Abstract
N-glycosylation is a post-translational modification that occurs across evolution. In the thermoacidophilic archaea Sulfolobus acidocaldarius, glycoproteins are modified by an N-linked tri-branched hexasaccharide reminiscent of the N-glycans assembled in Eukarya. Previously, hexose-bearing dolichol phosphate was detected in a S. acidocaldarius Bligh-Dyer lipid extract. Here, we used a specialized protocol for extracting lipid-linked oligosaccharides to detect a dolichol pyrophosphate bearing the intact hexasaccharide, as well as its biosynthetic intermediates. Furthermore, evidence for N-glycosylation of two S. acidocaldarius proteins by the same hexasaccharide and its derivatives was collected. These findings thus provide novel insight into archaeal N-glycosylation.
Keywords: Archaea, dolichol, N-glycosylation, Sulfolobus acidocaldarius, thermoacidophile
INTRODUCTION
N-glycosylation, namely the covalent linkage of glycans to select Asn residues of a target protein, is a post-translational modification performed across evolution. Nonetheless, domain-specific differences exist in terms of the composition and architecture of the N-linked glycans, the enzymes used to assemble and attach these glycans and the nature of the phosphorylated polyprenoid lipid carriers upon which N-linked glycans are initially assembled [1–4]. Moreover, it is becoming increasingly clear that archaeal N-glycosylation presents more diversity in each of these aspects of the process than seen in the parallel eukaryal or bacterial pathways [5,6].
In yeast and higher Eukarya, a dolichol pyrophosphate (DolPP)-linked heptasaccharide is assembled on the cytoplasmic face of the endoplasmic reticulum membrane. Once translocated across the membrane, seven more sugars are transferred from individual dolichol phosphate (DolP) carriers to yield the final 14-member glycan [7,8]. By contrast, in Bacteria, where N-glycosylation is apparently restricted to delta/epsilon proteobacterial species, simpler glycans are assembled on a cytoplasmically-oriented undecaprenol pyrophosphate carrier [9–11]. In Archaea, as in Eukarya, dolichol serves as the lipid carrier upon which N-linked glycans are assembled. While both versions of dolichol contain a saturated α-position isoprene subunit, all archaeal dolichols considered to date also present a saturated isoprene at the ω-position of the molecule [12–18]. Furthermore, dolichols in the thermophiles Archaeoglobus fulgidus, Pyrobaculum calidifontis, Pyrococcus furiosus, Sulfolobus acidocaldarius and Sulfolobus solfataricus have been shown to include saturated isoprenes at more internal positions [14,17,18].
In addition to invoking dolichols demonstrating variety in terms of the degree of isoprene subunit saturation, variability in dolichol phosphorylation is also seen in Archaea, where N-glycosylation seemingly relies on both DolP and DolPP carriers. In some cases, such as the halophile Haloferax volcanii and the methanogen Methanococcus maripaludis, species for which N-glycosylation pathways have been delineated, N-linked glycans are assembled from precursors derived from one or more DolP carriers [13,15,19]. In other species, DolP bearing complete or derivatives (i.e. precursors or breakdown products) of N-linked glycans decorating glycoproteins have been reported, including A. fulgidus, Haloferax mediterranei, Haloarcula marismortui, P. furiosus, S. acidocaldarius and S. solfataricus [14,16–18,20]. On the other hand, DolPP bearing glycans decorating glycoproteins in Methanothermus fervidus, P. calidifontis and S. solfataricus have been described [18,21]. In Halobacterium salinarum, where the surface (S)-layer glycoprotein is modified by two distinct N-linked glycans, one glycan is assembled on DolP while the other is constructed on DolPP [22,23]. Still, no evidence for the contribution of both DolP and DolPP carriers in the biogenesis of the same N-linked glycan, a scenario that occurs in Eukarya, has been demonstrated in Archaea.
Members of the genus Sulfolobus may offer examples of archaeal N-glycosylation systems that combine both DolP- and DolPP-linked sugars or glycans. In both S. acidocaldarius and S. solfataricus, the N-linked glycan corresponds to a complex tri-branched oligosaccharide attached to target Asn residues via a di-N-acetylglucosamine core, reminiscent of the N-linked glycan decorating eukaryal glycoproteins [24–26]. In the case of S. acidocaldarius, many of the steps involved in the biogenesis of the N-linked hexasaccharide decorating glycoproteins in this species have been described [6,27]. For instance, based on the similarities of the first three sugars assembled on the lipid carrier in S. acidocaldarius and eukaryotes, it is thought that the first steps of S. acidocaldarius N-glycosylation resemble the parallel steps of the eukaryal N-glycosylation pathway, where Alg7 and Alg13/14 catalyze the assembly of DolPP-N-acetylglucosamine2-mannose [4,8,28]. Accordingly, genes encoding homologues of Alg7 and Alg13/14 are found within the S. acidocaldarius genome. Recently, DolPP modified by the hexasaccharide precursor of the N-linked heptasaccharide as well as DolP modified by a hexose were observed in S. solfataricus [18]. Likewise, DolP-hexose was previously detected in S. acidocaldarius [14]. With these latest findings in mind, the involvement of DolPP-linked glycans in S. acidocaldarius N-glycosylation was considered in the present report. Furthermore, evidence for the N-glycosylation of two additional S. acidocaldarius proteins is provided.
MATERIALS AND METHODS
Strain and growth
S. acidocaldarius (MW001) [29] were grown at 75°C in Brock’s medium [30], pH-adjusted to 3 using sulphuric acid, supplemented with 0.1% (w/v) NZ-amine, 0.2% (w/v) dextrin and 10 μg/ml uracil, under constant shaking. Cell growth was monitored by measuring optical density at 600 nm.
Isolation of S. acidocaldarius DolPP-linked glycans
To isolate DolPP-linked glycans, the protocol described by Kelleher et al. [31] was employed, with minor modifications. Frozen S. acidocaldarius cells were gently thawed and sonicated on ice at room temperature (2 s on, 5 s off, for a total of 1 min; Vibracell VCX750 ultrasonic cell disrupter, Sonics, Newtown, CT). After sonication, the cell suspension was centrifuged for 30 min at 10,000 rpm in a SW-41 rotor at 4°C to clear non-broken cells and other debris. The supernatant was transferred into fresh tubes and centrifuged for an additional 45 min at 36,000 rpm in a SW-41 rotor at 4°C. The resulting supernatant was removed and the pellet was re-suspended in 35 ml homogenization buffer (150 mM NaCl, 50 mM Tris-HCl, pH 8) containing 50 ml CHCl3:CH3OH (3:2) at 4°C and homogenized using a Pyrex Potter-Elvehjem tissue grinder (Thomas Scientific). After homogenization, 65 ml cold (4°C) CHCl3:CH3OH (3:2) were added and the homogenate was mixed by vigorous shaking before centrifugation (3,400 g, 15 min, 4°C). The resulting clear upper aqueous and lowest organic phases were removed and the middle (solid) phase was resuspended in 75 ml of CHCl3:CH3OH (3:2) containing 1 mM MgCl2 at room temperature. After vigorous re-homogenization, the suspension was adjusted to a total volume of 150 ml and centrifuged for 15 min (3,400 g, 4°C). The supernatant was removed and the pellet was suspended in 150 ml CH3OH containing 4 mM MgCl2 before centrifugation (3,400 g, 15 min, 4°C). These steps were repeated and the resulting pellet was suspended in 150 ml CHCl3:CH3OH:DDW (10:10:3) and centrifuged in a swing-out rotor (1,000 rpm) for 15 min at 22°C. The supernatant was removed and stored while the pellet were re-extracted with 100 ml CHCl3:CH3OH:DDW (10:10:3) at 37°C and centrifuged as above. The supernatants obtained from the first and second extractions were combined and the ensuing solution was evaporated at 30°C. Thereafter, any remaining solvents were removed using a stream of nitrogen. The dried extracts were then subjected to analysis by liquid chromatography coupled with mass spectrometry.
Liquid chromatography-electrospray ionization mass spectrometry (LC-ESI MS) analysis of S. acidocaldarius DolPP-linked glycans
Normal phase LC-ESI MS of the S. acidocaldarius DolPP-glycan-containing extract was performed using an Agilent 1200 Quaternary LC system coupled to a high resolution TripleTOF5600 mass spectrometer (Sciex, Framingham, MA). A Unison UK-Amino column (3 μm, 25 cm × 2 mm) (Imtakt USA, Portland, OR) was used. Mobile phase A consisted of chloroform/methanol/aqueous ammonium hydroxide (800:195:5, v/v/v). Mobile phase B consisted of chloroform/methanol/water/aqueous ammonium hydroxide (600:340:50:5, v/v/v/v). Mobile phase C consisted of chloroform/methanol/water/aqueous ammonium hydroxide (450:450:95:5, v/v/v/v). The elution program consisted of the following: 100% mobile phase A was held isocratically for 2 min and then linearly increased to 100% mobile phase B over 14 min and held at 100% B for 11 min. The LC gradient was then changed to 100% mobile phase C over 3 min and held at 100% C for 3 min, and finally returned to 100% A over 0.5 min and held at 100% A for 5 min. The total LC flow rate was 300 μl/min. The post-column splitter diverted ~10% of the LC flow to the ESI source of the TF5600 mass spectrometer, with MS settings as follows: Ion spray voltage (IS) = −4500 V, Curtain gas (CUR) = 20 psi, Ion source gas 1 (GS1) = 20 psi, De-clustering potential (DP) = −55 V, and Focusing Potential (FP) = −150 V. Nitrogen was used as the collision gas for tandem mass spectrometry (MS/MS) experiments. Data acquisition and analysis were performed using Analyst TF1.5 software (Sciex, Framingham, MA).
Isolation of S. acidocaldarius FlaB and SlaB
For isolation of the S. acidocaldarius surface (S)-layer that includes SlaB, cell pellets of freshly grown cells (100 ml culture of OD600 0.8) were re-suspended in 35 ml buffer A (10 mM NaCl, 0.5% (w/v) N-lauroylsarcosine, pH 5.5). The mixture was incubated for 30 min in a rotary shaker at 45°C, complemented with a small amount of DNAse. Subsequent centrifugation at 5,000 x g for 20 min resulted in a brownish pellet with a white layer (the S-layer) on top. The S-layer was carefully removed and transferred to a 1.5 ml reaction tube, re-suspended in 1 ml buffer A and incubated at 45°C for 20 min under constant shaking. Samples were then centrifuged at maximum speed for 10 min in a tabletop centrifuge and the supernatant was discarded. Repetitive washing with buffer A together with incubation at 45°C resulted in a white S-layer pellet, free of contaminants. The S-layer was re-suspended in buffer A and stored at 4°C. Before loading onto a SDS-PAGE gel, the S-layer, comprising SlaA and SlaB, was diluted two-fold with sodium carbonate buffer (12 mM Na2CO3, pH 10) and incubated at 42°C for two hours.
To isolate the archaellin FlaB, exponentially growing cells were transferred to Brock’s medium lacking NZ-amine and dextrin. After starving the cells overnight, the culture was harvested and re-suspended in 35 ml Brock’s medium without NZ-amine and dextrin. The cell suspension was pumped ten times through a 0.8 mm diameter injection needle on ice using a peristaltic pump (Gilson/Abimed Miniplus 3). Next, the cells were pumped through an injection needle (0.4 mm diameter) ten times. Samples were centrifuged at 4,500 x g for 20 min at 4°C. The supernatant was subjected to another round of centrifugation. The cleared supernatant was centrifuged at 240,000 x g for 12 h at 4°C to pellet the archaella, containing FlaB.
LC-ESI MS analysis of S. acidocaldarius FlaB and SlaB
For LC-ESI MS analysis of S. acidocaldarius FlaB and SlaB, the fractions containing these proteins were separated on 11% polyacrylamide gels and stained with Coomassie R-250 (Roth). For in-gel digestion, the individual protein bands were excised, destained in 400 μl of 50% (vol/vol) acetonitrile (Sigma) in 40 mM NH4HCO3, pH 8.4, dehydrated with 100% acetonitrile, and dried using a SpeedVac drying apparatus. The proteins were reduced with 10 mM dithiothreitol (Sigma) in 40 mM NH4HCO3 at 56°C for 60 min and then alkylated for 45 min at room temperature with 55 mM iodoacetamide in 40 mM NH4HCO3. The gel pieces were washed with 40 mM NH4HCO3 for 15 min, dehydrated with 100% acetonitrile, and SpeedVac-dried. The gel slices were rehydrated with 12.5 ng/μl of mass spectrometry (MS)-grade Trypsin Gold (Promega) in 40 mM NH4HCO3. The protease-generated peptides were extracted with 0.1% (v/v) formic acid in 20 mM NH4HCO3, followed by sonication for 20 min at room temperature, dehydration with 50% (v/v) acetonitrile, and additional sonication. After three rounds of extraction, the gel pieces were dehydrated with 100% acetonitrile, dried completely with a SpeedVac, resuspended in 5% (v/v) acetonitrile containing 1% formic acid (v/v) and infused into the mass spectrometer using static nanospray Econotips (New Objective, Woburn, MA). The protein digests were separated on-line by nano-flow reverse-phase liquid chromatography by loading onto a 150-mm by 75-μm (internal diameter) by 365-μm (external diameter) Jupifer pre-packed fused silica 5-μm C18 300Å reverse-phase column (Thermo Fisher Scientific, Bremen, Germany). The sample was eluted into the LTQ Orbitrap XL mass spectrometer (Thermo Fisher Scientific) using a 60-min linear gradient of 0.1% formic acid (v/v) in acetonitrile/0.1% formic acid (1:19, by volume) to 0.1% formic acid in acetonitrile/0.1% formic acid (4:1, by volume) at a flow rate of 300 nl/min.
RESULTS
S. acidocaldarius contains glycan-charged DolP and DolPP
In earlier attempts at identifying lipid-linked glycans putatively involved in S. acidocaldarius N-glycosylation, both dolichol and DolP were identified [14]. The extremely short dolichol was shown to be saturated not only at the α- and ω-positions, as holds true for dolichol in all Archaea considered to date, but also at more internal positions. When, however, glycosylated versions of this polyprenoid were sought, only hexose-charged DolP were detected. As such, it was proposed at the time that a different method than the Bligh and Dyer [32] protocol used might be needed for extracting such lipids containing higher-ordered glycans. Accordingly, in the present study, the extraction protocol developed by Kelleher et al. [31] was adopted.
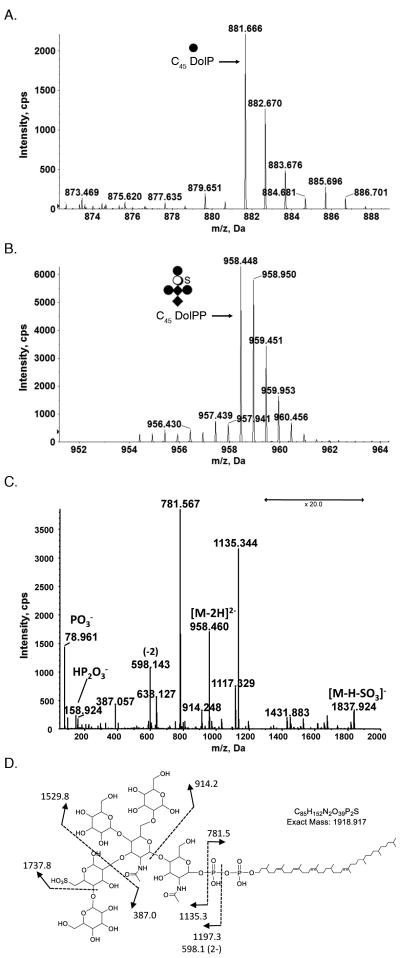
Following such extraction, the [M-H]− ion of hexose-charged C45DolP was identified by LC-ESI MS; its [M-H]− monoisotopic ion peak was observed at m/z 881.666 (Fig. 1A), as first described by Guan et al. [14]. In addition, a doubly charged ion peak of m/z 958.448, corresponding to the monoisotopic [M-2H]2− ion peak of C45DolPP bearing the hexasaccharide N-linked to S. acidocaldarius glycoproteins was also detected (Fig 1B) [24,25]. The deduced monoisotopic mass (1918.912 Da) is in agreement with the calculated mass (1917.917 Da) of C45DolPP linked to a N-acetylglucosamine2-mannose2-sulfated quinovose-glucose hexasaccharide. The identification was further verified by MS/MS. The product spectrum of the [M-2H]2− ion at m/z 958.448 showed peaks consistent with DolPP bearing the previously defined N-linked hexasaccharide decorating S. acidocaldarius glycoproteins (Fig 1C). Specifically fragments corresponding to DolPP and the hexasaccharide were detected.
Fig 1.
Glycan-charged phosphorylated dolichol species in S. acidocaldarius. LC-ESI MS analysis of a S. acidocaldarius lipid extract revealed the presence of (A) C45 DolP modified by a hexose and (B) C45 DolPP modified by a hexasaccharide comprising N-acetylhexosamine2-hexose3-sulfated quinovose (QuiS). C. MS/MS spectrum of [M-2H]2− at m/z 958.4. D. Fragmentation scheme for the observed product ions in C. The depicted DolPP-linked glycan structure is based on the previously reported protein N-linked glycan [24,25]. The arrows indicating x20 reflect magnification of the ion peaks in the corresponding region of the m/z values on the graph. Where the position of a double bond is only speculated, a dotted line is drawn. Diamonds represent N-acetylhexosamines, full circles represent hexoses, open circles represent quinovose and S represents sulfation.
In addition to hexasaccharide-modified DolPP, the S. acidocaldarius lipid extract also included C45DolPP bearing a pentasaccharide comprising N-acetylhexosamine2-hexose2-sulfated quinovose (Fig 2A) and a tetrasaccharide comprising N-acetylhexosamine2-hexose-sulfated quinovose (Fig 2B). DolPP modified by N-acetylhexosamine2-hexose, N-acetylhexosamine2 or N-acetylhexosamine were not detected.
Fig 2.
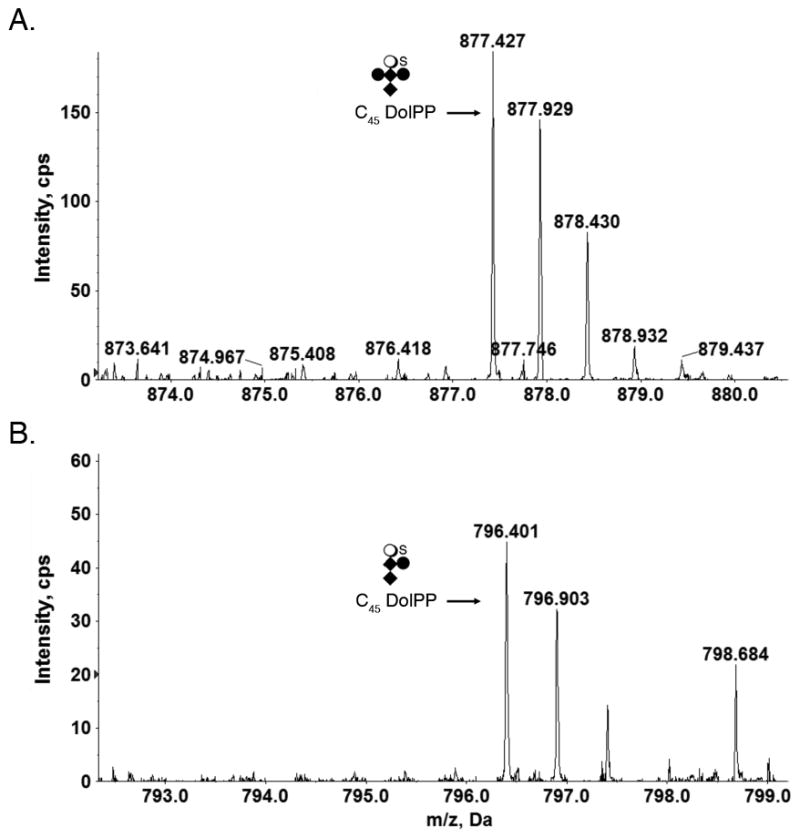
Glycan-charged S. acidocaldarius DolPP species. (A) C45 DolP modified by a pentasaccharide comprising N-acetylhexosamine2-hexose2-sulfated quinovose. (B) C45 DolP modified by a tetrasaccharide comprising N-acetylhexosamine2-hexose-sulfated quinovose. Diamonds represent N-acetylhexosamines, full circles represent hexoses, open circles represent quinovose and S represents sulfation.
LC-ESI MS analysis of S. acidocaldarius glycoproteins reveals novel sites of N-glycosylation
To date, N-glycosylation by a hexasaccharide comprising N-acetylhexosamine2-hexose3-sulfated quinovose hexasaccharide has been demonstrated for two S. acidocaldarius glycoproteins, namely cytochrome b558/566 [24] and SlaA, a component of the surface layer [25]. In addition, site-directed modification of predicted N-glycosylation sites of FlaB, a component of the archaellum, led to changes in the SDS-PAGE migration protein, providing support for it also undergoing such post-translational modification [33]. In the present study, LC-ESI MS was performed on S. acidocaldarius glycoproteins to confirm previous assignments, to identify new N-glycosylation targets and to gain additional insight into the process of N-glycosylation in this organism.
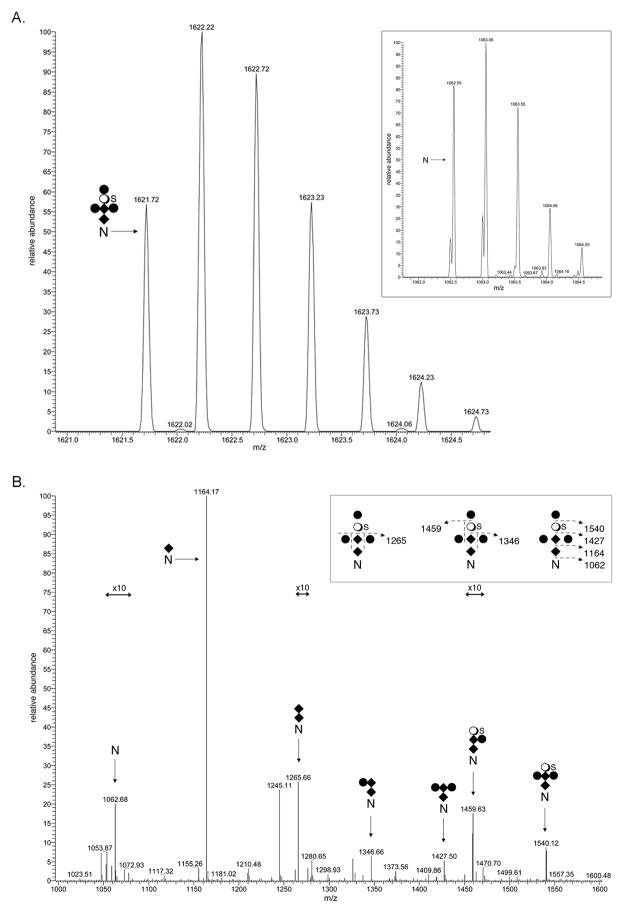
The membrane protein SlaB is one of the two glycoproteins comprising the S. acidocaldarius S-layer [25]. The LC-ESI MS profile of a SlaB-derived Asn-278-containing peptide generated by trypsin treatment (265VTTAPVSAQVYYPNGTQTVK284) was considered. Such analysis detected a [M+2H]2+ peak at m/z 1062.55 (Fig 3A, inset). This value is in excellent agreement with the calculated [M+2H]2+ mass of the peptide, 1062.55 Da. The same mass spectrometry profile also included a [M+2H]2+ peak at m/z 1621.72 (Fig 3A), likely corresponding to the same peptide modified by N-acetylhexosamine2-hexose3-sulfated quinovose (calculated mass 1621.85 Da). In addition, [M+2H]2+ peaks at m/z 1164.09 (Fig S1A), 1265.63 (Fig S1B), 1346.66 (Fig S1C), 1427.69 (Fig S1D), 1459.67 (Fig S1E) and 1540.69 (Fig S1F) were observed. These values are in good agreement with the predicted [M+2H]2+ values of the Asn-278-containing peptide respectively modified by N-acetylhexosamine (calculated mass 1164.09 Da), N-acetylhexosamine2 (calculated mass 1265.63 Da), N-acetylhexosamine2-hexose (calculated mass 1346.70 Da), N-acetylhexosamine2-hexose2 (calculated mass 1427.77 Da), N-acetylhexosamine2-hexose-sulfated quinovose (calculated mass 1459.71 Da) and N-acetylhexosamine2-hexose2-sulfated quinovose (calculated mass 1540.78 Da). To confirm this assessment, the [M+2H]2+ base peak at m/z 1621.72, thought to correspond to the peptide modified by the complete hexasaccharide, was subjected to MS/MS. The fragmentation pattern obtained was consistent with this previously described N-linked glycan (Fig 3B). Experimental evidence for the similar modification of SlaB Asn-357, Asn-376 and Asn-397 was also obtained (Table 1). In addition, a trypsin-generated peptide containing both Asn-425 and Asn-430 was shown to be modified at only one of these positions, although it was not possible to determine which. Thus, of 13 potential N-glycosylation sites in SlaB, five were determined as being modified and one was deemed as not being processed.
Fig 3.
N-glycosylation of S. acidocaldarius SlaB. A. LC-ESI MS analysis of a tryptic fragment of SlaB that includes Asn-278 revealed the peptide (inset) and its modification by a hexasaccharide comprising N-acetylhexosamine2-hexose3-sulfated quinovose. [M+2H]2+ ion peaks are presented. B. MS/MS profile of the [M+2H]2+ base peak of the peptide observed at m/z 1621.72. The inset schematically represents the fragmentation scheme. The N represents Asn-278, diamonds represent N-acetylhexosamines, full circles represent hexoses, open circles represent quinovose and S represents sulfation. The arrows indicating x10 reflect magnification of the ion peaks in the corresponding region of the m/z values on the graph.
Table 1.
N-glycosylated positions in S. acidocaldarius FlaB and SlaB, as identified by LC-ESI MS
| protein | peptide | observed mass (m/z) | calculated mass (m/z) | glycan |
|---|---|---|---|---|
| FlaB | GGQLTSSPLYIIS195NT | |||
| SIVASKPWLK | 1260.273+ | 1260.36 | HexNAc2Hex3QuiS | |
| 1206.263+ | 1206.31 | HexNAc2Hex2QuiS | ||
| 1152.243+ | 1152.26 | HexNAc2HexQuiS | ||
| 1130.923+ | 1130.97 | HexNAc2Hex2 | ||
| 1076.903+ | 1076.92 | HexNAc2Hex | ||
| 1022.883+ | 1022.87 | HexNAc2 | ||
| 955.193+ | 955.18 | HexNAc | ||
| SlaB | VTTAPVSAQVYYP278 | |||
| NGTQTVK | 1621.722+ | 1621.85 | HexNAc2Hex3QuiS | |
| 1540.692+ | 1540.78 | HexNAc2Hex2QuiS | ||
| 1459.672+ | 1459.71 | HexNAc2HexQuiS | ||
| 1427.692+ | 1427.77 | HexNAc2Hex2 | ||
| 1346.662+ | 1346.70 | HexNAc2Hex | ||
| 1265.632+ | 1265.63 | HexNAc2 | ||
| 1164.092+ | 1164.09 | HexNAc | ||
| EISSLVQ357NITNLEK | 1353.602+ | 1353.73 | HexNAc2Hex3QuiS | |
| 1272.572+ | 1272.66 | HexNAc2Hex2QuiS | ||
| 1191.552+ | 1191.59 | HexNAc2HexQuiS | ||
| 1159.572+ | 1159.65 | HexNAc2Hex2 | ||
| 1078.542+ | 1078.58 | HexNAc2Hex | ||
| 997.512+ | 997.51 | HexNAc2 | ||
| 895.972+ | 895.97 | HexNAc | ||
| TVNNLQTQISTL376NS | ||||
| SLSSLSQR | 1170.533+ | 1755.43 | HexNAc2Hex3QuiS | |
| 1116.513+ | 1116.57 | HexNAc2Hex2QuiS | ||
| 1062.503+ | 1062.52 | HexNAc2HexQuiS | ||
| 1041.183+ | 1561.34 | HexNAc2Hex2 | ||
| 987.163+ | 987.18 | HexNAc2Hex | ||
| 933.113+ | 933.13 | HexNAc2 | ||
| 865.453+ | 865.45 | HexNAc | ||
| ITSLQNTLASL397NSTI SSLSGTASSLSSQLNA |
||||
| LQGK | 1537.733+ | 1537.82 | HexNAc2Hex3QuiS | |
| 1483.713+ | 1483.77 | HexNAc2Hex2QuiS | ||
| 1429.673+ | 1429.72 | HexNAc2HexQuiS | ||
| n.d. | 1408.43 | HexNAc2Hex2 | ||
| 1354.363+ | 1354.38 | HexNAc2Hex | ||
| 1300.333+ | 1300.33 | HexNAc2 | ||
| 1232.663+ | 1232.64 | HexNAc | ||
| ISSL425NSSIT430NLSGQ | ||||
| LNSLQSK* | 1103.843+ | 1103.93 | HexNAc2Hex3QuiS | |
| 1049.823+ | 1049.88 | HexNAc2Hex2QuiS | ||
| 995.803+ | 995.83 | HexNAc2HexQuiS | ||
| n.d. | 974.54 | HexNAc2Hex2 | ||
| 920.463+ | 920.49 | HexNAc2Hex | ||
| 866.443+ | 866.43 | HexNAc2 | ||
| 798.753+ | 798.75 | HexNAc | ||
n.d. – not detected
Asn-425 or Asn-430 is modified but not both
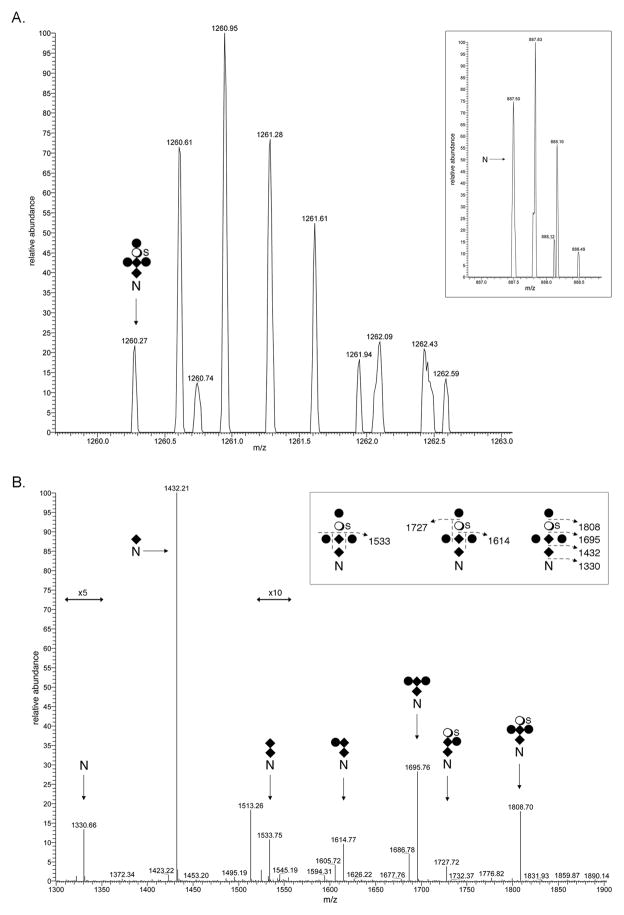
In the case of FlaB, the filament protein of the archaellum, the archaeal motility structure [34], direct evidence for modification of one of the six putative N-glycosylation sites in the protein by the same hexasaccharide was provided. LC-ESI MS analysis of a Asn-195-containing tryptic peptide (182GGQLTSSPLYIISNTSIVASKPWLK206) revealed a [M+3H]3+ peak at m/z 887.50, a value in excellent agreement with the calculated [M+3H]3+ mass of the peptide, 887.49 Da (Fig 4A, inset). A [M+3H]3+ peak at m/z 1260.27 was also observed (Fig 4A), likely corresponding to the same peptide modified by N-acetylhexosamine2-hexose3-sulfated quinovose (calculated mass 1260.36 Da). The same mass spectrometry profile also contained [M+3H]3+ peaks at m/z 955.19, likely corresponding to the peptide modified by N-acetylhexosamine (calculated mass 955.18 Da)(Fig S2A), at m/z 1022.88, likely corresponding to the peptide modified by N-acetylhexosamine2 (calculated mass 1022.87 Da)(Fig S2B), at m/z 1076.90, likely corresponding to the peptide modified by N-acetylhexosamine2-hexose (calculated mass 1022.87 Da)(Fig S2C), at m/z 1130.92, likely corresponding to the peptide modified by N-acetylhexosamine2-hexose2 (calculated mass 1130.97 Da)(Fig S2D), at m/z 1152.24, likely corresponding to the peptide modified by N-acetylhexosamine2-hexose-sulfated quinovose (calculated mass 1152.26 Da)(Fig S2E), and at m/z 1206.26, likely corresponding to the peptide modified by N-acetylhexosamine2-hexose2-sulfated quinovose (calculated mass 1206.31 Da)(Fig S2F). MS/MS analysis of the 13C1-containing isotopic [M+2H]2+ peak at m/z 1890.291, thought to correspond to the peptide modified by the complete hexasaccharide, yielded a fragmentation pattern consistent with these predictions (Fig 4B).
Fig 4.
N-glycosylation of S. acidocaldarius FlaB. A. LC-ESI MS analysis of a tryptic fragment of FlaB that includes Asn-195 revealed the peptide (inset) and its modification by a hexasaccharide comprising N-acetylhexosamine2-hexose3-sulfated quinovose. [M+3H]3+ ion peaks are presented. B. MS/MS profile of the 13C1-containing isotopic [M+2H]2+ peak at m/z 1890.291. The inset schematically represents the fragmentation scheme. The N represents Asn-195, diamonds represent N-acetylhexosamines, full circles represent hexoses, open circles represent quinovose and S represents sulfation. The arrows indicating x5 and x10 reflect magnification of the ion peaks in the corresponding region of the m/z values on the graph.
DISCUSSION
When compared to the considerable body of data available on eukaryal and bacterial N-glycosylation, the archaeal version of this post-translational modification remains poorly understood. In recent years, however, considerable progress in closing this gap has been realized. Today, information is available on a number of N-linked glycan structures, on enzymes involved in the biosynthesis of these glycans, and on the lipids upon which these glycans are assembled (for recent review, see ref. [6]). Still, concerted efforts to describe all of these aspects of the N-glycosylation process in a single archaeal species have thus far focused on a limited number of organisms, namely Hfx. volcanii, M. maripaludis and S. acidocaldarius. In the present study, novel insight into S. acidocaldarius N-glycosylation was obtained.
A major finding of this study was that the complete hexasaccharide N-linked to S. acidocaldarius glycoproteins is assembled on a single DolPP carrier, prior to its transfer to target Asn residues. This apparently differs from the situation in S. solfataricus, where it was reported that only the hexasaccharide precursor of the N-linked heptasaccharide, was detected as DolPP-bound, with the seventh sugar being possibly derived from a DolP carrier, perhaps after delivery of the hexasaccharide to target Asn residues [18]. If so, then the role played by the DolP-bound hexose detected in earlier studies of S. acidocaldarius is unclear [14]. It is possible that in S. acidocaldarius, DolP-hexose provides one or more hexoses to the growing DolPP-linked hexasaccharide. Alternatively, DolP-hexose may participate in glycolipid biogenesis in this organism. Indeed, S. acidocaldarius membranes contain lipids bearing phosphomyoinositol, glucopyranose, galactylpyranosyl-glucopyranose [35].
It was further shown that the hexasaccharide N-linked to FlaB and SlaB is identical to that previously reported to decorate SlaA and cytochrome b558/566 [24,25]. Although DolPP bearing glycans comprising less than four of the sugars comprising the N-linked hexasaccharide sugars was not detected, assessing FlaB- and SlaB-derived glycopeptides nonetheless offered novel insight into the S. acidocaldarius N-glycosylation pathway. The detection of proteins bearing various precursors of the N-linked hexasaccharide argues that DolPP bearing such precursors can be processed by AglB, the oligosaccharyltransferase responsible for transferring glycans from DolPP carriers to target Asn residues, as well as by the currently unidentified flippase responsible for translocating glycan-charged DolPP across the membrane. The fact that certain derivatives of the N-linked hexasaccharide, such as N-acetylglucosamine2-sulfoquinovose, were not detected argues that these glycopeptides bore precursors rather than breakdown products of the hexasaccharide. As such, and in agreement with previous reports [25], the results presented here show that the two N-acetylglucosamines of the N-linked hexasaccharide are first loaded onto the DolPP carrier, followed by a mannose. The fact that the glycopeptides bearing both N-acetylglucosamine2-mannose-sulfoquinovose and N-acetylglucosamine2-mannose2 were detected argues that S. acidocaldarius can add both sulfoquinovose and mannose to the DolPP-linked trisaccharide precursor, although the present results cannot determine which of the lipid-linked tetrasaccharides is further processed or whether both are. However, earlier work involving the deletion of the glycosyltransferase-encoding agl3 gene supported N-acetylglucosamine2-mannose-sulfoquinovose as being the precursor of the hexasaccharide [36]. This scenario is reminiscent of what occurs in Hfx. volcanii, where two different trisaccharide-modified DolP species are seemingly generated, with only one serving as the precursor of the N-linked pentasaccharide and the other not undergoing further processing before being delivered to target Asn residues [37].
Still, the detection of hexasaccharide-charged DolPP raises new questions concerning the S. acidocaldarius N-glycosylation pathway. For instance, it remains to be determined whether DolPP is charged with N-acetylglucosamine or whether a phosphorylated version of the sugar is added to a DolP carrier to yield DolPP bearing this first subunit of the hexasaccharide. Accordingly, S. acidocaldarius aglH is a homologue of eukaryal agl7, encoding a UDP- N-acetylglucosamine:DolP N-acetylglucosamine-1-phosphotransferase [38]. Although S. acidocaldarius aglH could not be deleted, the introduced gene could rescue a yeast agl7 conditional mutant (B.H. Meyer and S.V. Albers, unpublished). This argues for DolP being charged with nucleotide-activated N-acetylglucosamine to yield DolPP-N-acetylglucosamine. At the same time, when the same experiment was performed using Methanococcus voltae aglH, where DolP is thought to be the lipid glycan carrier [15], complementation of the same yeast mutant was realized [39].
With glycan-charged lipid carriers, N-linked glycan composition, sites of modification in reporter glycoproteins, and several enzymes participating in glycan assembly and attachment already known, together with gene deletion techniques and mass spectrometry protocols that will allow for the identification of additional pathway components, the stage is now set for detailed delineation of the S. acidocaldarius N-glycosylation process.
Supplementary Material
Acknowledgments
The mass spectrometry facility in the Department of Biochemistry of the Duke University Medical Center and Z.G. are supported by the LIPID MAPS Large Scale Collaborative Grant number GM-069338 and EY023666 from NIH. A.D., P.N., S.V.A. and J.E. are supported by a grant from the German-Israeli Foundation for Scientific Research and Development (grant I-1290 416.13/2015). B.M. received support from an ERC starting grant (311523, ARCHAELLUM). The funding agencies played no role in the collection, analysis and interpretation of data, in the writing of the report, or in the decision to submit the article for publication.
ABBREVIATIONS
- DolP
dolichol phosphate
- DolPP
dolichol pyrophosphate
- LC-ESI MS
liquid chromatography-electrospray ionization mass spectrometry
- MS/MS
tandem mass spectrometry
- S-layer
surface layer
Footnotes
AUTHOR CONTRIBUTIONS
ZG, AD, PN and BM performed the experiments; ZG, AD, SVA and JE analyzed the data; JE wrote the manuscript.
References
- 1.Swiezewska E, Danikiewicz W. Polyisoprenoids: structure, biosynthesis and function. Prog Lipid Res. 2005;44:235–258. doi: 10.1016/j.plipres.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 2.Jones MB, Rosenberg JN, Betenbaugh MJ, Krag SS. Structure and synthesis of polyisoprenoids used in N-glycosylation across the three domains of life. Biochim Biophys Acta. 2009;1790:485–494. doi: 10.1016/j.bbagen.2009.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guan Z, Eichler J. Liquid chromatography/tandem mass spectrometry of dolichols and polyprenols, lipid sugar carriers across evolution. Biochim Biophys Acta. 2011;1811:800–806. doi: 10.1016/j.bbalip.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hartley MD, Imperiali B. At the membrane frontier: a prospectus on the remarkable evolutionary conservation of polyprenols and polyprenyl-phosphates. Arch Biochem Biophys. 2012;517:83–97. doi: 10.1016/j.abb.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eichler J. Extreme sweetness: protein glycosylation in Archaea. Nat Rev Microbiol. 2013;11:151–156. doi: 10.1038/nrmicro2957. [DOI] [PubMed] [Google Scholar]
- 6.Jarrell KF, Ding Y, Meyer BH, Albers SV, Kaminski L, Eichler J. N-linked glycosylation in Archaea: A structural, functional and genetic analysis. Microbiol Mol Biol Rev. 2014;78:304–341. doi: 10.1128/MMBR.00052-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weerapana E, Imperiali B. Asparagine-linked protein glycosylation: from eukaryotic to prokaryotic systems. Glycobiology. 2006;16:91R–101R. doi: 10.1093/glycob/cwj099. [DOI] [PubMed] [Google Scholar]
- 8.Aebi M. N-linked protein glycosylation in the ER. Biochim Biophys Acta. 2013;1833:2430–2437. doi: 10.1016/j.bbamcr.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 9.Szymanski CM, Wren BW. Protein glycosylation in bacterial mucosal pathogens. Nat Rev Microbiol. 2005;3:225–237. doi: 10.1038/nrmicro1100. [DOI] [PubMed] [Google Scholar]
- 10.Abu-Qarn M, Eichler J, Sharon N. Not just for Eukarya anymore: N-glycosylation in Bacteria and Archaea. Curr Opin Struct Biol. 2008;18:544–550. doi: 10.1016/j.sbi.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 11.Nothaft H, Szymanski CM. Bacterial protein N-glycosylation: new perspectives and applications. J Biol Chem. 2013;288:6912–6920. doi: 10.1074/jbc.R112.417857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuntz C, Sonnenbichler J, Sonnenbichler I, Sumper M, Zeitler R. Isolation and characterization of dolichol-linked oligosaccharides from Haloferax volcanii. Glycobiology. 1997;7:897–904. doi: 10.1093/glycob/7.7.897. [DOI] [PubMed] [Google Scholar]
- 13.Guan Z, Naparstek S, Kaminski L, Konrad Z, Eichler J. Distinct glycan-charged phosphodolichol carriers are required for the assembly of the pentasaccharide N-linked to the Haloferax volcanii S-layer glycoprotein. Mol Microbiol. 2010;78:1294–1303. doi: 10.1111/j.1365-2958.2010.07405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guan Z, Meyer BH, Albers SV, Eichler J. The thermoacidophilic archaeon Sulfolobus acidocaldarius contains an unusually short, highly reduced dolichyl phosphate. Biochim Biophys Acta. 2011;1811:607–616. doi: 10.1016/j.bbalip.2011.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Larkin A, Chang MM, Whitworth GE, Imperiali B. Biochemical evidence for an alternate pathway in N-linked glycoprotein biosynthesis. Nat Chem Biol. 2013;9:367–373. doi: 10.1038/nchembio.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohen-Rosenzweig C, Guan Z, Shaanan B, Eichler J. Substrate promiscuity: AglB, the archaeal oligosaccharyltransferase, can process a variety of lipid-linked glycans. Appl Environ Microbiol. 2014;80:486–496. doi: 10.1128/AEM.03191-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang MM, Imperiali B, Eichler J, Guan Z. N-linked glycans are assembled on highly reduced dolichol phosphate carriers in the hyperthermophilic archaea Pyrococcus furiosus. PLoS One. 2015;10:e0130482. doi: 10.1371/journal.pone.0130482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taguchi Y, Fujinami D, Kohda D. Comparative analysis of archaeal lipid-linked oligosaccharides that serve as oligosaccharide donors for Asn glycosylation. J Biol Chem. 2016;291:11042–11054. doi: 10.1074/jbc.M115.713156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaminski L, Guan Z, Yurist-Doutsch S, Eichler J. Two distinct N-glycosylation pathways process the Haloferax volcanii S-layer glycoprotein upon changes in environmental salinity. mBio. 2013;4:e00716–e00713. doi: 10.1128/mBio.00716-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Calo D, Guan Z, Naparstek S, Eichler J. Different routes to the same ending: comparing the N-glycosylation processes of Haloferax volcanii and Haloarcula marismortui, two halophilic archaea from the Dead Sea. Mol Microbiol. 2011;81:1166–1177. doi: 10.1111/j.1365-2958.2011.07781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hartmann E, Konig H. Uridine and dolichyl diphosphate activated oligosaccharides are intermediates in the biosynthesis of the S-layer glycoprotein of Methanothermus fervidus. Arch Microbiol. 1989;151:274–281. [Google Scholar]
- 22.Mescher MF, Hansen U, Strominger JL. Formation of lipid-linked sugar compounds in Halobacterium salinarium. Presumed intermediates in glycoprotein synthesis. J Biol Chem. 1976;251:7289–7294. [PubMed] [Google Scholar]
- 23.Lechner J, Wieland F, Sumper M. Biosynthesis of sulfated saccharides N-glycosidically linked to the protein via glucose. Purification and identification of sulfated dolichyl monophosphoryl tetrasaccharides from halobacteria. J Biol Chem. 1985;260:860–866. [PubMed] [Google Scholar]
- 24.Zahringer U, Moll H, Hettmann T, Knirel YA, Schafer G. Cytochrome b558/566 from the archaeon Sulfolobus acidocaldarius has a unique Asn-linked highly branched hexasaccharide chain containing 6-sulfoquinovose. Eur J Biochem. 2000;267:4144–4149. doi: 10.1046/j.1432-1327.2000.01446.x. [DOI] [PubMed] [Google Scholar]
- 25.Peyfoon E, Meyer B, Hitchen PG, Panico M, Morris HR, Haslam SM, Albers SV, Dell A. The S-layer glycoprotein of the crenarchaeote Sulfolobus acidocaldarius is glycosylated at multiple sites with the chitobiose-linked N-glycans. Archaea. 2010;2010:754101. doi: 10.1155/2010/754101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palmieri G, Balestrieri M, Peter-Katalinić J, Pohlentz G, Rossi M, Fiume I, Pocsfalvi G. Surface-exposed glycoproteins of hyperthermophilic Sulfolobus solfataricus P2 show a common N-glycosylation profile. J Proteome Res. 2013;12:2779–2790. doi: 10.1021/pr400123z. [DOI] [PubMed] [Google Scholar]
- 27.Meyer BH, Albers SV. Hot and sweet: protein glycosylation in Crenarchaeota. Biochem Soc Trans. 2013;41:384–392. doi: 10.1042/BST20120296. [DOI] [PubMed] [Google Scholar]
- 28.Burda P, Aebi M. The dolichol pathway of N-linked glycosylation. Biochim Biophys Acta. 1999;1426:239–257. doi: 10.1016/s0304-4165(98)00127-5. [DOI] [PubMed] [Google Scholar]
- 29.Wagner M, van Wolferen M, Wagner A, Lassak K, Meyer BH, Reimann J, Albers SV. Versatile genetic toolbox for the crenarchaeote Sulfolobus acidocaldarius. Front Microbiol. 2012;3:214. doi: 10.3389/fmicb.2012.00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brock TD, Brock KM, Belly RT, Weiss RL. Sulfolobus: a new genus of sulfur-oxidizing bacteria living at low pH and high temperature. Arch Mikrobiol. 1972;84:54–68. doi: 10.1007/BF00408082. [DOI] [PubMed] [Google Scholar]
- 31.Kelleher DJ, Karaoglu D, Gilmore R. Large-scale isolation of dolichol-linked oligosaccharides with homogeneous oligosaccharide structures: determination of steady-state dolichol-linked oligosaccharide compositions. Glycobiology. 2001;11:321–333. doi: 10.1093/glycob/11.4.321. [DOI] [PubMed] [Google Scholar]
- 32.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 33.Meyer BH, Birich A, Albers SV. N-Glycosylation of the archaellum filament is not important for archaella assembly and motility, although N-glycosylation is essential for motility in Sulfolobus acidocaldarius. Biochimie. 2015;118:294–301. doi: 10.1016/j.biochi.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 34.Albers SV, Jarrell KF. The archaellum: how Archaea swim. Front Microbiol. 2015;6:23. doi: 10.3389/fmicb.2015.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gulik A, Luzzati V, DeRosa M, Gambacorta A. Tetraether lipid components from a thermoacidophilic archaebacterium. Chemical structure and physical polymorphism. J Mol Biol. 1988;201:429–435. doi: 10.1016/0022-2836(88)90149-0. [DOI] [PubMed] [Google Scholar]
- 36.Meyer BH, Zolghadr B, Peyfoon E, Pabst M, Panico M, Morris HR, Haslam SM, Messner P, Schäffer C, Dell A, Albers SV. Sulfoquinovose synthase - an important enzyme in the N-glycosylation pathway of Sulfolobus acidocaldarius. Mol Microbiol. 2011;82:1150–1163. doi: 10.1111/j.1365-2958.2011.07875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kandiba L, Lin C-w, Aebi M, Eichler J, Guerardel Y. Structural characterization of the N-linked pentasaccharide decorating glycoproteins of the halophilic archaeon Haloferax volcanii. Glycobiology. 2016 doi: 10.1093/glycob/cww014. in press. [DOI] [PubMed] [Google Scholar]
- 38.Lehrman MA. Biosynthesis of N-acetylglucosamine-P-P-dolichol, the committed step of asparagine-linked oligosaccharide assembly. Glycobiology. 1991;1:553–562. doi: 10.1093/glycob/1.6.553. [DOI] [PubMed] [Google Scholar]
- 39.Shams-Eldin H, Chaban B, Niehus S, Schwarz RT, Jarrell KF. Identification of the archaeal alg7 gene homolog (encoding N-acetylglucosamine-1-phosphate transferase) of the N-linked glycosylation system by cross-domain complementation in Saccharomyces cerevisiae. J Bacteriol. 2008;190:2217–2220. doi: 10.1128/JB.01778-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.