Abstract
Objectives
Food fussiness (FF), or the frequent rejection of both familiar and unfamiliar foods, is common among children and given its link to poor diet quality, may contribute to the onset and/or maintenance of childhood obesity. The current study examined child FF in association with anthropometric variables and diet in children with overweight/obesity participating in family-based behavioral weight loss treatment (FBT). Change in FF was assessed in relation to FBT outcome, including whether change in diet quality mediated the relation between change in FF and change in child weight.
Methods
Child (N=170; age=9.41 ± 1.23) height and weight were measured and parents completed FF questionnaires and three 24-hour recalls of child diet at baseline and post-treatment. Healthy Eating Index-2005 (HEI) scores were calculated.
Results
At baseline, child FF was related to lower vegetable intake. Average child FF decreased from start to end of FBT. Greater decreases in FF were associated with greater reductions in child zBMI and improved overall diet quality. Overall diet quality change through FBT mediated the relation between child FF change and zBMI change.
Conclusions
Children with high FF can benefit from FBT and addressing FF may be important in childhood obesity treatment to maximize weight outcomes.
Keywords: food fussiness, childhood obesity, family-based treatment, diet quality
Introduction
Food fussiness (FF) refers to rejection of a substantial number of unfamiliar and even some familiar foods, resulting in consumption of a limited variety of food types or food items1. FF typically emerges by age 6 and is relatively stable through childhood, with estimated prevalence rates around 5–25% in infants and children2–6. FF is problematic because rejected foods tend to be low-calorie, nutrient-rich foods, such as fruits and vegetables7–9. As such, FF has been associated with children's lower intakes of certain healthy food groups, particularly vegetables9–11, lower intakes of vitamins and minerals7,12, and lower intakes of dietary fiber7. Indeed, many children with high FF fail to meet dietary recommendations for certain food and nutrient groups7. Additionally, FF appears to be associated with negative psychosocial implications, including eating disorder development13, higher rates of behavioral and emotional disorders6, and parental concern6
Prior research has found that FF is negatively related to both calorie consumption and child Body Mass Index (BMI)4,9,11,12,14; however, recent research contradicts these findings and suggests that FF may play a role in pediatric obesity development and maintenance. Faith and Hittner (2010) found that a negative reaction to food, as measured by the Colorado Childhood Temperament Inventory15 which these investigators liken to early FF, in female infants predicted greater likelihood of obesity development by age 6. Additionally, Finistrella and colleagues (2012) found that in a population of 2- to 6-year-olds, children with overweight or obesity were significantly more likely to demonstrate FF than children of normal weight16. These investigators explain their opposing results by reasoning that in an effort to get children with high FF to eat, parents may utilize coercive feeding strategies, which have been implicated in children's overconsumption of food (albeit of limited variety). Parents of children with high FF may also be feeding them highly palatable, but calorically-dense foods, instead of lower-calorie rejected items like fruits and vegetables17. In fact, children with high FF have been shown to consume more sweet foods than children with low FF12. Thus, additional research on the relation between FF and child weight status is warranted. If FF contributes to pediatric obesity development and sustainment, it may be a relevant target within pediatric obesity treatment.
Interventions that target FF do so through repeatedly exposing the child to novel and/or disliked foods and by encouraging parents to model the desired eating behaviors and use positive reinforcement when disliked foods are consumed18–22. Programs and strategies to reduce FF are efficacious, and are often contained within broader interventions for child eating and feeding issues20,22. Family-based treatment (FBT) approaches to pediatric obesity use these same techniques for increasing consumption of low energy-dense foods, such as fruits and vegetables, which are commonly rejected by children high in FF23. Thus, FBT may improve children's FF. Of note, rates of FF are higher in treatment-seeking than community samples of children with obesity24. Reasons for this discrepancy remain unknown; one proposed theory is that parents may self-select into treatment if they are having high levels of perceived difficulty feeding more healthful foods to their child with obesity. In light of the higher rates of FF among treatment-seeking populations of children with obesity, this may be an optimal group with which to intervene for both obesity and FF reduction. To date, it remains unclear how children's FF may change throughout such treatment, and what role FF may play in FBT success. If children who are obese with higher FF are able to reduce FF, diversify their diet, and consume greater amounts of fruits and vegetables within an identified calorie range, substituting these healthier options for the more calorically-dense, nutrient-poor foods may lead to improved weight outcomes. Understanding the role of FF in FBT may lead to treatment modification or better personalization of treatment content based on children's FF.
The current study aimed to examine associations between initial FF and child weight and diet quality (including food group intake) in a treatment-seeking sample of children with overweight/obesity. Additionally, this study examined baseline FF as a predictor of FBT response and, consequently, how changes in FF may contribute to child weight and diet change following treatment. Finally, given the link between dietary and weight change our data have previously indicated25,26, an exploratory mediation analysis assessed changes in food group consumption and diet quality as a mediator of the relation between children's FF change and weight change.
Methods
Overview / Study Design
This study is a pre- and post-comparison study, which used data collected as part of a multi-site randomized-controlled trial that examined different maintenance interventions following FBT. Data for the present study are limited to participants who engaged in FBT (n=170) and include assessments before FBT (baseline) and at post-FBT (prior to maintenance treatment randomization). Assessments consisted of anthropometrics, questionnaires, and 24-hour dietary recalls. Written informed consent and verbal assent were obtained from parents and children, respectively. The study was conducted at the Washington University School of Medicine and Seattle Children's Research Institute and was approved by each site's Institutional Review Board.
Participants
Children (ages 7–11 years) with a BMI ≥85th percentile for age and sex and at least one parent who had a BMI ≥25 kg/m2 were recruited via fliers, newspapers, television, radio, referrals from schools and community providers, and word of mouth. Exclusion criteria for both children and parents were participation in another weight control program, major psychiatric problems including an eating disorder diagnosis, and dietary limitations, such as severe food allergies, or physical activity limitations that restricted engaging with treatment recommendations.
Family-Based Behavioral Treatment (FBT)
FBT is an empirically-supported treatment for childhood obesity that targets diet, physical activity, behavioral modification, and parenting skills to support child weight loss23. Diet-related components, and those most likely to influence FF, include the Traffic Light Eating Plan, which aims to increase consumption of low-fat nutrient-dense foods (e.g. fruits and vegetables; 5 or more servings a day) and decrease consumption of high-fat, nutrient-poor foods (e.g. sugar-sweetened beverages, cookies;15 or fewer servings per week) and a child kilocalorie goal. Having both calorie goals and food quality goals encourages children to consume a healthy nutrient-rich diet while reducing calories to lose weight. Furthermore, the treatment simultaneously targets both the child and parent for behavioral changes, as active parent engagement and weight loss have been shown to be strong predictors of child weight loss27. Parents are thus asked to model healthy eating behavior changes in addition to making modifications to their parenting around food and changing the home environment to support healthy eating. Positive reinforcement in the form of parental praise and a structured points system based on goal attainment and corresponding tangible rewards is used to help children change eating behaviors.
Measures
Demographic questionnaires were completed only at baseline. All other measures were completed at baseline and post-FBT.
Demographics
Parents reported their child's age, sex, race/ethnicity, and their annual household income.
Anthropometrics
Height and weight were measured in triplicate using an electronic scale and wall-mounted stadiometer by research staff following a detailed protocol. Children wore light clothing and removed shoes for measurements. Child BMI was then calculated and BMI z-scores generated using the growth curves published by the Centers for Disease Control and Prevention, according to age and sex using the LMS method28.
Food Fussiness (FF)
Food fussiness was assessed using the Child Eating Behavior Questionnaire (CEBQ). The CEBQ was designed to measure children's eating style, reported by their parents, and demonstrates high internal validity and good test-retest reliability29. The FF scale is comprised of 6 items regarding child FF (e.g. “My child is difficult to please with meals”), and parents respond on a five-point Likert-type scale from 1 (never) to 5 (always). Items were averaged to generate a summary FF score.
Dietary Intake
Child dietary intake was assessed by trained, expert interviewers using three telephone-administered 24-hour recalls via the Nutrition Data System for Research (NDSR version 2009, Nutrition Coordinating Center, University of Minnesota). Parents reported child's intake for the previous day, and if the child was present, the child assisted in the recall. Recalls were conducted on non-consecutive random days following standard protocols using the multiple-pass method, and included at least one weekday and one weekend day. Twenty-four hour recalls by phone with parent report have been validated in children aged 4–11 and have shown to be highly reliable30. Mean servings were averaged across the three days for each food group at each time point. The food groups assessed in the present study were healthy vegetables (all vegetables excluding potatoes and fried vegetables), fruits, lean meats, whole grains, and high-calorie, low-nutrient sweet foods (e.g. cakes, cookies, ice creams, etc.). These food groups were chosen because they are all specifically targeted for change in FBT. During treatment, the goal is to increase consumption of healthy vegetables, lean meats, and whole grains (primarily GREEN foods according to the Traffic Light Plan), and to decrease consumption of the sweet foods (primarily RED foods according to the Traffic Light Plan).
Diet Quality
An overall diet quality score was calculated from the 24-hour dietary recall data using the Healthy Eating Index-2005 (HEI-2005). The measure is used to assess compliance with the U.S. Dietary Guidelines set forth by the U.S. Department of Agriculture and can be used to evaluate changes in dietary patterns31,32. The scores range from 0 to 100, with higher numbers indicating better diet quality. The HEI-2005 demonstrates good content validity and construct validity, as well as good reliability32.
Statistical Analysis
The relation between child age, household income, child race and ethnicity, and child sex were assessed in relation to baseline FF as potential confounders using either Pearson-product correlations (continuous variables) or ANOVAs (categorical variables). Baseline FF was not significantly associated (all p's > 0.05) with any of these baseline demographic variables; therefore, no demographic covariates were included in the subsequent analyses. Pearson correlations were conducted between baseline measures of food fussiness and zBMI, HEI-2005, and food group consumption. Changes from baseline to post-FBT (four-month) in zBMI, HEI-2005, and food group consumption were assessed using paired samples t-tests. To assess predictors of change through FBT, change variables were calculated by subtracting baseline values from four-month values (e.g. negative values of FF change indicating decreases in FF). Separate linear regressions were then conducted to examine change in FF as a predictor of change in zBMI, overall diet quality, and food group consumption.
Mediation analyses were performed using the Preacher and Hayes method33. Separate models assessed mediating effects of change in child diet variables on the relation between change in FF and change in zBMI. Diet variables were only assessed as mediators if they had been found to be related to change in FF in the linear regression analyses. Bootstrapping using 5,000 resamples was conducted to assess the 95% confidence intervals (CIs) of indirect effects. An effect was considered statistically significant if the CI did not contain zero. SPSS software, version 22 was used to conduct all statistical analysis, and an α level of p < 0.05 was set to determine significance.
Results
Sample Characteristics
Baseline sample characteristics are described in Table 1. About 60% of the children were female, about two-thirds were non-Hispanic White, and about three-fourths were from a family with an annual income above $50,000. The average zBMI of the sample was 2.16 ± 0.39, with a range of 0.99 to 2.85. All participants completed 24-hour recalls; however, one parent did not complete post-treatment assessments of their child's FF.
Table 1.
Baseline characteristics of the study sample.
| Baseline Characteristic | Participants (n=170) |
|---|---|
| Child age (years; mean ± SD) | 9.41 ± 1.23 |
| Child sex [n (%)] | |
| Male | 66 (38.8) |
| Female | 104 (61.2) |
| Child race [n (%)] | |
| White | 119 (70.0) |
| African-American | 29 (17.1) |
| Other or multiple races | 23 (12.9) |
| Child ethnicity [n (%)] | |
| Hispanic | 17 (10.0) |
| Non-Hispanic | 153 (90.0) |
| Annual household incomea [n (%)] | |
| <$50,000 | 42 (24.7) |
| ≥$50,000 | 128 (75.3) |
4 participants chose not to report
Baseline FF Associations with Baseline Weight and Diet Variables
Baseline FF was not related to baseline child zBMI. At baseline, higher levels of FF were associated with lower total healthy vegetable consumption (r(168)=−.29, p<.001) [Table 2]. There was no relation between baseline child FF and baseline overall diet quality, total fruit, whole grains, lean meats or low-nutrient sweet foods consumption.
Table 2.
Correlation coefficients of baseline FF and baseline weight and diet variables
| Baseline FF | |
|---|---|
| zBMI | −0.090 |
| HEI-2005 | −0.062 |
| Healthy Vegetables | −0 241*** |
| Fruit | −0.051 |
| Lean Meat | −0.145 |
| Whole Grain | −0.009 |
| Sweet Foods | −0.017 |
p<0.001
Changes in FF, Weight, and Diet Variables Post-FBT
Results showing changes in variables across treatment are described in Table 3. FF decreased significantly across treatment, as did child zBMI. Overall diet quality improved, as did most food group variables, with healthy vegetables, fruit, and lean meat increasing and sweet foods decreasing. No change from baseline to post-FBT was observed for whole grain intake.
Table 3.
Comparison of FF, weight, and diet variables pre- and post-FBT
| Variable | Pre-FBT M ± SD | Post-FBT M ± SD | p Value |
|---|---|---|---|
| FFa | 2.94 ± 0.95 | 2.85 ± 0.91 | 0.012 |
| Child zBMIb | 2.16 ± 0.39 | 1.87 ± 0.56 | <0.001 |
| HEI-2005 | 59.34 ± 8.79 | 74.54 ± 9.76 | <0.001 |
| Food Group Servings | |||
| Healthy Vegetables | 1.39 ± 0.99 | 1.67 ± 1.21 | 0.002 |
| Fruit | 1.11 ± 1.01 | 2.32 ± 1.73 | <0.001 |
| Lean Meat | 1.82 ± 1.41 | 2.25 ± 1.40 | 0.002 |
| Whole Grain | 1.21 ± 1.16 | 1.34 ± 1.16 | 0.262 |
| Sweet Foods | 2.57 ± 1.57 | 1.75 ± 1.26 | <0.001 |
Range: 1–5, higher score represents higher FF
zBMI = Standardized Body Mass Index
Associations between baseline FF and Change in Weight and Diet Variables Post-FBT
Baseline FF did not predict change in zBMI from baseline to post-FBT, nor did it predict change in total diet quality or any examined food groups [Table 4].
Table 4.
Regression coefficients of FF predicting change in weight and diet variables
| Baseline FF | Change in FF | |
|---|---|---|
| zBMI | −0.050 | 0.18* |
| HEI-2005 | 0.032 | −0.18* |
| Healthy Vegetables | −0.045 | −0.072 |
| Fruit | 0.075 | −0.15t |
| Lean Meat | −0.028 | 0.068 |
| Whole Grain | 0.015 | −0.104 |
| Sweet Foods | 0.011 | −0.040 |
p=0.053;
p<0.05
Associations between Change in FF and Change in Weight and Diet Variables Post-FBT
As shown in Table 4, reductions in FF across treatment were associated with decreases in child zBMI, β=.18, t(168)=2.38, p<.05. Decreases in FF from pre- to post-FBT were also associated with overall diet quality improvement (HEI-2005) β=−.18, t(168)=−2.19, p<.05, as well as increases in total fruit consumption at a trend-level, β=−.15, t(168)=−2.02, p=.053. Change in FF was not associated with changes in consumption of the other food group variables (i.e. healthy vegetables, lean meats, whole grains, and sweet foods).
Mediation of the Relation between Change in FF and Change in zBMI Post-FBT
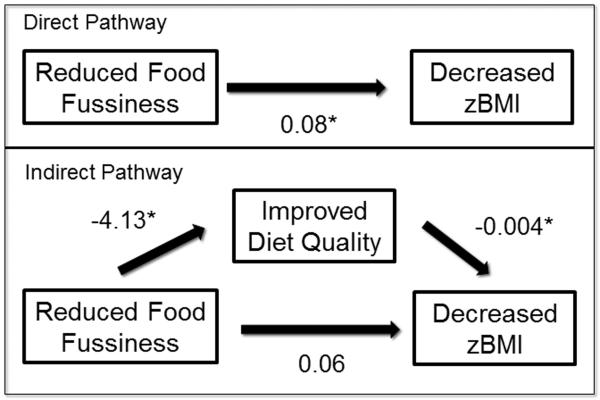
Diet variables were assessed as mediators if they were significantly related to change in FF post-treatment. To this end, change in overall diet quality was assessed as a mediator between change in FF and change in zBMI. In the model with change in overall diet quality as a mediator, the standardized regression coefficient between change in FF and change in diet quality was statistically significant (as noted above), as was the standardized regression coefficient between change in diet quality and change in child zBMI. Using bootstrapping procedures, we tested the significance of the indirect effect. Indirect effects were computed for each of 5,000 bootstrapped samples, and the 95% confidence interval was computed by determining the indirect effects at the 2.5th and 97.5th percentiles. The bootstrapped indirect effect was .15, 95% CI [0.0019, 0.0435], suggesting that the change in overall diet quality mediated the relation between change in FF and change in child zBMI. The associations between these variables are shown in Figure 1.
Figure 1.
Direct and indirect pathways for mediation model *p <.05
Discussion
The present results show that cross-sectionally, for children with obesity who are starting weight management treatment, those with higher FF were consuming fewer vegetables than corresponding children with lower FF. Additionally, when examining how FF may change and influence treatment, decreases in FF across treatment were found to be associated with decreases in zBMI, suggesting that FF may be a relevant treatment target for children involved in FBT. This relation was mediated by overall change in diet quality, highlighting the importance of focusing on quality, as well as quantity, of food consumption.
Levels of FF in this sample were found to be similar to levels of FF in other obesity treatment-seeking samples of children, but were higher than levels of FF documented in community samples of children with obesity. The higher rates of FF in treatment-seeking samples of children with obesity indicate that FF may be a relevant consideration during FBT. However, the lack of association between FF and child weight status at baseline signifies that FF was not driving differences in the level of overweight/obesity in the participants. Overall diet quality at baseline was also not predicted by level of FF; however, FF did predict consumption of certain food groups. Children with higher FF were less likely to eat healthy vegetables than children with lower FF, consistent with prior community samples which included children across the weight spectrum7. However, in contrast to existing literature examining children of various weight ranges7,9, consumption of sweet foods was not found to vary by FF in this population with overweight/obesity. Therefore, children with high FF entering obesity treatment may be getting fewer nutrients from vegetables than children with low FF; however, these high FF children with obesity are not necessarily consuming disproportionally more unhealthy sweet foods than their low FF counterparts.
Results also showed that baseline FF did not predict weight change or diet change across weight loss treatment for these children. Initial FF may not be a barrier to treatment success, given that the parenting strategies taught in FBT may help parents of children high in FF to address their children's limited food group intake and improve child FF. Indeed, results from the study also show overall decreases in levels of FF from before to immediately following FBT, as well as average improvements in diet quality and consumption of healthy vegetables, fruits, and lean meats across FBT. As repeated exposure to rejected foods is an evidence-based intervention for changing food preferences18,19, the FBT focus on increasing fruit and vegetable consumption and continued exposure to more healthful foods may be helping to reduce FF. Additionally, FBT targets parent strategies for establishing healthy home eating patterns as well as parent modeling of healthy behaviors, which may shift children's eating patterns and the impact of FF. Child diet mirrors parental diet34, and thus, by asking parents to increase their own consumption of foods that their child normally rejects, it may encourage child intake of these healthy items. Finally, peer modeling of food consumption has been shown to change child preferences and consumption patterns35,36. Child participants attended weekly child group sessions, and thus, by discussing healthy dietary changes of peers, children may be more motivated to change their own diets.
While small in magnitude, changes in FF across treatment were significantly associated with pre- to post-FBT changes in child zBMI and diet quality, such that greater decreases in FF predicted greater decreases in zBMI and greater improvements in diet quality, suggesting that even small changes in FF may have clinical significance. However, changes in FF were not associated with changes in any individual food group, suggesting changes in FF may not contribute to meaningful changes in one specific food group, but contribute to overall improved diet quality when assessed as a whole. Finally, diet quality was assessed as a mediator of change in FF and change in zBMI and was found to mediate this relation, suggesting that greater decreases in FF led to greater improvements in diet quality, which then led to more success in reducing weight status. Although expected, this strengthens the importance of continuing to have FBT focus on increasing (healthy) food diversity (e.g. through goals for increasing fruit/vegetable consumption) while also reducing consumption of more unhealthy foods for which FF is not a problem. It also suggests that FBT might benefit from expanding to incorporate specific additional strategies for reducing FF, such as pairing unfamiliar and/or disliked foods with liked foods37, in an attempt to improve children's food diversity and resulting in better weight outcomes.
This study has many strengths. It is the first to examine FF and its relations to weight and dietary change in children engaging in weight management treatment. Given the association of FF with poor diet quality, it is important to understand how it may affect children of all sizes. Additionally, this study used high-quality dietary measures (three 24-hour dietary recalls) at both baseline and post-FBT timepoints30, providing reliable and valid data. Limitations of the study should also be acknowledged. Without a control group, it is difficult to say for certain that observed changes in FF, zBMI, and diet are only the result of FBT. However, data demonstrate that FF has a tendency to persist over time5,38, and would thus be unlikely to change substantially without intervention. Additionally, given the demographics of the sample and the lack of follow-up, further study should be completed in underserved ethnically diverse children and should be tracked over time to ensure generalizability and maintenance. Finally, the mediation analysis in the current study utilized non-experimental data (i.e. mediators were not pre-selected and tested against a control), which may increase bias of the results39. However, similar approaches are commonly used within the literature25,26,40, and serve to provide an initial test of causal models that can be confirmed with a follow-up study designed specifically to address the challenges of mediation analysis.
Conclusion
While FF has been studied broadly in children, specific research on FF among children with obesity is limited. The current study highlights differences in dietary patterns of children with high and low FF who are seeking obesity treatment. Results provide evidence that addressing FF is key in the context of obesity treatment, as improvements in FF are linked to improvements in diet quality, which may ultimately lead to greater weight loss. These findings help to illuminate FF as a potential mechanism underlying treatment success of FBT for childhood obesity and highlight the importance of focusing on the improvement of overall diet quality, which may naturally promote calorie reduction.
What is already known about this subject?
Food fussiness in children is associated with lower intakes of healthy food groups, vitamins and minerals
While commonly examined in children with normal or low weights, food fussiness has recently been associated with childhood obesity prospectively and cross-sectionally
Effective interventions for food fussiness exist, and many of these intervention components are similar to those included in family-based childhood obesity treatment
What does this study add?
Children with obesity who are high in food fussiness eat fewer vegetables than children with overweight/obesity who are low in food fussiness
Reductions in food fussiness during family-based childhood obesity treatment are associated with greater weight loss, potentially through improvements in diet quality
Food fussiness is a potential mechanism underlying treatment success of family-based childhood obesity treatment
Acknowledgments
Funding: This work was supported by several National Institutes of Health (NIH) Grants: 5R01HD036904 (National Institute of Child Health and Human Development), 5K24MH070446 (National Institute of Mental Health), and 5T32HL007456 (National Heart, Lung, and Blood Institute). JF Hayes, M Altman, RP Kolko, KN Balantekin, and J Cahill Holland were supposed by NIH Grant 5T32HL007456 (NHLBI), and RI Stein was supported by NIH Grant KL2RR024994 (National Center for Research Resources). This work was also made possible by NIH Grant UL1 RR024992 (National Center for Research Resources).
Footnotes
Disclosure: Dr. Epstein reports involvement with Kurbo/Datri Health outside the submitted work; Dr. Wilfley reports consulting for Shire pharmaceuticals outside the submitted work.
References
- 1.Dovey TM, Staples PA, Gibson EL, Halford JC. Food neophobia and `picky/fussy'eating in children: A review. Appetite. 2008;50(2):181–193. doi: 10.1016/j.appet.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 2.Chatoor I. Feeding disorders in infants and toddlers: diagnosis and treatment. Child and adolescent psychiatric clinics of North America. 2002;11(2):163–183. doi: 10.1016/s1056-4993(01)00002-5. [DOI] [PubMed] [Google Scholar]
- 3.Esparo G, Canals J, Jane C, Ballespi S, Vinas F, Domenech E. Feeding problems in nursery children: prevalence and psychosocial factors. Acta Paediatrica. 2004;93(5):663–668. [PubMed] [Google Scholar]
- 4.Wright CM, Parkinson KN, Shipton D, Drewett RF. How do toddler eating problems relate to their eating behavior, food preferences, and growth? Pediatrics. 2007;120(4):e1069–e1075. doi: 10.1542/peds.2006-2961. [DOI] [PubMed] [Google Scholar]
- 5.Mascola AJ, Bryson SW, Agras WS. Picky eating during childhood: A longitudinal study to age 11years. Eating behaviors. 2010;11(4):253–257. doi: 10.1016/j.eatbeh.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Micali N, Simonoff E, Elberling H, Rask CU, Olsen EM, Skovgaard AM. Eating patterns in a population-based sample of children aged 5 to 7 years: association with psychopathology and parentally perceived impairment. Journal of Developmental & Behavioral Pediatrics. 2011;32(8):572–580. doi: 10.1097/DBP.0b013e31822bc7b7. [DOI] [PubMed] [Google Scholar]
- 7.Galloway AT, Fiorito L, Lee Y, Birch LL. Parental pressure, dietary patterns, and weight status among girls who are “picky eaters”. Journal of the American Dietetic Association. 2005;105(4):541–548. doi: 10.1016/j.jada.2005.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacobi C, Schmitz G, Agras WS. Is picky eating an eating disorder? International Journal of Eating Disorders. 2008;41(7):626–634. doi: 10.1002/eat.20545. [DOI] [PubMed] [Google Scholar]
- 9.Tharner A, Jansen PW, Kiefte-de Jong JC, et al. Toward an operative diagnosis of fussy/picky eating: a latent profile approach in a population-based cohort. Int J Behav Nutr Phys Act. 2014;11(1):14. doi: 10.1186/1479-5868-11-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perry RA, Mallan KM, Koo J, Mauch CE, Daniels LA, Magarey AM. Food neophobia and its association with diet quality and weight in children aged 24 months: a cross sectional study. International Journal of Behavioral Nutrition and Physical Activity. 2015;12(1):13. doi: 10.1186/s12966-015-0184-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dubois L, Diasparra M, Bédard B, et al. Genetic and environmental influences on eating behaviors in 2.5-and 9-year-old children: a longitudinal twin study. International Journal of Behavioral Nutrition and Physical Activity. 2013;10(1):134. doi: 10.1186/1479-5868-10-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carruth BR, Ziegler PJ, Gordon A, Barr SI. Prevalence of picky eaters among infants and toddlers and their caregivers' decisions about offering a new food. Journal of the American Dietetic Association. 2004;104:57–64. doi: 10.1016/j.jada.2003.10.024. [DOI] [PubMed] [Google Scholar]
- 13.Marchi M, Cohen P. Early childhood eating behaviors and adolescent eating disorders. Journal of the American Academy of Child & Adolescent Psychiatry. 1990;29(1):112–117. doi: 10.1097/00004583-199001000-00017. [DOI] [PubMed] [Google Scholar]
- 14.Webber L, Hill C, Saxton J, Van Jaarsveld C, Wardle J. Eating behaviour and weight in children. International Journal of Obesity. 2009;33(1):21–28. doi: 10.1038/ijo.2008.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rowe DC, Plomin R. Temperament in early childhood. Journal of personality assessment. 1977;41(2):150–156. doi: 10.1207/s15327752jpa4102_5. [DOI] [PubMed] [Google Scholar]
- 16.Finistrella V, Manco M, Ferrara A, Rustico C, Presaghi F, Morino G. Cross-sectional exploration of maternal reports of food neophobia and pickiness in preschooler-mother dyads. Journal of the American College of Nutrition. 2012;31(3):152–159. doi: 10.1080/07315724.2012.10720022. [DOI] [PubMed] [Google Scholar]
- 17.Faith M, Hittner J. Infant temperament and eating style predict change in standardized weight status and obesity risk at 6 years of age. International Journal of Obesity. 2010;34(10):1515–1523. doi: 10.1038/ijo.2010.156. [DOI] [PubMed] [Google Scholar]
- 18.Daniels LA, Mallan KM, Nicholson JM, Battistutta D, Magarey A. Outcomes of an early feeding practices intervention to prevent childhood obesity. Pediatrics. 2013;132(1):e109–e118. doi: 10.1542/peds.2012-2882. [DOI] [PubMed] [Google Scholar]
- 19.Wardle J, Herrera ML, Cooke L, Gibson EL. Modifying children's food preferences: the effects of exposure and reward on acceptance of an unfamiliar vegetable. European journal of clinical nutrition. 2003;57(2):341–348. doi: 10.1038/sj.ejcn.1601541. [DOI] [PubMed] [Google Scholar]
- 20.Mitchell GL, Farrow C, Haycraft E, Meyer C. Parental influences on children's eating behaviour and characteristics of successful parent-focussed interventions. Appetite. 2013;60:85–94. doi: 10.1016/j.appet.2012.09.014. [DOI] [PubMed] [Google Scholar]
- 21.Cooke L. The importance of exposure for healthy eating in childhood: a review. Journal of human nutrition and dietetics. 2007;20(4):294–301. doi: 10.1111/j.1365-277X.2007.00804.x. [DOI] [PubMed] [Google Scholar]
- 22.Fraser K, Wallis M, St John W. Improving children's problem eating and mealtime behaviours: An evaluative study of a single session parent education programme. Health Education Journal. 2004;63(3):229–241. [Google Scholar]
- 23.Epstein LH, Paluch RA, Roemmich JN, Beecher MD. Family-based obesity treatment, then and now: twenty-five years of pediatric obesity treatment. Health Psychology. 2007;26(4):381. doi: 10.1037/0278-6133.26.4.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Croker H, Cooke L, Wardle J. Appetitive behaviours of children attending obesity treatment. Appetite. 2011;57(2):525–529. doi: 10.1016/j.appet.2011.05.320. [DOI] [PubMed] [Google Scholar]
- 25.Altman M, Holland JC, Lundeen D, et al. Reduction in Food Away from Home Is Associated with Improved Child Relative Weight and Body Composition Outcomes and This Relation Is Mediated by Changes in Diet Quality. Journal of the Academy of Nutrition and Dietetics. 2015 doi: 10.1016/j.jand.2015.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holland JC, Kolko RP, Stein RI, et al. Modifications in parent feeding practices and child diet during family–based behavioral treatment improve child zBMI. Obesity. 2014;22(5):E119–E126. doi: 10.1002/oby.20708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wrotniak BH, Epstein LH, Paluch RA, Roemmich JN. Parent weight change as a predictor of child weight change in family-based behavioral obesity treatment. Archives of pediatrics & adolescent medicine. 2004;158(4):342–347. doi: 10.1001/archpedi.158.4.342. [DOI] [PubMed] [Google Scholar]
- 28.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. CDC growth charts: United States. Advance data. 2000;(314):1–27. [PubMed] [Google Scholar]
- 29.Wardle J, Guthrie CA, Sanderson S, Rapoport L. Development of the children's eating behaviour questionnaire. Journal of Child Psychology and Psychiatry. 2001;42(07):963–970. doi: 10.1111/1469-7610.00792. [DOI] [PubMed] [Google Scholar]
- 30.Burrows TL, Martin RJ, Collins CE. A systematic review of the validity of dietary assessment methods in children when compared with the method of doubly labeled water. Journal of the American Dietetic Association. 2010;110(10):1501–1510. doi: 10.1016/j.jada.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 31.Guenther PM, Reedy J, Krebs-Smith SM. Development of the healthy eating index-2005. Journal of the American Dietetic Association. 2008;108(11):1896–1901. doi: 10.1016/j.jada.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 32.Guenther PM, Reedy J, Krebs-Smith SM, Reeve BB. Evaluation of the healthy eating index-2005. Journal of the American Dietetic Association. 2008;108(11):1854–1864. doi: 10.1016/j.jada.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 33.Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behavior research methods. 2008;40(3):879–891. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- 34.Galloway AT, Lee Y, Birch LL. Predictors and consequences of food neophobia and pickiness in young girls. Journal of the American Dietetic Association. 2003;103(6):692–698. doi: 10.1053/jada.2003.50134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marinho H. Social influence in the formation of enduring preferences. The Journal of Abnormal and Social Psychology. 1942;37(4):448. [Google Scholar]
- 36.Salvy S-J, De La Haye K, Bowker JC, Hermans RC. Influence of peers and friends on children's and adolescents' eating and activity behaviors. Physiology & behavior. 2012;106(3):369–378. doi: 10.1016/j.physbeh.2012.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pliner P, Stallberg-White C. “Pass the ketchup, please”: familiar flavors increase children's willingness to taste novel foods. Appetite. 2000;34(1):95–103. doi: 10.1006/appe.1999.0290. [DOI] [PubMed] [Google Scholar]
- 38.Ashcroft J, Semmler C, Carnell S, Van Jaarsveld C, Wardle J. Continuity and stability of eating behaviour traits in children. European Journal of Clinical Nutrition. 2008;62(8):985–990. doi: 10.1038/sj.ejcn.1602855. [DOI] [PubMed] [Google Scholar]
- 39.Bullock JG, Green DP, Ha SE. Yes, but what's the mechanism?(don't expect an easy answer) Journal of personality and social psychology. 2010;98(4):550. doi: 10.1037/a0018933. [DOI] [PubMed] [Google Scholar]
- 40.Van den Berg L, Pieterse K, Malik J, et al. Association between impulsivity, reward responsiveness and body mass index in children. International Journal of Obesity. 2011;35(10):1301–1307. doi: 10.1038/ijo.2011.116. [DOI] [PubMed] [Google Scholar]