear translocation was absent. |
Yuan et. al. [71] |
| GQD-COOH: Diameter = 15 nm; UV-Vis peak = 362 nm; Ex/Em = 400/440 nm; FTIR peaks at 1388 and 1571 cm−1 (COO-), |
|||||||
| GQD-CO- N(CH3)2: Diameter = 3–10 nm; UV-Vis peak = 300 nm; Ex/Em = 400/500 nm; FTIR peaks at 1400 and 1304 cm−1 (C-N) |
|||||||
| GO and rGO |
AFM, EDX, Aerodyna mic diameter (dae) |
Lateral dimension = 100nm – 5µm; Height = 1.1– 15nm; dae = 20– 200nm; Oxygen content = 40% in GO and 10% in rGO |
0.0125–12.5 µg/cm2 |
A549 and RAW 264.7 cells |
MTT, DNA assay, FMCA assay, apoptosis, ROS, cell TEM |
Dose dependent cytotoxicity. Cellular internalization of GO inside phagoendosomes was observed |
Horváth et. al. [72] |
| PEG- amine functionali zed GO |
TEM, AFM, FTIR, DLS |
Thickness = 1.8nm; Hydrodynamic size = 10–120nm; |
75µg/ml | Saos-2 osteob lasts, MC3t 3-E1 preost eoblas t, RAW- 264.7 macro phages |
ROS, Hoechst 33258, Gen- Probe Diaclone kit |
After internalization, nanosheets are localized on F-actin filaments inducing cell-cycle alterations, apoptosis, and oxidative stress. |
Matesanz et. al. [70] |
| GO, rGO | AFM, TEM, XPS, Raman spectrosco py |
Flake size = 0.4– 0.8µm; Thickness = 1 nm, |
1–10µg/ml | HUVE C |
MTT, LDH, ROS, FACS, RT-PCR, Comet assay |
Oxidation state, dose and size dependent cytotoxicity. GO exhibits higher toxicity than rGO due to ROS generation. Small flake size graphene exhibit greater cytotoxicity compared to larger sheets due to intracellular accumulation of graphene. |
Das et. al. [73] |
| GQD-PEG | AFM, TEM, FTIR, TGA, XPS, Elemental analysis |
Diameter = 3–5 nm; Height = 0.5– 1 nm; 1–2 graphene layers; Oxygen content 36% |
10–640 µg/ml |
HeLa, A549 |
WST-1, annexin V and PI, LDH, ROS, |
No cytotoxicity; (HeLa cells treated with 160 µg/ml and A549 cells with 320 µg/ml doses show no cytotoxicity) |
Chong et. al. [74] |
| TRGO-Cl, TRGO-Br, TRGO-I |
Raman Spectrosc opy, Elemental analysis, XPS |
TRGO-Cl: Crystallite size/nm = 14.4, Amt. of halogen = 2.1, C/O = 16.77; TRGO-Br: Crystallite size/nm = 15.4, Amt. of halogen = 1.6, C/O = 20.37; TRGO-I: Crystallite size/nm = 22.3, Amt. of halogen = 0.2, C/O = 11.75. |
0–200 µg/ml | A549 | MTT, WST-8 | Dose-dependent cytotoxicity between 3.125–200 µg/ml. Cytotoxicity depends on the amount of halogen content and follows the trend: TRGO-Cl > TRGOO- Br > TRGO-I. |
Teo et. al. [75] |
| Fluorinate d Graphene (F-G) |
SEM, Elemental analysis, XPS, FTIR, XRD |
Three different formulations with varying F content – 1.5%, 42.6%, 50.7%, C=C (284.5 eV), C-F (289 eV), C-F2 (284.5 eV), C-CF (286.1 eV), C- CF2 (287.3 eV), CF-CF2 (290.5 eV), C-F3 (293.5 eV); FTIR peaks at 1150 cm−1 (C- F) |
0–400 µg/ml | A549 | MTT, WST-8 | Dose-dependent cytotoxicity of fluorinated graphene with greater cytotoxicity for graphene containing higher mono-fluoro substituted carbon atoms. |
Teo et. al. [76] |
| Highly hydrogena ted graphene (HHG) and GO |
XPS, Elemental analysis |
HHG: C/O ratio (8.79), H (37.42%), O (10.41%); GO: C/O ratio (2.78), H (25.72%), O (37.65%) |
0–400 µg/ml | A549 | MTT, WST-8 | Greater cytotoxicity was observed due to increased adsorption of micronutrients on hydrophobic surface of HHG sheets limiting their availability. |
Chng et. al. [77] |
| rGO, rGO+Arg, rGO+Pro |
TEM, FTIR, Zeta ζ potential |
Size: 100 nm – 1.5 µm. ζ = 19.5 (rGO), 32.5 (rGO+Arg), 39.8 (rGO+Pro) FTIR: rGO: 1769 cm−1 (C=O), 1602 cm−1 (C=C) and 1289 cm−1 (C-O); rGO+Arg and rGO+Pro: 3500– 3140 cm−1 (O-H, N-H), 1570 cm−1 (C-O, C-N), 890- 810 cm−1 (N-H), 1725 cm−1 (C=O) |
50 µg/ml | U87 | Trypan blue, XTT, gene expression |
Reduction in GBM tumor volume was observed. rGO+Arg shows anti-angiogenic and pro-apoptotic characteristics and has potential for GBM therapy. |
Sawosz et. al. [78] |
| GONR (PEG- DSPE dispersed) |
TEM, Raman |
Width = 125–220 nm, Length = 500–2500 nm; ID/IG = 1.3 |
10–400 µg/ml |
HeLa, MCF- 7, SKBR , NIH3 T3 |
Alamar blue, Neutral red, Trypan blue, LDH, ROS |
Cell type dose, and time dependent cytotoxicity. Significant cell death observed for HeLa cells. |
Mullick Chowdhu ry et. al. [36] |
| rGONP, GONP |
AFM, XPS, Raman, |
GONP: Length = 3.8±0.4µm; Thickness = 0.7nm; |
0.01–100 µg/ml |
hMSC s |
FDA, ROS, RNA efflux, comet, |
Size dependent cytotoxic response with smaller particles eliciting lower cytotoxicity compared to larger particles. Oxidative stress and direct contact interaction of extremely sharp edges of graphene were determined as most likely mechanisms for cytotoxicity of sheets and nanoplatelets. |
Akhavan, et. al. [79] |
| rGONP: Length = 418±56nm; Thickness = 1.1– 2.3nm |
|||||||
| GO | TEM, AFM, FTIR, Raman spectrosco py, XPS, Particle- size distributio n and ζ- potential |
Large (l-GO): Size = 780±410 nm, Thickness = 0.9 nm, Hydrodynamic diameter = 556 nm, FTIR peaks at 1720cm−1 and band at 3400cm−1, Oxygen content = 33.1%, ID/IG = 1.27, ζ-potential = −72.9 |
10–200 µg/ml |
A549 cells |
CCK-8, Trypan Blue, LDH, FITC-annexin V apoptosis, ROS |
Cell viability and ROS generation is dependent on the size of GO sheets. Smaller GO sheet exhibit greater cell viability and less ROS generation. |
Chang et. al. [80] |
| Small (s- GO):Size = 160±90 nm, Thickness = 0.9 nm, Hydrodynamic diameter = 148 nm, FTIR peaks at 1720cm−1 and band at 3400cm−1, Oxygen content = 37%, ID/IG = 1.26, ζ-potential = −51.9 |
|||||||
| Mixture (m-GO): Size = 430±300 nm, Thickness = 0.9 nm, Hydrodynamic diameter = 588 nm, FTIR peaks at 1720cm−1 and band at 3400cm−1, Oxygen content = 35.8%, ID/IG = 1.25, ζ-potential = −59.2 |
|||||||
| GO | AFM, stability and dispersion capacity, carboxyl group assay, TEM |
GO: Length = 350nm and 2µm; Height = 3.9 and 4.05nm; Thickness = 1nm; |
0–20µg/ml | PMØ, J774A .1, LLC, MCF- 7, HepG 2, HUVE C |
LIVE/DEAD, CCK8, Coomassie Blue, CLSM, Cytokine assay |
Cellular internalization independent of graphene size due to differential uptake mechanisms. Removal of Mn from Graphene sheets resulted in reduction of toxicity. Micron sized graphene induced stronger inflammatory response and release of cytokines. |
Yue et. al. [81] |
| GO, PVP- GO |
AFM, UV-Vis, FTIR |
GO: Thickness = 1.7 nm; UV absorption peaks at 230 nm and 300 nm; FTIR peaks at 3395 cm−1, 1726 cm−1, 1620 cm−1, 1410 cm−1, 1226cm−1, 1052cm−1 |
25–100µg/ml | Dendri tic cells, Macro phages , T lymph ocytes |
MTT assay, Phenotype assessment, Apoptosis assay |
PVP functionalized GO sheets are immunocompatible and may be used as adjuvants to improve vaccine therapy |
Zhi et. al. [82] |
| PVP-GO: Thickness = 2.5 nm; UV absorption peak at 265 nm; FTIR peaks at 3395 cm−1, 1726 cm−1, 1620 cm−1 |
|||||||
| Pristine Graphene in 1% F108 pluronic |
SEM, XRD, Raman |
GO: ID/IG = 1.23; Thickness = 2– 3nm; Size = 500– 1000nm; |
0–100µg/ml | RAW 264.7 macro phages |
ROS, MMP, Apoptosis, TEM, Western- blotting, PCR, |
Pristine Graphene can induce cytotoxicity through the depletion of mitochondrial membrane potential resulting in the increase of ROS leading to the activation of MAPK and TGF-β that in turn activate caspase- 3 and PARP proteins resulting in apoptosis. |
Li et. al. [83] |
| GO | AFM, HRXPS, ATR- FTIR, DLS |
GO: Thickness = 1–1.2nm; Hydrodynamic diameter = 2.4µm and 350nm; |
5 or 100µg/ml |
SNY- 449, Mahla vu, A549, HEK2 93, RAW 264.7 |
TEM, Immunofluores cence, Western- blotting, RT- PCR, Flow Cytometry, siRNA |
GO simultaneously triggers autophagy and activates toll-like receptors TLR4/TLR9 resulting in inflammatory responses. |
Chen et. al. [84] |
| GO, C60, C60-tris |
TEM, SEM, AFM, microscop y, spectrosco py, X-ray diffraction |
GO: z-potential = −32.4mV; C60- Fullerenes: z- potential = −13.6; Sizes = 45.2±25.3nm; C60-TRIS Fullerenes: z- potential = −26.1; Size = 45.6±18.8nm |
1.0, 6.25, 25.0µg/ml |
B3Z T cells |
LAL, FITC/Lucifer Yellow, |
Both C60 and graphene capable of modulating antigen- specific T cell responses due to ability to directly affect functional activity of dendritic cells. GO suppresses antigen processing machinery of DCs. |
Tkach et. al. [85] |
| GO, GS | X-ray diffraction , AFM, XPS, DLS, Z- potential |
GO: Diameter = 765nm; z- potential = −40.6; GS: Diameter = 3018nm; z- potential = − 37.2mV |
3–200µg/ml | Suspe nded human RBCs and adhere nt skin fibrobl asts. |
MTT, WST-8, Trypan blue, ROS |
Individually dispersed GO leads to greater RBC membrane damage compared to aggregated graphene sheets. Chitosan coated GO show no hemolytic activity |
Liao et. al. [86] |
| GO, rGO | HR-TEM, 2DFFT, FTIR |
Size = 0.2–5µm | 0–20µg/ml | Huma n platele ts |
FITC, immunoblottin g, LDH, ROS, Electron Microscopy |
GO can evoke strong aggregatory response in platelets comparable to that elicited by thrombin. |
Singh et. al. [87] |
| G-NH2 | FTIR, HR-TEM, FFT, Raman, z- potential, FSC, SSC |
Size = 2µm | 0–10µg/ml | Huma n platele ts |
ROS, MTT | G-NH2 is not associated with any pro-thrombotic characteristics and does not induce platelet-stimulating response. Membrane integrity of RBCs is maintained. |
Singh et. al. [95] |
| GO, rGO | HR-TEM, Raman, UV/vis, XRD |
Size = 100– 350nm |
3–100µg/ml | L929 mice fibrobl ast cells |
WST-1 | Dose and surfactant dependent cytotoxicity of GO and rGO. Good cytocompatibility observed for concentrations between 3.125 and 12.5 µg/ml of GO and rGO dispersed in PEG. |
Wojtonis zak et. al. [91] |
| GNP-Dex | AFM | Diameter = 60– 100 nm; Thickness = 2–4 nm |
1–10 mg/ml | RBL- 2H3 mast cells, human platele ts |
Histamine release, platelet activation, complement activation, cytokine release, blood cell hemolysis |
Dextran coated graphene oxide nanoplatelets exhibit no hematological toxicity |
Mullick Chowdhu ry et. al. [89] |
| FBS-GO | TEM, AFM |
Thickness = 1nm | 20 and 100µg/ml |
A549 cells |
MTT, Bradford Protein Assay |
FBS coating of GO attenuates cytotoxicity |
Hu et al. [92] |
| BSA-GO | FITC- BSA, Steady State fluorescen ce spectra, AFM, Z- potential, SEM, CLSM, Flow cytometry , TEM, |
Flake size: 50 nm or 1µm; z- potential = − 10mV; Thickness = 9.1±7.1nm |
50µg/ml | C2C1 2 cells |
TEM, SEM, confocal microscopy, WST-1 |
BSA coated GO sheets exhibit size- dependent internalization. Small GO sheets are internalized by clathrin-mediated endocytosis and large GO sheets are internalized by phagocytosis. |
Mu et. al. [93] |
| FBS and human plasma serum functionali zed GONRs |
AFM, XPS, Raman spectrosco py, FTIR, Mass spectrome try |
Width = 100 nm; Height = 1 nm; FTIR peaks at 3400 cm−1 (O-H), 1760 cm−1 (C=O), 1300 cm−1 (C- OH), 1080 cm−1 (C-O); O/C ratio = 0.54; ID/IG = 1.38 |
10–100µg/ml | A549 cells |
Trypan blue and Apoptosis assay |
Dose dependent cytotoxicity of protein-functionalized GONRs was observed. Concentrations below 50µg/ml did not exhibit cytotoxic effects. |
Mbeh et. al. [94] |
3.1 Dose, time, and morphology dependent cytotoxicity
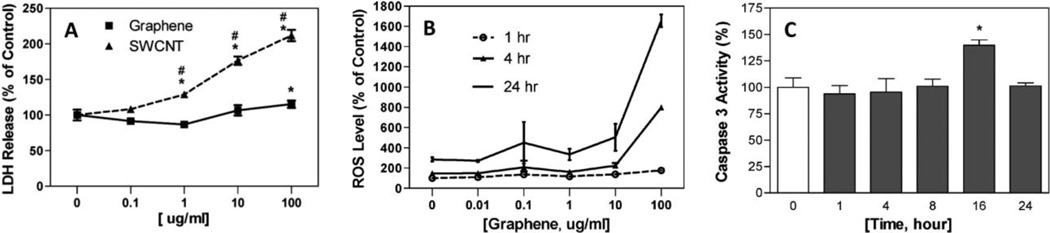
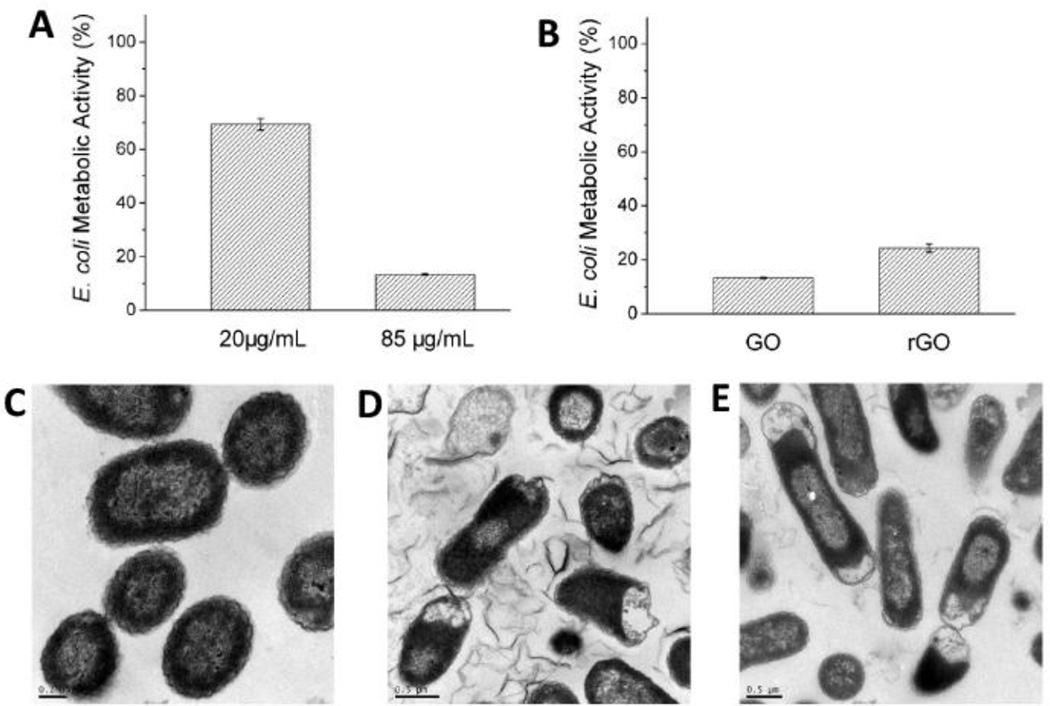
Zhang et. al. investigated the interactions of graphene (diameter 100–110 nm, thickness 3–5 nm) with rat pheochromocytoma PC12 cells using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and Lactate Dehydrogenase (LDH) assays and compared the results with single-walled carbon nanotubes (SWCNTs) [61]. More than 70% cell death was observed for 100 µg/ml treatment concentration of SWCNTs whereas no cell death was observed for 0.01–10 µg/ml concentrations of graphene (Figure 4A). Nearly 15–20% cell death was observed for graphene treatment at 100 µg/ml. The observed cytotoxicity was attributed to the agglomeration of graphene, generation of reactive oxygen species (Figure 4B) and an increased caspase-3 activation (Figure 4C) resulting in apoptosis. These results show a dose dependent cytotoxicity trend that is dependent on the morphology (shape and composition) of the nanomaterial, with graphene exhibiting an overall lower toxicity compared to single-walled carbon nanotubes (SWCNTs). Vallabani et. al. investigated the toxicity of graphene oxide using normal human lung cells (BEAS-2B) after 24 and 48 hours of exposure at concentrations between 10–100 µg/ml. A significant dose- and time- dependent decrease in cell viability and an increase of early and late apoptotic cells was observed using MTT assay [62].
Figure 4.
Effects on (A) lactate dehydrogenase release, (B) reactive oxygen species generation and (C) caspase-3 activity (apoptosis marker) of PC12 cells treated with 0.1–100 µg/ml of graphene and single-walled carbon nanotubes. Adapted from Reference [61] with permission, copyright © American Chemical Society, 2010.
Yuan et. al. evaluated the cytotoxicity of graphene oxide on human hepatoma HepG2 cells using MTT assay, DFDA fluorescence analysis and 2D LC-MS proteome analysis [63]. After 48 hours of exposure to GO at 1 µg/ml concentration, HepG2 cells showed 6% mitochondrial damage, 8% increase in ROS generation and no significant changes in apoptotic cell population, cell cycle, and expression of metabolic and cytoskeletal proteins. Cells treated with oxidized-SWCNTs (ox-SWCNTs) showed ~20% mitochondrial damage, >100% increase in ROS generation, ~26% increase in apoptotic cell population, and ~30 differentially expressed proteins involved in metabolic pathway, redox regulation, cytoskeleton formation, and cell growth. These results suggest that GO may be less cytotoxic compared to ox-SWCNTs. In another study, Lv et. al. show that GO does not elicit cytotoxic or apoptotic effects in human neuroblastoma SH-SY5Y cells at low concentrations (<80 µg/ml) [64]. Interestingly, GO enhances the retinoic acid induced differentiation of SH-SY5Y cells, improving neurite length and expression of MAP2 (neuronal marker), suggesting that GO may be suitable for applications in neurodegenerative diseases.
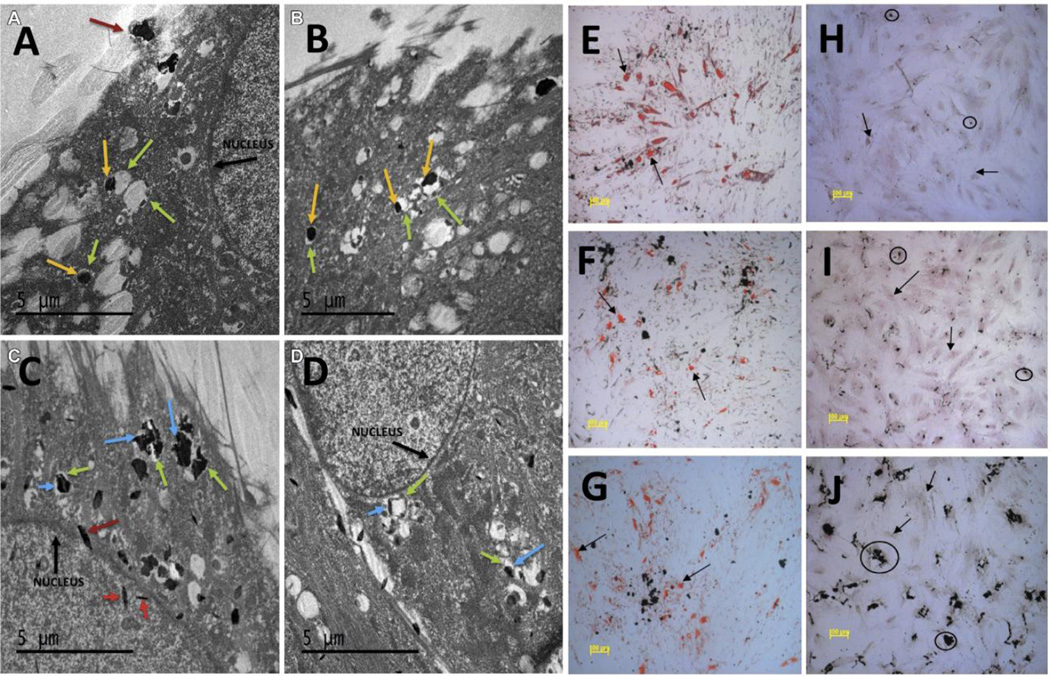
Talukdar et. al. have investigated the effects of graphene nanostructures of various morphologies (such as oxidized-nanoribbons (GONRs), oxidized-nanoplatelets (GONPs), and nanoonions (GNOs)) on the toxicity and stem cell differentiation potential of human mesenchymal stem cells (hMSCs) [65]. hMSCs (derived from bone marrow and adipose tissue) were treated with various concentrations (5–300 µg/ml) of GONRs, GONPs and GNOs for 24 or 72 hours and cytotoxicity was evaluated using Alamar blue and CalceinAM assays. Results show a dose –dependent (no time-dependent) cytotoxicity of various 2D graphene nanostructures with concentrations >50 µg/ml showing no cytotoxicity. TEM imaging shows cellular and nuclear uptake of GNOs and GONPs (Figure 5 A–D). Furthermore, results show that all graphene nanostructures did not induce any changes in the adipogenic and osteogenic differentiation of hMSCs (Figure 5 E–J) suggesting the used of graphene as labels for stem cell imaging and therapy.
Figure 5.
Representative transmission electron microscopy images of mesenchymal stem cells (MSC) treated with graphene nanoonions (GNOs, A&B) and oxidized-graphene nanoplatelets (GONPs, C&D) at 50 µg/ml for 24 hours. Yellow arrows correspond to aggregates of GNO visualized in vacuoles (green arrows). No nuclear uptake of GNOs was observed. Blue arrows correspond to aggregates of GONPs. GONPs were observed inside the nucleus (red arrows). Oil red O staining after adipogenic differentiation of MSC treated with 50 µg/ml of (E) GNO, (F) GONR and (G) GONP. Alizarin Red staining after osteogenic differentiation of MSC treated with 50 µg/ml of (H) GNO, (I) GONR and (J) GONP. No changes in the adipogenic and osteogenic differentiation of MSCs were observed. Adapted from Reference [44] with permission, copyright © Elsevier, 2014.
Chng et. al. have reported a comparative study on the cytotoxicity of GONRs and GONPs [66]. GONRs were synthesized from the longitudinal unzipping of CNTs and GONPs were synthesized from stacked graphene nanofibers. In vitro cytotoxicity evaluated using MTT and WST-8 assays using human epithelial (A549 cells) show that GONRs exhibit a significantly higher cytotoxic response than GONPs over all concentrations (3–400 µg/ml). The increased cytotoxicity of GONRs was attributed to the presence of a greater amount of carbonyl groups (28.22% on GONRs vs. 11.06% on GONPs) and the high aspect ratio (width × length of GONRs ~310 × 5000 nm and GONPs ~ 100 × 100 nm) of GONRs.
Akhavan et. al. have reported the cyto- and geno-toxicity of reduced GONRs and reduced graphene oxide sheets (rGOS) using human MSCs derived from umbilical cord blood [67]. Cell viability measured by fluorescein diacetate (FDA) test shows that rGONRs are toxic, significant cytotoxicity was observed after 1 hour of exposure with rGONRs at 10 µg/ml, while the same cytotoxicity was observed upon incubation with 100 µg/ml of rGOS after 96 hours. The cytotoxicity of rGOS was attributed to the generation of oxidative stress whereas the cytotoxicity of rGONRs was attributed to DNA fragmentation and chromosomal aberrations (observed even at low concentrations of ~ 1 µg/ml after 1 hour) due to penetration of rGONRs inside the cells. These results suggest that the cytotoxicity and genotoxicity of graphene is dependent on the dose and shape of the nanomaterial (sheets vs. nanoribbons).
Jaworski et. al. have reported the interactions of graphene platelets with human glioblastoma U87 and U118 cells [68]. After 24 hours of incubation with 100 µg/ml graphene, 42% and 52% cell mortality was observed for U87 and U118 cells, respectively. However, graphene activated apoptosis only in U118 cells not in U87 cells where apoptosis and necrosis both were activated. These results suggest the potential application of graphene in anticancer therapy.
3.2 Functionalization dependent cytotoxicity
Sasidharan et. al. investigated the cytotoxicity of pristine graphene and carboxylated GO (GO-COOH) using monkey renal cells at concentrations between 10–300 µg/ml treatment concentrations to assess the differences between cellular interactions of hydrophobic and hydrophilic graphene derivatives [69]. Pristine graphene accumulated on the cell membrane leading to the destabilization of F-actin alignment whereas GO-COOH was internalized by cells and accumulated in the perinuclear region without any membrane destabilization even at 300 µg/ml doses. These results suggest that hydrophilic (more oxidized) graphene nanoparticles may be more cytocompatible and efficient intracellular delivery systems. In another study, Matesanz et. al. observed internalization and localization of poly(ethylene glycol amine)-functionalized GO sheets on F-actin filaments resulting in cell-cycle alterations, oxidative stress and apoptosis in MC3T3-E1 murine pre-osteoblasts, Saos-2 osteoblasts and RAW-264.7 macrophage cells [70].
Yuan et. al have investigated the cytotoxicity and distribution of three kinds of GQD (NH2, COOH and CO-N(CH3)2 functionalized) in human neural glioma C6 and A549 lung carcinoma cells using MTT and Trypan blue assay [71]. Results show the absence of mortality and apoptosis or necrosis at all treatment concentrations (10–200 µg/ml) after 24 hours for all three GQD groups. Furthermore, Raman spectroscopic analysis showed the intracellular accumulation of all three GQDs; nuclear translocation was absent.
Horváth et. al. have evaluated the toxicity of GO and rGO in A549 human lung epithelial cells and RAW 264.7 mouse peritoneal macrophages using MTT assay, fluorometric DNA assay and fluorometric microculture cytotoxicity assay (FMCA) [72]. Cells treated with 0.0125–12.5 µg/cm2 of GO or rGO for 5 days showed a dose dependent cytotoxicity. Significant differences in cell death between control and GO or rGO treated cells were observed from day 2 in A549 cells and day 3 in RAW 264.7 macrophages for two higher concentrations of 1.25–12.5 µg/cm2. Cells treated with lower concentrations of GO (0.0125–0.125 µg/cm2) did not lead to increases in ROS production. Cellular internalization of GO was observed in phagoendosomes without signs of any intracellular damage.
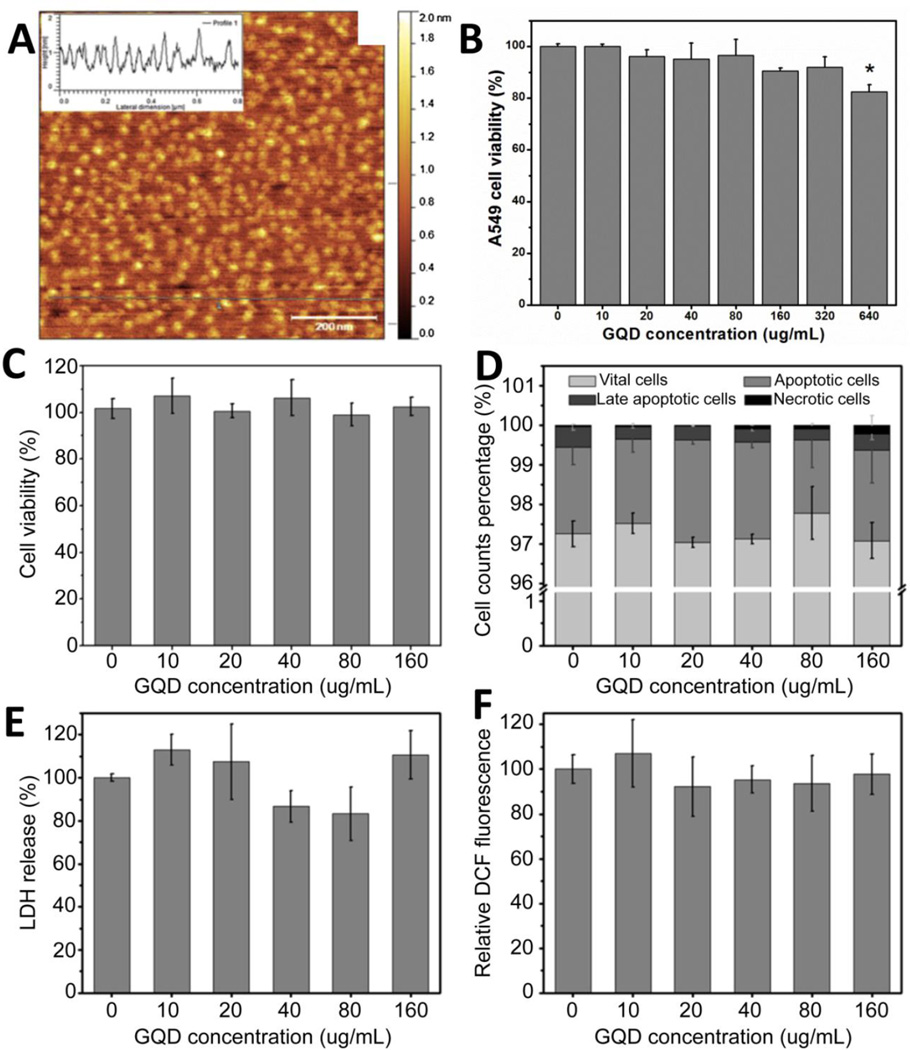
Aggregation of pristine graphene in biological buffers could result in greater cytotoxicity in comparison to oxidized graphene derivatives that can be readily dispersed without aggregation during the duration of cytotoxicity studies. Das et. al. have reported higher cytotoxicity of GO sheets compared to reduced graphene oxide sheets of similar dimensions, an effect attributed to the presence of high density of oxidative functional groups on the surface of GO which lead to the generation of reactive oxygen species [73]. HUVEC cells treated with 1, 5 or 10 µg/ml concentration of GO and rGO showed a dose and functionalization state dependent cytotoxicity. Furthermore, a size dependent cytotoxicity was also observed for both GO and rGO. Upon a 10 fold reduction in sizes of oxidized and reduced graphene sheets, smaller graphene nanosheets showed a higher toxicity compared to non-sonicated larger GO or rGO sheets which was attributed to an increased intracellular interaction and uptake of small sized graphene. However, chong et. al. have reported the low cytotoxicity of PEG dispersed graphene quantum dots (>30nm diameter stacks of 1–10 graphene layers) upto 160 µg/ml for HeLa cells and 320µg/ml for A549 cells (Figure 6) [74].
Figure 6.
(A) Representative atomic force microscopy (AFM) image of graphene quantum dots (GQDs). Inset in image A depicts AFM height profile. (B) Cell viability of A549 cells assessed by WST-1 assay. Data reported as means ± SE. No significant differences in cell viability were observed upto a treatment concentration of 320 µg/ml. (C) Cell viability assessed by WST-1 assay, (D) cell apoptosis and necrosis (E) LDH assay and (F) ROS generation by HeLa cells upon treatment with 0–160 µg/ml of GQDs. No toxicity upto 160 µg/ml concentration was observed. Adapted from Reference [74] with permission, copyright © Elsevier, 2014.
Teo et. al. have investigated the cytotoxicity of halogenated graphene sheets [75]. GO sheets prepared by oxidation of graphite were thermally reduced with chlorine, bromine, and iodine vapor to form chlorine-, bromine-, and iodine-doped graphene, respectively (TRGO-Cl, TRGO-Br, and TRGO-I). A549 cells were treated with 0–200 µg/ml concentration of halogenated graphene for 24 hours and cell viability was analyzed using MTT and WST-8 assays. Results show that all halogenated graphene nanoparticles exhibit a dose-dependent cytotoxicity between 3.125–200 µg/ml with TRGO-Cl exhibiting highest cytotoxicity (~25.7% cell viability at maximum treatment concentration of 200 µg/ml). The levels of observed cytotoxicity follows the trend: TRGO-Cl > TRGO-Br > TRGO-I and is dependent on the amount of halogen functionalization. In another study, Teo et. al. have reported the cytotoxicity of fluorinated graphene (FG) [76]. Three types of graphene derivatives with varying amount of fluorine content were prepared (1.5%, 42.6%, and 50.7%). A549 cells were treated with 0–400 µg/ml of fluorinated graphene and cytotoxicity was analyzed using MTT and WST-8 assays. Results show a dose-dependent cytotoxicity response with greater cytotoxicity observed for graphene with higher mono-fluoro substituted carbon atoms. In a similar study, Chng et. al. synthesized highly hydrogenated graphene (HHG) and evaluated their in vitro cytotoxicity profile against A548 cells [77]. After 24 hours of exposure, MTT and WST-8 assay results show a dose-dependent cytotoxicity of HHG compared to GO controls at all treatment concentrations (0–400 µg/ml). The increased cytotoxicity of HHG was hypothetically attributed to the preferential adsorption of essential micronutrients on the hydrophobic surfaces of HHG compared to hydrophilic surfaces of GO sheets, thereby limiting nutrient availability.
Sawosz et. al. have investigated the cytotoxicity of arginine (Arg) and proline (Pro) functionalized rGO using U87 glioblastoma multiforme (GBM) cells and tumors in vitro [78]. Cells were treated with 50 µg/ml of rGO, rGO+Arg and rGO+Pro for 24 hours and cell viability was evaluated using Trypan blue and XTT assay. Results show ~40% cell death for rGO group and ~15% cell death for rGO+Arg and rGO+Pro groups greater than the controls. GBM tumors cultured on chorioallantoic membrane of chicken embryo were injected with rGO, rGO+Arg and rGO+Pro for 3 days. A greater reduction in tumor volume was observed for rGO compared to rGO+Arg and rGO+Pro groups, which also reduced the tumor volume albeit lower than rGO. Histological analysis of tumors showed the presence of white gaps and rupture sites indicating necrosis and endothelial proliferation. rGO+Arg were observed close to microglial cells and small blood vessels whereas rGO+Pro were aligned outside the cells in the tissue rather than inside the cells. Tumor cells require arginine for aggressive growth, therefore rGO+Arg were present in the outer layers of tumor – site for active angiogenesis. Gene expression analysis suggests that rGO+Arg, leads to the down regulation of MDM2 expression and increased expression of NQO1. Furthermore, no change in the expression of COX6 and CASP3 mRNA expression were observed. These results suggest that rGO+Arg is anti-angiogenic and pro-apoptotic and has potential for GBM therapy.
3.3 Cell dependent cytotoxicity
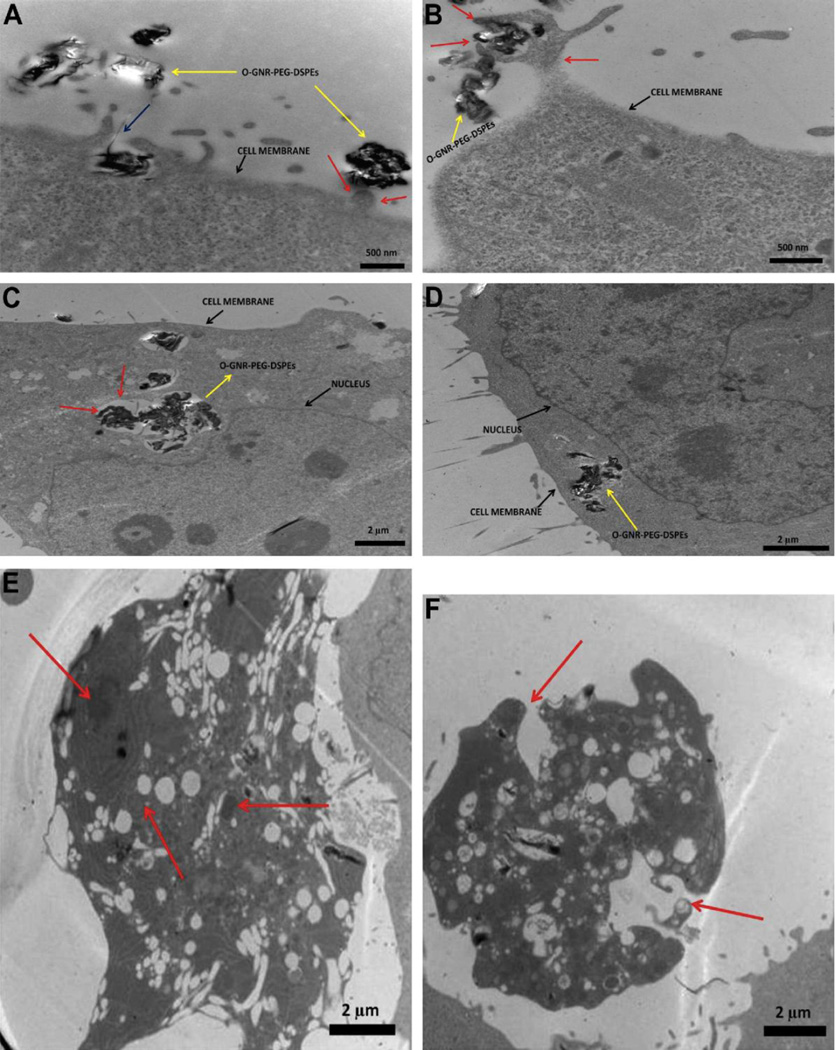
Cytotoxicity of graphene nanoparticles is dependent on cell type. Mullick-Chowdhury et. al. reported the cytotoxicity screening of graphene oxide nanoribbons (GONRs) dispersed in DSPE-PEG (1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[amino(polyethylene glycol)]) using six different assays and four representative cell lines: NIH-3T3 mouse fibroblast cells (NIH-3T3), Henrietta Lacks cells (HeLa) derived from cervical cancer tissue, Michigan cancer foundation-7 breast cancer cells (MCF7), and Sloan Kettering breast cancer cells (SKBR3)[36]. All cell lines exhibit a dose dependent (10–400 µg/ml) and time dependent (12–48 hours) decrease in cell viability. HeLa cells showed the least cell viability (5–25%), compared to other cell types (78–100%), depending on the treatment concentration and exposure time. An increased cellular uptake of GONRs was observed and attributed to an increased cytotoxic response in HeLa cells. TEM imaging (Figure 7) shows the formation of cytoplasmic vesicles to facilitate intracellular uptake. Swollen and ruptured plasma membrane was observed suggesting necrotic cell death.
Figure 7.
Representative transmission electron microscopy images of HeLa cells treated with 20 µg/ml of PEG-DSPE dispersed graphene oxide nanoribbons for 3 hours. (A) Presence of GONR aggregates towards cell periphery (blue arrows), (B) cell membrane protrusion and internalization of GONRs (red arrows), (C & D) GONR aggregates enclosed in large cytoplasmic vesicles or endosomes (red arrows), (E and F) HeLa cells showing ruptured plasma membrane and swollen vesicles suggesting necrotic cell death after 24 hours of exposure to 20 µg/ml DSPE-PEG dispersed GONRs. Adapted from Reference [36] with permission, copyright © Elsevier, 2013.
3.4 Size dependent cytotoxicity
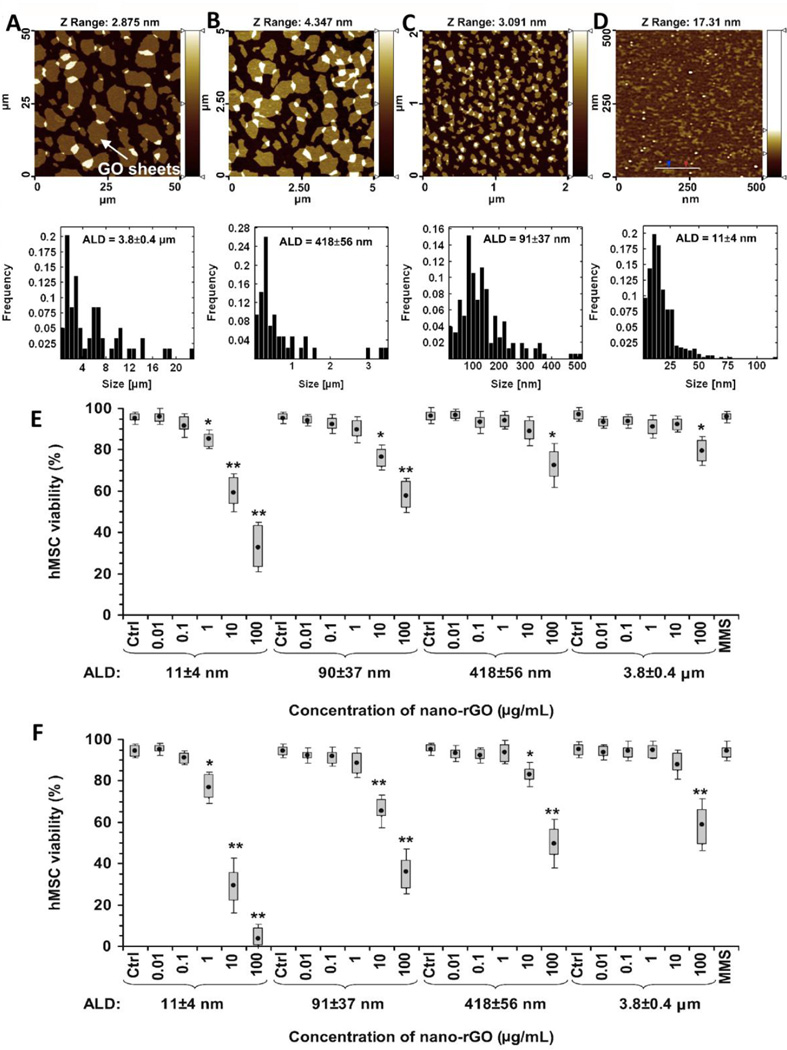
Akhavan et. al. investigated the cytotoxicity of reduced graphene oxide nanoplatelets (rGONPs) of various sizes (11±4 nm, 91±37 nm and 418±56 nm) and as prepared GO (3.8±0.4 µm) using human mesenchymal stem cells (hMSCs) [79]. The cytotoxicity and cell viability was assessed using fluorescein diacetate (FDA) assay, ROS assay, RNA efflux and Comet assay. Results showed a significant size-dependent cytotoxicity; a treatment concentration of 100 µg/ml rGONPs (11±4 nm) showed >95% cell death which reduced with increasing lateral size dimensions (Figure 8). As-prepared GO with largest lateral size dimensions (3.8±0.4 µm) showed lowest (~20%) cell death. Results show that rGONPs can also induce DNA fragmentation even at low concentration of 0.1 µg/ml.
Figure 8.
Representative atomic force microscopy (AFM) images of (A) as-prepared rGO (3.8±0.4 µm), (B) sonicated rGO (418±56 nm), (C) large rGONPs (91±37 nm) and (D) small rGONPs (11±4 nm). Corresponding lateral size distributions are shown below. Images (E and F) show human mesenchymal stem cell viability after treatment with 0.01–100 µg/ml concentration of rGONPs for 1 and 24 hours, respectively. Adapted from Reference [79] with permission, copyright © Elsevier, 2012.
Chang et. al. investigated the cytotoxicity of GO of various sizes (160±90 nm, 430±300 nm and 780±410 nm) using A549 (human lung adenocarcinoma) cells [80]. Cell viability was assessed using CCK-8 assay after 24–72 hours of incubation at GO concentrations 10–200 µg/ml. Small GO sheets (160±90 nm) showed lower cell viability of ~67% compared to large GO sheets (430±300 nm and 780±410 nm) that showed >80% cell viability. However, GO sheets of dimensions 780±410 nm show >50% higher ROS generation compared to GO of dimensions 160±90 nm and 430±300 nm. These results suggest that the cell viability and ROS generation potential of GO is dependent on the size of graphene sheets.
Dasgupta et. al. have reported the size dependent cytotoxicity of graphene oxide nanoribbons (GONRs) after post processing sonication steps that result in a size reduction of nanoparticles [60]. GONRs were dispersed in cell culture media by bath sonication (5 or 20 minutes) or probe sonication (1, 5 or 10 minutes) and MCF-7 and A549 cells were exposed to GONR containing media at 20 µg/ml concentrations. LDH assay, presto blue assay and ROS generation showed that GONR solutions prepared via probe sonication results in a decrease of metabolic stress of cells in vitro. No adverse effects were observed when cells were exposed to non-sonicated and bath sonicated solutions of GONRs. TEM analysis showed presence of smaller GONR fragments and carbonaceous debris after probe sonication, which may be the cause of observed cytotoxicity.
Yue et. al. report that cellular internalization and regulation of cellular responses are directly dependent on the lateral dimension of GO [81]. In this study, six representative cell lines (peritoneal macrophage PMØ, murine macrophage J774A.1, murine Lewis lung carcinoma LLC, human breast cancer MCF-7, human hepatocarcinoma cells HepG2, and human umbilical vein endothelial cells HUVEC) were exposed to GO sheets of different sizes (350 nm and 2 µm) at a concentration of 20 µg/ml for cell viability analysis (LIVE/DEAD assay). After 48 hours of incubation, a significant cytotoxicity (~40–60% cell death) was detected for all six cell types. However, cell viability was restored upon the removal of manganese (Mn), an impurity present during the oxidative synthesis of GO. Cells upon treatment with Mn-free GO at 20 µg/ml showed ~80–100% cell viability. These results highlight the importance of purification steps involved during the synthesis of GO to eliminate false positive contributions from metal ions. PMØ and J774A.1 macrophage cells were treated with 2–6 µg/ml of nano- and micro-sized GO. Cellular uptake studies show that internalization of GO was independent of size and both nano- and micro-sized GO (350 nm and 2 µm) had similar intracellular accumulation. The analyses of uptake mechanisms showed that GO of size 350 nm was wrapped by filopodia of macrophages and internalized whereas GO of 2 µm was internalized via direct penetration. Post cellular internalization, the micron sized GO developed wrinkle formations and appeared to be sequestered into lysosomes. Furthermore, the micron sized GO induced a stronger inflammatory response and release of cytokines. These results suggest that cytokine release and inflammatory response are dependent on the size of GO sheets.
3.5 Immunotoxicity of graphene
Zhi et. al. have reported the immunotoxicity of GO with and without functionalization with poly(vinyl pyrrolidone) (PVP) against human immune cells such as T lymphocytes, dendritic cells and macrophages [82]. Results show that PVP-coated GO (PVP-GO) exhibit lower immunogenicity compared to pristine GO at concentrations between 25–100 µg/ml. The differentiation and maturation of dendritic cells was unaffected upon incubation with PVP-GO; the levels of secreted TNF- α and IL-1β showed no significant differences between GO and PVP-GO groups, yet the secretion of IL-6 was maintained in PVP-GO group. Incubation with PVP-GO also delayed the apoptosis of T lymphocytes and stimulated and enhanced the physiological activity of macrophages.
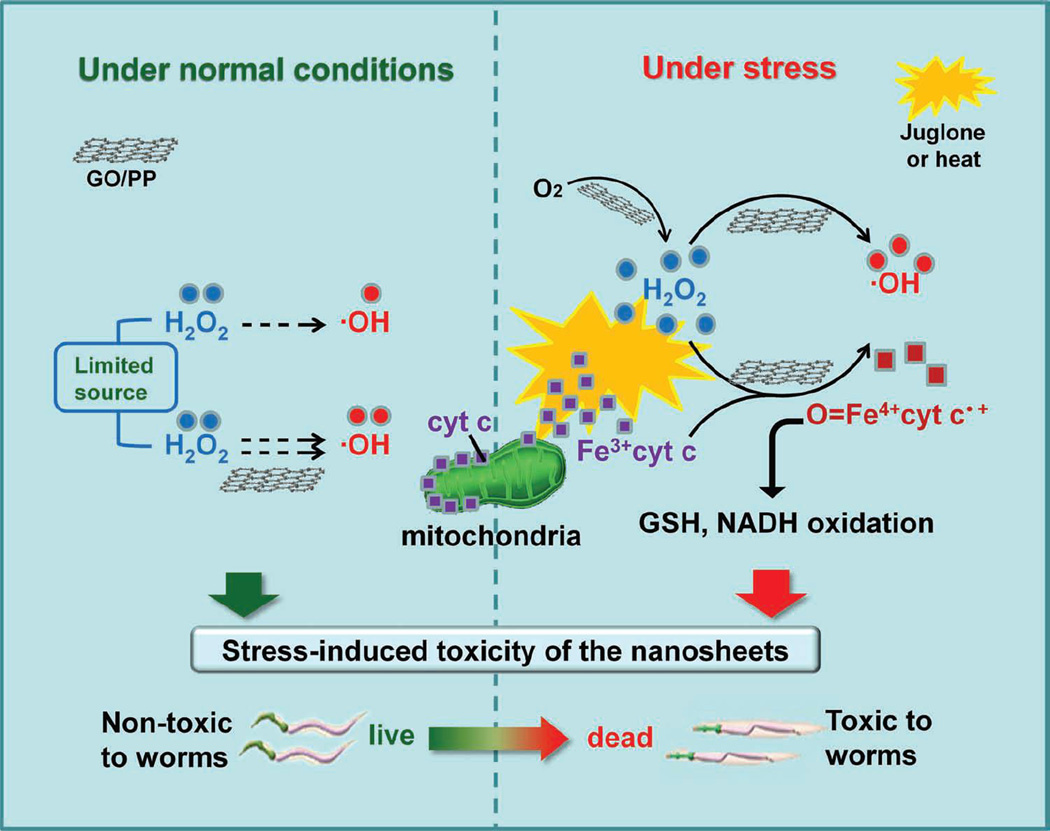
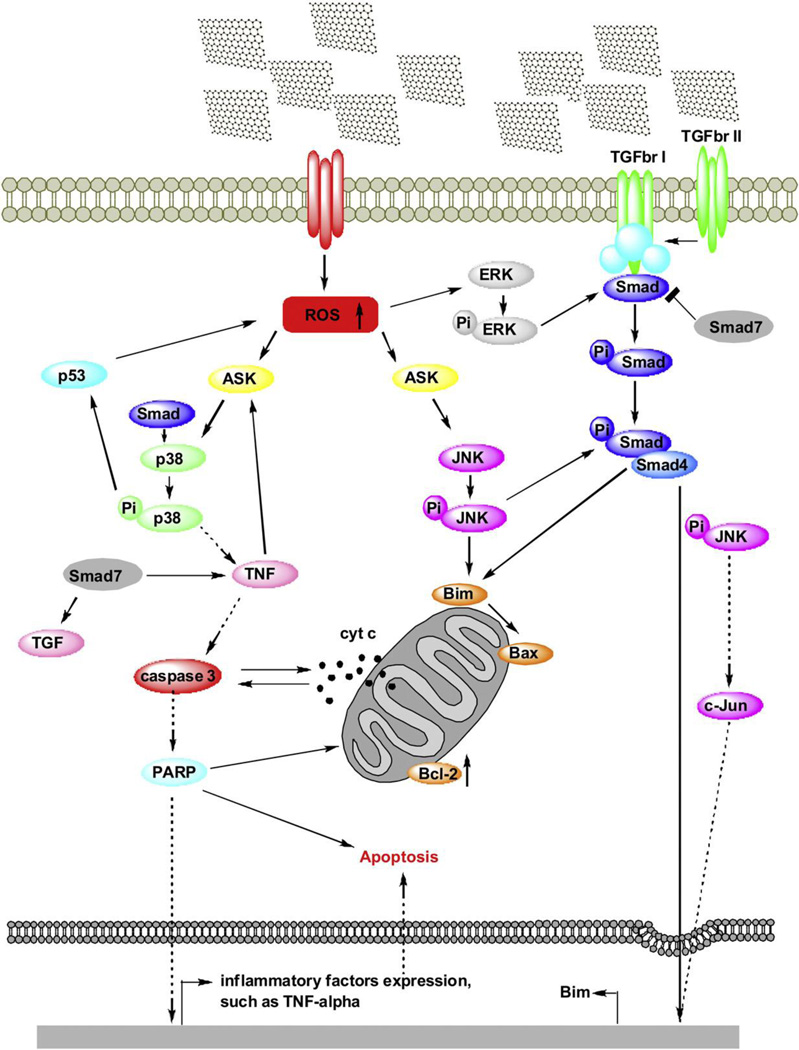
Li et. al. investigated the interactions of pristine graphene with RAW 264.7 macrophage cells at concentrations between 5–100 µg/ml (dispersed in 1% pluronic F108 surfactant).[83] Pristine graphene shows dose-dependent cytotoxicity in RAW 264.7 cells; ~ 78% cell death was observed at 100 µg/ml treatment concentrations. Further investigation of the mechanisms of cytotoxicity showed that interaction of pristine graphene with macrophage cell membrane leads to depletion of mitochondrial membrane potential thereby increasing ROS leading to the activation of apoptotic cascade. MAPK and TGF- β signaling pathways were activated which in turn activated two pro-apoptotic proteins (Bim and Bax). Consequently, caspase-3 and PARP proteins were activated triggering apoptosis. The identification of mechanisms of cytotoxicity is extremely important and provides information towards development of strategies to control graphene-induced apoptosis.
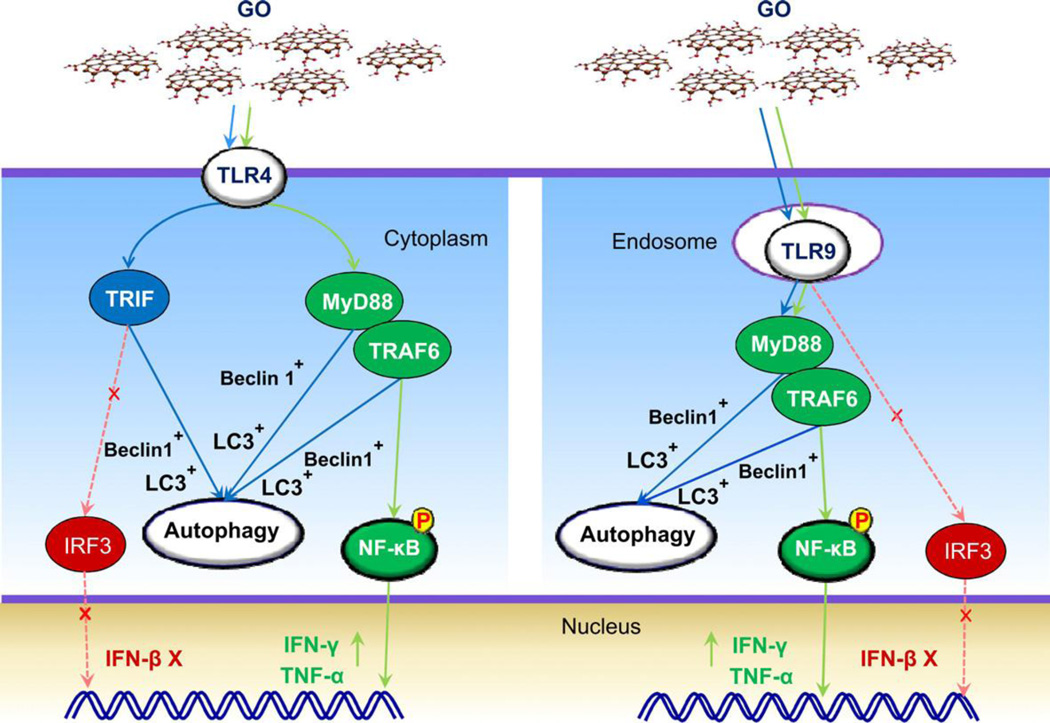
Chen et. al. showed that GO triggers autophagy (catabolic self-destruction of dysfunctional cellular components) in liver cancer cells (SNU-449 and mahlavu), lung cancer cells (A549), human embryonic kidney cell (HEK293), and RAW 264.7 macrophages by stimulating toll-like receptor signaling cascade (release of cytokines such as IL-2, IFN-γ, IL-10 and TNF-α) at treatment concentrations of 5 or 100 µg/ml [84]. Toll like receptors - TLR4 and TLR9 were activated resulting in GO-mediated inflammatory responses. The results of this study show that GO exposure to cells simultaneously triggers autophagy and TLR4/9 mediated inflammatory responses.
Tkach et. al. showed that treatment of dendritic cells (DCs) with GO at 6.25 µg/ml results in an impaired stimulatory potential of DCs (activation of T-cells); treatment with similar concentrations of fullerenes (C60 and C60-tris) promotes the ability of DCs to activate T-cells [85]. Further analysis showed that GO did not alter antigen uptake by DCs nor inhibit antigen peptide presenting abilities of DCs. However, exposure of DCs to GO resulted in suppression of an immunoproteosome subunit (LMP-7), which is a critical component of MHC-I antigen processing machinery (APM) illustrating the mechanism of inactivation of DCs by GO. These results suggest that GO may modulate antigen-specific T-cell response and emphasize the importance of elaborate assessment of immunomodulatory effects of graphene nanoparticles.
3.6 Hemolytic toxicity of Graphene
Hemolytic potential of graphene is dependent on the size and aggregation state of individual nanosheets. Liao et. al. investigated the cytotoxicity of graphene and GO using human erythrocytes (RBCs) [86]. Hemolysis was quantified by measuring the amount of hemoglobin released due to RBC membrane damage upon incubation with graphene and GO at 3–200 µg/ml for 3 hours. At 200 µg/ml, individually dispersed GO sheets showed ~60% hemolysis, significantly higher than graphene dispersions which showed ~20% hemolysis. The aggregation of graphene in DI water results in fewer cell-contractable ROS groups on the surface of graphene. However, cells interact with several ROS species present on the surface of individually dispersed GO, leading to greater hemolysis. Chitosan coated GO aggregate in DI water due to pH dependent conformational change of chitosan resulting in no hemolytic toxicity of GO.
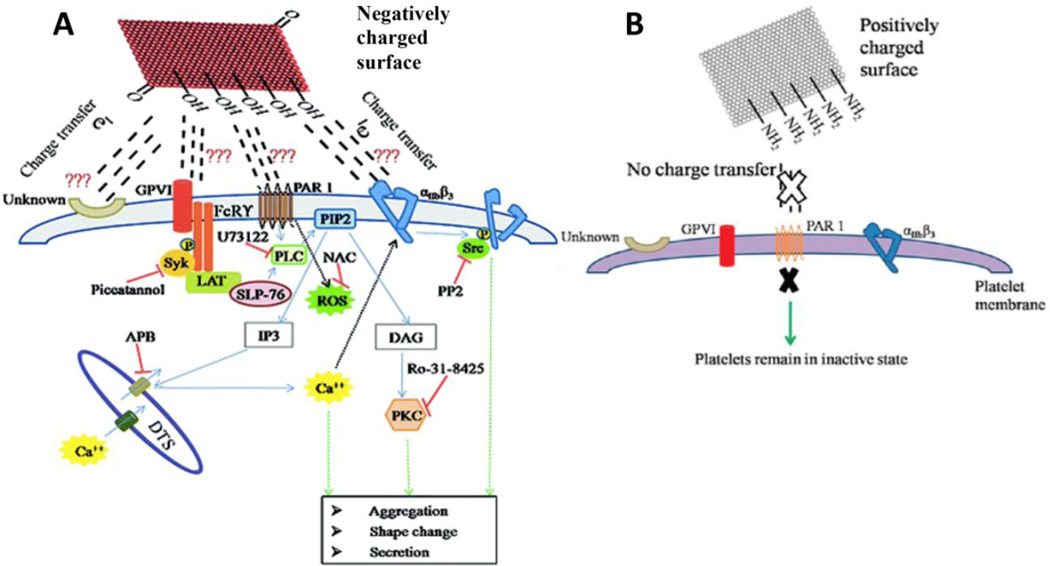
Singh et. al. have reported the in vitro hemolytic toxicity of GO and rGO using human platelets [87]. Freshly isolated suspension of platelets exposed to GO (2 µg/ml) show aggregation and platelet activation at levels greater than induction by thrombin (1 U/ml, a strong platelet agonist). Exposure of platelets to GO resulted in the activation of Src kinases and release of calcium, leading to thrombus formation. In comparison, rGO at 2 µg/ml induced minor platelet aggregation, only 10% of aggregation induced by GO. In another study, Singh et. al. showed that amine functionalized GO does not induce lysis of erythrocytes and has no stimulatory effects on platelets highlighting their non-thrombotoxic properties [88]. These results suggest that surface modifications of graphene nanoparticles play an important role towards defining their hemolytic activity.
Mullick Chowdhury et. al. show that graphene oxide nanoplatelets (GONPs) functionalized with biocompatible polymer dextran (GNP-Dex) exhibit no hematological toxicity [89]. RBL-2H3 mast cells and human platelets showed no histamine release, platelet activation or blood cell hemolysis upon treatment with GNP-Dex at concentrations ranging from 1–10 mg/ml. At concentrations >7 mg/ml, RBL-2H3 cells showed 12–20% increase in complement protein expression. However, cytokine TNF-Alpha and IL-10 levels remained within physiological levels. In another study, Mullick Chowdhury et. al. have investigated the interactions of DSPE-PEG functionalized graphene oxide nanoribbons (GONRs) with blood vascular system components [90]. No release of histamine, platelet PF4 activation and complement activation was observed from mast cells upto treatment concentrations of 80 µg/ml. TEM imaging shows significant uptake of GONRs into endothelial cells and exhibit a concentration dependent reduction of cell viability. Results show that DSPE-PEG functionalized GONRs are hemocompatible upto a concentration of 80 µg/ml.
3.7 Surfactant/coating dependent cytotoxicity
Wojtoniszak et. al. investigated the cytotoxicity of GO and rGO dispersed using three surfactants (polyethylene glycol (PEG), polyethylene glycol–polypropylene glycol–polyethylene glycol (Pluronic P123), and sodium deoxycholate (DOC)) at concentrations between 3.12–100 µg/ml using mice fibroblasts L929 cells [91]. Cytotoxicity analysis using WST-1 assay showed that the cell viability is dependent on the surfactant used to stabilize the suspension, chemical state of material (oxidized or reduced), and the treatment concentration. GO functionalized with PEG exhibits the lowest toxicity (cell viability ~ 36.3% at 100 µg/ml) whereas GO functionalized with DOC and Pluronic P123 shows 15.5% and 6.3% cell viability, respectively. L929 cells exposed to the PEG dispersed rGO between 3.125–25 µg/ml show ~95−60% cell viability. Similar results are observed for rGO functionalized with DOC, however rGO functionalized with Pluronic P123 showed least cell viability. Both, GO and rGO show good cytocompatibility between 3.125–12.5 µg/ml. GO dispersed in PEG shows the best cytocompatibility. These results suggest that GO and rGO exhibit a dose- and surfactant-dependent cytotoxicity.
Hu et. al. investigated the cytotoxic effects of fetal bovine serum (FBS) coated GO using A549 cells [92]. At 100 µg/ml exposure, FBS coated GO showed ~90% cell viability whereas GO without FBS coating showed only ~50% cell viability. TEM analysis showed irreversible cell membrane damage after 2 hours of exposure to GO. FBS coated GO did not induce any membrane damage. These results suggest that cytotoxicity of GO is a result of direct physical interactions with cell membrane that can be mitigated by coating GO with FBS.
Mu et. al. investigated the cellular uptake of bovine serum albumin (BSA) coated GO (flake size ~ 500 nm or ~1 µm) by mouse mesenchymal progenitor C2C12 cells [93]. Results show that small GO (~500 nm) are internalized by clathrin-mediated endocytosis whereas large GO (~1 µm) are internalized by phagocytosis. Large GO sheets translocate into the reticuloendothelial system and small GO sheets are accumulated in various organelles.
Mbeh et. al. have reported the cytotoxicity of albumin functionalized GONRs against A549 cells evaluated using Trypan blue and apoptosis (hoechst and propidium iodide staining) [94]. A dose-dependent cytotoxicity was observed wherein albumin functionalized GONRs at concentrations < 50 µg/ml did not exhibit significant cytotoxicity, whereas incubation of A549 cells with higher concentrations (100 µg/ml) resulted in loss of cell proliferation and induction of apoptosis.
4. In vivo toxicology
A crucial step in the toxicological assessment of graphene-based formulations is their dose- and/or time-dependent safety pharmacological assessment in small and large animal models under various modes of administration (e.g. intravenous. intraperitoneal, oral). Table 2 summarizes the cytotoxicity of graphene and graphene oxide (GO) assessed in animal models.
Table 2.
In vivo toxicity of graphene
| Intravenous Administration | ||||||
| Material |
Characte rization |
Properties |
Animal Model |
Dose, Route and Duration |
Conclusion | References |
| GO, rGO | HR-TEM, Zeta- potential, UV-Vis NIR Spectrosc opy, flow cytometry and fluorescen ce spectrosco py |
GO: Size = 0.2–5 µm, Bilayer GO sheets with intersheet distance of 0.40±0.02 nanometer. FFT diffraction pattern confirms single and bi-layer GO. FTIR peak at 1735 cm−1 confirming C=O groups. rGO : Size = 0.2–5 µm, UV Vis: Red shift to 260 nm. FTIR confirms removal of oxygenated functional groups. |
Swiss male mice (8–12 weeks old) |
Intravenous administration. 250µg/kg dose injected via tail vein. Lungs collected for histological analysis (H&E staining) after 15 minutes. |
GO: Thrombotoxicity, pulmonary embolism and human platelet aggregation observed. Nearly 48 % lung vessels totally or partially obstructed by platelet thrombosis. rGO: Limited platelet aggression and reduced thromboembolism compared to GO. rGO induce ~ 8% total or partial obstruction of lung blood vessels. |
Singh et. al. [87] |
| Amine Graphene Oxide (NH2- GO) |
HR-TEM, FTIR, Raman spectrosco py, Zeta- potential, UV-Vis NIR spectrosco py, Flow cytometry |
Sheet size: 2 µm, FTIR peaks: 950 cm−1, 1250 cm−1, 1573 cm−1; Raman spectroscopy: G band = 1580 cm−1, D band = 1350 cm−1. High absorbance in visible and NIR region. No detectable fluorescence properties. |
Swiss male mice (8–12 weeks old) |
Intravenous administration. 250µg/kg dose injected via tail vein. Lungs collected for histological analysis (H&E staining) after 15 minutes. |
NH2-GO No thrombotoxicity. Blood vessels appear normal with no indication of occlusive pathology |
Singh et. al. [95] |
| FLG, FLG- COOH, FLG- PEG, 99mTc- FLG- COOH, 99mTc- FLG- PEG |
HR-TEM, AFM, Raman spectrosco py, FTIR, BET |
Graphene sheets with 2 – 4 layers; Size: 128 ± 37.6 nm; Thickness: 0.7 ± 0.31 nm; Raman peaks at 1325 cm−1, 1575 cm−1, and 2640 cm−1 corresponding to D, G, and 2D bands, respectively; BET surface area = 210 – 650 m2/g. FTIR peaks at 3400 cm−1, 1715 cm−1 |
Swiss albino mice (4–5 weeks old) |
Intravenous administration. A single dose of 20 mg/kg injected via tail vein. Brain, kidney, lungs, liver, spleen, intestine, heart and testis were collected after 1, 8, 30, and 90 days. |
Mice survived after 90 days of graphene administration. Body Weight of mice on days 60, 70, 80, and 90 were significantly lower than untreated mice. |
Sasidharan et. al. [96] |
| GO-Dex, 125I-GO- Dex |
AFM, FTIR, TGA, UV-Vis |
Size: 50–100 nm; Thickness: 2.8 nm; UV-VIS absorbance peak at 230 – 240nm |
Female Balb/c Mice |
Intravenous administration. 20 mg/kg GO-Dex injected into tail vein. Major organs collected for histology after 1, 3 and 7 days post injection. 125I-GO- Dex injected at a dose of 4mg/kg. Blood collected for pharmacokinetics and biodistribution study at 4, 24, 72, and 168 hours post injection. |
No short-term toxicity, excretion via renal and fecal pathways. |
Zhang et. al. [97] |
| GO, 188Re-GO |
AFM, Raman spectrosco py, zeta potential |
Size: 100–800nm; Thickness: 1nm; Single layered sheets; Zeta potential = −29.87 (GO), −20.47 (188Re- GO) |
Kun Ming Mice (Spragu e- Dawley Rats, 6– 8 weeks old) |
Intravenous administration. GO: 1 and 10 mg/kg dose. Histopathological analysis of lung, liver, spleen and kidneys performed after 14 days post injection. 188Re- GO: 200µl (50 µCi) dose. Biodistribution measured after 1, 3, 6, 12, 24, and 48 hours post injection. |
Dose dependent toxicity. No significant pathological changes were observed after day 1 whereas inflammation, cell infiltration, pulmonary edema and granuloma formation were observed after 14 days. GO exhibits high blood half life (5.3 ± 1.2 hours) and low RES uptake. Maximum uptake was observed in lungs. |
Zhang et. al. [98] |
| GO | AFM, TEM, FTIR |
Monolayer sheet; Thickness: 1nm; FTIR peaks at 3395 cm−1 (O-H), 1726 cm−1 (C=O), 1426 cm−1 (O-H), 1226 cm−1 (C-O), 1052 cm−1 (C-O) |
Female Kunmin g Mice (Spragu e- Dawley Rats, 4– 5 weeks old) |
Intravenous administration. Doses: 0 mg, 0.1, mg, 0.25 mg and 0.4 mg per mouse injected via tail vein. Histology and biodistribution analysis performed after 1, 7, and 30 days post injection. |
No toxicity observed upto 0.25 mg dose. Chronic toxicity observed for 0.4 mg dose with 4/9 mice death. Long-term accumulation observed in liver, kidneys and spleen along with granuloma formation in lungs. No accumulation in brain - GO cannot pass blood brain barrier. |
Wang et. al. [99] |
| GO, 125I- GO |
AFM, TEM, Raman Spectrosc opy, Infrared Spectrosc opy, particle size distributio n, zeta potential, DLS |
Size: Large GO = 1– 5µm, Small GO = 110–500nm; Thickness: 0.9 nm (single layer); Dh for large GO = 914nm, Dh for small GO = 243nm. |
Male ICR Mice |
Intravenous administration. Single dose of 1– 10 mg/kg administered via tail vein injection for biodistribution and pharmacokinetics studies. Tissue samples collected after 2–180 min post injection. For ultrastructural observation, 10 mg/kg dose was administered and lungs and liver collected 10 min post injection. |
GO elimination from blood was observed. Large GO sheets accumulated in lungs and small GO accumulated in liver. Small GO has a longer blood half-life than large GO. |
Liu et. al. [100] |
| NanoGra phene Sheets functiona lized with polyethyl ene glycol (NGS- PEG) |
AFM, FTIR, UV-Vis, NIR fluorescen ce |
Size: 10–50 nm; Single or bi-layered sheets; FTIR peaks at 2800 cm−1 (C-H) and 1100–1500 cm−1 (C-O) |
Tumor bearing Balb/c Mice |
Intravenous administration. Single dose of 20 mg/kg. Organs harvested after 1, 6, and 24 hours post injection. |
High tumor build up, no sign of abnormalities on the kidney, spleen, heart, liver and lung. Gradual elimination. Low uptake by RES. Photothermal therapy resulted in disappearance of tumor after 1-day treatment and an increase in the longevity of mouse by at least 24 days. |
Yang et. al. [101] |
| NanoGra phene Sheets functiona lized with polyethyl ene glycol (NGS- PEG), 125I- NGS- PEG |
AFM, FTIR, XPS |
Size: 10–30 nm; Single or bi-layered GO sheets; FTIR peaks at 2800 cm−1 (C-H) and 1100– 1500 cm−1 (C-O) |
Balb/c Mice |
Intravenous administration. Pharmacokinetics study: Mice injected with 4- mg/kg doses of 125I-NGS-PEG and blood drawn between 0 – 25 hours. Biodistribution study: 4mg/kg dose injected then sacrificed at 1 h, 6 h, 1 d, 3 d, 7 d, 15 d, 30 d, 60 d. Blood biochemistry/Hem atology study: injected intravenously 20 mg/kg sacrificed at 3, 7, 20, 40, and 90 days. |
NGS-PEG mainly accumulates in the reticuloendothelial system and can be gradually cleared by renal and fecal excretion. |
Yang et. al. [102] |
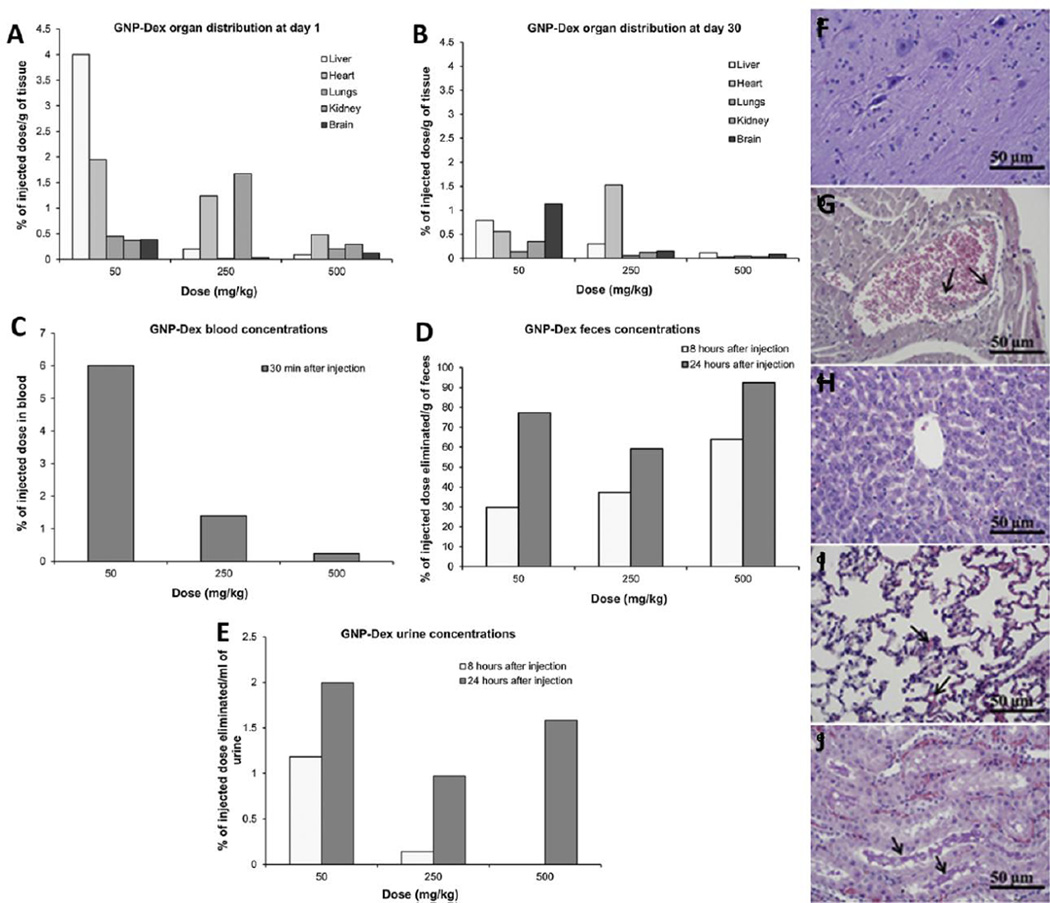
| GNP- Dex |
TEM, AFM |
Disc Shaped; Diameter: 100 nm; Thickness: 3 nm |
Wistar Rats |
Intravenous administration. Dose: 1, 50, and 100 mg/kg 3 times a week for 3 weeks. Heart, liver, kidney and brain harvested for histology. |
1 mg/kg and 50 mg/kg doses show no sign of toxicity. All vital parameters such as body weight, blood pressure, breathing and heart rate were normal. Two out of 8 animals died for 100mg/kg dose after 2 weeks. |
Kanakia et. al. [103] |
| GNP- Dex |
AFM | Diameter range: 60 – 100 nm. Thickness: 2 – 4 nm. Soluble in water up to 100 mg/ml |
Male hamster s |
Hamsters' left cheek pouch was exteriorized, pinned across a Lucite pedestal, and cleared of connective tissue. GNP-Dex was micropipetted at dosages of 0, 0.1, 0.5, 2.6, 10, 50 mg/ml at 30 seconds exposure and 5-minute washout between dosages. |
GNP-Dex showed no signs of arteriole dilation upto concentrations of 50mg/ml. |
Chowdhury et. al. [89] |
| GNP- Dex |
TEM, AFM |
Disc Shaped; Diameter: 100 nm; Thickness: 3 nm |
Wistar Male Rats |
Intravenous administration. Single doses of 1, 25, 50, 125, 250 and 500 mg/kg were injected via tail vein. Organs were harvested 1 and 30 days post injection of GNP- Dex for biodistribution and histopathological analysis. |
Maximum Tolerable dosage is between 50 mg/kg and 125 mg/kg. No changes in brain, neither cardiovascular, nor hematological factors at dosages less than 125 mg/kg. |
Kanakia et. al. [104] |
| GO, GO- NH2, GO- DOTA, 111In- DOTA- GO |
TEM, AFM, FTIR, XPS, TGA, Raman spectrosco py |
Size: 50–2000 nm; Single or bilayered GO sheets, FTIR peaks at 3400 cm−1 (OH), 1729 cm−1 (C=O), 1624 cm−1 (H-O-H), 1374 cm−1 (O-H), 1225 cm−1 (C-O-C); ID/IG = 1.34 (GO-DOTA), 1.21 (GO), 0.31 (Graphite) |
C57BL/ 6 mice (6–8 weeks) |
Intravenous administration. A dose of 200 µl of 111In-GO-DOTA (5–6 MBq) was injected via tail vein (t = 1, 4, 24 hours) for SPECT/CT study. For biodistribution study, animals were injected with 1–2 MBq equivalent dose for 1, 4, and 24 hours. |
Maximum accumulation was observed in liver and spleen, however, GO translocation from liver to spleen was also observed. No bilary excretion or metabolism by hepatocytes was observed. GO was eliminated via urine. These results suggest that chemical functionalization of GO sheets can alter their biodistribution. |
Jasim et. al. [105] |
| Intraperitoneal Administration | ||||||
|
125I labeled GO, nGO- PEG, RGO- PEG and nRGO- PEG |
AFM | Size: Diameters of GO, nGO- PEG, RGO- PEG and nRGO-PEG are 450, 25, 50 and 27 nm, respectively. Average thickness of GO, GO-PEG, RGO-PEG, and nRGO-PEG are 0.94, 1.22, 4.43 and 5.66 nm, respectively. |
Female Balb/c Mice |
Intraperitoneal administration: 80 mice were intraperitoneally injected with GO, nGO- PEG, RGO-PEG, and nRGO-PEG at 50 mg/kg (20µCi) and euthanized 1, 7, 30, and 90 days post administration. Oral administration: 15 mice were orally injected with nGO-PEG at 100 mg/kg dose and euthanized at 1, 7, and 30 days post injection. Blood was collected for serum biochemistry and blood panel analysis. All major organs were collected for histology and biodistribution. |
High accumulation in liver and spleen after intraperitoneal administration. No accumulation was observed after oral administration. PEG GO derivatives were phagocytized in the RES system in a size and surface coating dependent manner. Despite long-term retention, no toxicity was observed in blood analysis, serum biochemistry and histology analysis. Toxicity and biodistribution of graphene is dependent on size, surface coatings and route of administration. |
Yang et. al. [106] |
| cGO and pGO (highly pure GO) |
TEM, AFM, FTIR, Raman spectrosc opy, UV- Vis |
Size: cGO: > 0.1 µm2, pGO: 0.01–0.02 µm2; Thickness ~1 nm. UV absorbance peak at 230 nm. Raman spectroscopy: D Band = 1350 cm−1, G Band = 1590 cm−1 |
C57BL/ 6 Mice (6–8 weeks old) |
Intraperitoneal administration: Dose 50 µg in 0.5 ml saline. Animals euthanized 24 hours and 7 days post administration and assessment of Inflammatory reactions, protein levels in peritoneal lavage and histology of diaphragm was performed. |
Highly purified single layered GO sheets show no signs of inflammation or granuloma formation upto 50-µg/animal dose administered intraperitoneally. |
Ali- Boucetta et. al. [107] |
| GO- pluronic hydrogel |
TEM, SEM, Rheolog y |
Size: 100–500 nm. Gelation at pH = 7, absorbs IR light |
Female Balb/c mice (6–7 weeks old) |
Intraperitoneal administration. Gel composition: 0.4% GO- 0.25–1% pluronic. GO- pluronic gel implanted in subcutaneous cavity of mice. After 3 and 8 weeks post implantation, dermal tissue was analyzed by histology. |
No toxicity was observed. Mild inflammation observed after 3 weeks of implantation. After 8 weeks, the number of macrophages reduced. No tissue necrosis, acute inflammation or tissue hemorrhaging was observed suggesting a good biocompatibility of GO- pluronic gels. |
Sahu et. al. [108] |
| GO, graphite, nanodia monds (ND) |
TEM, Zeta potential |
Size: 8–25 nm (GO), 3–4 nm (graphite and ND); Zeta potential: −83.8 ± 0.25 mV (GO), 12.5 ± 0.43 (graphite), −15.8 ± 0.55 mV (ND) |
Female Wistar rats (6 weeks old) |
Intraperitoneal administration: 40 mice were injected with a dose of 4 mg/kg administered for 4 or 12 weeks at 3- day intervals. Liver and blood collected post euthanasia for analysis of blood serum biochemical indices, blood morphology and liver physiology. |
No toxicity was observed. Blood biochemical indices and liver enzymes were within physiological range. Large aggregates of nanomaterials were observed at the injection site, smaller aggregates were observed in liver serosa and mesentery. |
Strojny et. al. [109] |
| Oral Administration | ||||||
| GO | TEM, AFM, XRD, FTIR, XPS, UV-Vis and Raman spectrosc opy, |
Size: 0.2 µm; Thickness = 1.8 nm; XRD peaks at 12° and 43°; UV-Vis peaks at 232 nm and 280 nm; FTIR peaks at 1715 cm−1 (C=O), 1120 cm−1 (C-O-C), 3400 and 1620 cm−1 (O-H); XPS - C:O molar ratio 2:11, C1s peaks at 285 eV (C=C), 286.1 eV (C-O), 287.5 eV (C=O), 288.5 eV (C=O); Raman peaks at 1355 cm−1 (D band), 1588 cm−1 (G band) and 2680 cm−1 (2D band). |
Female ICR mice (6–8 weeks old) |
Oral administration. Dose: 0.5 and 0.05 mg/ml. GO mixed with drinking water. Days 1–38 for maternal mice and 1–21 for filial mice. After 21 and 38 days, blood was collected for biochemistry analysis and organs were harvested for H&E staining. |
Decrease in body weight, body length and tail length for high concentration group. No significant differences in blood biochemistry. Pathological examination shows severe atrophy of all major organs. H&E staining of intestine showed increased villi and duodenum width. Results show that GO is toxic to developmental offsprings in mice. |
Fu et. al. [110] |
| 125I-rGO | TEM, Particle size distributi on |
Size: Small GO (~100nm), Large GO (~450nm) |
Male C57b/6 mice (6–8 weeks old) |
Oral administration. Dose 60mg/kg, administered via oral gavage every 24 hours for 5 days. |
Initial decrease neuromuscular coordination and locomotor activity, which were restored to normal levels at later time points (15 and 60 days post administration). No significant differences in blood biochemistry, liver function, kidney function, blood enzyme levels, learning, memory, anxiety, and spatial and exploratory behaviors. rGO administered via oral administration is non-toxic. |
Zhang et. al. [111] |
| GO | TEM, AFM, FTIR, Raman spectrosc opy and Particle size tracking |
Size: 72 ± 11 nm; Thickness: 1 nm, FTIR peaks: 3425 cm−1 (O-H), 1749 cm−1 (C=O), 1630 cm−1 (C=O), 1130 cm−1 (C-O). Raman peaks at 1377 cm−1 (D band) and 1609 cm−1 (G band); Zeta potential = −20.2 mV. |
Caenor habditis elegans |
Oral administration. GO mixed with food (K medium) at 0.1– 100 mg/L. Acute exposure (24 hours) and prolonged exposure (larvae to adult). Lethality, growth, reproduction and locomotion behavior was analyzed. |
Prolonged exposure to 0.5– 100 mg/L of GO caused damage in primary (intestine) and secondary (neurons and reproductive) organs. GO translocated into intestinal wall due to loss of villi and were distributed surrounding mitochondria. Additional observations were increased mean defecation cycle length and hyper- permeable state of intestinal barrier. Results suggest that GO exposure to environmental organisms is toxic. |
Wu et. al. [112] |
| Pulmonary Administration | ||||||
| GNPs | SEM, EPR, BET, density measure ment, Aerodyn amic diameter, ICP-MS |
Size: Diameter = 5.64 ± 4.56 nm, Layers = 1–10; Surface area = ~100m2/g; Density = ~2; EPR = 867.3± 77.5 a.u. |
Female C57BL/ 6 mice (9 weeks old) |
Pharyngeal administration. Dose - 50 µg/mouse. Mice sacrificed after 24 hours and 1-week post exposure. BAL fluid was extracted. Intrapleural injection: Dose - 5µg /mouse. Mice sacrificed after 24 hours and 7 days. Pleural space was lavaged and the surrounding tissue was excised for histology. |
GNPs upto 25 µm are respirable and deposit beyond ciliary airways post inhalation. GNPs induced inflammation in lung and pleural space with an increase in the levels of MIP-1α, MCP-1, MIP-2, IL-8 and IL-1β. SEM images revealed signs of frustrated phagocytosis. Inflammatory response decreased one-week post exposure. |
Schinwald et. al. [113] |
| Graphene (aggregat ed in water), Graphene (2% pluronic) , GO |
AFM, XPS, Raman spectrosc opy, optical absorban ce |
Optical absorbance: 600nm; Size: 40,000 nm2 (Graphene), 200,000 nm2 (GO); Thickness: 1.2–5 nm (Graphene), 0.5–2 nm (GO) |
Male C56BL/ 6 mice (8–12 weeks old) |
Pulmonary administration. Dose - 50 µg/mouse administered via intratracheal instillation. GO is injected directly into the lungs. Lung assessment post 24 hours using histology and electron microscopy |
Inflammation, apoptosis, increase of mitochondrial respiration and pulmonary inflammation were observed. |
Duch et. al. [114] |
| 125I-GO | TEM, SEM, AFM, FTIR, Raman spectrosc opy |
Size: 10–800 nm; Thickness: 1 nm; FTIR peaks at 1731 cm−1 (O-H), 1628 cm−1 (C=C), 1078 cm−1 (C-O); Raman peaks: 1333 cm−1 (D band), 1594 cm−1 (G band). |
Male Kunmin g mice (biodist ribution ) and male C57BL/ 6 mice (pulmo nary toxicity ) |
Pulmonary administration. Dose: 1, 5, 10 mg/kg for dose dependent acute and chronic pulmonary toxicity assessed after 24 hours. 10mg/kg for time dependent toxicity assessed after 0, 24, 48, 72 hours and 1 week. 10 mg/kg for long-term chronic toxicity assessed after 1 and 3 months. Biodistribution evaluated by SPECT imaging, pulmonary toxicity by histology and cell injury, lung edema and neutrophil infiltration assays. |
Acute lung injury, thickening of alveolar septa, increased neutrophil counts and oxidative damage were observed. GO can pass through air-blood barrier albeit in smaller amounts and care must be taken to ensure minimal exposure by inhalation during large- scale production of GO. |
Li et. al. [115] |
| Intravitreal administration | ||||||
| GO | AFM, FTIR, Raman spectroscopy |
Size: 50–500 nm; Thickness: 1 nm; FTIR peaks at 3430 cm−1, 1720 cm−1, and 1000 cm−1; Raman Spectroscopy: D band at 1370 cm−1, G band at 1590 cm−1. ID/IG = 0.75 |
Japanese White Rabbits (2–3 kgs) |
Intravitreal administration. Dose: 0.1, 0.2, or 0.3 mg. Eye function was measured using electroretinography (ERG) after 2, 7, 28 and 49 days post injection. After 49 days, animals were euthanized and eyes were collected for histological examination. Balanced sale solution was used as the controls. |
No significant differences in ERG amplitudes compared to controls. H&E staining showed small amounts of GO residue, however, no retinal abnormality was observed. |
Yan et. al. [116] |
4.1 Intravenous administration
Intravenous (IV) administration is a widely employed method wherein a needle is inserted into the vein and formulation is administered through that needle. It is the preferred mode of systemically introducing pharmaceutical formulations for imaging, drug delivery or therapy. Singh et. al have investigated the in vivo platelet aggregation of GO and rGO nanosheets. GO and rGO sheets were administered intravenously via tail vein injection to Swiss male mice (8–12 weeks old) at 250 µg/kg dose for 15 minutes [87]. A collagen-epinephrine mixture was administered as positive control whereas saline was used as the negative control. After 15 minutes post injection, the mice were euthanized and lungs were harvested for histological analysis. Hematoxylin and eosin (H&E) staining showed ~48% thromboembolism whereas the collagen – epinephrine control solution resulted in ~64% occlusion of blood vessels. rGO was not as effective as GO towards platelet activation; rGO administration resulted in ~ 8% blood vessel blockage, significantly less than GO. These results show that GO induces severe pulmonary thromboembolism that may be attributed to the greater surface charge density of graphene surface upon oxidation. In a follow-up study, Singh et. al. investigated the in vivo thrombogenic properties of amine-modified GO (NH2-GO) [95]. Compared to GO which induces platelet aggregation, NH2-GO does not elicit any stimulatory effects on platelets or pulmonary thromboembolism. H&E staining revealed that GO resulted in ~46% blockage of pulmonary blood vessels while NH2-GO showed no signs of obstruction.
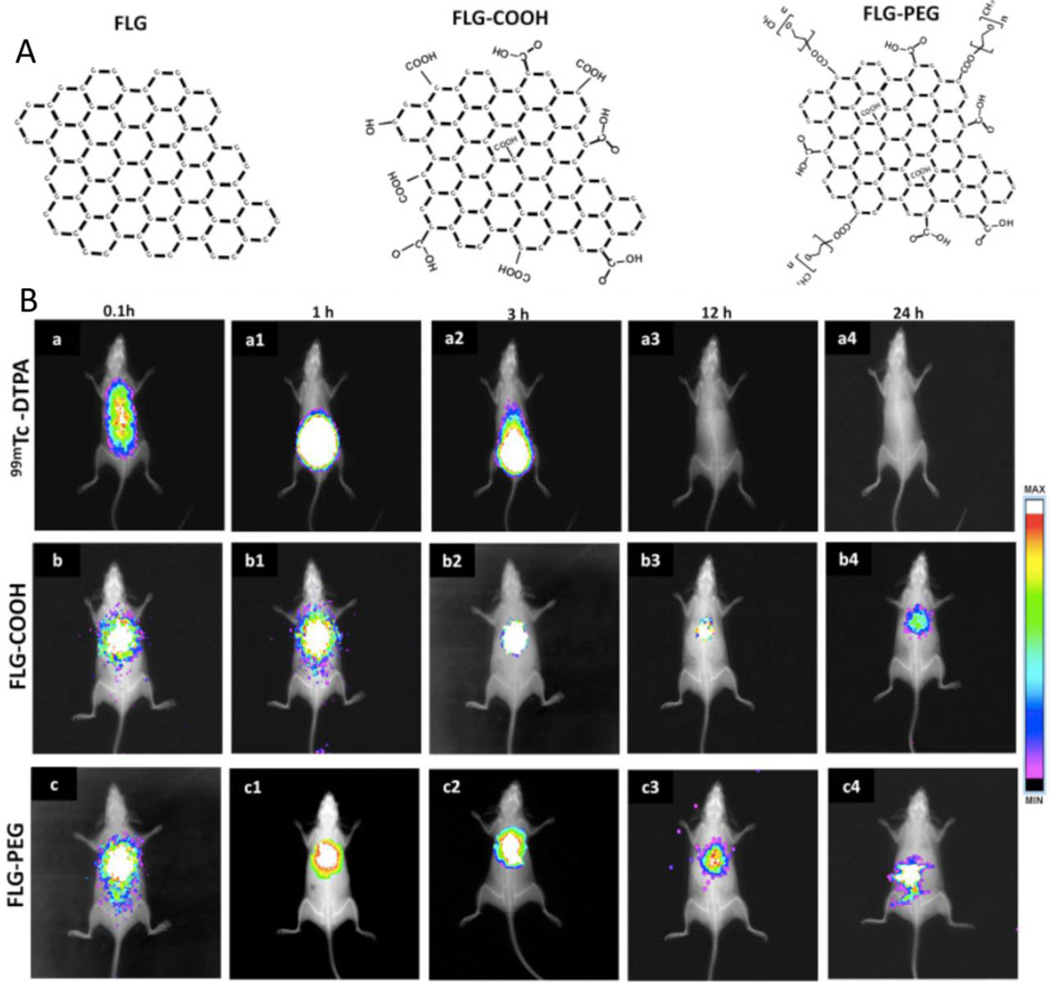
Sasidharan et. al. have reported the long term in vivo toxicology of pristine and functionalized few layered graphene (FLG), FLG-COOH and FLG-PEG (Figure 9A) administered intravenously to Swiss albino mice at 20 mg/kg for 1, 8, 30, and 90 days [96]. Sterile physiological saline was administered to control animals. All animals survived over the course of 90 days, however, the body weight of FLG, FLG-COOH and FLG-PEG treated mice was lower on days 60–90 compared to control mice. To dynamically track the in vivo biodistribution, 99mTc labeled FLG-COOH and FLG-PEG were injected and whole body images were captured at 0.1, 1, 3, 12, and 24 hours (Figure 9B). 99mTc-FLG-COOH showed accumulation and retention in lungs over 24 hours. However, after 12 hours, 99mTc-FLG-PEG was redistributed to RES system such as spleen and liver. FLG-COOH accumulated in the lungs resulting in thicker alveolar walls. Injection of FLG and FLG-COOH resulted in extensive spleen damage including the loss of dividing line between red pulp and marginal zone, abundance of megakaryocytes in the red pulp 90 days post injection, and lack of lymphocytes in the white pulp. On the contrary, FLG-PEG did not result in any injury to the marginal zone and only several black spots in the red pulp were observed. Liver tissue analysis revealed that FLG and FLG-COOH induced liver tissue degeneration while FLG-PEG did not and was observed as black spots. However, kidney necrosis was observed upon administration of both FLG and FLG-COOH as early as the first day of administration. FLG-PEG was present as black spots on the tissue but did not exhibit any signs of necrosis. FLG, FLG-COOH, and FLG-PEG did not damage brain, heart, or testis suggesting that graphene cannot pass through the blood-brain barrier.
Figure 9.
(A) Schematic illustrating structural depiction of few layered graphene (FLG), FLG-COOH and FLG-PEG. (B) Real time in vivo biodistribution of 99Tc labeled FLG, FLG-COOH, FLG-PEG, signal accrued for 24 hours. Adapted from Reference [96] with permission, copyright © Elsevier, 2015.
Zhang et. al. have reported the toxicity of dextran functionalized graphene oxide (GO-Dex) intravenously administered via tail vein injection to female Balb/c mice at 20mg/kg dose for 1, 3, and 7 days [97]. H&E staining of liver sections showed a significant increase in black spots – areas of GO aggregation - after 7 days indicating clearance of GO-Dex from mouse liver. For biodistribution and pharmacokinetic studies, 125I labeled GO-Dex (125I-GO-Dex) was injected via tail vein at 4 mg/kg concentration and blood was collected after 4, 24, 72, and 168 hours. After 4 hours of administration, 125I-GO-Dex was found in liver, spleen, stomach, lungs, kidney, and intestine. At later time points, 125I-GO-Dex was predominantly found in the liver and spleen. Histological sections of liver and kidney confirm the presence of 125I-GO-Dex as black dots that were abundant at day 1 and decreased at later time points suggesting the excretion of 125I-GO-Dex via renal and fecal pathways. Since GO-Dex has a wide size distribution, small GO-Dex sheets could pass through glomerulus for renal excretion and large GO-Dex sheets accumulated in RES organs could be excreted out in feces via biliary pathway.
Zhang et. al. have reported the distribution and biocompatibility of GO after intravenous administration to male Sprague Dawley rats at 1 and 10 mg/kg dose [98]. Histopathological analysis of lung, liver, spleen and kidneys performed 14 days post injection shows no pathological changes for all organs examined for 1 mg/kg dose. For 10 mg/kg treatment, all organs except lung showed normal pathophysiology. However, due to high accumulation and slow clearance, GO accumulated in lungs resulted in pulmonary edema, granulomatous lesions, inflammatory cell infiltration, and fibrosis. These results suggest that GO is biocompatible, however, accumulation in lungs at higher concentration may lead to safety concerns. Biodistribution of GO was assessed by tracking intravenously administered 188Re labeled GO (188Re-GO) after 1, 3, 6, 12, 24, and 48 hours. It was observed that GO cleared from blood, accumulated in lungs, liver and spleen and was up taken by mononuclear phagocytes in the reticuloendothelial system.
Wang et. al. have reported the biocompatibility of GO administered via tail vein injections to 4–5 week old female kunming mice (Sprague Dawley rats) at 0, 0.1 mg (low), 0.25 mg (medium), and 0.4 mg (high) doses [99]. No toxicity was observed for low and medium doses. However, for high dose, 4 out of 9 mice died after 1 week due to airway blockage caused by accumulation of GO. Histology analysis after 1, 7, and 30 days shows long-term accumulation of graphene in liver, kidney and spleen. Granuloma formation along with the presence of neutrophils and foamy alveolar macrophages was observed in lungs suggesting a foreign body immune response. No accumulation was observed in brain suggesting that GO cannot pass the blood brain barrier. These results suggest that GO is non-toxic at low concentrations and results in irreversible airway damage and chronic pulmonary toxicity at high concentrations.
Liu et. al. have reported the dose- and size- dependent toxicity and biodistribution of GO sheets [100]. Male ICR mice were intravenously injected with small and large GO sheets (s-GO and l-GO) labeled with 125I to enable radioactive tracking of tissue biodistribution, organ accumulation and blood clearance of GO after 2–180 min post injection at 1–10 mg/kg doses. It was observed that s-GO mainly accumulated in the liver with some aggregates present in lungs and spleen, however, after 180 minutes, clearance of s-GO was observed with a residual accumulation of ~11% in liver and <1% in lungs. On the contrary, in comparison to s-GO, l-GO showed higher accumulation in lungs with a residual accumulation of ~19% after 180 minutes. TEM analysis of lung sections show intracellular accumulation of s-GO in phagocytic cells while l-GO particles (bigger than 1 µm) was lodged in cell gaps of lungs. The size-regulated biodistribution of s-GO and l-GO was attributed to the different aggregation states of the nanoparticles. The less dispersed GO resulted in the formation of larger GO-protein complexes, which were filtered by the pulmonary blood vessels. At higher doses, s-GO aggregated to large particulates resulting in the entrapment in lungs. The blood half-life of s-GO was 2.2 minutes (T1/2 alpha) and 170 minutes (T1/2 beta). For l-GO, T1/2 alpha was 1.8 minutes and T1/2 beta was 102 minutes. These results suggest that s-GO possesses longer blood retention time than l-GO.
Yang et. al. have reported in vivo biodistribution and photothermal activity of PEG functionalized nano graphene sheets (NGS-PEG) [101]. Cy7 dye labeled NGS-PEG was intravenously injected into tumor bearing Balb/C mice at a dose of 20 mg/kg and organs were harvested after 1, 6, and 24 hours. Significant accumulation of NGS-PEG was observed in tumors due to leaky vasculature along with low accumulation in RES organs. After 24 hours post injection, the kidneys showed strong fluorescence (Figure 10) attributed to the renal excretion of small sized NGS particles. NGS-PEG showed no toxicity - neither death nor significant weight loss was observed in all animals. After NGS-PEG administration, the tumors on the right shoulder of 4T1 Balb/c mice were exposed to 808 nm laser until the surface temperature reached ~50°C. The tumors disappeared 1-day post laser treatment leaving a black scar that disappeared after one week. No tumor regrowth was observed after 40 days. These results suggest that PEG functionalized graphene can be suitable for in vivo photothermal therapy applications. In another study, Yang et. al. have reported the long term in vivo biodistribution and pharmacokinetics of 125I-labled NGS-PEG intravenously administered in Balb/c mice at 4 mg/kg dose [102]. For pharmacokinetics study, blood was drawn after 0–25 hours and measured by a gamma counter. To examine the biodistribution of 125I-NGS-PEG, 4 mg/kg was administered intravenously and organs were harvested at various time points: 1 hour −60 days post injection. NGS-PEG initially accumulated in several organs, however accumulation at later time points was observed in liver and spleen. H&E staining of liver and spleen sections showed a reducing number of NGS-PEG aggregates over time suggesting removal of NGS-PEG from RES system. Renal pathway cleared out smaller sized NGS-PEG (10 nm diameter) while larger NGS-PEG aggregates were excreted through biliary pathway into the feces. Blood biochemistry and hematology analysis showed normal levels of urea, blood cells, hemoglobin and other factors suggesting that there were no toxic effects of NGS-PEG to liver and kidneys. These results suggest that NGS-PEG does not exhibit long-term in vivo toxicity in mice.
Figure 10.
Biodistribution analysis of Cy7 labeled PEG functionalized nano graphene sheets (NGS-PEG-Cy7). Tumor bearing 4T1 mice were sacrificed after 1, 6, and 24 hours of NGS-PEG-Cy7 administration. (A) Spectrally resolved ex vivo fluorescence images of SK-skin, M-muscle, I-intestine, H-heart, LU-lung, LI-liver, K-kidney, SP-spleen, ST-stomach, and T-tumor. (B) Chart depicting semi quantitative biodistribution of each organ for n=3 mice per group. Adapted from Reference [101] with permission, copyright © American Chemical Society, 2010.
Kanakia et. al. have reported the sub acute toxicity of dextran functionalized graphene nanoplatelets (GNP-Dex) administered via intravenous injections to Wistar rats at 1, 50, and 100 mg/kg doses 3 times a week for three weeks [103]. No signs of toxicity were observed for 1 mg/kg and 50 mg/kg doses. All vital parameters such as body weight, blood pressure, breathing and heart rate were normal. However, for 100 mg/kg dose, 2 out of 8 animals died after 2 weeks. A complete blood count analysis showed physiological levels of blood urea nitrogen and creatinine indicating normal kidney function. ALT and ALP levels were elevated, however, blood glucose was normal. Histology analysis after 3 weeks showed the presence of GNP-Dex in hepatic kuppfer cells and pulmonary alveolar macrophages, which increased with increasing dose of GNP-Dex (Figure 11). No adverse effects or inflammation were observed in brain, heart, spleen and kidney.
Figure 11.
Representative H&E staining of lung and liver sections post GNP-Dex administration at 1, 50, and 100 mg/kg in Wistar rats. Pigmentation (arrows, A–C) was observed within alveolar macrophages in lungs at all GNP-Dex administration concentrations indicating the presence of graphene nanoparticles. (D) Sham lungs showed no diagnostic abnormalities. Liver sections at 1 mg/kg (E) showed minimal at liver steatosis, at 50 mg/kg (F) showed pigmented macrophages in Kupffer cells indicating the presence of graphene. No signs of inflammation were observed. At 100 mg/kg dose (G), an increase in pigmentation was observed. (H) Sham liver sections showed no diagnostic abnormality. Adapted from Reference [103] with permission, copyright © Kanakia et. al. (open access, Nature Scientific Reports), 2015.
Mullick Chowdhury et. al. have reported the in vivo vasoactivity of GNP-Dex using male hamsters cheek pouch model [89]. GNP-Dex was administered at doses ranging from 1–50 mg/ml to the excised left cheek pouch tissue of hamsters using a micropipette. The arcade-terminal arteriolar network junction was the microvascular observation site. The baseline diameters of arcade and terminal arterioles were 23 µm and 8 µm, respectively. The administration of 0.1 mg/ml and 0.5 mg/ml GNP-Dex had no significant effect on the arteriole diameters. No significant differences in the dilation of arterioles were observed at higher doses of 10 mg/ml and 50 mg/ml. However, the administration of FDA-approved natural biopolymer dextran at 35 mg/ml resulted in ~23% dilation of arcade arterioles and ~ 63% dilation of terminal arterioles. The lack of dilation post GNP-Dex administration and an increased dilation due to dextran suggests that the observed minor vasoactive effects of GNP-Dex could be due to the dextran coating of GNPs.
In another study, Kanakia et. al. have evaluated the histopathology and biodistribution of GNP-Dex administered via intravenous injections in male Wistar rats at doses between 1–500 mg/kg after 1 and 30 days [104]. The results show that the maximum tolerable dose (MTD) of GNP-Dex is between 50–125 mg/kg. Blood half-life of GNP-Dex is ~30 minutes. Maximum accumulation of GNP-Dex after day 1 was found in liver and kidney, which reduced (at least 2–4 folds) after 30 days of administration suggesting a clearance of GNP-Dex via RES system (Figure 12 A&B). ICP analysis showed that GNP-Dex administered at 50 mg/kg had a higher blood concentration than 500 mg/kg doses 30 minutes post-administration (Figure 12C). Majority of GNP-Dex nanoparticles were excreted via feces (~60–90%) within 24 hours (Figure 12D), small amounts were excreted via urine (Figure 12E). Histopathological changes (Figure 12 F–J) were observed in heart, lung, liver, kidney and spleen at high treatment concentrations (250 µg/ml). No adverse effects were observed in brain. Hematological factors and cardiovascular parameters remained at physiological levels upto 125 mg/ml treatment doses. These results suggest that GNP-Dex is non-toxic with a MTD of 125 mg/kg.
Figure 12.
(A&B) Tissue biodistribution, (C) blood half life, (D) elimination via feces and (E) urine after GNP-Dex administration at doses 50–500 mg/kg to Wistar rats analyzed via ICP-MS. Liver and kidney showed maximum uptake after 24 hours of administration. Majority of GNP-Dex was excreted via feces; small amounts were cleared via urine. Histological sections of (F) cerebral cortex, (G) myocardium, (H) liver, (I) pulmonary parenchyma and (J) renal cortex after 24 hours of GNP-Dex administration at 250 mg/kg dose. No diagnostic abnormalities were observed in cerebral cortex and liver. Vascular congestion of myocardium was observed. Arrows in (G) show dilated vein containing debris of GNP-Dex. Mild focal congestion was observed in the alveolar capillaries of pulmonary parenchyma. Vascular congestion and proteinaceous casts were observed in renal tubules of renal cortex. Adapted from Reference [104] with permission, copyright © Elsevier, 2014.
Jasim et. al. have reported the in vivo biodistribution of chemically functionalized graphene (GO-DOTA) labeled with 111In after intravenous injections in C57BL/6 mice at 200 µl dosage [105]. Post 1, 2, and 24 hours of administration, 111In-DOTA-GO was accumulated in bladder and excreted via urine. No fecal elimination was observed. Maximum accumulation was observed in liver and spleen. Furthermore, at later time points, translocation of GO from liver to spleen was also observed. No organ damage was observed at all time points. These results show that chemically functionalized GO sheets are non-toxic and possess distinctly different physiological behavior (biodistribution and excretion characteristics) than pristine or non-covalently functionalized graphene sheets.
4.2 Intraperitoneal administration
Intraperitoneal (IP) administration is the injection of the formulation into the peritoneum (or body cavity). Yang et. al. have reported the in vivo toxicity of PEG functionalized GO administered intraperitoneally and orally in female balb/c mice [106]. PEG functionalized and 125I labeled nano-graphene oxide (nGO-PEG), reduced graphene oxide (rGO – PEG), and nano reduced graphene oxide (nrGO – PEG) of diameters 25, 50, and 27 nm, respectively, were administered intraperitoneally at 50 mg/kg dose and orally at 100mg/kg. Animals were euthanized post 1, 7, 30 and 90 days post intraperitoneal administration and 1, 7, and 30 days post oral injections. All major organs were collected for histology and biodistribution analysis and blood was collected from the orbital for complete blood panel and serum biochemistry analysis. The radioactivity of GO formulations after oral administration was undetectable after 1 week suggesting negligible uptake of PEGylated GO administered orally. However, after intraperitoneal administration, PEGylated GO showed high accumulation in RES organs (black colored liver and spleen) after 1 and 7 days. Larger sized RGO-PEG showed higher uptake (> 2 fold, determined by radioactivity measurements) than smaller nGO-PEG and nrGO-PEG formulation. No animal death, body weight loss, inflammation, or significant changes in bloody panel or serum biochemistry were observed after 90 days post intraperitoneal administration indicating no signs of toxicity. These results suggest that PEGylated GO do not elicit any adverse effects under the above conditions in rodents, and the biodistribution and clearance profiles depend on the size, surface coating and route of administration.
Ali-Boucetta et. al. have investigated the in vivo pathogenicity of highly pure, colloidally stable dispersions of GO [107]. Conventional GO (cGO, size > 0.10 µm2) prepared using Hummer’s method was subjected to several purification steps to obtain highly pure GO (pGO, size 0.01 µm2 to 0.02 µm2). Both, cGO and pGO had similar chemical functional groups (carbonyls, hydroxyls and epoxides). pGO sheets were administered intraperitoneally at a dose of 50 µg/animal for 1 and 7 days. CNTs were used as positive controls. The inflammatory response was investigated by observing the change in protein levels and the change in the number of polymorphonuclear leucocytes 1 and 7 days post administration. After 1 day, pGO did not show a change in polymorphonuclear leucocyte (PMN) and protein levels whereas CNT controls induced at least 2-fold increase in total PMN count. After 7 days, there was accumulation of macrophages and giant cells with a deposition of collagen on the mesothelial membrane for CNT controls; pGO groups did not show any such effects. These results show that highly pure single layered GO sheets show no signs of inflammation or granuloma formation upto 50 µg/animal dose administered intraperitoneally.
Sahu et. al. have investigated the in vivo biocompatibility of GO dispersed pluronic gels administered intraperitoneally via implantation in subcutaneous pockets in 6–7 weeks old balb/c mice [108]. Mild inflammation was observed 3 weeks post implantation. After 8 weeks, the number of macrophages reduced and no chronic inflammation, tissue necrosis or hemorrhaging was observed. Furthermore, no gel degradation or degradation products were observed in the surrounding tissues.
Strojny et. al. have reported the intraperitoneal toxicity of GO, graphite and nanodiamonds administered to 6 weeks old female Wistar rats [109]. Nanoparticle suspensions were injected at a dose of 4 mg/kg for 4 or 12 weeks at three-day intervals. After 4 or 12 weeks, rats were euthanized and liver and blood were collected. Results show the presence of nanoparticle aggregates in the peritoneal cavity close to the injection site. Smaller aggregates were observed in the mesentery and liver serosa suggesting transportation and accumulation of nanoparticles in liver. No adverse health effects were observed for all nanoparticles (GO, graphite or nanodiamonds) at all time points (4 or 12 weeks). Blood analysis and liver enzyme levels were normal suggesting good liver biocompatibility.
4.3 Oral administration
In oral administration a formulation/substance is administered via mouth in cases where a systemic effect is desired. Fu et. al. have investigated the development of mice offsprings after oral administration of graphene oxide at 0.5 and 0.05 mg/ml to maternal mice [110]. GO suspension in drinking water was administered to female ICR mice (8–9 weeks old) from 1–38 postnatal days (PND). Filial mice were administered GO water during the suckling period from 1–21 PND and normal water during the weaning period from 22–38 PND. After 21 and 38 days, pups were weighed and euthanized. Compared to the control groups that received normal water, significant decrease in body weight, body length and tail length of filial mice were observed for 0.5 mg/ml treatment group. Blood biochemistry analysis showed no significant differences in the levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), blood urea nitrogen (BUN) and creatinine (CREA) for both the GO groups. Pathological examination of heart, lung, spleen, kidney and liver of filial mice administered with 0.5 mg/ml GO suspension showed severe atrophy (Figure 13 A). H&E staining of duodenum, ileum, jejunum (parts of small intestine) showed increase in villi length and duodenum width post GO administration (Figure 13 B). These results show that GO can have significant negative effects on the development of filial mice during the lactation period.
Figure 13.
(A) Pathological examination of lungs, heart, kidney, spleen and liver collected from control and GO administered mice (0.5 mg/ml) after 38 days showing severe atrophy of all major organs. (B) H&E staining of duodenum, jejunum and ileum of GO treated filial mice at 0.05 mg/ml for 21 days and 0.5 mg/ml for 21 and 38 days. The length, width and height of villi of GO administered groups were longer than control groups. Scale bars represent 100 µm. Adapted from Reference [110] with permission, copyright © Elsevier, 2015.
Zhang et. al. have investigated the short-term and long-term effects of reduced GO (rGO) on general locomotor activity, neuromuscular coordination, balance, anxiety, learning and memory of male C57b/6 mice (6–8 weeks old) using rotarod, open field and Morris water maze tests [111]. HEPES Buffer dispersed rGO were administered via oral gavage every 24 hours for 5 days at 60mg/kg dose. rGO treated mice maintained normal body weight, organ weight, and instinctive behaviors (eating etc.) compared to control mice administered with chow and HEPES buffer. However, initial 3–4 days post treatment, mice showed decreased neuromuscular coordination and locomotor activity failing the rotarod and open field tests. At later time points (15 and 60 days post administration), all these parameters returned to their normal state. No significant differences in blood biochemistry, liver function and kidney function and aging parameters were observed. The morphologies of neurons in the hippocampus and neuroglia cells as well as choline acetyl transferase and hippocampal acetylcholine esterase (enzymes involved in memory and learning) levels also remained normal post rGO administration. These results show that exposure to high concentration of rGO sheets via oral administration results in a short term decrease in neuromuscular coordination and locomotor activity which return to normalcy a few days post exposure; it does not affect learning, memory, anxiety, spatial and exploratory behaviors.
Wu et. al. have investigated the toxicity of graphene oxide at doses between 0.1–100 mg/L administered orally on nematode Caenorhabditis elegans after acute (24 hours) and prolonged exposure (larva to adult) [112]. GO was mixed with nematode food (K medium) and lethality, growth, reproduction and locomotion were analyzed. Results show that prolonged exposure at concentrations 0.5 mg/L and greater lead to significant primary (intestine) and secondary (neurons and reproductive) organ damage. Additionally, GO induces loss of villi and trans locates into the intestinal walls. Other adverse events noted were increased defecation cycle and hyper permeable intestinal barrier. These results show that GO upon exposure to environment would come into contact with nematodes, worms and other environmental organisms and may induce long-term adverse effects in the environmental flora.
4.4 Pulmonary administration
Schinwald et. al. have reported the in vivo toxicity of pristine GNPs after intrapleural and pharyngeal administration in 9 weeks old female C57BL/6 mice at 5 µg and 50 µg per mouse doses, respectively [113]. After 24 hours and 1 week of administration, a differential cell count of lavage fluid showed that mice exposed to GP had elevated levels (2× of physiological levels) of eosinophils and neutrophils. The chemokine and cytokine protein levels (MIP-1α, MCP-1, MIP-2, IL-8 and IL-1β) were also elevated. Microscopy imaging showed that pleural macrophages were not able to fully phagocytize GNPs due to their size and shape; multiple macrophages surrounding a single GNP forming a rosette-like cell/particle aggregation suggested frustrated phagocytosis. Histological evaluation showed extended retention of GNPs in pleural space and the formation of ganulomatous lesions in bronchiolar lumen. The initial inflammatory response to GNPs reduced after 1-week post administration; reduction in the number of inflammatory cells in the parietal pleura was observed. Clearance of GNPs from the pleural space to cranial mediastinal lymph nodes was observed. 1-week post administration, numerous small sized GNP fragments were observed in lymph nodes. This study shows that the toxicity of GNPs is dependent on the nanomaterial shape and size. The 2D size of GNPs leads to frustrated phagocytosis in lungs.
Duch et. al. have reported the pulmonary toxicity of three types of graphene (aggregated pristine graphene in water, pristine graphene in 2% pluronic and graphene oxide) administered via intratracheal instillation to male C56BL/6 mice at 50 µg/mouse dose [114]. After 24 hours of administration, mice were euthanized and lungs were analyzed by histology and electron microscopy. Results show that the pulmonary toxicity of graphene varies as a function of dispersion and oxidation state. Highly dispersed pristine graphene in pluronic co-polymer solution induces an acute non-fibrotic lung inflammation, which is significantly lower compared to the local fibrotic response induced by aggregated graphene. Pristine graphene in dispersed or aggregated form does not induce apoptosis or ROS generation in lung macrophages. However, GO formulations lead to persistent lung injury that lasts > 21 days (Figure 14). These results suggest that compared to GO, the use of pristine graphene may reduce potential health risks associated with pulmonary exposure.
Figure 14.
Aggregated graphene induces patchy fibrosis in mice. Mice were treated with highly purified and dispersed preparations of graphene in 2% Pluronic (Dispersed), aggregates of graphene in water (Aggregated) or GO in water (Oxide) by intratracheal instillation and 21 days later, the lungs were examined for markers of fibrosis. (a) Trichrome stained lung sections. (b) Sirius Red stained lung sections. (c) Total lung collagen determined by picrosirius red precipitation of whole lung homogenates (GD; dispersed graphene, GA; aggregated graphene, GO; graphene oxide). Adapted from Reference [114] with permission, copyright © American Chemical Society, 2011.
Li et. al. have analyzed the in vivo biodistribution and pulmonary toxicity of GO after intratracheal instillation in kunming mice at a dose of 0, 1, 5 or 10 mg/kg to evaluate dose-dependent acute and chronic pulmonary toxicity for 24 hours [115]. The authors have also evaluated time-dependent pulmonary toxicity by administering 10 mg/kg GO for 0, 24, 48, 72 hours and 1 week and chronic pulmonary toxicity at 10 mg/kg dose evaluated 1 and 3 months post instillation. Biodistribution was evaluated using SPECT imaging and pulmonary toxicity was assessed using histology and assays for cell injury, lung edema and neutrophil infiltration. Results show that GO was localized in the lungs even after 3 months of administration. Furthermore, GO induced a dose-dependent acute lung injury and resulted in chronic pulmonary fibrosis. A dose-dependent increase in neutrophils was observed in bronchoalveolar lavage fluid. Lung histopathological analysis showed alveolar septa thickening, extensive hemorrhage, changes in alveolar architecture and moderate interstitial edema. Furthermore, increases in the levels of superoxide dismutase and glutathione peroxidase were observed suggesting oxidative stress post 48 hours of GO administration. SPECT imaging showed that GO was mainly localized in the lungs with minor presence in other organs such as liver and intestines suggesting that GO can pass through the air-blood barrier. These results show that GO possesses severe pulmonary toxicity and appropriate steps must be taken to minimize human exposure to GO sources, especially during large-scale production.
4.5 Intravitreal administration
In intravitreal administration, a substance/formulation is administered directly into the eye using a needle. Yan et. al. have evaluated the ocular toxicity of GO after intravitreal administration in Japanese white rabbits at 0.1, 0.2, or 0.3 mg doses [116]. Eyes were reviewed for the effects of GO using a slit-lamp biomicroscopy and fundoscopy. Results show that GO did not have any effect on the corneas, interior media, posterior media, and the retina compared to the control group. The intraocular pressure showed no difference between the control and the experimental eye. Electroretinography (ERG) was performed to assess changes in the electrical impulse conduction in the eye. Compared to the controls, GO administration did not result in any significant changes in ERG amplitudes after 2, 7, 28 or 49 days of administration. H&E staining of eyes harvested 49 days post administration showed small amounts of GO residue, however, no retinal abnormality was observed.
5. Antimicrobial toxicity
Table 3 summarizes the studies assessing antimicrobial toxicity of graphene. Sawangphruk et. al. have investigated the antifungal activity of reduced graphene oxide (rGO) against A. niger, A. oryzae, and F. oxysporum between 0–500 µg/ml treatment concentrations [117]. Antifungal effects of rGO were assessed by quantifying mycelial growth inhibition. It was found that the rGO was effective against all three fungi with IC50 value between 50–100 µg/ml indicating a good antifungal activity of rGO (Figure 15). A. niger and F. oxysporum are pathogenic strains of fungi whereas A. oryzae is non-pathogenic, therefore; antifungal activity of rGO against A. oryzae could be a concern towards the development of graphene based broad spectrum antifungal agents. Akhavan et. al. have reported the antibacterial activity of graphene oxide nanowalls (GONWs) and reduced graphene oxide nanowalls (rGONWs) against E. coli and S. aureus [118]. Results show that bacterial cells are damaged by the direct contact of the cell membrane with extremely sharp edges of GO. Gram positive S. aureus without cell membrane showed greater cell death compared to gram positive E. coli which was more resistant due to the presence of outer membrane. Additionally, rGONWs were more toxic to bacterial cells than GONWs due to effective charge transfer between bacteria and edges of nanowalls during bacterial cell contact.
Table 3.
Antibacterial toxicity of graphene
| Material | Charact erization |
Properties | Dose | Cell line |
Assays | Conclusions | References |
|---|---|---|---|---|---|---|---|
| rGO | FTIR, Raman, TEM, SEM |
Size: < 5µm; Raman peaks: 1342 cm−1 (D band), 1576 cm−1 (G band), IG/ID = 0.85; FTIR peaks at 3200–3400 cm−1 (O-H), 1718 cm−1 (C=O), 1579 cm−1 (C=C), 1226 cm−1 (C-OH), 1070 cm−1 (C-O) |
0– 500µg/ ml |
A. niger, F. oxysp orum, A. oryzae |
Antifun gal Assay |
IC50 value is between 50– 100 µg/ml. rGO shows good antifungal activity. |
Sawangphruk et. al. [117] |
| GONW, rGONW |
SEM, XPS, Raman |
XPS peaks at 285 eV (C-C, C=C, C- H), 285.8 eV (C- OH), 287.6 (C=O), 289.1 eV (O=C- OH); ACOH/ACC = 1.35 (GONW) and 0.08 (rGONW). ID/IG: 1.78 (GONW) and 1.26 (rGONW) |
1mg/ml | E. coli, S. aureus |
Efflux of RNA |
Sharp edges of the nanowalls resulted in efflux of RNA. S. aureus bacteria was further damaged as compared to E. coli. |
Akhavan et. al. [118] |
| PEI-rGO, PEI-rGO- AgNPs |
z- potential, XPS, TEM, UV/vis, AFM, FTIR, XRD |
Thickness: 0.6nm, Size: 5–15nm, Zeta potential = −46.7 mV. |
0–958 mg/L |
E. coli, S. aureus |
Antibac terial Test |
PEI-rGO shows ~14– 20% antibacterial activity which increases to ~90% for PEI-rGO-AgNPs. Blade like edges of PEI- rGO-AgNP causes cell disruption leading to long-term antibacterial effect |
Cai et. al. [119] |
| rGO, GO | DLS, AFM, UV-Vis, TEM, Raman, FTIR |
Size: 300–600 nm; Thickness: 0.76 nm (GO), 1.6 nm (rGO); Raman peaks at 1350 cm−1 1590 cm−1; UV-Vis peak: 230 nm (GO), 267 nm (rGO); FTIR peaks at 3423 cm−1 (O- H), 1750 cm−1 (C=O), 1200 cm−1 (C-OH), 1050 cm−1 (C-O) for GO and 3400 cm−1 and 1047 cm−1 for rGO. |
50– 250µg/ ml |
Xanth omona s oryzae pv. Oryza e, Xoo |
Antibac terial Activity |
GO has extremely prominent dose- dependent inhibitory effect on cell growth due to combination of membrane damage and oxidative stress. |
Chen et. al. [120] |
| PVK-GNP thin films |
TGA, AFM, UV-Vis |
Thickness: 150nm; Roughness: 24.7nm, UV Vis peaks at 240, 250, 295, 331 and 344 nm |
1, 0.5, 0.05, 0.01mg /ml |
E. coli, B. subtili s |
Metabo lic Activity Assay, Bacteri al Viabilit y Assay |
Antimicrobial property of PVK-GNP nanocomposite is dependent on the concentration of GNPs. PVK-GNP films do not show cytotoxicity to NIH3T3 fibroblasts. |
Santos et. al. [121] |
| PVK-GO | SEM | Size: ~ 1µm; Thickness: 150nm; Roughness: 24.7nm, UV Vis peaks at 240, 250, 295, 331 and 344 nm |
10– 1000µg /ml |
Escher ichia coli, Cupria vidus metalli durans , Bacill us subtili s, Rhodo coccus opacus |
Metabo lic Activity Assay |
The addition of PVK to GO enhances antimicrobial properties of the nanocomposite in a dose-dependent manner. PVK-GO nanocomposite has low toxicity towards mammalian cells. |
Carpio et. al. [122] |
| GO, rGO and GO or rGO antibacteria l paper |
SEM, AFM, TEM |
Thickness: 1.1nm (GO), 1 nm (rGO); 1.5µm (GO paper), 4.6µm (rGO paper); |
0, 20, 85 µg/ml |
E. coli | ATP assay |
GO and rGO demonstrate superior antibacterial effect with only mild cytotoxicity towards mammalian cells (A549). rGO showed slightly greater toxicity towards A549 cells than GO |
Hu et. al. [123] |
| Pristine graphene, GO, rGO |
TEM, SEM, Zeta potential, FTIR |
Pristine graphene: Size = 1.86 ± 0.6 µm, Zeta potential = − 17.7± 4.3 mV, C=C. GO: Size = 1.27 ± 0.1 µm, Zeta potential = − 49.8± 1 mV, O- H, C=C, C=O, C- O, C-H. rGO: Size = 2.53 ± 0.2 µm, Zeta potential = − 25.1± 2.6 mV, C=C, C-O, C=O. |
25 and 250 µg/ml |
Listeri a monoc ytogen es and Salmo nella enteric a |
Bacteri al growth inhibiti on |
GO inhibits (~100% cell death) both bacterial strains at high and low treatment concentration. Pristine graphene and rGO exhibit variable antibacterial response. Presence of oxidative functional groups is important for bacterial cell attachment to graphene |
Kurantowicz et. al. [127] |
| GO | AFM, SEM, XPS |
Size: 0.753, 0.127 0.065 0.035 0.013 0.010 µm2, Thickness: 1nm; XPS peaks at 283.17 eV (C=C), 285.21 eV (C-O), 286.9 eV (C=O). |
0–80 µg/ml |
E. coli | Antibac terial Activity |
Size dependent antibacterial activity of GO sheets. Larger sheets exhibit greater antibacterial activity |
Liu et. al. [129] |
| GO, rGO | AFM, SEM, FTIR, XPS |
Size: 300 nm (GO), 2.71 µm (rGO); Thickness: 1nm (GO) and few microns (rGO, aggregated); FTIR peaks at 3430 cm−1 (O-H), 1720 cm−1 (C=O), 1215 cm−1 (C-OH), 1050 cm−1 (C-O), for rGO no peaks at 1720 cm−1, 1050 cm−1 and 1215 cm−1 |
5– 80µg/m l |
E. coli | Bacteri al Membr ane, Oxidati ve Stress |
GO has a higher antibacterial activity than other materials including reduced graphene oxide, graphite oxide and reduced graphite oxide that can be attributed to membrane and oxidative stresses. |
Liu et. al. [128] |
Figure 15.
(A) Mycelial growth inhibition of A. niger on media containing 0–500 µg/ml of rGO. (B) Plot of rGO concentration (µg/ml) vs. mycelial growth inhibitory activity (%) of A. niger, A. oryzae and F. oxysporum. Adapted from Reference [117] with permission, copyright © Elsevier, 2012.
Cai et. al. have investigated the antibacterial activity of polyethyleneimine-modified reduced graphene oxide (PEI-rGO) and sliver nanoparticles functionalized PEI-rGO (PEI-rGO-AgNPs) against E. coli and S. aureus between 0–958 mg/L treatment concentration [119]. The results show that PEI-rGO-AgNPs are extremely effective in killing bacteria, >90% reduction in cell viability was observed for both E. Coli and S. aureus colonies at 958 mg/L concentration. The long term antibacterial activity of PEI-rGO-AgNPs was attributed to the damage of bacterial cell due to interactions with sharp blade like edges of GO which may facilitate effective interactions of Ag+ ions with the intracellular contents, eventually killing bacteria. Chen et. al. have reported the dose-dependent antibacterial activity of GO and rGO against a rod shaped, gram negative phytopathogenic bacterium Xanthomonas oryzae pv. Orzae (Xoo) [120]. GO exhibited a greater antibacterial effect with ~94.5 and 86.4% cell mortality in DI water and 0.9% NaCl dispersions at 250µg/ml treatment concentration. rGO at 250µg/ml concentration resulted in 36.1 and 22.3% cell mortality Furthermore, an increased incubation time from 1 hour to 4 hours resulted in significant increases in the antibacterial activity of GO (from 19.4% to 66.1%) and rGO (13.8% to 30.5%). Further analysis by TEM and thiol quantification assay showed that GO resulted in physical damage and increased oxidative stress to bacterial cells. These results suggest that GO has a significantly greater dose- and time-dependent antibacterial activity compared to rGO.
Santos et. al. have investigated the antibacterial activity of poly(N-vinylcarbazole) graphene (PVK-G) solutions and thin films against E. coli and B. subtilis at concentrations between 0.01–1 mg/ml [121]. Results show a dose-dependent antibacterial effect of PVK-G solutions with~ 80% reduction in the percentage of metabolically active cells at 1 mg/ml treatment concentration. AFM imaging showed less bacterial coverage on PVK-G films compared to PVK and ITO (control) surfaces after 24 hours of incubation with E coli. Furthermore, PVK-G solutions at 1 mg/ml concentration showed ~ 80% cell viability of NIH3T3 fibroblast cells. These results show good antibacterial activity of PVK-G composites and thin films and suggest a potential use of PVK-G nanocomposites for a wide variety of antibacterial applications where bactericidal properties along with good biocompatibility are desired. In another study, Carpio, Santos et. al. have investigated the antibacterial properties of PVK-graphene oxide (PVK-GO) nanocomposites between 10–1000 µg/ml treatment concentrations against E coli, C metallidurans, B subtilis, and R opacus [122]. The results of this study show a similar effect wherein addition of GO to PVK enhances the antimicrobial properties of the nanocomposite. PVK-GO nanocomposites in solution appear to effectively encapsulate the bacterial cells leading to reduction in microbial metabolic potential and eventual cell death. AFM imaging shows significant reduction in E. coli biomass after 48 hours of culture on PVK-GO films in comparison to PVK and ITO (controls) surfaces. Additionally, similar to PVK-G nanocomposites, PVK-GO nanocomposites are also cytocompatible (~90% cell viability of NIH3T3 cells was observed after 48 hours of exposure to PVK-GO solutions at 1000 µg/ml concentration).
Hu et. al. have investigated the antibacterial activity of GO and rGO nanosheets against E. coli cells at concentrations of 0, 20, and 85 µg/ml [123]. After 2 hours of incubation with GO at 20 and 85 µg/ml, the metabolic activity of E. coli cells (measured by luciferase-based ATP assay) decreased to ~70% and ~13%, respectively (Figure 16A). rGO exhibited antibacterial activity, 2 hours of incubation of E. coli cells with rGO solutions at 85 µg/ml resulted in only ~24% cell viability (Figure 16B). TEM studies show loss of cell integrity via physical damages to the cell membrane upon exposure to GO and rGO (Figure 16 C–E). Furthermore, cells cultured on GO and rGO paper also showed damages to cell membrane of bacteria. However, Mangadlao et. al. [124], Hui et. al. [125], and Li et. al. [126] have reported that antibacterial effect of GO films is not due to cell membrane rupture by graphene edges. The antibacterial effect is observed due to charge transfer between basal plane of graphene and bacterial cell body leading to inactivation of bacteria.
Figure 16.
(A) Metabolic activity of E. coli cells upon exposure to GO at 20 and 85 µg/ml concentration for 2 hours. (B) Comparative metabolic activity of GO and rGO at 85 µg/ml concentration for 2 hours. GO shows greater antibacterial activity than rGO. Transmission electron microscopy images of E. coli cells - (C) control (D) after exposure to GO and (E) rGO at 85 µg/ml. Loss of membrane integrity are observed. Adapted from Reference [123] with permission, copyright © American Chemical Society, 2010.
Kurantowicz et. al. have investigated the interactions of pristine graphene, graphene oxide (GO) and reduced graphene oxide (rGO) against food borne bacterial pathogens – Listeria monocytogenes and Salmonella enterica [127]. Bacteria were incubated with high (250 µg/ml) and low (25 µg/ml) treatment concentrations of pristine graphene, GO and rGO for 18 hours. At 250 µg/ml concentration, all nanomaterials consistently inhibited 100% growth of S. enterica and L monocytogenes. However, at lower concentration (25 µg/ml), only GO showed 100% inhibition of both bacteria. Pristine graphene inhibited the growth of S. enterica by 96.5% and L. monocytogenes by 54.5% whereas rGO inhibited the growth of L. monocytogenes by 91% and S. enterica by 46%. TEM results showed a uniform distribution of bacterial cells over the surface of GO whereas on the surface of pristine graphene and rGO, bacterial cells adhered to the edges and wrinkles of the graphene sheets. The authors hypothesize that the presence of oxidative functional groups throughout the surface of GO and on the edges of pristine graphene and rGO act as bait for attracting bacteria. After attaching to the flakes, cell may be damaged via direct contact or destabilization of the phospholipid cell membrane. These results show a functionalization state dependent antibacterial effect of graphene and GO.
Liu et. al. have investigated the time- and dose-dependent antibacterial activity of four types of graphene-based materials (graphite (Gt), graphite oxide (GtO), graphene oxide (GO), and reduced graphene oxide (rGO)) against E. coli [128]. At 40 µg/ml treatment concentration after 2 hours of incubation, Gt, GtO, GO and rGO showed ~ 26.1 ± 4.8%, 15.0 ± 3.7%, 69.3 ± 6.1% and 45.9 ± 4.8%, respectively. After 4 hours, GO and rGO dispersions lead to ~ 89.7 ± 3.1% and 74.9 ± 4.8% inhibition of E coli. GO and rGO exhibit a concentration dependent antibacterial activity, cell mortality increases from 10.5 ± 6.6% to 91.6 ± 3.2% by increasing the GO concentration from 5 µg/ml to 80 µg/ml. Similarly, increases in rGO concentration from 5 µg/ml to 80 µg/ml leads to increased E. coli mortality from 8.4 ± 7.3% to 76.8 ± 3.1%. The antibacterial activity of GO and rGO was primarily due to inactivation of cellular functions due to loss of cell integrity. SEM imaging showed that direct contact of E coli cells with GO and rGO disrupts cell membrane (Figure 17). Additionally, graphene-based materials also oxidize glutathione, which is a redox mediator in bacterial cells, leading to oxidative stress. In another study, Liu et. al. investigated the lateral-dimension dependent antibacterial activity of GO [129]. The sizes of GO sheets used in this study were 0.753, 0.127, 0.065, 0.035, 0.013, and 0.010 µm2, respectively. Results show that large GO sheets exhibit significantly greater antibacterial activity compared to small GO sheets. The results of both these investigations taken together suggest that GO and rGO are effective antibacterial agents and physiochemical properties such as functional group density, size, and morphology play an important role in influencing the antibacterial potential of graphene-based materials.
Figure 17.
Scanning electron microscopy images of E. coli after 2 hours of incubation with (A, B) saline solution, (C, D) GO dispersions 40 µg/ml, (E, F) rGO dispersions at 40 µg/ml. Loss of membrane integrity is clearly observed. Adapted from Reference [128] with permission, copyright © American Chemical Society, 2011.
6. Environmental toxicity
The use of graphene for various industrial and healthcare applications would lead to increased environmental exposure and its disposal into waste streams. Therefore, it is important to assess the short- and long-term environmental toxicity of graphene and graphene-based materials and develop effective strategies to minimize any potential deleterious impact to flora and fauna. Table 4 summarizes the studies assessing environmental toxicity of graphene. Begum et. al. have investigated the phytotoxicity of graphene and its effects on root and shoot growth and shape, cell death and biomass by incubating seedlings of cabbage, tomatoes, red spinach and lettuce with 500–2000 mg/L for 20 days [130]. The results of physiological and morphological analysis show that graphene significantly inhibited plant growth and biomass production (Figure 18) and led to a reduction in the number and size of leaves in a dose-dependent manner. At 2000 mg/L concentration, ~18–78% root growth inhibition was observed depending on the plant species. Furthermore, leaves show wilting, necrotic lesions and reduction in leaf area. Graphene at high treatment concentrations (>500 mg/L) led to the production of reactive oxygen species leading to necrosis, loss of plasma membrane, and eventual cell death. No toxic effects were observed on lettuce at similar treatment concentrations. These results show that the phytotoxicity of graphene depends on the concentration, exposure time and plant species.
Table 4.
Environmental toxicity of graphene
| Material | Characterization | Properties | Conclusion | References |
|---|---|---|---|---|
| Graphene | AFM, SEM, TEM | Height: 1 nanometer. TEM reveals typical wrinkled structure. Range of length × breadth: 0.5 × 0.6 – 1.5 × 6.5 µm |
Cotyledons and root system growth were slowed down with increasing concentration on tomatoes, cabbage, and red spinach; had no effect on lettuce. Primary roots were shorter and disappeared root hairs compared to the control. Graphene caused decreased root and shoot weight. Decreased number of leaves. |
Begum et. al. [130] |
| Few Layer Graphene |
TEM, AFM | Thickness: 2–5 nm; Diameter: 100–200 nm |
No Significant effect on growth of tomato plants. |
Khodakovskaya et. al. [131] |
| GONRs | AFM, FTIR, Raman spectroscopy |
Bath sonication: 700– 900 nm (20 mins) Probe sonication: 300–400 nm (1 min), <300 nm (5 mins), <200 nm (10 mins) ID/IG ratio: 1.30 (bath sonication) – 2.30 (probe sonication) |
Post processing high energy sonication leads to reduction in size of GONRs. Probe sonicated solutions of GONRs show greater medaka embryo mortality compared to non sonicated or bath sonicated solutions. |
Mullick Chowdhury et. al. [60] |
| GO | AFM, XPS, SEM | Interlayer Spacing: 1 nm. |
Inhibition of metabolic activity at all concentrations. GO is biodegraded by at least 50% after 5h. Inhibition of nitrifying bacteria. |
Ahmed et. al. [132] |
| Graphene | TEM, AFM, Raman spectroscopy |
No Holes or Defects, Basic Hexagonal Lattice. Height: 0.34 nm |
Post Hydrogen Peroxide Treatment: Randomly distributed holes in Graphene. Diameter of holes increase with higher concentration of H2O2. |
Xing et. al. [133] |
| GONRs and rGONRs |
TEM, Raman Spectroscopy, UV-Vis |
Flat, Smooth, and uniform multi- layered sheets. |
Lignin peroxidase Activity: GONR: Structure completely degraded by 96 hours. rGONR: Holes from outer to inner layers in the sheets. Both materials eventually degrade, but there is a delay in degradation for rGONR compared to GONR |
Lalwani et. al. [134] |
Figure 18.
Effect of graphene on growth and development of (A–C) seedling and (D–F) cotyledons and root systems of cabbage, tomato and red spinach after exposure to 500–2000 mg/L concentration for 20 and 4 days, respectively. A dose-dependent reduction in the plant growth and biomass production is observed. Adapted from Reference [130] with permission, copyright © Elsevier, 2011.
Khodakovskaya et. al. have investigated the toxicity of various carbon nanomaterials (activated carbon, graphene, single- and multi-walled carbon nanotubes) on the germination of tomato seedlings [131]. All nanomaterials were mixed with Murashige and Skoog (MS) growth medium at 50 µg/mL used to grow surface sterilized tomato seedlings. Examination of leaves and roots show that graphene (out of all materials tested) induced lowest activation of stress-related LeAqp2 gene (tomato water-channel protein); highest activation was observed for CNT groups. Photothermal and photoacoustic imaging studies show that graphene did not affect the plant growth rate due to the inability to penetrate plant tissues.
Mullick Chowdhury et. al. have evaluated the post-processing effects of graphene oxide nanoribbons (GONRs) dispersed in biological buffers using various sonication steps (bath sonication for 5 or 20 minutes or probe sonication for 5 or 10 minutes) on Medaka embryos [60]. Results show precocious hatching of the embryos when exposed to GONR solutions prepared by bath sonication. However, significant mortality (~50% increase in cell death) of the embryos was observed for GONR solutions prepared by probe sonication. AFM imaging showed the presence of smaller GONR particles and carbonaceous debris after probe sonication. Probe sonicated GONR solutions lead to structural damage of the chorionic membrane of embryos. These results suggest that post-processing steps of graphene such as high-energy sonication may lead to variable environmental toxicity.
Ahmed et. al. have investigated the effects of graphene oxide on the microbial community present in wastewater [132]. Efficient biological wastewater treatment requires functioning of diverse microbial species. Active sludge samples were incubated with 10–300 mg/L concentration of GO for 5 hours at room temperature to observe short-term toxicity. Results show a dose-dependent toxicity with significant reduction in bacterial metabolic activity, viability, and their capacity to effectively remove nutrients such as organics, phosphorous and nitrogen from activated sludge in the presence of GO. A dose-dependent reduction in the conversion of ammonia to nitrate was observed suggesting a reduction in the concentration of nitrifying bacteria. It was also observed that the presence of GO in wastewater led to deterioration of the quality of final wastewater effluent (increased turbidity was observed). Results also show that interaction of GO with wastewater sludge induced production of reactive oxygen species. These results show that the presence of GO loads in wastewater treatment sludge disrupts the functioning of antimicrobial community, which may lead to compromised treatment performance.
Hydrogen Peroxide (H2O2) is a naturally occurring ubiquitous compound found in rain and surface water, and in biological systems at concentrations ranging between 1 µM to 10000 µM. Xing et. al. have investigated the effects of hydrogen peroxide on the biodegradation of graphene [133]. TEM and AFM imaging studies show the presence of randomly distributed holes on graphene sheets in the presence of physiologically and environmentally relevant concentrations of H2O2 (Figure 19). After 10 hours of incubation with H2O2, the diameter of holes was between 1– 15 nm. An increase in the concentration of H2O2 from 1 µM to 10000 µM induced the formation of holes with significantly greater diameters (10–30 nm) suggesting a concentration dependent biodegradation of graphene. AFM studies show the depth of holes between 9.4–13.5 nm; greater than the height of a single graphene sheet (~0.34 nm). Therefore, based on these results, it was concluded that H2O2 attacked the inner layers of graphene along with the outer surface layers. Raman spectroscopy results show a progressive time- and concentration-dependent decrease in the intensity for both D and G band for all H2O2 treatment groups. The biodegradation of graphene by H2O2 may further be accelerated by the presence of trace elements such as nickel or iron (used as catalysts during the synthesis of graphene) by catalyzing the conversion of hydrogen peroxide to hydroxyl radicals via the Haber-Weiss reaction. The results of this study show that multilayered graphene can undergo effective biodegradation at environmental and physiological concentrations of H2O2.
Figure 19.
(A–D) Representative transmission electron microscopy images of multilayered graphene treated with (A) DI water, (B) 1 µM H2O2, (C) 100 µM H2O2 and (D) 10000 µM H2O2 for 10 hours. Arrows in (B) indicate the formation of holes on graphene sheets and in (C) indicate the formation of lighter (few graphene layers) and darker regions (multiple graphene layers) suggesting the degradation of multilayered graphene. (E–J) Representative atomic force microscopy images of multilayered graphene on Ni wafer. (E and G) are topographical scans of graphene incubated with DI water for 25 hours. (G and H) show graphene after 25 hours of incubation with 10000 µM H2O2. Inset in images (G and H) are corresponding height profiles. (I and J) are 3D representations of images G and H. Adapted from Reference [133] with permission, copyright © John Wiley and Sons Inc., 2014.
Lalwani et. al. have investigated the oxidative biodegradation of graphene oxide nanoribbons (GONRs) and reduced graphene oxide nanoribbons (rGONRs) by lignin peroxidase (LiP), an enzyme released by white rot fungi (Phanerochaete chrysosporium) distributed worldwide in forests soils with dead and decaying organic matter [134]. LiP degrades lignin – a component of plant cell wall. TEM (Figure 20) and Raman spectroscopy analysis of GONRs and rGONRs treated with LiP for 4–96 hours show the formation of holes confirming the structural degradation of graphene sheets. It was observed that GONRs showed a higher rate of biodegradation compared to rGONRs; numerous holes (1–5 nm diameter) were detected on GONR sheets within 4 hours of treatment which increased to ~300–350 nm after 48 hours. The diameter of holed on rGONRs was between 5–30 nm after 48 hours of enzymatic treatment. After 96 hours, GONRs appeared to have completely degraded whereas numerous holes extending throughout the width of rGONRs were observed. These results suggest that oxidized and reduced graphene nanoribbons released in the environment may undergo oxidative biodegradation by lignin peroxidase.
Figure 20.
Representative transmission electron microscopy images of oxidized and reduced graphene oxide nanoribbons (GONRs – A–D) and (rGONRs – E–H) after 0, 4, 48, and 96 hours of treatment with lignin peroxidase. Arrows in B, D and G indicate the formation of holes on graphene sheets. Extensive biodegradation of GONRs whereas the formation of holey rGONRs is observed after 96 hours of incubation. (I) Ribbon diagram of lignin peroxidase, (J) Enzymatic cycle of lignin peroxidase and (K) Schematic representation of degradation of graphene in the presence of lignin peroxidase. Adapted from Reference [134] with permission, copyright © Royal Society of Chemistry, 2014.
7. Mechanisms of toxicity
The interactions of graphene with cells, proteins, and other biomolecules is influenced by its physiochemical properties such as shape, size, functional group density and charge transfer abilities. The main mechanism of graphene toxicity is associated with the generation of intracellular reactive oxygen species that cause damage to proteins and DNA leading to cell death via apoptotic or necrotic pathways [83, 135, 136]. Graphene can be internalized into cells via passive internalization (endocytosis)[137, 138] or active internalization (clathrin mediated energy dependent endocytosis[139] or actin-dependent macropinocytosis[36]). Studies have elucidated two mechanism of graphene mediated ROS damage: (1) Upon cellular internalization, GO interferes with the electron transport system, induces overproduction of H2O2 and hydroxyl radicals. This leads to the oxidization of cardiolipin and the release and translocation of hemoprotein from mitochondrial inner membrane to the cytoplasm. This triggers release of cytochrome c complex (cyt c) which induces calcium release from endoplasmic reticulum and activates caspase 9 which in turn activates caspase 3 and 7 leading to cell death (Figure 21) [136]. (2) GO induces the activation of MAPK (JNK, ERK, p38) and TGF- β signaling pathways that lead to activation of Bcl-2 proteins which in turn activate mitochondria-induced apoptosis (Figure 22) [83]. In addition to ROS induced cell death, GO may also lead to the activation of toll-like receptors and induce autophagy via inflammatory pathways (Figure 23) [84]. Post internalization; graphene may induce DNA cleavage due to interactions such as pi-pi stacking, hydrophobicity, and electrostatic interactions [140–142]. Singh et. al. have shown that surface charge distribution on graphene sheets plays an important role in the activation of src kinases and release of calcium eventually leading to platelet aggregation (Figure 24) [87, 95].
Figure 21.
Schematic representation of the proposed mechanism of oxidative stress induced toxicity by graphene oxide. Adapted from Reference [136] with permission, copyright © John Wiley & Sons Inc., 2012.
Figure 22.
Schematic illustrating the signaling pathways involved in pristine-graphene induced cell apoptosis via ROS mediated MAPK and TGF-beta pathways (mitochondria dependent apoptotic cascades). Adapted from Reference [83] with permission, copyright © Elsevier, 2012.
Figure 23.
Overview of the GO-induced cytokine response and autophagy mediated by the TLR4/TLR9 signaling pathway. GO treatment led to the activation of TLR4 and TLR9, which relayed signals through MyD88-TRAF6-NF-kB and ultimately gave rise to cytokine expression. However, GO-induced TLRs signaling neither elicited IFN-b expression nor activated IRF3, suggesting that TRIF and IRF3 were dispensable in the inflammatory response. Conversely, GO-induced TLR4-MyD88-TRAF6 and TLR4-TRIF signaling cascades signaled through Beclin 1 to initiate autophagy. GO engagement of TLR9 also activated MyD88 and TRAF6, leading to Beclin 1 and LC3 activation and subsequent autophagy. Adapted from Reference [84] with permission, copyright © Elsevier, 2012.
Figure 24.
Schematic illustrating the interaction of (A) graphene oxide (negative surface charge) and (B) amine-modified graphene (positive surface charge) on platelet function. Surface charge distribution determines the interactions of graphene with different agonist receptors on platelet membrane. (A) Adapted from Reference [87] and (B) adapted from Reference [88] with permissions, copyright © American Chemical Society, 2011 and 2012.
Several studies have reported that extremely sharp edges of graphene lead to membrane destabilization and loss of cell integrity by direct contact [67, 118]. Wang et. al. have shown that adsorption of GO on RBCs leads to the loss of cell membrane resulting in hemolysis [137]. Long sheets of graphene have also been observed to wrap around bacterial cells thereby inhibiting their growth [122]. Single layered GONRs exhibit greater cyto- and geno-toxicity due to the interactions between cells and sharp edges of nanoribbons resulting in extensive chromosomal aberration and DNA fragmentation [67]. Li et. al. have shown that graphene micro sheets enter cells through spontaneous membrane penetration at corner sites and edge asperities [143]. Molecular dynamics simulation studies have shown that graphene has a strong affinity for phospholipids and can be localized into the hydrophobic interior of biological membranes [144]. Tu et. al. have shown that due to strong interactions between graphene and lipids, graphene penetrates into and extracts significantly large amounts of phospholipids from cell membrane leading to cytotoxicity (Figure 25) [145]. Graphene quantum dots affect cellular function by inserting into cell membrane [146]; pristine GO has been reported to form aggregates on cell membrane thereby affecting cellular morphology [69]. rGO sheets inhibit the growth of fungal mycelium due to their direct insertion into the membrane of fungal cells [117].
Figure 25.
Representative simulated trajectories of graphene nanosheets insertion and lipid extraction in the outer membrane (pure palmitoyloleoylphosphatidylethanolamine, POPE) and inner membrane (mixed POPE-POPG) of E. coli. Water is represented in violet and phospholipids in tan lines with hydrophilic charged atoms as colored spheres (hydrogen – white, oxygen – red, nitrogen – dark blue, carbon – cyan and phosphorus – orange). Graphene is shown as yellow sheet with a large sphere marked at one corner representing restrained atom in simulations. Extracted phospholipids are shown as large spheres. Adapted from Reference [145] with permission, copyright © Macmillan Publishers Limited, 2013.
8. Conclusion and Future Perspective
The studies till date indicate that toxicity of graphene could be dependent on the shape, size, purity, post-production processing steps, oxidative state, functional groups, dispersion state, synthesis methods, route and dose of administration, and exposure times. The morphology, shape and size of graphene nanoparticles could influence their cellular uptake characteristics whereas presence of functional groups can alter their interactions with proteins, biomolecules and micronutrients. The initial starting materials and the methods used in the production of oxidized graphene can result in the presence of metallic impurities and oxidative debris in the final product, which could result in variable toxicity effects. The post synthesis processing steps employed to disperse the nanoparticles in aqueous media could also influence toxicity. Reactive oxidation species mediated cell damage has been postulated as a primary cytotoxicity mechanism of graphene. Graphene sheets with sharp edges could induce direct physical damage and interact with phospholipids leading to membrane destabilization. Surface coating of graphene with several biocompatible moieties (e.g. natural polymers) can mitigate these cytotoxicity effects.
The studies taken together provide information on dosaging, biodistribution and pharmacology of various graphene-based formulations. It must be noted that even though there are many types of graphene nanoparticles, GO have been the most widely used for biomedical applications and studies that employ GO dominate the review. While majority of published literature on toxicity of other members of the graphene family have been reviewed herein, more toxicological studies on formulations of other types of graphene nanoparticles are warranted. Additionally, for all types of graphene nanoparticles, it is important to investigate and critically evaluate the potential short- and long-term health risks and toxicity hazards after acute, sub-acute and chronic exposures using in vitro and in vivo (small and large animal) models. Towards clinical translation of any graphene-based biomedical application that requires its systemic administration, formulations with high purity, dispersibility in aqueous media, and controlled physiochemical properties are highly desirable. For each of these formulations, regulatory compliance would require mapping of their chemistry, manufacturing and control (CMC) process and completing new drug (IND)-enabling preclinical studies. With advancements in the synthesis methods and establishment of several commercial ventures for large-scale industrial production of graphene, the widespread use of graphene for several consumer products is becoming a reality. This ubiquitous use would lead to an increased environmental exposure of graphene. Therefore, more studies assessing the long-term environmental impact of graphene are required. Recent efforts have also involved incorporation of graphene nanoparticles in polymer matrices or their assembly in coating, films and porous scaffolds for bio-sensing, localized drug delivery or tissue engineering applications [147, 148]. For these applications, additional in vitro and in vivo toxicological studies specific to biomedical devices and implants would be needed. Finally, advances in graphene-like inorganic nanoparticles for biomedical applications allow opportunities to compare the biological response of graphene and its inorganic analogues [41, 43, 149–152]. All these studies will further advance the knowledge required to develop safe graphene-based technologies and products suitable for healthcare applications and to minimize the risks to human health.
Acknowledgments
The authors acknowledge the financial supp ort of National Institutes of Health (grant no. 1DP2OD007394-01) and Wallace H. Coulter Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dresselhaus MS, Dresselhaus G, Eklund PC. Science of fullerenes and carbon nanotubes: their properties and applications. Academic press; 1996. [Google Scholar]
- 2.Geim AK. Graphene: status and prospects. science. 2009;324(5934):1530–1534. doi: 10.1126/science.1158877. [DOI] [PubMed] [Google Scholar]
- 3.Lalwani G, Sitharaman B. Multifunctional Fullerene- and Metallofullerene-Based Nanobiomaterials. Nano LIFE. 2013;3(3):1342003-1–1342003-22. [Google Scholar]
- 4.The rise and rise of graphene. Nat Nano. 2010;5(11):755–755. doi: 10.1038/nnano.2010.224. [DOI] [PubMed] [Google Scholar]
- 5.The Kavli Prize. Kavli Prize Laureates. Nanoscience. 2008 http://www.kavliprize.org/prizes-and-laureates/prizes/2008-kavli-prize-laureates-nanoscience. [Google Scholar]
- 6.Geim AK, Novoselov KS. The rise of graphene. Nature materials. 2007;6(3):183–191. doi: 10.1038/nmat1849. [DOI] [PubMed] [Google Scholar]
- 7.Slonczewski J, Weiss P. Band structure of graphite. Physical Review. 1958;109(2):272. [Google Scholar]
- 8.Peierls R. Quelques propriétés typiques des corps solides. Annales de l'institut Henri Poincaré. 1935 [Google Scholar]
- 9.Landau L. Zur Theorie der phasenumwandlungen II. Phys. Z. Sowjetunion. 1937;11:26–35. [Google Scholar]
- 10.Landau LD, Lifshitz E. Statistical physics, part I. Course of theoretical physics. 1980;5:468. [Google Scholar]
- 11.Mermin ND. Crystalline order in two dimensions. Physical Review. 1968;176(1):250. [Google Scholar]
- 12.Novoselov KS, Geim AK, Morozov S, Jiang D, Zhang Y, Dubonos Sa, Grigorieva I, Firsov A. Electric field effect in atomically thin carbon films. science. 2004;306(5696):666–669. doi: 10.1126/science.1102896. [DOI] [PubMed] [Google Scholar]
- 13.Novoselov K, Geim AK, Morozov S, Jiang D, Katsnelson M, Grigorieva I, Dubonos S, Firsov A. Two-dimensional gas of massless Dirac fermions in graphene. nature. 2005;438(7065):197–200. doi: 10.1038/nature04233. [DOI] [PubMed] [Google Scholar]
- 14.Novoselov K, Jiang D, Schedin F, Booth T, Khotkevich V, Morozov S, Geim A. Two-dimensional atomic crystals. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(30):10451–10453. doi: 10.1073/pnas.0502848102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson D. Europe Invests €1 Billion to Become “Graphene Valley”. IEEE Spectrum. 2013 [Google Scholar]
- 16.Spasenovic M. South Korea To Spend US$40 Million On Graphene Development. Graphene Tracker. 2013 [Google Scholar]
- 17.Brown M. Government announces £50m funding hub for UK graphene research. WIRED. 2011 [Google Scholar]
- 18.HUAWEI. Huawei announces partnership with The University of Manchester to develop graphene-based technologies. 2015 [Google Scholar]
- 19.Bolotin KI, Sikes K, Jiang Z, Klima M, Fudenberg G, Hone J, Kim P, Stormer H. Ultrahigh electron mobility in suspended graphene. Solid State Communications. 2008;146(9):351–355. [Google Scholar]
- 20.Du X, Skachko I, Barker A, Andrei EY. Approaching ballistic transport in suspended graphene. Nature nanotechnology. 2008;3(8):491–495. doi: 10.1038/nnano.2008.199. [DOI] [PubMed] [Google Scholar]
- 21.Stoller MD, Park S, Zhu Y, An J, Ruoff RS. Graphene-based ultracapacitors. Nano letters. 2008;8(10):3498–3502. doi: 10.1021/nl802558y. [DOI] [PubMed] [Google Scholar]
- 22.Ponomarenko L, Geim A, Zhukov A, Jalil R, Morozov S, Novoselov K, Grigorieva I, Hill E, Cheianov V, Fal’Ko V. Tunable metal-insulator transition in double-layer graphene heterostructures. Nature Physics. 2011;7(12):958–961. [Google Scholar]
- 23.Zhang Y, Tan Y-W, Stormer HL, Kim P. Experimental observation of the quantum Hall effect and Berry's phase in graphene. Nature. 2005;438(7065):201–204. doi: 10.1038/nature04235. [DOI] [PubMed] [Google Scholar]
- 24.Balandin AA, Ghosh S, Bao W, Calizo I, Teweldebrhan D, Miao F, Lau CN. Superior thermal conductivity of single-layer graphene. Nano letters. 2008;8(3):902–907. doi: 10.1021/nl0731872. [DOI] [PubMed] [Google Scholar]
- 25.Lee C, Wei X, Kysar JW, Hone J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. science. 2008;321(5887):385–388. doi: 10.1126/science.1157996. [DOI] [PubMed] [Google Scholar]
- 26.Nair R, Blake P, Grigorenko A, Novoselov K, Booth T, Stauber T, Peres N, Geim A. Fine structure constant defines visual transparency of graphene. Science. 2008;320(5881):1308–1308. doi: 10.1126/science.1156965. [DOI] [PubMed] [Google Scholar]
- 27.Avouris P, Dimitrakopoulos C. Graphene: synthesis and applications. Materials today. 2012;15(3):86–97. [Google Scholar]
- 28.Subrahmanyam K, Panchakarla L, Govindaraj A, Rao C. Simple method of preparing graphene flakes by an arc-discharge method. The Journal of Physical Chemistry C. 2009;113(11):4257–4259. [Google Scholar]
- 29.Kim KS, Zhao Y, Jang H, Lee SY, Kim JM, Kim KS, Ahn J-H, Kim P, Choi J-Y, Hong BH. Large-scale pattern growth of graphene films for stretchable transparent electrodes. Nature. 2009;457(7230):706–710. doi: 10.1038/nature07719. [DOI] [PubMed] [Google Scholar]
- 30.Stankovich S, Dikin DA, Piner RD, Kohlhaas KA, Kleinhammes A, Jia Y, Wu Y, Nguyen ST, Ruoff RS. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon. 2007;45(7):1558–1565. [Google Scholar]
- 31.Kosynkin DV, Higginbotham AL, Sinitskii A, Lomeda JR, Dimiev A, Price BK, Tour JM. Longitudinal unzipping of carbon nanotubes to form graphene nanoribbons. Nature. 2009;458(7240):872–876. doi: 10.1038/nature07872. [DOI] [PubMed] [Google Scholar]
- 32.Pei S, Cheng H-M. The reduction of graphene oxide. Carbon. 2012;50(9):3210–3228. [Google Scholar]
- 33.Kanakia S, Toussaint JD, Chowdhury SM, Lalwani G, Tembulkar T, Button T, Shroyer KR, Moore W, Sitharaman B. Physicochemical characterization of a novel graphene-based magnetic resonance imaging contrast agent. International journal of nanomedicine. 2013;8:2821. doi: 10.2147/IJN.S47062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lalwani G, Cai X, Nie L, Wang LV, Sitharaman B. Graphene-based contrast agents for photoacoustic and thermoacoustic tomography. Photoacoustics. 2013;1(3):62–67. doi: 10.1016/j.pacs.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lalwani G, Sundararaj JL, Schaefer K, Button T, Sitharaman B. Synthesis, characterization, in vitro phantom imaging, and cytotoxicity of a novel graphene-based multimodal magnetic resonance imaging-X-ray computed tomography contrast agent. Journal of Materials Chemistry B. 2014;2(22):3519–3530. doi: 10.1039/C4TB00326H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mullick Chowdhury S, Lalwani G, Zhang K, Yang JY, Neville K, Sitharaman B. Cell specific cytotoxicity and uptake of graphene nanoribbons. Biomaterials. 2013;34(1):283–293. doi: 10.1016/j.biomaterials.2012.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chowdhury SM, Surhland C, Sanchez Z, Chaudhary P, Kumar MS, Lee S, Peña LA, Waring M, Sitharaman B, Naidu M. Graphene nanoribbons as a drug delivery agent for lucanthone mediated therapy of glioblastoma multiforme. Nanomedicine: Nanotechnology, Biology and Medicine. 2015;11(1):109–118. doi: 10.1016/j.nano.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Feng L, Zhang S, Liu Z. Graphene based gene transfection. Nanoscale. 2011;3(3):1252–1257. doi: 10.1039/c0nr00680g. [DOI] [PubMed] [Google Scholar]
- 39.Tian B, Wang C, Zhang S, Feng L, Liu Z. Photothermally enhanced photodynamic therapy delivered by nano-graphene oxide. ACS nano. 2011;5(9):7000–7009. doi: 10.1021/nn201560b. [DOI] [PubMed] [Google Scholar]
- 40.Huang P, Xu C, Lin J, Wang C, Wang X, Zhang C, Zhou X, Guo S, Cui D. Folic acid-conjugated graphene oxide loaded with photosensitizers for targeting photodynamic therapy. Theranostics. 2011;1:240. doi: 10.7150/thno/v01p0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lalwani G, Henslee AM, Farshid B, Lin L, Kasper FK, Qin Y-X, Mikos AG, Sitharaman B. Two-Dimensional Nanostructure-Reinforced Biodegradable Polymeric Nanocomposites for Bone Tissue Engineering. Biomacromolecules. 2013;14(3):900–909. doi: 10.1021/bm301995s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patel SC, Lalwani G, Grover K, Qin Y-X, Sitharaman B. Fabrication and cytocompatibility of in situ crosslinked carbon nanomaterial films. Scientific Reports. 2015;5 doi: 10.1038/srep10261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Farshid B, Lalwani G, Sitharaman B. In vitro cytocompatibility of one-dimensional and two - dimensional nanostructure - reinforced biodegradable polymeric nanocomposites. Journal of Biomedical Materials Research Part A. 2015;103(7):2309–2321. doi: 10.1002/jbm.a.35363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Talukdar Y, Rashkow JT, Lalwani G, Kanakia S, Sitharaman B. The effects of graphene nanostructures on mesenchymal stem cells. Biomaterials. 2014;35(18):4863–4877. doi: 10.1016/j.biomaterials.2014.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nayak TR, Andersen H, Makam VS, Khaw C, Bae S, Xu X, Ee P-LR, Ahn J-H, Hong BH, Pastorin G. Graphene for controlled and accelerated osteogenic differentiation of human mesenchymal stem cells. ACS nano. 2011;5(6):4670–4678. doi: 10.1021/nn200500h. [DOI] [PubMed] [Google Scholar]
- 46.Yang Y, Asiri AM, Tang Z, Du D, Lin Y. Graphene based materials for biomedical applications. Materials today. 2013;16(10):365–373. [Google Scholar]
- 47.Yang K, Feng L, Shi X, Liu Z. Nano-graphene in biomedicine: theranostic applications. Chemical Society Reviews. 2013;42(2):530–547. doi: 10.1039/c2cs35342c. [DOI] [PubMed] [Google Scholar]
- 48.Hu X, Zhou Q. Health and ecosystem risks of graphene. Chemical reviews. 2013;113(5):3815–3835. doi: 10.1021/cr300045n. [DOI] [PubMed] [Google Scholar]
- 49.Seabra AB, Paula AJ, de Lima R, Alves OL, Durán N. Nanotoxicity of graphene and graphene oxide. Chemical research in toxicology. 2014;27(2):159–168. doi: 10.1021/tx400385x. [DOI] [PubMed] [Google Scholar]
- 50.Zhao J, Wang Z, White JC, Xing B. Graphene in the aquatic environment: adsorption, dispersion, toxicity and transformation. Environmental science & technology. 2014;48(17):9995–10009. doi: 10.1021/es5022679. [DOI] [PubMed] [Google Scholar]
- 51.Tonelli FM, Goulart VA, Gomes KN, Ladeira MS, Santos AK, Lorençon E, Ladeira LO, Resende RR. Graphene-based nanomaterials: biological and medical applications and toxicity. Nanomedicine. 2015;10(15):2423–2450. doi: 10.2217/nnm.15.65. [DOI] [PubMed] [Google Scholar]
- 52.Bitounis D, Ali-Boucetta H, Hong BH, Min DH, Kostarelos K. Prospects and challenges of graphene in biomedical applications. Advanced Materials. 2013;25(16):2258–2268. doi: 10.1002/adma.201203700. [DOI] [PubMed] [Google Scholar]
- 53.Bianco A. Graphene: safe or toxic? The two faces of the medal. Angewandte Chemie International Edition. 2013;52(19):4986–4997. doi: 10.1002/anie.201209099. [DOI] [PubMed] [Google Scholar]
- 54.Yang K, Li Y, Tan X, Peng R, Liu Z. Behavior and toxicity of graphene and its functionalized derivatives in biological systems. Small. 2013;9(9 – 10):1492–1503. doi: 10.1002/smll.201201417. [DOI] [PubMed] [Google Scholar]
- 55.Sanchez VC, Jachak A, Hurt RH, Kane AB. Biological interactions of graphene-family nanomaterials: an interdisciplinary review. Chemical research in toxicology. 2011;25(1):15–34. doi: 10.1021/tx200339h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jastrzębska AM, Kurtycz P, Olszyna AR. Recent advances in graphene family materials toxicity investigations. Journal of Nanoparticle Research. 2012;14(12):1–21. doi: 10.1007/s11051-012-1320-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang B, Wang Y, Zhai G. Biomedical applications of the graphene-based materials. Materials Science and Engineering: C. 2015 doi: 10.1016/j.msec.2015.12.073. [DOI] [PubMed] [Google Scholar]
- 58.Wong CHA, Sofer Z, Kubešová M, Kučera J, Matějková S, Pumera M. Synthetic routes contaminate graphene materials with a whole spectrum of unanticipated metallic elements. Proceedings of the National Academy of Sciences. 2014;111(38):13774–13779. doi: 10.1073/pnas.1413389111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ambrosi A, Chua CK, Khezri B, Sofer Z, Webster RD, Pumera M. Chemically reduced graphene contains inherent metallic impurities present in parent natural and synthetic graphite. Proceedings of the National Academy of Sciences. 2012;109(32):12899–12904. doi: 10.1073/pnas.1205388109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mullick Chowdhury S, Dasgupta S, McElroy AE, Sitharaman B. Structural disruption increases toxicity of graphene nanoribbons. Journal of Applied Toxicology. 2014;34(11):1235–1246. doi: 10.1002/jat.3066. [DOI] [PubMed] [Google Scholar]
- 61.Zhang Y, Ali SF, Dervishi E, Xu Y, Li Z, Casciano D, Biris AS. Cytotoxicity effects of graphene and single-wall carbon nanotubes in neural phaeochromocytoma-derived PC12 cells. ACS Nano. 2010;4(6):3181–3186. doi: 10.1021/nn1007176. [DOI] [PubMed] [Google Scholar]
- 62.Vallabani NV, Mittal S, Shukla RK, Pandey AK, Dhakate SR, Pasricha R, Dhawan A. Toxicity of graphene in normal human lung cells (BEAS-2B) J Biomed Nanotechnol. 2011;7(1):106–107. doi: 10.1166/jbn.2011.1224. [DOI] [PubMed] [Google Scholar]
- 63.Yuan J, Gao H, Sui J, Duan H, Chen WN, Ching CB. Cytotoxicity evaluation of oxidized single-walled carbon nanotubes and graphene oxide on human hepatoma HepG2 cells: an iTRAQ-coupled 2D LC-MS/MS proteome analysis. Toxicol Sci. 2012;126(1):149–161. doi: 10.1093/toxsci/kfr332. [DOI] [PubMed] [Google Scholar]
- 64.Lv M, Zhang Y, Liang L, Wei M, Hu W, Li X, Huang Q. Effect of graphene oxide on undifferentiated and retinoic acid-differentiated SH-SY5Y cells line. Nanoscale. 2012;4(13):3861–3866. doi: 10.1039/c2nr30407d. [DOI] [PubMed] [Google Scholar]
- 65.Talukdar Y, Rashkow JT, Lalwani G, Kanakia S, Sitharaman B. The effects of graphene nanostructures on mesenchymal stem cells. Biomaterials. 2014;35(18):4863–4877. doi: 10.1016/j.biomaterials.2014.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chng ELK, Chua CK, Pumera M. Graphene oxide nanoribbons exhibit significantly greater toxicity than graphene oxide nanoplatelets. Nanoscale. 2014;6(18):10792–10797. doi: 10.1039/c4nr03608e. [DOI] [PubMed] [Google Scholar]
- 67.Akhavan O, Ghaderi E, Emamy H, Akhavan F. Genotoxicity of graphene nanoribbons in human mesenchymal stem cells. Carbon. 2013;54:419–431. [Google Scholar]
- 68.Jaworski S, Sawosz E, Grodzik M, Winnicka A, Prasek M, Wierzbicki M, Chwalibog A. In vitro evaluation of the effects of graphene platelets on glioblastoma multiforme cells. Int J Nanomedicine. 2013;8:413–420. doi: 10.2147/IJN.S39456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sasidharan A, Panchakarla LS, Chandran P, Menon D, Nair S, Rao CN, Koyakutty M. Differential nano-bio interactions and toxicity effects of pristine versus functionalized graphene. Nanoscale. 2011;3(6):2461–2464. doi: 10.1039/c1nr10172b. [DOI] [PubMed] [Google Scholar]
- 70.Matesanz MC, Vila M, Feito MJ, Linares J, Goncalves G, Vallet-Regi M, Marques PA, Portoles MT. The effects of graphene oxide nanosheets localized on F-actin filaments on cell-cycle alterations. Biomaterials. 2013;34(5):1562–1569. doi: 10.1016/j.biomaterials.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 71.Yuan X, Liu Z, Guo Z, Ji Y, Jin M, Wang X. Cellular distribution and cytotoxicity of graphene quantum dots with different functional groups. Nanoscale research letters. 2014;9(1):1–9. doi: 10.1186/1556-276X-9-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Horváth L, Magrez A, Burghard M, Kern K, Forró L, Schwaller B. Evaluation of the toxicity of graphene derivatives on cells of the lung luminal surface. Carbon. 2013;64:45–60. [Google Scholar]
- 73.Das S, Singh S, Singh V, Joung D, Dowding JM, Reid D, Anderson J, Zhai L, Khondaker SI, Self WT. Oxygenated functional group density on graphene oxide: its effect on cell toxicity. Particle & Particle Systems Characterization. 2013;30(2):148–157. [Google Scholar]
- 74.Chong Y, Ma Y, Shen H, Tu X, Zhou X, Xu J, Dai J, Fan S, Zhang Z. The in vitro and in vivo toxicity of graphene quantum dots. Biomaterials. 2014;35(19):5041–5048. doi: 10.1016/j.biomaterials.2014.03.021. [DOI] [PubMed] [Google Scholar]
- 75.Teo WZ, Chng ELK, Sofer Z, Pumera M. Cytotoxicity of halogenated graphenes. Nanoscale. 2014;6(2):1173–1180. doi: 10.1039/c3nr05275c. [DOI] [PubMed] [Google Scholar]
- 76.Teo WZ, Sofer Z, Šembera F, Janoušek Z, Pumera M. Cytotoxicity of fluorographene. RSC Advances. 2015;5(129):107158–107165. [Google Scholar]
- 77.Chng ELK, Sofer Z, Pumera M. Cytotoxicity profile of highly hydrogenated graphene. Chemistry–A European Journal. 2014;20(21):6366–6373. doi: 10.1002/chem.201304911. [DOI] [PubMed] [Google Scholar]
- 78.Sawosz E, Jaworski S, Kutwin M, Vadalasetty KP, Grodzik M, Wierzbicki M, Kurantowicz N, Strojny B, Hotowy A, Lipińska L. Graphene Functionalized with Arginine Decreases the Development of Glioblastoma Multiforme Tumor in a Gene-Dependent Manner. International journal of molecular sciences. 2015;16(10):25214–25233. doi: 10.3390/ijms161025214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Akhavan O, Ghaderi E, Akhavan A. Size-dependent genotoxicity of graphene nanoplatelets in human stem cells. Biomaterials. 2012;33(32):8017–8025. doi: 10.1016/j.biomaterials.2012.07.040. [DOI] [PubMed] [Google Scholar]
- 80.Chang Y, Yang ST, Liu JH, Dong E, Wang Y, Cao A, Liu Y, Wang H. In vitro toxicity evaluation of graphene oxide on A549 cells. Toxicol Lett. 2011;200(3):201–210. doi: 10.1016/j.toxlet.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 81.Yue H, Wei W, Yue Z, Wang B, Luo N, Gao Y, Ma D, Ma G, Su Z. The role of the lateral dimension of graphene oxide in the regulation of cellular responses. Biomaterials. 2012;33(16):4013–4021. doi: 10.1016/j.biomaterials.2012.02.021. [DOI] [PubMed] [Google Scholar]
- 82.Zhi X, Fang H, Bao C, Shen G, Zhang J, Wang K, Guo S, Wan T, Cui D. The immunotoxicity of graphene oxides and the effect of PVP-coating. Biomaterials. 2013;34(21):5254–5261. doi: 10.1016/j.biomaterials.2013.03.024. [DOI] [PubMed] [Google Scholar]
- 83.Li Y, Liu Y, Fu Y, Wei T, Le Guyader L, Gao G, Liu RS, Chang YZ, Chen C. The triggering of apoptosis in macrophages by pristine graphene through the MAPK and TGF-beta signaling pathways. Biomaterials. 2012;33(2):402–411. doi: 10.1016/j.biomaterials.2011.09.091. [DOI] [PubMed] [Google Scholar]
- 84.Chen GY, Yang HJ, Lu CH, Chao YC, Hwang SM, Chen CL, Lo KW, Sung LY, Luo WY, Tuan HY, Hu YC. Simultaneous induction of autophagy and toll-like receptor signaling pathways by graphene oxide. Biomaterials. 2012;33(27):6559–6569. doi: 10.1016/j.biomaterials.2012.05.064. [DOI] [PubMed] [Google Scholar]
- 85.Tkach AV, Yanamala N, Stanley S, Shurin MR, Shurin GV, Kisin ER, Murray AR, Pareso S, Khaliullin T, Kotchey GP, Castranova V, Mathur S, Fadeel B, Star A, Kagan VE, Shvedova AA. Graphene oxide, but not fullerenes, targets immunoproteasomes and suppresses antigen presentation by dendritic cells. Small. 2013;9(9–10):1686–1690. doi: 10.1002/smll.201201546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liao KH, Lin YS, Macosko CW, Haynes CL. Cytotoxicity of graphene oxide and graphene in human erythrocytes and skin fibroblasts. ACS Appl Mater Interfaces. 2011;3(7):2607–2615. doi: 10.1021/am200428v. [DOI] [PubMed] [Google Scholar]
- 87.Singh SK, Singh MK, Nayak MK, Kumari S, Shrivastava S, Gracio JJ, Dash D. Thrombus inducing property of atomically thin graphene oxide sheets. ACS Nano. 2011;5(6):4987–4996. doi: 10.1021/nn201092p. [DOI] [PubMed] [Google Scholar]
- 88.Singh SK, Singh MK, Kulkarni PP, Sonkar VK, Gracio JJ, Dash D. Amine-modified graphene: thrombo-protective safer alternative to graphene oxide for biomedical applications. ACS Nano. 2012;6(3):2731–2740. doi: 10.1021/nn300172t. [DOI] [PubMed] [Google Scholar]
- 89.Chowdhury SM, Kanakia S, Toussaint JD, Frame MD, Dewar AM, Shroyer KR, Moore W, Sitharaman B. In vitro hematological and in vivo vasoactivity assessment of dextran functionalized graphene. Scientific reports. 2013;3 doi: 10.1038/srep02584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chowdhury SM, Fang J, Sitharaman B. Interaction of graphene nanoribbons with components of the blood vascular system. Future Science OA. 2015;1(3) doi: 10.4155/fso.15.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wojtoniszak M, Chen X, Kalenczuk RJ, Wajda A, Lapczuk J, Kurzewski M, Drozdzik M, Chu PK, Borowiak-Palen E. Synthesis, dispersion, and cytocompatibility of graphene oxide and reduced graphene oxide. Colloids Surf B Biointerfaces. 2012;89:79–85. doi: 10.1016/j.colsurfb.2011.08.026. [DOI] [PubMed] [Google Scholar]
- 92.Hu W, Peng C, Lv M, Li X, Zhang Y, Chen N, Fan C, Huang Q. Protein corona-mediated mitigation of cytotoxicity of graphene oxide. ACS Nano. 2011;5(5):3693–3700. doi: 10.1021/nn200021j. [DOI] [PubMed] [Google Scholar]
- 93.Mu Q, Su G, Li L, Gilbertson BO, Yu LH, Zhang Q, Sun YP, Yan B. Size-dependent cell uptake of protein-coated graphene oxide nanosheets. ACS Appl Mater Interfaces. 2012;4(4):2259–2266. doi: 10.1021/am300253c. [DOI] [PubMed] [Google Scholar]
- 94.Mbeh DA, Akhavan O, Javanbakht T, Mahmoudi M, Yahia LH. Cytotoxicity of protein corona-graphene oxide nanoribbons on human epithelial cells. Applied Surface Science. 2014;320:596–601. [Google Scholar]
- 95.Singh SK, Singh MK, Kulkarni PP, Sonkar VK, Grácio JJ, Dash D. Amine-modified graphene: thrombo-protective safer alternative to graphene oxide for biomedical applications. Acs Nano. 2012;6(3):2731–2740. doi: 10.1021/nn300172t. [DOI] [PubMed] [Google Scholar]
- 96.Sasidharan A, Swaroop S, Koduri CK, Girish CM, Chandran P, Panchakarla L, Somasundaram VH, Gowd GS, Nair S, Koyakutty M. Comparative in vivo toxicity, organ biodistribution and immune response of pristine, carboxylated and PEGylated few-layer graphene sheets in Swiss albino mice: A three month study. Carbon. 2015;95:511–524. [Google Scholar]
- 97.Zhang S, Yang K, Feng L, Liu Z. In vitro and in vivo behaviors of dextran functionalized graphene. Carbon. 2011;49(12):4040–4049. [Google Scholar]
- 98.Zhang X, Yin J, Peng C, Hu W, Zhu Z, Li W, Fan C, Huang Q. Distribution and biocompatibility studies of graphene oxide in mice after intravenous administration. Carbon. 2011;49(3):986–995. [Google Scholar]
- 99.Wang K, Ruan J, Song H, Zhang J, Wo Y, Guo S, Cui D. Biocompatibility of graphene oxide. Nanoscale Res Lett. 2011;6(8):1–8. doi: 10.1007/s11671-010-9751-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Liu J-H, Yang S-T, Wang H, Chang Y, Cao A, Liu Y. Effect of size and dose on the biodistribution of graphene oxide in mice. Nanomedicine. 2012;7(12):1801–1812. doi: 10.2217/nnm.12.60. [DOI] [PubMed] [Google Scholar]
- 101.Yang K, Zhang S, Zhang G, Sun X, Lee S-T, Liu Z. Graphene in mice: ultrahigh in vivo tumor uptake and efficient photothermal therapy. Nano letters. 2010;10(9):3318–3323. doi: 10.1021/nl100996u. [DOI] [PubMed] [Google Scholar]
- 102.Yang K, Wan J, Zhang S, Zhang Y, Lee S-T, Liu Z. In vivo pharmacokinetics, long-term biodistribution, and toxicology of PEGylated graphene in mice. ACS nano. 2010;5(1):516–522. doi: 10.1021/nn1024303. [DOI] [PubMed] [Google Scholar]
- 103.Kanakia S, Toussaint J, Hoang DM, Mullick Chowdhury S, Lee S, Shroyer KR, Moore W, Wadghiri YZ, Sitharaman B. Towards An Advanced Graphene-Based Magnetic Resonance Imaging Contrast Agent: Sub-acute Toxicity and Efficacy Studies in Small Animals. Scientific Reports. 2015;5:17182. doi: 10.1038/srep17182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kanakia S, Toussaint JD, Chowdhury SM, Tembulkar T, Lee S, Jiang Y-P, Lin RZ, Shroyer KR, Moore W, Sitharaman B. Dose ranging, expanded acute toxicity and safety pharmacology studies for intravenously administered functionalized graphene nanoparticle formulations. Biomaterials. 2014;35(25):7022–7031. doi: 10.1016/j.biomaterials.2014.04.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jasim DA, Ménard-Moyon C, Bégin D, Bianco A, Kostarelos K. Tissue distribution and urinary excretion of intravenously administered chemically functionalized graphene oxide sheets. Chemical Science. 2015 doi: 10.1039/c5sc00114e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yang K, Gong H, Shi X, Wan J, Zhang Y, Liu Z. In vivo biodistribution and toxicology of functionalized nano-graphene oxide in mice after oral and intraperitoneal administration. Biomaterials. 2013;34(11):2787–2795. doi: 10.1016/j.biomaterials.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 107.Ali-Boucetta H, Bitounis D, Raveendran-Nair R, Servant A, Van den Bossche J, Kostarelos K. Purified graphene oxide dispersions lack in vitro cytotoxicity and in vivo pathogenicity. Advanced healthcare materials. 2013;2(3):433–441. doi: 10.1002/adhm.201200248. [DOI] [PubMed] [Google Scholar]
- 108.Sahu A, Choi WI, Tae G. A stimuli-sensitive injectable graphene oxide composite hydrogel. Chemical Communications. 2012;48(47):5820–5822. doi: 10.1039/c2cc31862h. [DOI] [PubMed] [Google Scholar]
- 109.Strojny B, Kurantowicz N, Sawosz E, Grodzik M, Jaworski S, Kutwin M, Wierzbicki M, Hotowy A, Lipińska L, Chwalibog A. Long Term Influence of Carbon Nanoparticles on Health and Liver Status in Rats. PloS one. 2015;10(12):e0144821. doi: 10.1371/journal.pone.0144821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fu C, Liu T, Li L, Liu H, Liang Q, Meng X. Effects of graphene oxide on the development of offspring mice in lactation period. Biomaterials. 2015;40:23–31. doi: 10.1016/j.biomaterials.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 111.Zhang D, Zhang Z, Liu Y, Chu M, Yang C, Li W, Shao Y, Yue Y, Xu R. The short- and long-term effects of orally administered high-dose reduced graphene oxide nanosheets on mouse behaviors. Biomaterials. 2015;68:100–113. doi: 10.1016/j.biomaterials.2015.07.060. [DOI] [PubMed] [Google Scholar]
- 112.Wu Q, Yin L, Li X, Tang M, Zhang T, Wang D. Contributions of altered permeability of intestinal barrier and defecation behavior to toxicity formation from graphene oxide in nematode Caenorhabditis elegans. Nanoscale. 2013;5(20):9934–9943. doi: 10.1039/c3nr02084c. [DOI] [PubMed] [Google Scholar]
- 113.Schinwald A, Murphy FA, Jones A, MacNee W, Donaldson K. Graphene-based nanoplatelets: a new risk to the respiratory system as a consequence of their unusual aerodynamic properties. ACS nano. 2012;6(1):736–746. doi: 10.1021/nn204229f. [DOI] [PubMed] [Google Scholar]
- 114.Duch MC, Budinger GS, Liang YT, Soberanes S, Urich D, Chiarella SE, Campochiaro LA, Gonzalez A, Chandel NS, Hersam MC. Minimizing oxidation and stable nanoscale dispersion improves the biocompatibility of graphene in the lung. Nano letters. 2011;11(12):5201–5207. doi: 10.1021/nl202515a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Li B, Yang J, Huang Q, Zhang Y, Peng C, Zhang Y, He Y, Shi J, Li W, Hu J. Biodistribution and pulmonary toxicity of intratracheally instilled graphene oxide in mice. NPG Asia Materials. 2013;5(4):e44. [Google Scholar]
- 116.Yan L, Wang Y, Xu X, Zeng C, Hou J, Lin M, Xu J, Sun F, Huang X, Dai L. Can graphene oxide cause damage to eyesight? Chemical research in toxicology. 2012;25(6):1265–1270. doi: 10.1021/tx300129f. [DOI] [PubMed] [Google Scholar]
- 117.Sawangphruk M, Srimuk P, Chiochan P, Sangsri T, Siwayaprahm P. Synthesis and antifungal activity of reduced graphene oxide nanosheets. Carbon. 2012;50(14):5156–5161. [Google Scholar]
- 118.Akhavan O, Ghaderi E. Toxicity of graphene and graphene oxide nanowalls against bacteria. ACS nano. 2010;4(10):5731–5736. doi: 10.1021/nn101390x. [DOI] [PubMed] [Google Scholar]
- 119.Cai X, Lin M, Tan S, Mai W, Zhang Y, Liang Z, Lin Z, Zhang X. The use of polyethyleneimine-modified reduced graphene oxide as a substrate for silver nanoparticles to produce a material with lower cytotoxicity and long-term antibacterial activity. Carbon. 2012;50(10):3407–3415. [Google Scholar]
- 120.Chen J, Wang X, Han H. A new function of graphene oxide emerges: inactivating phytopathogenic bacterium Xanthomonas oryzae pv. Oryzae. Journal of nanoparticle research. 2013;15(5):1–14. [Google Scholar]
- 121.Santos CM, Mangadlao J, Ahmed F, Leon A, Advincula RC, Rodrigues DF. Graphene nanocomposite for biomedical applications: fabrication, antimicrobial and cytotoxic investigations. Nanotechnology. 2012;23(39):395101. doi: 10.1088/0957-4484/23/39/395101. [DOI] [PubMed] [Google Scholar]
- 122.Carpio IEM, Santos CM, Wei X, Rodrigues DF. Toxicity of a polymer–graphene oxide composite against bacterial planktonic cells, biofilms, and mammalian cells. Nanoscale. 2012;4(15):4746–4756. doi: 10.1039/c2nr30774j. [DOI] [PubMed] [Google Scholar]
- 123.Hu W, Peng C, Luo W, Lv M, Li X, Li D, Huang Q, Fan C. Graphene-based antibacterial paper. Acs Nano. 2010;4(7):4317–4323. doi: 10.1021/nn101097v. [DOI] [PubMed] [Google Scholar]
- 124.áde Leon A. On the antibacterial mechanism of graphene oxide (GO) Langmuir– Blodgett films. Chemical Communications. 2015;51(14):2886–2889. doi: 10.1039/c4cc07836e. [DOI] [PubMed] [Google Scholar]
- 125.Hui L, Piao JG, Auletta J, Hu K, Zhu Y, Meyer T, Liu H, Yang L. Availability of the basal planes of graphene oxide determines whether it is antibacterial. ACS Appl Mater Interfaces. 2014;6(15):13183–13190. doi: 10.1021/am503070z. [DOI] [PubMed] [Google Scholar]
- 126.Li J, Wang G, Zhu H, Zhang M, Zheng X, Di Z, Liu X, Wang X. Antibacterial activity of large-area monolayer graphene film manipulated by charge transfer. Sci Rep. 2014;4:4359. doi: 10.1038/srep04359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kurantowicz N, Sawosz E, Jaworski S, Kutwin M, Strojny B, Wierzbicki M, Szeliga J, Hotowy A, Lipińska L, Koziński R. Interaction of graphene family materials with Listeria monocytogenes and Salmonella enterica. Nanoscale research letters. 2015;10(1):1–12. doi: 10.1186/s11671-015-0749-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Liu S, Zeng TH, Hofmann M, Burcombe E, Wei J, Jiang R, Kong J, Chen Y. Antibacterial activity of graphite, graphite oxide, graphene oxide, and reduced graphene oxide: membrane and oxidative stress. Acs Nano. 2011;5(9):6971–6980. doi: 10.1021/nn202451x. [DOI] [PubMed] [Google Scholar]
- 129.Liu S, Hu M, Zeng TH, Wu R, Jiang R, Wei J, Wang L, Kong J, Chen Y. Lateral dimension-dependent antibacterial activity of graphene oxide sheets. Langmuir. 2012;28(33):12364–12372. doi: 10.1021/la3023908. [DOI] [PubMed] [Google Scholar]
- 130.Begum P, Ikhtiari R, Fugetsu B. Graphene phytotoxicity in the seedling stage of cabbage, tomato, red spinach, and lettuce. Carbon. 2011;49(12):3907–3919. [Google Scholar]
- 131.Khodakovskaya MV, de Silva K, Nedosekin DA, Dervishi E, Biris AS, Shashkov EV, Galanzha EI, Zharov VP. Complex genetic, photothermal, and photoacoustic analysis of nanoparticle-plant interactions. Proceedings of the National Academy of Sciences. 2011;108(3):1028–1033. doi: 10.1073/pnas.1008856108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ahmed F, Rodrigues DF. Investigation of acute effects of graphene oxide on wastewater microbial community: a case study. Journal of hazardous materials. 2013;256:33–39. doi: 10.1016/j.jhazmat.2013.03.064. [DOI] [PubMed] [Google Scholar]
- 133.Xing W, Lalwani G, Rusakova I, Sitharaman B. Degradation of Graphene by Hydrogen Peroxide. Particle & Particle Systems Characterization. 2014;31(7):745–750. [Google Scholar]
- 134.Lalwani G, Xing W, Sitharaman B. Enzymatic degradation of oxidized and reduced graphene nanoribbons by lignin peroxidase. Journal of Materials Chemistry B. 2014;2(37):6354–6362. doi: 10.1039/C4TB00976B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ma Y, Shen H, Tu X, Zhang Z. Assessing in vivo toxicity of graphene materials: current methods and future outlook. Nanomedicine. 2014;9(10):1565–1580. doi: 10.2217/nnm.14.68. [DOI] [PubMed] [Google Scholar]
- 136.Zhang W, Wang C, Li Z, Lu Z, Li Y, Yin JJ, Zhou YT, Gao X, Fang Y, Nie G. Unraveling Stress-Induced Toxicity Properties of Graphene Oxide and the Underlying Mechanism. Advanced Materials. 2012;24(39):5391–5397. doi: 10.1002/adma.201202678. [DOI] [PubMed] [Google Scholar]
- 137.Wang T, Zhu S, Jiang X. Toxicity mechanism of graphene oxide and nitrogen-doped graphene quantum dots in RBCs revealed by surface-enhanced infrared absorption spectroscopy. Toxicology Research. 2015 [Google Scholar]
- 138.Liu K, Zhang J-J, Cheng F-F, Zheng T-T, Wang C, Zhu J-J. Green and facile synthesis of highly biocompatible graphene nanosheets and its application for cellular imaging and drug delivery. Journal of Materials Chemistry. 2011;21(32):12034–12040. [Google Scholar]
- 139.Huang J, Zong C, Shen H, Liu M, Chen B, Ren B, Zhang Z. Mechanism of Cellular Uptake of Graphene Oxide Studied by Surface - Enhanced Raman Spectroscopy. Small. 2012;8(16):2577–2584. doi: 10.1002/smll.201102743. [DOI] [PubMed] [Google Scholar]
- 140.Ren H, Wang C, Zhang J, Zhou X, Xu D, Zheng J, Guo S, Zhang J. DNA cleavage system of nanosized graphene oxide sheets and copper ions. ACS nano. 2010;4(12):7169–7174. doi: 10.1021/nn101696r. [DOI] [PubMed] [Google Scholar]
- 141.Zhang L, Wang Z, Lu Z, Shen H, Huang J, Zhao Q, Liu M, He N, Zhang Z. PEGylated reduced graphene oxide as a superior ssRNA delivery system. Journal of Materials Chemistry B. 2013;1(6):749–755. doi: 10.1039/c2tb00096b. [DOI] [PubMed] [Google Scholar]
- 142.Wu M, Kempaiah R, Huang P-JJ, Maheshwari V, Liu J. Adsorption and desorption of DNA on graphene oxide studied by fluorescently labeled oligonucleotides. Langmuir. 2011;27(6):2731–2738. doi: 10.1021/la1037926. [DOI] [PubMed] [Google Scholar]
- 143.Li Y, Yuan H, von dem Bussche A, Creighton M, Hurt RH, Kane AB, Gao H. Graphene microsheets enter cells through spontaneous membrane penetration at edge asperities and corner sites. Proceedings of the National Academy of Sciences. 2013;110(30):12295–12300. doi: 10.1073/pnas.1222276110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Titov AV, Král P, Pearson R. Sandwiched graphene– membrane superstructures. ACS nano. 2009;4(1):229–234. doi: 10.1021/nn9015778. [DOI] [PubMed] [Google Scholar]
- 145.Tu Y, Lv M, Xiu P, Huynh T, Zhang M, Castelli M, Liu Z, Huang Q, Fan C, Fang H. Destructive extraction of phospholipids from Escherichia coli membranes by graphene nanosheets. Nature nanotechnology. 2013;8(8):594–601. doi: 10.1038/nnano.2013.125. [DOI] [PubMed] [Google Scholar]
- 146.Nurunnabi M, Khatun Z, Huh KM, Park SY, Lee DY, Cho KJ, Lee Y-k. In vivo biodistribution and toxicology of carboxylated graphene quantum dots. ACS nano. 2013;7(8):6858–6867. doi: 10.1021/nn402043c. [DOI] [PubMed] [Google Scholar]
- 147.Lalwani G, Patel SC, Sitharaman B. Two- and Three-Dimensional All-Carbon Nanomaterial Assemblies for Tissue Engineering and Regenerative Medicine. Annals of Biomedical Engineering - Accepted Manuscript. 2016 doi: 10.1007/s10439-016-1623-5. [DOI] [PubMed] [Google Scholar]
- 148.Lalwani G, D'Agati M, Gopalan A, Rao M, Schneller J, Sitharaman B. Three-Dimensional Macroporous Graphene Scaffolds for Tissue Engineering. Journal of Biomedical Materials Research Part A - Accepted Manuscript. 2016 doi: 10.1002/jbm.a.35867. [DOI] [PubMed] [Google Scholar]
- 149.Farshid B, Lalwani G, Shir Mohammadi M, Simonsen J, Sitharaman B. Boron nitride nanotubes and nanoplatelets as reinforcing agents of polymeric matrices for bone tissue engineering. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2015 doi: 10.1002/jbm.b.33565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Rashkow JT, Talukdar Y, Lalwani G, Sitharaman B. Interactions of 1D-and 2D-layered inorganic nanoparticles with fibroblasts and human mesenchymal stem cells. Nanomedicine. 2015;10(11):1693–1706. doi: 10.2217/nnm.15.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Teo WZ, Chng ELK, Sofer Z, Pumera M. Cytotoxicity of Exfoliated Transition - Metal Dichalcogenides (MoS2, WS2, and WSe2) is Lower Than That of Graphene and its Analogues. Chemistry–A European Journal. 2014;20(31):9627–9632. doi: 10.1002/chem.201402680. [DOI] [PubMed] [Google Scholar]
- 152.Chng ELK, Sofer Z, Pumera M. MoS 2 exhibits stronger toxicity with increased exfoliation. Nanoscale. 2014;6(23):14412–14418. doi: 10.1039/c4nr04907a. [DOI] [PubMed] [Google Scholar]