Summary
The decision to engage in food-seeking behavior depends not only on homeostatic signals related to energy balance [1] but also on the presence of competing motivational drives [2] and learned cues signaling food availability [3]. Agouti-related peptide (AgRP) neurons in the arcuate nucleus of the hypothalamus (ARC) are critical for homeostatic feeding behavior. Selective ablation or silencing of AgRP neurons causes anorexia [4,6] while selective stimulation in fed mice promotes feeding and learned instrumental actions to obtain food rewards [5–8]. However, it remains unknown whether AgRP neuron stimulation is sufficient to drive food-seeking behavior in the continued presence of a competing motivational drive, such as threat avoidance, or whether it can drive discrimination between learned sensory cues associated with food rewards and other outcomes. Here, we trained mice to perform a sensory discrimination task involving appetitive and aversive visual cues. Food-restricted mice exhibited selective operant responding to food-predicting cues but largely failed to avoid cued shocks by moving onto a safety platform. The opposite was true following re-feeding. Strikingly, AgRP neuron photostimulation did not restore operant responding in fed mice when initiated within the threat-containing arena, but did when initiated in the home cage, prior to arena entry. These data suggest that the choice to pursue certain behaviors and not others (e.g. food seeking vs. shock avoidance) can depend on the temporal primacy of one motivational drive relative to the onset of a competing drive.
eTOC blurb
Jikomes et al. show that hypothalamic AgRP neuron stimulation drives a learned food-seeking behavior when stimulation begins prior to, but not following, entry into a threat-containing context. Thus, competing motivational stimuli can moderate the effects of AgRP neuron activation in driving learned behaviors in complex environments.
Results
We tested whether stimulation of AgRP neurons in fed mice is sufficient to recapitulate a learned behavior involving sensory discrimination between cues of opposite valence, using a previously described optogenetic photostimulation paradigm in AgRP-IRES-Cre mice [5,7] (Figure 1A; see Supplemental Experimental Procedures). First, we confirmed that photostimulation induced consumption of standard chow in ad libitum fed mice during the early light cycle (Figure 1B–D; p<0.001, Laser On vs. Laser Off periods). Food intake using this stimulation protocol was quantitatively similar to that observed in mice following fasting for 24 hours (Figure 1D). In addition, we investigated the effect of photostimulation on in vivo AgRP neuron firing rates by analyzing previously recorded electrophysiology data [8]. We found that photostimulation of identified AgRP neurons produced modest yet reliable increases in firing rate (Figure S1) that were well within the range of previously reported endogenous AgRP neuron firing rates [8].
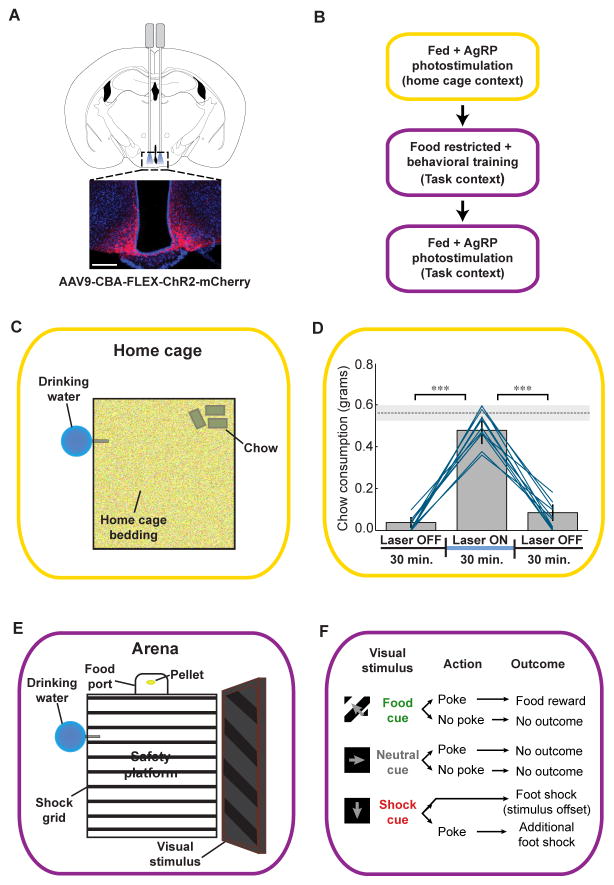
Figure 1. Experimental design for AgRP neuron stimulation in freely moving mice in the home cage and during a visual discrimination task.
(A) Selective expression of ChR2-mCherry in AgRP neurons in the arcuate nucleus of the hypothalamus. Optic fibers were implanted either uni- or bilaterally (see Supplemental Experimental Procedures and Figure S2C). Scale bar, 200 μm. (B) Flow chart illustrating order of experiments. Once photostimulation-evoked feeding in the home cage was confirmed in fed mice, mice were food-restricted, trained on a visual discrimination task, returned to ad libitum access to food, and then tested for photostimulation-evoked discrimination behavior. (C) Schematic showing the home cage setting used to test for AgRP stimulation-evoked feeding. (D) Food intake before, during, and after unilateral AgRP neuron photostimulation in the home cage setting. Lines represent mean intake for all individual mice used in all subsequent photostimulation experiments (n = 11); bars represent grand average ± SE. Paired t-tests, ***p < 0.001; dashed line represents mean ± SE chow consumption in mice fasted for 24 hours. (E) Schematic showing the behavioral arena used for visual discrimination training. (F) Schematic of visual discrimination task stimulus-response contingencies. See also Figure S1 and Movie S1.
Once robust photostimulation-induced home cage feeding was confirmed (see Supplemental Experimental Procedures), mice were food-restricted and trained to perform a visual discrimination task (Figure S2; see Supplemental Experimental Procedures). In this task, head entry into a food port during a 5-second window following termination of one of three randomly presented visual stimuli (grating patterns drifting in one of three directions, each 5 seconds in duration) resulted in either food reward, foot shock, or no outcome (Figure 1E,F and Supplemental Experimental Procedures). Importantly, the shock-associated cue was also followed by a shock that did not depend on head entry, but that could be avoided by occupying a safety platform in the arena center (Figure 1E).
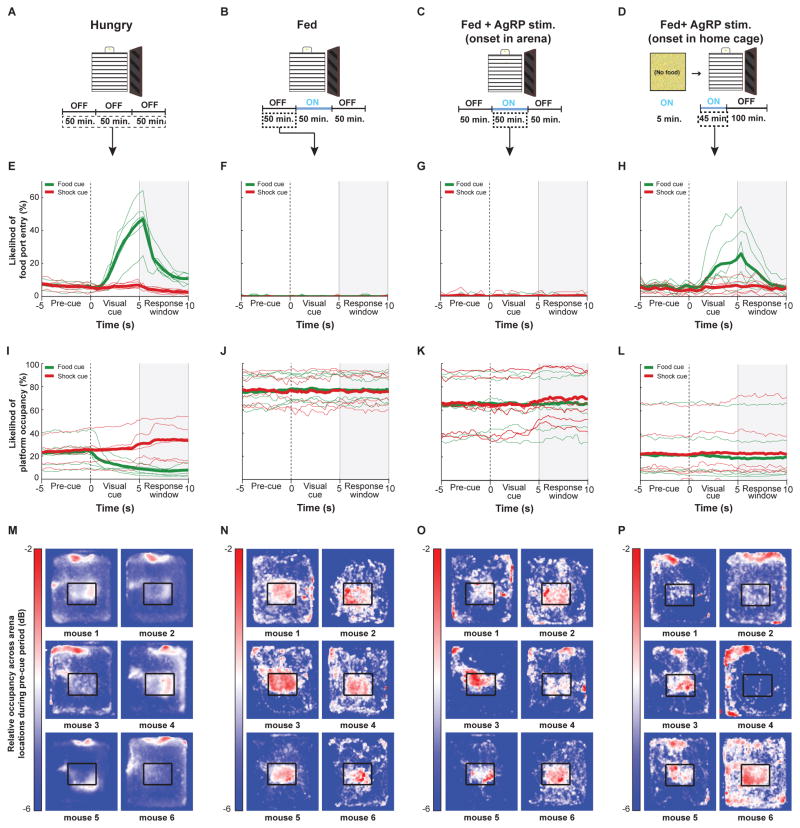
Example behavioral data are shown in Figure 2 and further quantified in Figures 3–4. Once food-restricted mice reached stable performance criteria (see Figure S2 and Supplemental Experimental Procedures), they reliably discriminated the different visual cues and displayed robust food cue-triggered head poking for hundreds of trials per session (Figure 2A,E). These mice often remained near the food port, off of the safety platform (Figure 2I,M), and thus received a majority of the shocks that followed shock cues, despite avoiding additional shocks triggered by operant responding (Figure S3A–B). After two days of ad libitum access to food in the home cage, fed mice no longer engaged in operant head poking (Figure 2B,F) and instead occupied the safety platform (Figure 2J,N), avoiding most shocks (Figure S3; Supplemental Videos 1–2).
Figure 2. Performance of an operant visual discrimination task depends on hunger state and AgRP neuron photostimulation protocols.
(A–D): Schematics illustrating basic design of behavioral experiments across conditions. (A) Food-restricted mice did not experience photostimulation. Experiments in well-trained mice typically lasted 150 minutes (600 trials). (B) Behavior of fed mice was assessed in a 50-minute block in the absence of any photostimulation. (C) In the first AgRP photostimulation protocol, stimulation in fed mice occurred during the second 50-minute block. (D) In the second photostimulation protocol, stimulation began in the home cage (5 minutes), and continued during and for 45 minutes after mouse placement into the behavioral arena. (E–H): Mean peri-stimulus time courses of the probability of head poking into the food port during food-cue trials (green) and shock-cue trials (red). Thin lines: 6 example mice; thick lines: average across all mice (for additional quantification, see Figure 3). (E) Food-restricted mice engage in this discrimination task, and make more food port entries following the food cue as compared to following the shock cue. (F) Prior to photostimulation, fed mice almost never entered the food port. (G) AgRP photostimulation, when initiated in the middle of a behavioral session, did not cause cue-evoked increases in food port entry. (H) AgRP neuron photostimulation for 5 minutes in the home cage and continuing during and after placement in the arena led to a clear increase in cue-evoked food port entries, especially during food-cue trials. (I–L): Mean peristimulus time courses of the probability of being on the safety platform during food (green) and shock (red) cues. Thin lines: 6 example mice; thick lines: average across all mice. (I) Food-restricted mice are unlikely to occupy the safety platform, especially following food cues. (J) Fed mice are much more likely to occupy the safety platform. (K) Mice are still likely to occupy the safety platform during AgRP photostimulation initiated within the arena. (L) When AgRP photostimulation is initiated in the home cage, mice are much less likely to occupy the safety platform. (M–P): Cameras mounted above the behavioral arena enabled tracking of mouse location, further illustrating the clear differences in average mouse location prior to visual cue onset. Heat maps show relative occupancy in various areas of the arena for six example mice (same orientation as in panel A, with food port located at top). For additional quantification, see Figure 4. (M) Food-restricted mice were relatively more likely to be found near the food port compared to other areas, such as the safety platform. (N) The same mice, when fed, now spent more time on the safety platform compared to the food port area. (O) During the AgRP photostimulation block of trials, fed mice still largely remained on the safety platform. (P) In sessions in fed mice with AgRP photostimulation beginning in the home cage and continuing after arena placement, most mice spent more time near the food port rather than on the safety platform (see Figure 4 and S3). See also Figures S1, S2 and S4, and Movie S2.
Surprisingly, AgRP neuron photostimulation in fed mice, beginning midway through a session, did not cause increases in operant head poking or food port approach behavior (Figure 2C,G), and mice still avoided the majority of unconditional shocks by remaining on the safety platform (Figure 2K,O; Figure S3B). This lack of task engagement could not be explained by extinction of learned behavior, as mice still performed the task upon subsequent food restriction (Figure S3C). Indeed, we were careful to limit photostimulation periods to 50 minutes, and to always follow photostimulation with a Laser OFF period in order to avoid photostimulation-induced extinction [7]. In addition, the lack of task engagement could not be explained by diminished efficacy of AgRP photostimulation, as mice still displayed photostimulation-induced home cage chow consumption on the following day (Figure S3D), and displayed strong ChR2-mCherry expression localized to the ventromedial ARC (Figure S3E). However, the lack of engagement did appear related to the presence of shocks: arena-onset AgRP photostimulation in fed mice trained on a variant of the task which did not include shock trials did partially rescue visual cue-induced food-port entry, albeit with poor discrimination between food cues and neutral cues due to lack of negative reinforcement (Figure S4A–F).
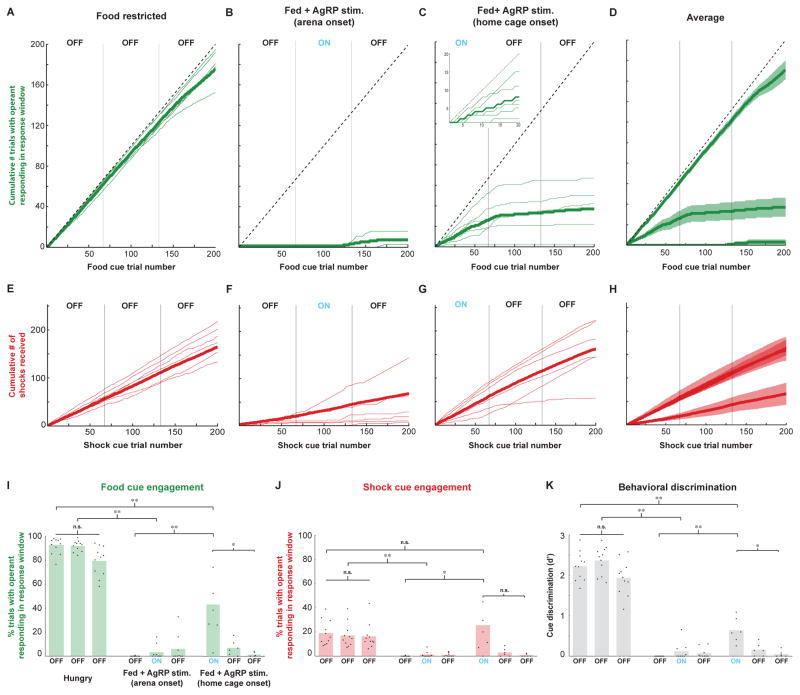
Previous work in rodents has shown that acute stress induces hypophagia [9], while food restriction can block the induction of responses to acute stress [10]. These findings, together with our observation that food-restricted mice avoided shocks much less frequently than fed mice (Figure S3A–B; p<0.001), led us to hypothesize that AgRP neuron activation may simultaneously suppress behavioral responses to threats while promoting food-seeking, perhaps as a means of prioritizing certain motivated behaviors over others [11]. Conversely, the continued presence of threats during satiety might facilitate a state of threat avoidance that, in turn, suppresses subsequent initiation of food seeking by AgRP photostimulation. We therefore tested whether AgRP photostimulation in fed mice, prior to placement in the shock-containing arena, might restore operant behavior. Strikingly, we found that AgRP photostimulation for five minutes in the home cage (in the absence of food) and continuing during transfer into the arena (Figure 2D) caused fed mice to engage in cue-triggered operant behavior (Figure 2H) and to remain near the food port, off of the safety platform (Figure 2L,P), thus receiving most cued shocks (Figure S3A–B; p<0.001). For the six mice that underwent both arena and home cage onset photostimulation, the timing-dependent differences in effects of photostimulation can be seen by comparing, across these conditions, the average cumulative number of trials for which mice displayed operant head poking during the response window (Figure 3A–D; see also Figure S4 for analysis of head-poking during the visual cue rather than during the subsequent response window). Note that, for the home cage onset condition, task engagement already began within the first few trials (Figure 3C, inset), continued at similar levels throughout the photostimulation block, and could persist for several trials following photostimulation offset (Figure 3C, middle block). Similarly, plots of cumulative shocks received (Figure 3E–H) revealed that, similar to food-restricted mice, fed mice with home cage photostimulation onset failed to avoid shocks – a behavior that persisted long after cessation of photostimulation. These behavioral observations were further quantified below (Figures 3I–K and 4), across all natural and artificial hunger and satiety states.
Figure 3. Quantification of engagement in the visual discrimination task across hunger states and AgRP neuron photostimulation protocols.
(A–D): Cumulative number of pokes during the response window, following termination of the food cue, obtained throughout 600 trial sessions in the food-restricted (A), arena photostimulation onset (B), and home cage photostimulation onset (C) conditions, together with the means ± SE across mice for each condition (D). (E–H): Similar to A–D, but for cumulative shocks received. Note that, for each shock cue presentation, it was possible to receive up to two shocks, one for poking following shock cue offset, and one for being off of the safety platform at shock cue offset. (I) Food cue engagement, defined as the percentage of food cue trials in which mice received a food reward, is high in food-restricted (FR) mice (left), and low both in both fed mice and in fed mice receiving AgRP neuron photostimulation that began after arena placement (middle). In contrast, fed mice that received AgRP neuron photostimulation beginning in the home cage, prior to arena entry, showed an intermediate level of food cue engagement (right). Dots: mean engagement for each mouse; bars: grand average across mice. (J) Mice also displayed differential engagement following the shock cue across experimental protocols. (K) The discriminability index, d′, which measures a mouse’s ability to perform selective operant responding following the food cue but not following the other cues, was also significantly elevated in food-restricted mice, and in fed mice that received AgRP photostimulation prior to arena entry, as compared to fed mice in all other conditions. **p<0.01/n, *p<0.05/n, n.s., not significant; Bonferroni correction for multiple comparisons (n = 9); Wilcoxon rank-sum test for independent samples. See also Figures S3 and S4.
In Figure 3I–K, we calculated task engagement as the fraction of trials in which mice poked during the 5-s response window following each visual cue. Food-restricted mice and fed mice that received AgRP photostimulation prior to arena entry demonstrated significantly more cue-evoked head poking than mice in other fed conditions, particularly in response to the food cue (Figure 3I–J). While 5/6 mice tested during home cage-initiated photostimulation displayed a significant increase in visual cue-triggered poking compared to the fed condition (Figure 3I; Tukey’s HSD p<0.01, fed vs. fed + home cage photostimulation onset), food cue engagement did not typically reach the same level displayed by food-restricted mice (p<0.01). To calculate the behavioral discriminability of food cues from other cues, we used a standard discriminability index (d′, [12]; see Experimental Procedures). While discrimination during home cage-initiated photostimulation was lower than during food restriction (p<0.01; Figure 3K), it was nevertheless significantly higher than in fed mice during the block of trials prior to arena-onset photostimulation (p<0.01; Figure 3K).
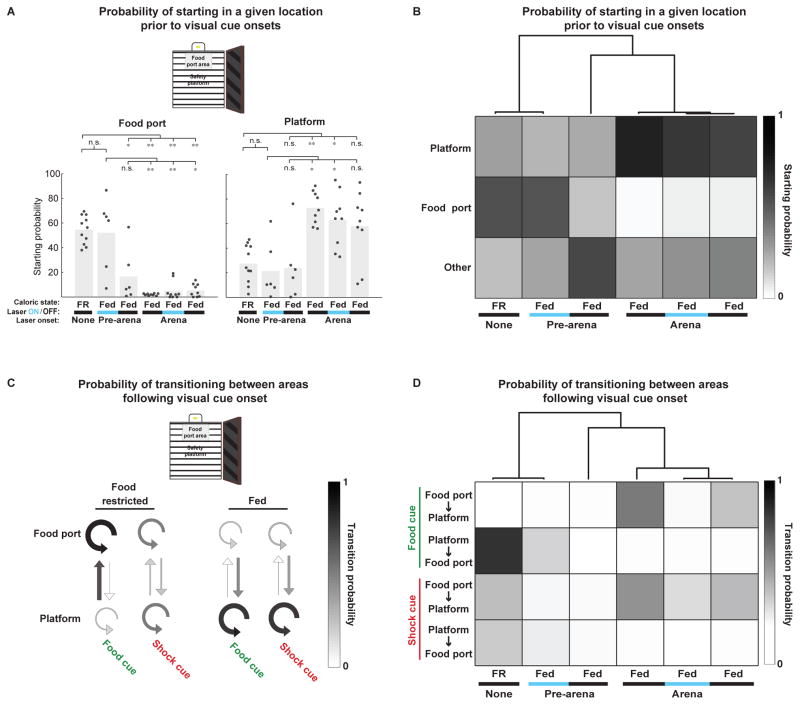
Additional videography analyses enabled a more in-depth comparison of behavior across conditions. These analyses further illustrated a greater similarity of AgRP photostimulation-induced behavior in fed mice to either the food-restricted state or the fed state, depending on the context in which photostimulation was first initiated. We classified mice as being located in one of three sections of the arena (food port area, safety platform, or elsewhere; Figure 4A, schematic). Food-restricted mice and fed mice with home cage-initiated AgRP photostimulation spent a majority of trials within the food port area prior to visual cue onset (Figure 4A, left), while fed mice, both prior to and after receiving arena-initiated AgRP photostimulation, spent a majority of trials on the safety platform (Figure 4A, right; see Supplemental Experimental Procedures). By comparing these pre-cue probabilities across all experimental conditions and performing unsupervised hierarchical clustering [13], we confirmed that home cage-initiated and arena-initiated photostimulation elicited pre-stimulus arena occupancy that more closely resembled food-restricted and fed states, respectively (Figure 4B).
Figure 4. Quantification of non-operant measures of freely moving behavior across hunger states and AgRP neuron photostimulation protocols.
(A) Mice displayed major differences in their tendency to begin trials within the food port area, safety platform, or other areas (see schematic) across experimental conditions. Left: food-restricted mice, and fed mice receiving AgRP photostimulation beginning in the home cage (columns 1–2), were often located in the food port area prior to trial onset, but this was not the case for all other conditions (columns 3–6; see also Figure 2M–P). Right: By contrast, food-restricted mice, and fed mice receiving AgRP photostimulation beginning in the home cage (columns 1–3), were less likely to begin trials on the safety platform compared to fed mice in other conditions (columns 4–6). **p<0.01/n, *p<0.05/n; Bonferroni correction for multiple comparisons (n=6); Wilcoxon rank-sum test for independent samples. (B) Same data as in (A), represented as a probability matrix across all conditions. Top: Hierarchical clustering of the pre-cue starting probabilities revealed that the starting location of fed mice receiving AgRP neuron photostimulation prior to arena entry most closely resembles that of food-restricted mice, while fed mice receiving AgRP photostimulation within the arena more closely resembled fed mice. (C) Visual cue-evoked transitions between arena locations differed between food-restricted and fed mice. The thickness and shading of each arrow is proportional to the probability of mice making the indicated transition following visual cue onset. Food-restricted mice were very likely to move to, or remain at, the food port following food cue onset. Fed mice were most likely to transition to, or remain at the safety platform following food or shock cue onset. (D) The same data as in (C), represented as a probability matrix across all conditions. Top: Hierarchical clustering of food and shock cue-evoked transition probabilities between the food port and safety platform revealed that fed mice receiving AgRP neuron photostimulation prior to arena entry closely resembled food-restricted mice, while fed mice receiving AgRP photostimulation within the arena more closely resembled fed mice. See also Figure S3.
Analysis of the probability of visual cue-evoked transitions between locations yielded similar results. Food-restricted mice were most likely to transition to, or remain at, the food port area upon food cue presentation, while fed mice were most likely to transition to, or remain at, the safety platform upon presentation of any cue (Figure 4C; p<0.001, food port transition probability, food restricted vs. fed). Hierarchical clustering again confirmed that home cage-initiated or arena-initiated photostimulation elicited cue-evoked transition behaviors that more closely resembled food-restricted and fed states, respectively (Figure 4D).
Discussion
We have shown that initiation of AgRP neuron photostimulation in the presence of both food- and threat-predicting cues is not sufficient to drive a learned food-seeking behavior in fed mice This finding stands in contrast to previous reports in which stimulation of AgRP neurons was sufficient to drive learned food-seeking behaviors in contexts that lacked overt threats [5–8]. Given that the photostimulation protocol we used produces voracious home cage feeding comparable to what is observed in 24-hour fasted mice (Figure 1D; [5]), and that we stimulated AgRP neurons bilaterally, it is unlikely that this observation can be explained by insufficient stimulation of AgRP neurons. Remarkably, initiating an identical AgRP photostimulation protocol in the home cage, prior to entering the shock-containing arena, drove fed mice to subsequently engage in this sensory discrimination task. This observation expands the set of complex motivated behaviors orchestrated by this remarkably small neuronal population [6–7, 14]. Thus, our findings provide a useful starting point for the investigation of neural circuits downstream of AgRP neurons, which are likely regulated by competing drives, including food seeking and threat avoidance.
It is worth noting that, in the home cage onset condition, mice typically began accumulating food rewards within the first few trials after arena entry, ~5 minutes after onset of home cage photostimulation (Figure 3C, inset). This is consistent with previous work showing that AgRP neuron photostimulation can begin to induce food-seeking behavior within minutes of onset [5,15]. This rapid induction of task engagement suggests that GABA and/or NPY likely mediate the effects of this experimental manipulation, as the AgRP peptide affects feeding only at substantially longer delays [16]. An important avenue for future work will be to dissect which transmitters and peptide systems contribute to the suppression of competing motivational drives.
It is intriguing that AgRP photostimulation in fed mice, when initiated prior to arena entry, restored not only operant discrimination behavior but also hunger-associated indifference to avoidable shocks delivered following termination of each shock cue. Given the risks of predation incurred during natural foraging [17], AgRP neurons may restore energy balance by coordinated activation of food-seeking circuitry at specific AgRP targets [18] together with suppression of circuits driving incompatible behaviors [11,19]. This might serve as a means to prioritize the threat of starvation over other potential threats. For example, AgRP neuron target regions such as the central amygdala and periaqueductal gray have a well-defined role in promoting avoidance behaviors [20], and stimulation of AgRP axon terminals in these targets does not increase feeding [18]. Indeed, a recent study found that AgRP neuron stimulation may be anxiolytic in the absence of food, and defined a disynaptic pathway by which a subset of AgRP neuron can suppress territorial aggression [11]. This suggests that certain AgRP subpopulations promote food seeking via inhibition of downstream circuits that promote and/or sustain motivational states that are not conducive to food seeking. Notably, the behavioral assays in [11] involved AgRP neuron stimulation protocols that begin before initial exposure to threats (e.g. resident intruders or anxiety-promoting contexts), leaving open the question of whether stimulation would have the same effects on anxiety and territorial aggression if initiated after exposure to threats. An important avenue of future work will be the systematic, combinatorial interrogation of multiple AgRP targets regions that likely underlie competitive interactions between incompatible motivational states. Antagonistic interactions between distinct behavioral states, often driven by mutual inhibition of competing circuits, have been observed or proposed for many other motivational circuits in the hypothalamus and limbic system [18, 21–22]. For example, the efficacy of a mounting sleep drive is mitigated by sustained, stressor-induced activation of arousal circuitry [21]. Similarly, placement of mice into our threat-containing arena in a fed state may permit induction of arena-induced, sustained activation of stress-related circuits, analogous to the persistent state of defensive arousal observed in a recent study in Drosophila [23]. As with sleep drive, a mounting food-seeking drive may not be able to overcome this stressor-induced defensive state once it is initiated. In contrast, entering the arena with AgRP neuron stimulation already underway may act to prevent such a defensive state from arising, potentially through a combination of reduced contextual fear [11] and hunger-induced analgesia [24]. Work in invertebrate systems has begun to elucidate the circuit mechanisms underlying such competitive interactions [25–26], and behavioral paradigms have recently been developed to investigate these interactions in Drosophila [23]. The paradigm we introduce here provides a similar starting point for probing such circuit mechanisms in an experimentally tractable mammalian model system. Our study provides a behavioral framework for understanding the coordinated actions of different AgRP projection neurons on food-seeking and other behaviors, which could yield new insights into the comorbidity between eating- and stress-related disorders [14,27]. The observation of different behavioral outcomes depending on temporal primacy of AgRP neuron stimulation — in the minutes prior to vs. following exposure to a threat-containing environment — further underscores the value of reversible and temporally precise in vivo approaches to understanding the function of neural circuits controlling complex behaviors [28].
Supplementary Material
Highlights.
Hungry mice work to obtain cued food rewards but fail to avoid cued shocks
Fed mice do not work to obtain cued food rewards but do avoid cued shocks
AgRP neuron stimulation in the task arena fails to drive cued food-seeking in fed mice
AgRP stimulation, if begun prior to arena entry, drives cued food-seeking in fed mice
Acknowledgments
We thank Guido Meijer and Paola Patella for technical assistance with behavioral training and video tracking. We thank Derek Woloszyn, Crista Carty, David Gonzalez-Cameron and Mary Dello Russo for assistance with mouse training and Kirsten Levandowski for assistance with mouse histology. We thank Drs. Bradford Lowell, Christian Burgess, Yoav Livneh, Todd Anthony, and Rachel Ross for helpful comments on the manuscript, and members of the Andermann lab for useful feedback and discussion. Support was provided the National Science Foundation (NSFGRFP) and Sackler Scholars in Psychobiology Programme (NJ), NIH NRSA F31 NIDDK (RNR), Charles King Trust (YMC), and by a NIH Director’s New Innovator Award DP2DK105570-01, R01DK109930-01, and grants from the Klarman Family Foundation, McKnight Foundation, Richard and Susan Smith Family Foundation, and the Pew Scholars Program in the Biomedical Sciences (MLA).
Footnotes
Supplemental information includes Supplemental Experimental Procedures and four figures and can be found online.
Author contributions
NJ performed all experiments and data analyses. YMC collected and RNR and YMC analyzed electrophysiology data in Figure S1. NJ and MA designed all experiments and wrote the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Williams KW, Elmquist JK. From neuroanatomy to behavior: central integration of peripheral signals regulating feeding behavior. Nat Neurosci. 2012;15:1350–1355. doi: 10.1038/nn.3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Palmiter R. Hunger logic. Nat Neurosci. 2015;18:789–791. doi: 10.1038/nn.4032. [DOI] [PubMed] [Google Scholar]
- 3.Reppucci CJ, Petrovich GD. Learned food-cue stimulates persistent feeding in sated rats. Appetite. 2012;59:437–447. doi: 10.1016/j.appet.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luquet S, Perez FA, Hnasko TS, Palmiter RD. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science. 2005;310:683–685. doi: 10.1126/science.1115524. [DOI] [PubMed] [Google Scholar]
- 5.Aponte Y, Atasoy D, Sternson SM. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat Neurosci. 2011;14:351–355. doi: 10.1038/nn.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krashes MJ, Koda S, Ye C, Rogan SC, Adams AC, Cusher DS, Maratos-Flier E, Roth BL, Lowell BB. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J Clin Invest. 2011;121:1424–1428. doi: 10.1172/JCI46229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Betley JN, Xu S, Cao ZF, Gong R, Magnus CJ, Yu Y, Sternson SM. Neurons for hunger and thirst transmit a negative-valence teaching signal. Nature. 2015;521:180–185. doi: 10.1038/nature14416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mandelblat-Cerf Y, Ramesh RN, Burgess CR, Patella P, Yang Z, Lowell BB, Andermann ML. Arcuate hypothalamic AgRP and putative POMC neurons show opposite changes in spiking across multiple timescales. Elife. 2015:4. doi: 10.7554/eLife.07122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calvez J, Fromentin G, Nadkarni N, Darcel N, Even P, Tome D, Ballet N, Chaumontet C. Inhibition of food intake induced by acute stress in rats is due to satiation effects. Physiol Behav. 2011;104:675–683. doi: 10.1016/j.physbeh.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 10.Maniscalco JW, Zheng H, Gordon PJ, Rinaman L. Negative Energy Balance Blocks Neural and Behavioral Responses to Acute Stress by ‘Silencing’ Central Glucagon-Like Peptide 1 Signaling in Rats. J Neurosci. 2015;35:10701–10714. doi: 10.1523/JNEUROSCI.3464-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Padilla LP, Qiu J, Soden ME, Sanz E, Nestor CC, Barker FD, Quintana A, Zweifel LS, Ronnekleiv OK, Kelly MJ, Palmiter RD. Agouti-related peptide neural circuits mediate adaptive behaviors in the starved state. Nature Neurosci. 2016;19:734–741. doi: 10.1038/nn.4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Green DM, Swets JA. Signal detection theory and psychophysics. New York: Wiley; 1966. [Google Scholar]
- 13.Cohen JY, Haesler S, Vong L, Lowell BB, Uchida N. Neuron-type-specific signals for reward and punishment in the ventral tegmental area. Nature. 2012;482:85–88. doi: 10.1038/nature10754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dietrich MO, Zimmer MR, Bober J, Horvath TL. Hypothalamic Agrp neurons drive stereotypic behaviors beyond feeding. Cell. 2015;160:1222–1232. doi: 10.1016/j.cell.2015.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Atasoy D, Betley JN, Su HH, Sternson SM. Deconstruction of a neural circuit for hunger. Nature. 2012;488:172–177. doi: 10.1038/nature11270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krashes MJ, Shah BP, Koda S, Lowell BB. Rapid versus delayed stimulation of feeding by the endogenously released AgRP neuron mediators GABA, NPY, and AgRP. Cell Metab. 2013;18:588–595. doi: 10.1016/j.cmet.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sih A. Optimal behavior: can foragers balance two conflicting demands? Science. 1980;210:1041–1043. doi: 10.1126/science.210.4473.1041. [DOI] [PubMed] [Google Scholar]
- 18.Betley JN, Cao ZF, Ritola KD, Sternson SM. Parallel, redundant circuit organization for homeostatic control of feeding behavior. Cell. 2013;155:1337–1350. doi: 10.1016/j.cell.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carter ME, Soden ME, Zweifel LS, Palmiter RD. Genetic identification of a neural circuit that suppresses appetite. Nature. 2013;503:111–114. doi: 10.1038/nature12596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johansen JP, Cain CK, Ostroff LE, LeDoux JE. Molecular mechanisms of fear learning and memory. Cell. 2011;147:509–524. doi: 10.1016/j.cell.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saper CB, Fuller PM, Pedersen NP, Lu J, Scammell TE. Sleep state switching. Neuron. 2010;68:1023–1042. doi: 10.1016/j.neuron.2010.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hong W, Kim DW, Anderson DJ. Antagonistic control of social versus repetitive self-grooming behaviors by separable amygdala neuronal subsets. Cell. 2014;158:1348–1361. doi: 10.1016/j.cell.2014.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gibson WT, Gonzalez CR, Fernandez C, Ramasamy L, Tabachnik T, Du RR, Felsen PD, Maire MR, Perona P, Anderson DJ. Behavioral responses to a repetitive visual threat stimulus express a persistent state of defensive arousal in Drosophila. Curr Biol. 2015;25:1401–1415. doi: 10.1016/j.cub.2015.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fields HL. Understanding how opioids contribute to reward and analgesia. Reg Anesth Pain Med. 2007;32:242–246. doi: 10.1016/j.rapm.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 25.Gaudry Q, Kristan WB. Behavioral choice by presynaptic inhibition of tactile sensory terminals. Nature Neurosci. 2009;12:1450–1457. doi: 10.1038/nn.2400. [DOI] [PubMed] [Google Scholar]
- 26.Kristan WB. Neuronal decision-making circuits. Curr Biol. 2008;18:928–932. doi: 10.1016/j.cub.2008.07.081. [DOI] [PubMed] [Google Scholar]
- 27.Ulrich-Lai YM, Ryan KK. Neuroendocrine circuits governing energy balance and stress regulation: functional overlap and therapeutic implications. Cell Metab. 2014;19:910–925. doi: 10.1016/j.cmet.2014.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen Y, Knight ZA. Making sense of the sensory regulation of hunger neurons. Bioessays. 2016;38:316–324. doi: 10.1002/bies.201500167. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.