Abstract
Background
Extracellular hemoglobin and cell-free heme are toxic breakdown products of hemolyzed erythrocytes. Mammals synthesize the scavenger proteins haptoglobin and hemopexin, which bind extracellular hemoglobin and heme respectively. Transfusion of packed red blood cells (PRBCs) is a life-saving therapy for patients with hemorrhagic shock. Because erythrocytes undergo progressive deleterious morphological and biochemical changes during storage, transfusion of PRBCs that have been stored for prolonged intervals (SRBCs; stored for 35–40 days in humans or 14 days in mice) increases plasma levels of cell-free hemoglobin and heme. Therefore, in patients with hemorrhagic shock, perfusion-sensitive organs such as the kidneys are challenged not only by hypoperfusion, but also by the high concentrations of plasma hemoglobin and heme that are associated with the transfusion of SRBCs.
Methods
To test whether treatment with exogenous human haptoglobin or hemopexin can ameliorate adverse effects of resuscitation with SRBCs after two hours of hemorrhagic shock, mice receiving SRBCs were given a co-infusion of haptoglobin, hemopexin or albumin.
Results
Treatment with haptoglobin or hemopexin but not albumin improved the survival rate and attenuated SRBC-induced inflammation. Treatment with haptoglobin retained free hemoglobin in the plasma and prevented SRBC-induced hemoglobinuria and kidney injury. In mice resuscitated with fresh PRBCs, treatment with haptoglobin, hemopexin, or albumin did not cause harmful effects.
Conclusions
In mice the adverse effects of transfusion with SRBCs after hemorrhagic shock are ameliorated by treatment with either haptoglobin or hemopexin. Haptoglobin infusion prevents kidney injury associated with high plasma hemoglobin concentrations after resuscitation with SRBCs. Treatment with the naturally occurring human plasma proteins haptoglobin or hemopexin may have beneficial effects in conditions of severe hemolysis following prolonged hypotension.
Journal Subject Terms: Animal Models of Human Disease, Basic Science Research, Physiology
Keywords: extracellular hemoglobin, hemopexin, stored blood transfusion, hemoglobin and heme scavengers, critical care, haptoglobin, hemorrhage, blood
Acute kidney injury (AKI) has been reported in up to 35% of patients admitted to the intensive care unit (ICU) and is accompanied by a marked increase in morbidity and mortality1–3. The etiology of AKI is multifactorial and often associated with general or regional renal hypoperfusion4,5. Common causes of inadequate renal perfusion are reduced cardiac output and/or increased vascular resistance. These conditions are frequently due to hypovolemia associated with fluid loss or hemorrhage3.
Transfusion of packed red blood cells (PRBCs) is a life-saving therapy in patients with hemorrhagic shock, restoring blood volume, blood oxygen carrying capacity, cardiac output, and vital organ perfusion6. PRBCs are produced by collecting whole blood from volunteer donors, removing leukocytes, concentrating the RBCs and storing the cells for up to 42 days in a nutrient additive solution at 4°C. Complex morphological and biochemical changes occur with increasing storage time including changes in red cell shape and plasticity, loss of 2,3-diphosphoglycerate, and release of potassium, iron, lactate dehydrogenase, microparticles, heme, and hemoglobin7–9. Studies of critically ill trauma patients and surgical ICU-patients revealed an association between the duration of PRBC storage and ICU length of stay, morbidity and mortality10–12. Recently, two clinical trials reported no difference in survival or severe adverse events in patients transfused with PRBCs that were stored for either seven to ten days or a median storage time of 21 days13,14. Unfortunately neither study had sufficient power to examine the effects of prolonged (30–42 days) PRBC storage; prolonged storage is associated with maximum red blood cell injury and hemolysis7,9.
Transfusion of PRBCs that have been stored for prolonged intervals (SRBCs; 35–40 days in humans or 14 days in mice) results in increased plasma hemoglobin levels in humans and animals15–21. Plasma hemoglobin scavenges nitric oxide and causes vasoconstriction, platelet aggregation and inflammation9,22–24. Toxicity of free hemoglobin is also caused by the release of cell-free heme, which produces lipid peroxidation and mitochondrial damage and increases the production of reactive oxygen species. Cell-free heme selectively triggers pro-inflammatory receptors such as TLR-4 and BACH-1, and activates proteasomes25.
Mammals possess two independent scavenger systems to remove extracellular heme and hemoglobin from plasma. Hemopexin is a glycoprotein that is synthesized in the liver and has the highest known binding-affinity for plasma heme. Binding to hemopexin blocks the pro-oxidant and pro-inflammatory effects of cell-free heme and facilitates receptor-mediated uptake of heme by the liver and spleen26. Haptoglobin binds extracellular hemoglobin with high-affinity to form the high-molecular weight haptoglobin-hemoglobin complex. In this complexed form, hemoglobin is sequestered within the vasculature and the passage of hemoglobin through the endothelial junctions of blood vessels, including filtration by renal glomeruli, is prevented25. Plasma-clearance of haptoglobin-hemoglobin complexes occurs via binding to the CD163 receptor, which is expressed predominantly by hepatic and splenic macrophages27.
Increased levels of plasma hemoglobin result in increased hemoglobin filtration in renal glomeruli and subsequent tubular injury28. Therefore, we hypothesized that renal damage, which is also induced by hemorrhagic shock, would be aggravated by resuscitation with SRBCs. In this study we developed a murine model of resuscitation after prolonged hemorrhagic shock to examine the effects of transfusion of fresh syngeneic murine PRBCs (FRBCs) and SRBCs on kidney injury and mortality. We examined whether co-infusion of either exogenous human hemopexin or haptoglobin with SRBCs could improve survival rates and ameliorate kidney injury due to resuscitation with SRBCs.
Methods
Animal studies
The Institutional Animal Care and Use Committee of Massachusetts General Hospital (Boston, MA) approved the murine studies. Blood collection, leukocyte reduction and erythrocyte storage were conducted as described previously29. Mice were subjected to hemorrhagic shock and resuscitated with either “fresh” PRBCs (FRBCs, stored for less than 24 hours after collection) or PRBCs “stored for prolonged intervals” (SRBCs, maintained at 4°C for two weeks, mimicking 42-day human blood storage30). Control mice (sham-treated) underwent three hours of anesthesia and vascular cannulation but were not subjected to hemorrhagic shock or PRBC-resuscitation. Three groups of mice receiving SRBCs were given a co-infusion of haptoglobin, hemopexin, or albumin during syngeneic transfusion.
Mouse hemorrhagic shock model
Mice were anesthetized with 1.5% isoflurane at FiO2 0.21 and both femoral arteries were cannulated with heparinized polyethylene-10 tubing (BD Diagnostics, Franklin Lakes, NJ). One line was used for blood withdrawal and resuscitation; the other was used to continuously monitor arterial blood pressure (Ponemah Physiology Platform, Valley View, OH). Anesthetized C57Bl/6-mice were subjected to hemorrhagic shock by withdrawing arterial blood over 10 minutes (approximately 55% of their total blood volume) until a mean arterial pressure (MAP) of 35 mmHg was reached. Hypotension at 35 mmHg was maintained for two hours. Further detailed description of the procedures is available in the Online Supplemental Material.
Renal function studies
Twenty-four-hour urine samples were collected using a metabolic cage (Tecniplast, West Chester, PA). Urinary KIM-1 was measured using a microbead-based assay (see Online Supplemental Methods). Urine levels of creatinine were measured by isotope dilution LC-MSMS (UAB O’Brien Center, Birmingham, AL).
Histologic Analysis
Renal pathologists (IR and RBC), blinded to the experimental groups evaluated the histologic sections for signs of acute tubular injury and graded the injury according to published criteria31. Details on immunohistochemistry staining are available in the Online Supplemental Material. Ten 20x-fields (1.088 diameter) were reviewed for each H&E stained section. For Ki-67 staining, the number of Ki-67 positive cells in five 20x-fields (1.088 diameter) was determined.
Prussian blue staining
Deparaffinized renal tissue sections were stained with Prussian blue and counterstained with nuclear fast red (Polysciences, Inc.).
Statistical analysis
Statistical analyses were performed with GraphPad Prism Software Version 6.0 (GraphPad Software Inc., La Jolla, CA). Two independent groups were compared using the Mann-Whitney U test. Statistical comparison between more than two groups was assessed by the Kruskal-Wallis test with post-hoc pairwise comparisons adjusted with Bonferroni correction for multiple testing. Hemodynamic data were analyzed using a two-way ANOVA with repeated measures to compute differences between groups at various time points. Survival rates were compared using the Kaplan-Meier method with a log-rank test. Data on KIM-1 were log-transformed and analyzed by a one-way ANOVA followed by post-hoc pairwise comparisons adjusted with Bonferroni correction for multiple testing.
Further details concerning the methods used in this study are reported in the online Supplemental Methods and Supplemental Table II.
Results
A murine model of resuscitation after prolonged hemorrhagic shock
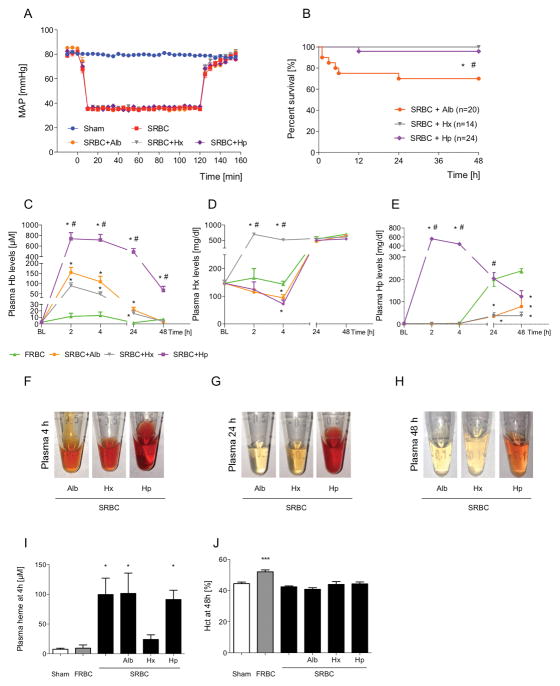
We developed a murine model of large volume blood resuscitation from hemorrhagic shock to investigate whether transfusion of SRBCs would worsen hypoperfusion-induced renal injury. Anesthetized C57Bl/6-mice were subjected to hemorrhagic shock and hypotension with a mean arterial pressure of 35 mmHg was maintained for two hours (Figure 1A). Mice that received FRBCs had a small, but significantly higher MAP immediately after transfusion as compared to mice receiving SRBCs (Figure 1A). Survival rates after hemorrhagic shock and resuscitation were measured to determine whether mortality after transfusion with SRBCs differed from mortality rates after resuscitation with FRBCs. After transfusion with FRBCs, 91% of the mice survived for at least 48 hours (Figure 1B). After resuscitation with SRBCs, only 58% of mice survived for 48 hours (SRBC vs. FRBC, P<0.05). All sham-treated mice survived for at least 48 hours.
Figure 1.
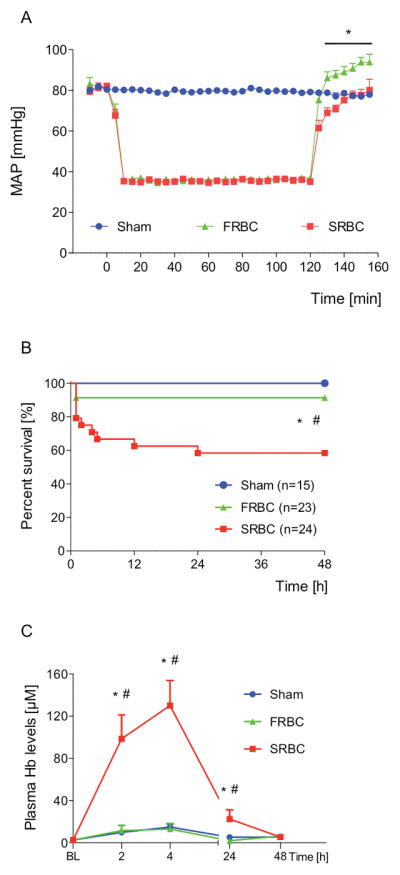
Hemodynamic measurements, plasma free hemoglobin levels and survival rates of mice resuscitated with PRBCs after prolonged hemorrhagic shock. (A) Mice resuscitated after two hours of hemorrhagic shock with fresh PBRCs (FRBC) had a higher mean arterial pressure (MAP) immediately after transfusion compared with mice receiving stored PBRCs (SRBC) (n=15, *P<0.001). (B) Percentages of mice surviving for 48 hours after hemorrhagic shock and resuscitation: Sham: 100%, FRBC: 91%, SRBC: 58% (*P<0.01, Sham vs. SRBC; #P<0.05, FRBC vs. SRBC). (C) Plasma extracellular hemoglobin (Hb) levels at two, four, 24, and 48 hours after resuscitation with PRBCs (*P<0.05 vs. Sham; #P<0.05 vs. FRBC, n=5–10 per time point per group). Data represent mean±SEM and were compared by 2-ANOVA (A) or Kruskall-Wallis test (C) followed by pairwise comparisons adjusted with Bonferroni correction for multiple testing or by log-rank test (B).
The mice that received FRBCs or SRBCs did not differ in body weight, shed blood volume, or the volumes of blood and lactated Ringer’s solution that were infused during resuscitation (Table S1). As reported in previous studies, SRBCs have a lower pH than FRBCs (SRBC vs. FRBC, pH: 6.44±0.03 vs. 6.98±0.01, P<0.001)17,29. In addition, the levels of cell-free hemoglobin were greater in units of SRBCs, as compared to FRBCs (Supplemental Table I).
In mice resuscitated with SRBCs, plasma hemoglobin levels were greater than in mice resuscitated with FRBCs or in sham-treated mice at two, four, and 24 hours after transfusion (Figure 1C). The plasma hemoglobin levels at two and four hours after SRBC-transfusion were greater than the cell-free hemoglobin levels in the supernatant of the SRBCs before transfusion (Figure 1C, Supplemental Table I), providing evidence that hemolysis occurs in vivo during and after transfusion of SRBCs. In contrast, plasma hemoglobin levels after resuscitation with FRBCs did not significantly differ from the cell-free hemoglobin levels in the supernatant of the FRBCs before transfusion.
Taken together, these results demonstrate that in this model, resuscitation from prolonged hemorrhagic shock with SRBCs as compared to FRBCs is associated with a higher mortality rate and increased plasma hemoglobin levels.
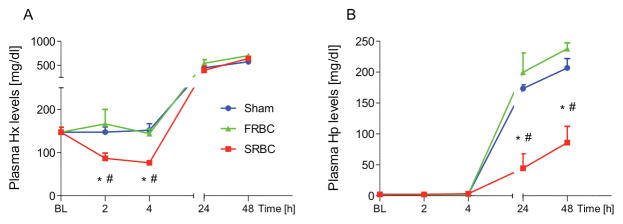
Plasma hemopexin and haptoglobin levels after hemorrhage and transfusion with stored or fresh PRBCs
To examine the effects of hemorrhage and resuscitation with FRBCs or SRBCs on murine hemopexin and haptoglobin levels, blood samples were obtained before transfusion and at intervals up to 48 hours after transfusion. In animals resuscitated with SRBCs, plasma hemopexin levels significantly decreased from 147.1±11.4 mg/dl at baseline to 86.7±12.2 and 76.3±5.4 mg/dl at two and four hours after resuscitation, respectively (p<0.05 for both, Figure 2A). This decrease in hemopexin levels was not observed in mice transfused with FRBCs or in sham-treated mice. Plasma haptoglobin levels were initially low in all mice prior to transfusion (Figure 2B). Consistent with hemopexin’s and haptoglobin’s function as acute phase reactants in mice, plasma levels of both proteins increased markedly in all mice at 24 and 48 hours after resuscitation compared to levels at baseline. In sham-treated animals, the trauma caused by incisions in both groins and femoral arterial cannulations is likely to have triggered the increase in hemopexin and haptoglobin plasma levels at 24 and 48 hours. The increase in plasma haptoglobin levels from baseline to 48 hours after resuscitation was less in mice transfused with SRBCs compared to mice transfused with FRBCs (P<0.001). These results are consistent with removal of hemopexin and haptoglobin from the circulation of SRBC-transfused mice, most likely in response to increased plasma heme and hemoglobin.
Figure 2.
Plasma hemopexin and haptoglobin levels after hemorrhage and resuscitation with stored or fresh PRBCs. Hemopexin (Hx) and haptoglobin (Hp) levels increased markedly at 24 and 48 hours (h) after anesthesia and surgery (P<0.05 vs. baseline (BL)). (A) Plasma Hx levels decreased at two and four h after resuscitation with stored PRBCs (SRBC) (P<0.01 vs. BL). (B) Plasma Hp levels were initially low in all mice prior to hemorrhagic shock and resuscitation. Hp levels increased to a lesser extent at 24 and 48 h after resuscitation with SRBC as compared to fresh PRBCs (FRBC). Data represent mean±SEM and were compared by Kruskall-Wallis test followed by Bonferroni-adjusted pairwise comparisons, n=5–7 per time point per group, *P<0.05 vs. Sham; #P<0.05, vs. FRBC.
The effects of treatment with hemopexin or haptoglobin during resuscitation with SRBCs on survival after hemorrhagic shock
We considered the possibility that administration of exogenous human hemopexin or haptoglobin might improve the survival rate after hemorrhage and resuscitation with SRBCs. Based on the changes in plasma hemopexin levels within four hours after resuscitation with SRBCs and the changes in plasma haptoglobin levels detected at 24–48 hours after hemorrhagic shock and SRBC-resuscitation (Figure 2), we estimated that a dose of 7.5 mg of either hemopexin or haptoglobin would be sufficient to replace the consumption of each scavenger protein.
To confirm that administration of exogenous human hemopexin or haptoglobin after hemorrhagic shock was safe, mice were subjected to hemorrhagic shock and resuscitated with FRBCs and a simultaneous infusion of 7.5 mg hemopexin or haptoglobin. A control group of mice was resuscitated with FRBCs and 7.5 mg of albumin. Survival rates seven days after hemorrhagic shock and resuscitation with FRBCs and hemopexin, haptoglobin, or albumin did not differ compared to mice resuscitated with FRBCs alone (Supplemental Figure I).
To test whether administration of exogenous hemopexin or haptoglobin improves the survival rate after hemorrhage and resuscitation with SRBCs, mice subjected to hemorrhagic shock were resuscitated with SRBCs alone or SRBCs and a simultaneous infusion of hemopexin, haptoglobin, or albumin (Figure 3A). The survival rate of mice resuscitated with SRBCs with co-infusion of albumin was 70% (Figure 3B). In contrast, all of the mice survived after SRBC-transfusion and treatment with hemopexin (SRBC+Hx vs. SRBC+Alb, P=0.018). Similarly the survival rate of mice resuscitated with SRBCs and co-infusion of haptoglobin was 96% (SRBC+Hp vs. SRBC+Alb, P=0.030).
Figure 3.
Effects of exogenous human hemopexin or haptoglobin on hemodynamics, survival, plasma free heme, and plasma extracellular hemoglobin concentrations after hemorrhagic shock and resuscitation with stored PRBCs. (A) Co-infusion of albumin (Alb), hemopexin (Hx), or haptoglobin (Hp) during transfusion of stored PRBCs (SRBC) did not alter the mean arterial pressure (MAP). (B) Survival rates 48 hours after resuscitation: SRBC+Hp: 96%, SRBC+Hx 100%, SRBC+Alb: 70%. (*P<0.05 SRBC+Hp vs. SRBC+Alb; #P<0.01 SRBC+Hx vs. SRBC+Alb). (C–E) Resuscitation with SRBC+Alb did not alter the plasma levels of hemoglobin (Hb), Hx, or Hp compared to hemorrhagic shock and resuscitation with SRBC alone. Infusion of SRBC+Hx resulted in increased levels of plasma Hx at two and four hours after transfusion as compared to baseline (BL) (n=4–7 per time point per group, P<0.001 vs. BL). Resuscitation with SRBC+Hp resulted in increased plasma Hp levels at two and four hours after transfusion (n=5–10, P<0.001 vs. BL). Resuscitation with SRBC+Hp induced a marked and prolonged increase in plasma Hb levels (n=5–10, *P<0.05 vs. FRBC; #P<0.05 vs. SRBC+Alb). (F–H) Representative plasma samples obtained at four, 24, and 48 hours after hemorrhagic shock and resuscitation. (I) Resuscitation with SRBC+Hx was associated with decreased plasma free heme levels (n=5–10, *P<0.05 vs. Sham). (J) Hematocrit (Hct) of resuscitated mice at 48 hours after hemorrhagic shock and transfusion (n=5–8, ***P<0.001 vs. Sham). Data represent mean±SEM and were compared by Kruskall-Wallis test followed by Bonferroni-adjusted pairwise comparisons (A, C–E, I and J) or log-rank test (B).
These results indicate that, in mice with hemorrhagic shock and resuscitation with FRBCs, co-infusion of either hemopexin or haptoglobin was safe and did not affect seven-day survival rates. In mice resuscitated with SRBCs after hemorrhagic shock, co-infusion of either hemopexin or haptoglobin significantly reduced the mortality rate during the first 48 hours after transfusion.
The effects of hemopexin or haptoglobin infusion on plasma concentrations of extracellular heme and hemoglobin after transfusion with SRBCs
To investigate the effects of infusion of hemopexin or haptoglobin during resuscitation with SRBCs, we measured plasma levels of heme and hemoglobin after infusion of hemopexin, haptoglobin, or albumin. Administration of hemopexin, haptoglobin, or albumin together with SRBCs did not affect the MAP during resuscitation (Figure 3A). Resuscitation with SRBCs together with albumin did not alter plasma levels of hemoglobin, hemopexin, haptoglobin, or heme as compared to resuscitation with SRBCs alone (Figure 3C–E, I, Supplemental Material and Supplemental Figure II A–B). Infusion of hemopexin together with SRBCs resulted in a 6.4-fold (95% CI [5.2 – 7.6]) increase in plasma hemopexin levels at four hours after transfusion (Figure 3D). The increase in hemopexin was associated with decreased plasma heme levels (Figure 3I). Infusion of hemopexin had no effect on plasma levels of hemoglobin or haptoglobin (Figure 3C and E).
In mice treated with haptoglobin together with SRBCs, there was a marked increase in the level of total plasma haptoglobin (Figure 3E). Increased plasma haptoglobin was associated with increased plasma extracellular hemoglobin (Figure 3C and 3F–H). At two hours after transfusion, the level of plasma hemoglobin was 5.1 times (95% CI [3.1 – 7.1]) greater in mice transfused with SRBCs and haptoglobin than in mice transfused with SRBCs and albumin. The hematocrit of mice resuscitated with SRBCs and a simultaneous infusion of any treatment protein did not differ from the hematocrit after resuscitation with SRBCs alone (Figure 3J).
These results demonstrate that transfusion with SRBCs and simultaneous infusion of exogenous hemopexin results in decreased levels of circulating cell-free heme. Co-administration of haptoglobin with SRBCs results in a prolonged increase in plasma hemoglobin levels for more than 48 hours after transfusion. None of the treatments was associated with increased hemolysis.
Haptoglobin prevents murine hemoglobinuria and kidney injury after hemorrhagic shock and resuscitation with SRBCs
High plasma hemoglobin levels are associated with renal injury28. We used the murine model of hemorrhagic shock to compare kidney injury after resuscitation with SRBCs to resuscitation with FRBCs. Furthermore, we examined whether co-infusion of hemopexin or haptoglobin with SRBCs could ameliorate SRBC-associated kidney injury.
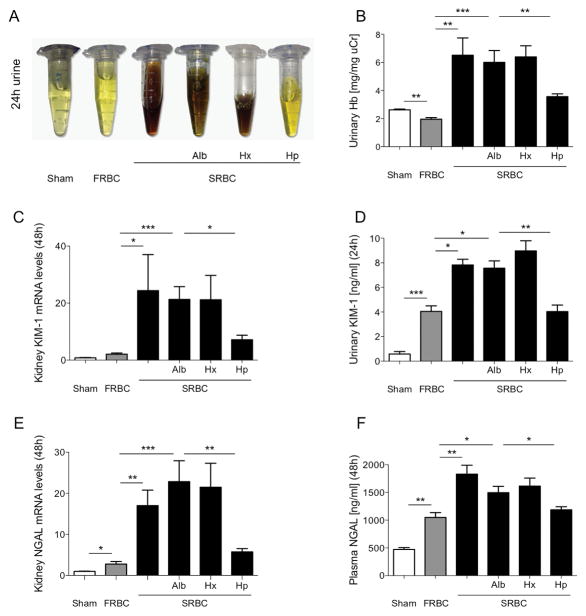
Urine output during the first 24 hours after resuscitation was reduced in mice transfused with SRBCs compared with FRBCs (SRBCs vs. FRBCs, urine output: 1.6±0.3 μl/h/g[BW] vs. 3.6±0.2 μl/h/g[BW], P<0.01). Hemoglobinuria was noted in mice resuscitated with SRBCs alone but not mice resuscitated with FRBCs or sham-treated mice (Figure 4A and B). Mice transfused with SRBCs and haptoglobin had a higher urine output than mice transfused with SRBCs and albumin (SRBC+Hp vs. SRBC+Alb, urine output: 2.2±0.1 μl/h/g[BW] vs. 1.7±0.1 μl/h/g[BW], P<0.05). Urine output did not differ between mice that received SRBCs and hemopexin and mice that received SRBCs and albumin (SRBC+Hx vs. SRBC+Alb urine output: 1.7±0.1 μl/h/g[BW] vs. 1.7±0.1 μl/h/g[BW], P=NS). Hemoglobinuria was present in mice resuscitated with SRBCs combined with albumin or hemopexin but not in mice resuscitated with SRBCs combined with haptoglobin (Figure 4A and B).
Figure 4.
Urine and plasma biochemical markers of renal injury after hemorrhagic shock and resuscitation with stored PRBCs. (A) Representative samples of urine collected for 24 hours after hemorrhagic shock and resuscitation. Hemoglobinuria was present in mice resuscitated with stored PRBCs (SRBC), SRBC+albumin (Alb) and SRBC+hemopexin (Hx) but not in mice resuscitated with fresh PRBCs (FRBC), or SRBC+haptoglobin (Hp), or in sham-operated mice (Sham). (B) Hemoglobin concentration in a 24-hour urine collection normalized for the level of urine creatinine (uCr) (n=5–9). (C) KIM-1 mRNA levels at 48 hours and (D) KIM-1 24-hour urine levels. (E) NGAL mRNA levels at 48 hours and (F) NGAL plasma concentrations at 48 hours after hemorrhagic shock and resuscitation (n=7–8). Data represent mean±SEM and were compared by Kruskall-Wallis test followed by Bonferroni-adjusted pairwise comparisons (B, E and F). KIM-1 data were log-transformed for analysis and compared by ANOVA followed by Bonferroni-adjusted pairwise comparisons (C and D). *P<0.05, **P<0.01, ***P<0.001.
Markers of renal damage, including kidney KIM-1 and NGAL mRNA levels, urinary KIM-1 protein levels, and plasma NGAL concentration, were greater in mice after resuscitation with SRBCs or SRBCs and albumin than in mice transfused with FRBCs (Figures 4C–F). Mice that were resuscitated with SRBCs and hemopexin had similar 48-hour KIM-1 and NGAL mRNA levels, urinary KIM-1 protein levels and NGAL plasma concentrations to mice resuscitated with SRBCs and albumin. However, kidney KIM-1 and NGAL mRNA levels, urinary KIM-1 protein levels and NGAL plasma concentrations in mice transfused with SRBCs and haptoglobin were lower than in mice transfused with SRBCs given a co-infusion of albumin (Figures 4C–F). Plasma creatinine and plasma urea nitrogen levels remained normal and unchanged under all conditions in all groups and treatments (Supplemental Figure III).
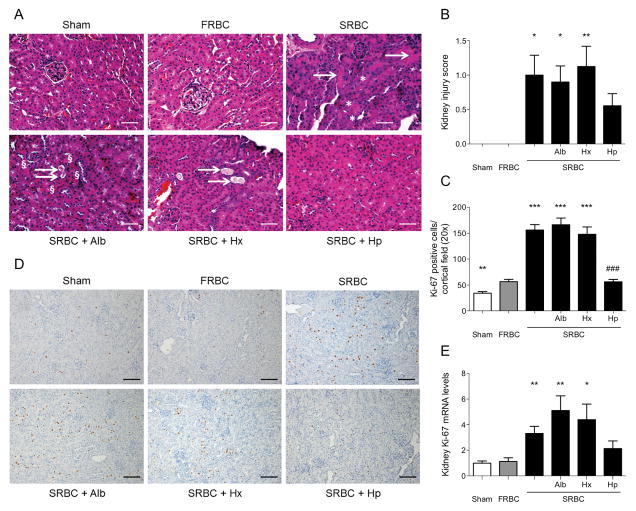
To further investigate the effect of co-infusion of haptoglobin on renal damage associated with hemorrhagic shock and transfusion of stored blood, we assessed histopathologic evidence of kidney injury. Forty-eight hours after resuscitation with SRBCs alone, SRBCs and albumin, or SRBCs and hemopexin, renal histopathology revealed dilated tubules, tubular casts and apoptosis in the renal cortex. In contrast, kidneys from mice resuscitated with FRBCs had no histologic signs of kidney damage (Figure 5A and B). There was no statistically significant difference in renal injury after hemorrhagic shock in mice resuscitated with SRBCs and haptoglobin compared to mice resuscitated with FRBCs.
Figure 5.
Renal injury after hemorrhagic shock and resuscitation with stored PRBCs, and the effects of co-treatment with hemopexin or haptoglobin. (A) Evidence of renal histopathology (H&E staining) with dilated tubules, increased mitotic activity (*) as well as tubular casts (→) and apoptosis (§) in the renal cortex at 48 hours after resuscitation with stored PRBCs (SRBC), SRBC+albumin (Alb), or SRBC+hemopexin (Hx) but not after resuscitation with fresh PRBCs (FRBC), SRBC+haptoglobin (Hp), or in sham-operated mice (Sham). (B) Kidney injury at 48 hours after hemorrhagic shock and resuscitation as quantified by kidney injury score. (C) Quantification of Ki-67 positive cells per 20×-field at 48 hours after hemorrhagic shock and resuscitation. (D) Kidney-tissue stained with the cell-cycle marker Ki-67 at 48 hours after hemorrhagic shock and resuscitation. (E) Gene expression of Ki-67 at 48 hours after hemorrhagic shock and resuscitation. Data represent mean±SEM and were compared by Kruskall-Wallis test followed by Bonferroni-adjusted pairwise comparisons, n=8–12, scale-bars: 25μm, *P<0.05, **P<0.01, ***P<0.001 vs. FRBC; ###P<0.001 vs. SRBC+Alb.
Tubular regeneration, an indirect marker of tubular injury, was assessed by quantification of the number of Ki-67-positive renal tubular cells at 48 hours after resuscitation and by measuring the level of Ki-67 mRNA in kidney tissue (Figures 5C–E). Kidney sections of mice resuscitated with SRBCs showed more cortical Ki-67 positive cells per 20× field (1.08 mm field diameter) than those of mice resuscitated with FRBCs (SRBC vs. FRBC: 156.2±10.4 vs. 56.4±4.7, p<0.01). The number of Ki-67-positive cells in the kidneys of mice resuscitated with SRBCs and albumin (166.3±12.8) or SRBCs and hemopexin (147.8±14.3) did not differ from the number in murine kidneys after SRBC-transfusion alone (Figure 5C and D). However, kidneys obtained from mice transfused with SRBCs and co-transfused with haptoglobin revealed significantly less mitotic activity than kidneys from mice transfused with SRBCs and albumin (SRBC+Alb vs. SRBC+Hp: 166.3±12.8 vs. 56.1±4.7, p<0.01). The number of Ki-67 positive cells in kidneys from mice that received SRBCs and haptoglobin was similar to the number of Ki-67 positive cells in kidneys from mice that received FRBCs. The quantification of Ki-67 positive cells was supported by comparable findings of Ki-67 gene expression measured 48 hours after resuscitation from hemorrhagic shock (Figure 5E).
These results demonstrate that resuscitation of hemorrhagic shock with stored blood is associated with hemoglobinuria and greater renal damage than resuscitation with FRBCs as reflected by alterations in urine output, urinary, plasma and tissue markers of kidney injury, renal histopathology, and renal tissue regeneration. Co-infusion of haptoglobin but not albumin or hemopexin prevented SRBC-induced hemoglobinuria and kidney injury at 48 hours after resuscitation from hemorrhagic shock.
The effects of resuscitation with SRBCs and co-infusion of hemopexin or haptoglobin on renal and splenic iron deposition and liver injury
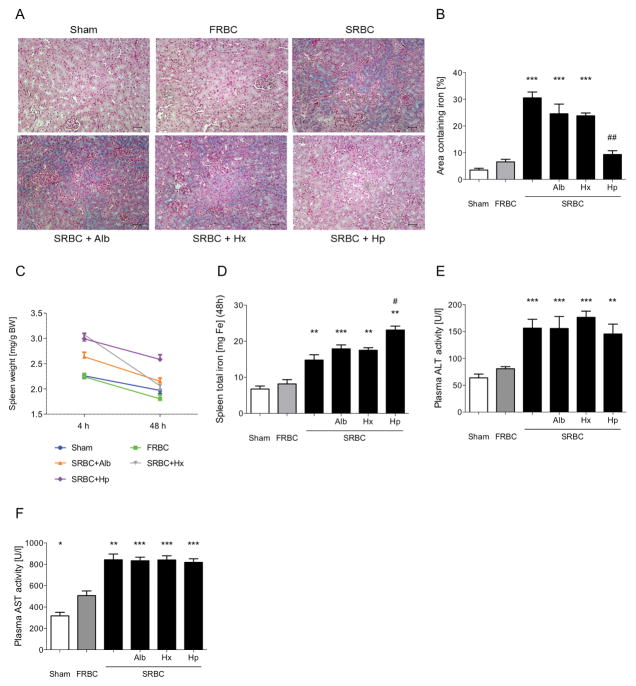
Renal iron accumulation was studied as an indicator of the kidney’s exposure to hemoglobin. Tissue iron staining revealed greater cortical iron accumulation in the kidneys of mice resuscitated with SRBCs, SRBCs combined with albumin and SRBCs combined with hemopexin than in the kidneys of mice transfused with FRBCs (Figure 6A and B). Only transfusion of SRBCs and haptoglobin prevented SRBC-induced renal iron accumulation (Figure 6A and B).
Figure 6.
Renal and splenic iron deposition and liver injury after hemorrhagic shock and PRBC-resuscitation. (A) Renal tissue sections stained with Prussian blue at 48 hours after hemorrhagic shock and resuscitation. There was increased cortical iron accumulation in mice resuscitated with stored PRBCs (SRBC), SRBC+albumin (Alb), and SRBC+hemopexin (Hx) compared to mice resuscitated with fresh PRBCs (FRBC) or SRBC+haptoglobin (Hp) or in sham-treated mice. (B) Percentage of iron-positive area in the renal cortex of Prussian-blue stained kidney slides at 48 hours after hemorrhagic shock (n=10, ***P<0.05 vs. FRBC, ##P<0.01 vs. SRBC+Alb). (C) Spleen to body weight ratio measured at four and 48 hours after resuscitation from hemorrhagic shock. (D) Splenic total iron content at 48 hours after hemorrhagic shock and resuscitation (n=8–10, **P<0.01, ***P<0.001 vs. FRBC, #P<0.05 vs. SRBC). (E–F) Plasma alanine transaminase (ALT) and plasma aspartate transaminase (AST) at 48 hours after hemorrhagic shock and resuscitation (n=8–10, *P<0.05, **P<0.01, ***P<0.001 vs. FRBC). Data represent mean±SEM and were compared by Kruskall-Wallis test followed by Bonferroni-adjusted pairwise comparisons, scale-bar: 25μm.
In addition to intravascular hemolysis, erythrocyte destruction of SRBCs occurs by the monocyte/macrophage system in the liver and the spleen32. To study splenic clearance of SRBCs, the ratio of spleen weight to body weight at four or 48 hours after resuscitation from hemorrhagic shock was determined (Figure 6C, Supplemental Results). Splenic iron content was greater in mice after resuscitation with SRBCs than in FRBC-transfused mice. In comparison to mice transfused with SRBCs alone, the spleens of mice resuscitated with SRBCs and haptoglobin contained more iron (Figure 6D).
Liver damage, measured by plasma levels of alanine transaminase (ALT) and aspartate transaminase (AST) at 48 hours after resuscitation, was greater after resuscitation with SRBCs compared to resuscitation with FRBCs (Figure 6E and F). The degree of SRBC-induced liver injury was not affected by co-infusion of any of the treatment proteins.
Taken together, these results indicate that haptoglobin co-transfusion prevented stored blood transfusion-associated renal iron uptake and led to increased weight and greater iron-accumulation in the spleens of mice. The ratios of spleen weight to body weight at 48 hours suggest that hemopexin might limit splenic erythrophagocytosis. Co-infusion of hemopexin or haptoglobin together with SRBCs did not decrease the hepatic injury associated with hemorrhagic shock and transfusion with SRBCs alone.
The effects of resuscitation with SRBCs and co-infusion of hemopexin or haptoglobin on inflammation in spleen, liver, kidney, and lungs
Extracellular hemoglobin and cell-free heme may be substantial contributors to the pro-inflammatory response after transfusion of stored blood32,33. To examine whether co-infusion of haptoglobin or hemopexin affects the SRBC-associated pro-inflammatory response after hemorrhagic shock, the expression of pro-inflammatory cytokines and enzymes were measured in spleen, liver, kidney, and lungs four hours after hemorrhagic shock and resuscitation (Figure 7 and Supplemental Figure IV).
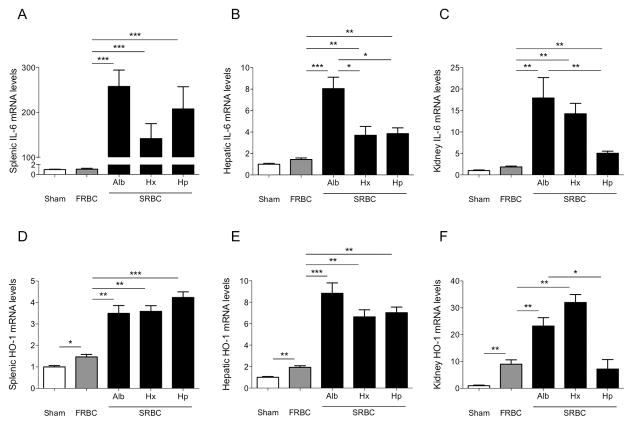
Figure 7.
IL-6 and hemoxygenase-1 (HO-1) mRNA levels in the spleen, liver, and kidney of mice after hemorrhagic shock and resuscitation. (A–C) Expression of the pro-inflammatory cytokine IL-6 in the spleen (left), liver (middle), and kidneys (right) at four hours after hemorrhagic shock and resuscitation with stored PRBCs (SRBC), fresh PRBCs (FRBC) or in sham-operated mice (Sham). Mice resuscitated with SRBC+hemopexin (Hx) showed attenuated hepatic IL-6 mRNA expression compared to mice resuscitated with SRBC+albumin (Alb). Mice resuscitated with SRBC+haptoglobin (Hp) revealed decreased hepatic and renal IL-6 mRNA expression as compared to mice resuscitated with SRBC+Alb. (D–F) HO-1 mRNA expression in the spleen, liver, and kidney at four hours after hemorrhagic shock and resuscitation. Data represent mean±SEM and were compared by Kruskall-Wallis test followed by Bonferroni-adjusted pairwise comparisons, n=7–10, *P<0.05, **P<0.01, ***P<0.001.
Survival rates 48 hours after hemorrhagic shock and resuscitation with SRBCs alone did not differ compared to mice resuscitated with SRBCs and albumin (SRBC vs. SRBC+Alb, P=0.39, Supplemental Figure IV A). Furthermore, both groups did not differ in organ damage and inflammation measured at 48 hours after hemorrhagic shock and resuscitation, and in plasma levels of extracellular hemoglobin, cell-free heme, hemopexin, and haptoglobin at four hours after hemorrhagic shock and resuscitation (Figures 4–6, Supplemental Figure IV). Therefore, we considered that either group could serve as a control to study the adverse inflammatory effects associated with resuscitation of SRBCs at four hours after hemorrhagic shock.
Resuscitation with SRBCs and albumin, SRBCs and hemopexin, and SRBCs and haptoglobin led to a marked increase in pro-inflammatory IL-6 in spleen, liver, and kidney when compared to resuscitation with FRBCs (Figures 7A–C). Mice that were resuscitated with SRBCs and hemopexin showed a significant attenuation of hepatic IL-6 expression (Figure 7B), while mice resuscitated with SRBCs and haptoglobin revealed decreased IL-6 expression in the liver and kidney as compared with mice resuscitated with SRBCs and albumin (Figures 7B and C).
Splenic, hepatic, renal, and pulmonary expression of the heme-processing enzyme hemoxygenase-1 (HO-1) increased at four hours after hemorrhagic shock and resuscitation with FRBCs as compared with sham-operated mice (Figures 7D–F, Supplemental Figure V B). Mice that were resuscitated with SRBCs and albumin or SRBCs and hemopexin showed a marked increase in HO-1 expression when compared to mice resuscitated with FRBCs. Resuscitation with SRBCs and haptoglobin prevented the increase in SRBC-induced renal HO-1 expression (Figure 7F).
To study whether prolonged, increased levels of circulating hemoglobin-haptoglobin complexes induced a tissue inflammatory response, we measured splenic, hepatic, renal, and pulmonary IL-6 gene expression at 48 hours after resuscitation. Splenic, hepatic, and pulmonary IL-6 mRNA levels did not differ in sham-operated, FRBC- or SRBC-transfused mice (Supplemental Figure VI A–C). The renal expression level of IL-6 was greater 48 hours after resuscitation with SRBCs alone, SRBC and albumin, and SRBCs and hemopexin when compared to resuscitation with FRBCs (Supplemental Figure VI D). Without adjusting for multiple comparison testing, IL-6 expression was lower in kidneys of mice resuscitated with SRBCs and haptoglobin compared to mice resuscitated with SRBCs and albumin (P<0.05). IL-6 mRNA expression at 48 hours in spleens, livers, and lungs was not affected by co-infusion of any of the proteins together with SRBCs.
Taken together, these results demonstrate that both hemopexin and haptoglobin can ameliorate the pro-inflammatory response after hemorrhagic shock and resuscitation with SRBCs. Furthermore, increased levels of circulating hemoglobin-haptoglobin complexes were not associated with greater inflammatory responses in the spleen, liver, kidneys, or lungs of mice resuscitated with SRBCs and haptoglobin.
Discussion
In this study we developed a murine model of hemorrhagic shock lasting two hours to investigate the adverse effects following resuscitation with PRBCs that were stored for a prolonged period of time. We tested two therapeutic interventions that involved the use of human plasma proteins that bind with high affinity to plasma heme and hemoglobin, hemopexin and haptoglobin respectively. Compared to murine hemorrhagic shock resuscitated with FRBCs, resuscitation with SRBCs produced higher mortality rates, greater plasma hemoglobin levels, hemoglobinuria, kidney injury, and diffuse tissue inflammation. We demonstrated that when resuscitating hemorrhagic shock with SRBCs, co-infusion of either exogenous human hemopexin or haptoglobin increased the survival rate and decreased tissue inflammation. However, only co-infusion of haptoglobin with SRBCs prevented hemoglobinuria and kidney injury.
Increased levels of extracellular hemoglobin and heme are important contributors to disease states associated with hemolysis. Plasma hemoglobin and heme are also acutely elevated during and after extracorporeal circulation and during and after transfusion of SRBCs25,34,35. In the current study, we developed a murine model of hemorrhagic shock to mimic the common clinical condition of an initial injury due to prolonged hypotension followed by a second noxious event consisting of resuscitation with SRBCs. We modified the previously described murine models of hemorrhagic shock and resuscitation with PRBCs by increasing the duration of hemorrhagic shock and by decreasing the MAP to 35 mmHg during shock17,36. These changes resulted in a higher mortality rate in mice resuscitated with SRBCs as compared to mice resuscitated with FRBCs (Figure 1B). We infused leukocyte-reduced murine PRBCs stored at 4°C for 14 days to mimic the biochemical and functional alterations of human PRBCs stored for 42 days30. After transfusion of SRBCs, plasma hemoglobin levels in the recipient increase markedly15,16. The extent of this increase in plasma hemoglobin was not solely due to transfusion of the free hemoglobin in the PRBC unit, suggesting that hemolysis of circulating stored blood occurs in vivo. Our findings are concordant with previous reports indicating that, compared to FRBCs, SRBCs undergo increased in vivo hemolysis within the first 24 hours after transfusion in animal models and humans16,18–21,28,29 (Figure 1C and Supplemental Table I). We were surprised to observe that mice receiving FRBCs for resuscitation of hemorrhagic shock had a small but significantly higher MAP immediately after transfusion as compared to mice that received SRBCs37,38. Acidosis is associated with vessel dilatation and decrease in systemic peripheral resistance39. When SRBCs are transfused for resuscitation of hemorrhagic shock, the vasodilatory effect of acidosis during and immediately after transfusion might overwhelm any vasoconstrictor effect caused by NO-scavenging by cell-free hemoglobin38,40.
In the current study, resuscitation with SRBCs but not FRBCs after hemorrhagic shock was associated with decreased endogenous murine plasma hemopexin and haptoglobin (Figure 2). Co-infusion of either hemopexin or haptoglobin with SRBCs led to markedly improved survival rates, while treatment with albumin did not alter the mortality rate produced by transfusing SRBCs (Figure 3B). Consistent with these findings, Larsen et al. used a mouse model of infection and sepsis to show that extracellular hemoglobin and heme increased tissue damage and inflammation; treatment with exogenous hemopexin improved survival and decreased tissue injury41. Extracellular heme and hemoglobin may contribute to the early death of mice after hemorrhagic shock and resuscitation with SRBCs, and co-infusion of either hemopexin or haptoglobin appear to be sufficient to protect against early death. Although albumin, like hemopexin, binds to heme, albumin binds cell-free heme with 106 times lower affinity than hemopexin42. Therefore, one would need to give significantly more albumin than hemopexin to achieve the same degree of heme binding. We did not investigate the precise mechanism by which either haptoglobin or hemopexin reduces acute mortality in our model of resuscitation with SRBCs. In addition, further investigation will be required to determine the potential benefits of both proteins in ischemia-reperfusion injury, independent of their effects on transfusion with SRBCs.
When haptoglobin binds to hemoglobin dimers, a large haptoglobin-hemoglobin complex is formed, which cannot transverse the glomerular basement membrane of the kidney19,43,44. Boretti and colleagues reported that stimulating the synthesis of endogenous haptoglobin in dogs by pre-treatment with corticosteroids prevented renal excretion of infused cell-free hemoglobin by retention of the hemoglobin-haptoglobin complex in plasma43. Furthermore, Baek et al. showed, that administration of exogenous human haptoglobin prevented renal damage after an exchange transfusion with 28-day-old blood in guinea pigs19. Additional studies suggest that cell-free hemoglobin causes significant renal damage when it acts as a secondary injury following an initial event that compromises renal function28,35,45,46. In the current study, we combined a murine model of hemorrhagic shock-induced renal damage with a transfusion model of stored blood to mimic the multifactorial genesis of kidney injury occurring in clinical settings. After hemorrhagic shock and resuscitation with SRBCs, the kidney has not only been challenged by prolonged hypotension but also by circulating and filtered free hemoglobin and heme30,36. Taken together, in the current study we demonstrate that preventing exposure of the tubules to extracellular hemoglobin prevented renal damage induced by resuscitation with SRBCs after prolonged hemorrhagic shock (Figure 4 and 5). Treatment with hemopexin, which decreased plasma heme levels but did not prevent hemoglobinuria, was not associated with renal protection after hemorrhagic shock and resuscitation with SRBCs.
In the current study, we demonstrated that treatment with hemopexin or haptoglobin during resuscitation with SRBCs after hemorrhagic shock attenuated the pro-inflammatory effect of stored blood on the liver and kidneys after resuscitation (Figure 7). Hemoglobin and heme synergize with multiple Toll-like receptor agonists to induce pro-inflammatory cytokines33,47. Hemopexin down-regulates endotoxin-induced production of pro-inflammatory cytokines in murine macrophages and prevents excessive and toxic heme accumulation in macrophages while haptoglobin prevents heme release from extracellular hemoglobin48,49. Hod et al. reported that rather than plasma heme and hemoglobin, the monocyte/macrophage-mediated erythrophagocytosis of senescent erythrocytes in the spleen and the liver is responsible for the inflammatory cytokine responses following SRBC-transfusion32. The findings in this study suggest that infusions of hemopexin and haptoglobin during SRBC-resuscitation might protect from intravascular as well as extravascular heme- and hemoglobin-mediated toxicity.
A potential limitation of this study is that the murine model does not perfectly mimic the situation of a patient in the intensive care setting. For example, we did not use intensive care therapeutic approaches such as mechanical ventilation or vasopressor therapy, forms of organ support that might be partly responsible for the lower mortality after hemorrhagic shock and SRBC-resuscitation seen in larger animal models such as the canine model used by Solomon and colleagues38. In addition, in this study, mice were resuscitated with PRBCs stored for 14 days (mimicking human blood stored for 35–42 days). In many medical facilities, patients that require large volume transfusion might receive a mixture of PBRCs that have been stored for different periods14. It is therefore possible that this model overestimates the adverse effects of transfusion of SRBCs.
In this study we infused purified human hemopexin and haptoglobin into mice. Although the ability of haptoglobin to prevent free hemoglobin filtration in the kidney is similar in mice and humans, it is possible that there could be species differences in the binding or clearance of hemoglobin or heme bound to the human proteins in the mouse50. In addition to renal excretion, both humans and mice have a second route for clearance of plasma hemoglobin via CD163-mediated uptake by hepatic and splenic macrophages25,27,51. Haptoglobin binding to cell-free hemoglobin in humans, in contrast to mice, leads to rapid CD163 receptor-mediated hepatic and splenic uptake51. Therefore, treatment with exogenous haptoglobin in humans might lead to rapid plasma clearance of free hemoglobin by hepatic uptake; prolonged circulation of the haptoglobin-hemoglobin complexes, as we observed in mice, may not occur. The possibility that increased hepatic and splenic iron uptake caused by haptoglobin-mediated clearance of plasma hemoglobin and hemopexin-driven clearance of cell-free heme might have a detrimental effect on patients requires further evaluation with studies designed to assess the long-term effects of hemopexin and haptoglobin.
Although many animal and clinical studies support the theory that prolonged storage of blood leads to an increased frequency of adverse outcomes, two recent prospective clinical trials did not report a mortality or morbidity difference between critically ill patients receiving transfusions of either PRBCs stored for 7 – 10 days or PRBCs stored for 21 days9–14,16–21,52,53. The last two studies did not examine patient subpopulations with comorbidities such as chronic renal disease or inflammation nor were they powered to examine patients who received transfusions with PRBCs that had been stored for longer periods, for example 30–42 days, when most red cell damage and hemolysis occurs7,9. It is likely that the risk of adverse outcomes after transfusion of stored blood will depend on the underlying disease and patient subpopulation in which transfusion occurs20,38. In previous preclinical studies, animals with bacterial infection that were transfused with SRBCs (42-days storage in dogs and 14-days storage in mice) had a significantly increased mortality compared to those transfused with FRBCs20,21,52,53. In conditions where tissues are compromised by infection, inflammation or ischemia, a second injury by transfusion of blood stored for a long duration might lead to increased organ damage and lethality. We were unable to determine the early cause of death in mice receiving SRBCs in this model. Future studies using larger animals would allow repetitive blood sampling and extended hemodynamic monitoring, and might help explain both the mechanism of early death after prolonged hemorrhagic shock and resuscitation with SRBCs and the protective effects of hemopexin and haptoglobin.
In conclusion, in the current study we developed a two-hit model of kidney injury in which mice were subjected to prolonged hemorrhagic shock and then resuscitated with PBRCs. In comparison to resuscitation with FRBCs, resuscitation with SRBCs was associated with an increased mortality rate as well as elevated plasma free hemoglobin levels, hemoglobinurina and increased kidney injury in surviving animals. Furthermore, co-infusion during SRBC-resuscitation of either hemopexin or haptoglobin increased the survival rate and reduced the early pro-inflammatory response after hemorrhagic shock and resuscitation with stored blood. Treatment with haptoglobin during transfusion of SRBCs retained free hemoglobin in the plasma and prevented SRBCs-induced hemoglobinuria and kidney injury. The salutary effects of transfusing these naturally occurring human plasma proteins should be further assessed in conditions of severe hemolysis following prolonged hypotension, as may occur after massive transfusion with SRBCs to treat hemorrhagic shock.
Supplementary Material
Clinical Perspective.
What Is New?
In a murine model of hemorrhagic shock, resuscitation with long stored packed red blood cells (SRBCs) produced higher mortality rates, greater plasma hemoglobin levels, hemoglobinuria, kidney injury, and diffuse tissue inflammation compared to resuscitation with fresh packed red blood cells (FRBCs).
When resuscitating hemorrhagic shock with SRBCs, co-infusion of either exogenous human hemopexin or haptoglobin increased the survival rate and decreased tissue inflammation.
Co-infusion of haptoglobin with SRBCs prevented hemoglobinuria and kidney injury.
What Are the Clinical Implications?
In conditions where tissues have been compromised by shock, infection, inflammation or ischemia, exposure to extracellular hemoglobin and cell-free heme augments organ damage and dysfunction.
Erythrocytes undergo progressive deleterious morphological and biochemical changes during storage.
In hemorrhagic shock, perfusion-sensitive organs such as the kidneys are challenged both by hypoperfusion and by high concentrations of plasma hemoglobin and cell-free heme resulting from transfusion of SRBCs.
The salutary effects of transfusing hemopexin and haptoglobin should be further assessed in conditions of severe hemolysis following prolonged hypotension, as may occur after massive transfusion with SRBCs to treat hemorrhagic shock.
Acknowledgments
Purified human hemopexin and haptoglobin were a generous gift of CSL Behring, USA. The authors thank Tim Houle, Ph.D., Department of Anesthesia, Critical Care and Pain Medicine, Massachusetts General Hospital, Boston, Massachusetts and Frederik Graw, Ph.D., Center for Quantitative Biology, University of Heidelberg, Germany for statistical assistance and help with data analysis.
Funding Sources: This research was supported by the Deutsche Forschungsgemeinschaft (DFG GR 4446/1-1) (JAG); the NIH (AI059010), the Shriners Hospital for Children (87200), and the Defense Advanced Research Projects Agency (W911NF-13-1-0070) (HSW); NIH Grant R01DK082971 and the LeDucq Foundation (DBB); and funds from the Department of Anesthesia, Critical Care and Pain Medicine at Massachusetts General Hospital (WMZ).
Footnotes
Conflict of Interest Disclosures: In accordance with institutional policy, HSW has declared hemopexin to MGH as a potential candidate molecule to help decrease inflammation, and the institution has filed for patent protection.
References
- 1.Ostermann M, Chang RW. Acute kidney injury in the intensive care unit according to RIFLE. Crit Care Med. 2007;35:1837–1843. doi: 10.1097/01.CCM.0000277041.13090.0A. [DOI] [PubMed] [Google Scholar]
- 2.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16:3365–3370. doi: 10.1681/ASN.2004090740. [DOI] [PubMed] [Google Scholar]
- 3.Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E, Gibney N, Tolwani A, Ronco C. Beginning and Ending Supportive Therapy for the Kidney I. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294:813–818. doi: 10.1001/jama.294.7.813. [DOI] [PubMed] [Google Scholar]
- 4.Bagshaw SM, George C, Bellomo R, Committee ADM. Early acute kidney injury and sepsis: a multicentre evaluation. Crit Care. 2008;12:R47. doi: 10.1186/cc6863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Basile DP, Anderson MD, Sutton TA. Pathophysiology of acute kidney injury. Comprehensive Physiology. 2012;2:1303–1353. doi: 10.1002/cphy.c110041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsai AG, Hofmann A, Cabrales P, Intaglietta M. Perfusion vs. oxygen delivery in transfusion with “fresh” and “old” red blood cells: the experimental evidence. Transfus Apher Sci. 2010;43:69–78. doi: 10.1016/j.transci.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bennett-Guerrero E, Veldman TH, Doctor A, Telen MJ, Ortel TL, Reid TS, Mulherin MA, Zhu H, Buck RD, Califf RM, McMahon TJ. Evolution of adverse changes in stored RBCs. Proc Natl Acad Sci U S A. 2007;104:17063–17068. doi: 10.1073/pnas.0708160104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hovav T, Yedgar S, Manny N, Barshtein G. Alteration of red cell aggregability and shape during blood storage. Transfusion. 1999;39:277–281. doi: 10.1046/j.1537-2995.1999.39399219284.x. [DOI] [PubMed] [Google Scholar]
- 9.Donadee C, Raat NJ, Kanias T, Tejero J, Lee JS, Kelley EE, Zhao X, Liu C, Reynolds H, Azarov I, Frizzell S, Meyer EM, Donnenberg AD, Qu L, Triulzi D, Kim-Shapiro DB, Gladwin MT. Nitric oxide scavenging by red blood cell microparticles and cell-free hemoglobin as a mechanism for the red cell storage lesion. Circulation. 2011;124:465–476. doi: 10.1161/CIRCULATIONAHA.110.008698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leal-Noval SR, Jara-Lopez I, Garcia-Garmendia JL, Marin-Niebla A, Herruzo-Aviles A, Camacho-Larana P, Loscertales J. Influence of erythrocyte concentrate storage time on postsurgical morbidity in cardiac surgery patients. Anesthesiology. 2003;98:815–822. doi: 10.1097/00000542-200304000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Zallen G, Offner PJ, Moore EE, Blackwell J, Ciesla DJ, Gabriel J, Denny C, Silliman CC. Age of transfused blood is an independent risk factor for postinjury multiple organ failure. Am J Surg. 1999;178:570–572. doi: 10.1016/s0002-9610(99)00239-1. [DOI] [PubMed] [Google Scholar]
- 12.Koch CG, Li L, Sessler DI, Figueroa P, Hoeltge GA, Mihaljevic T, Blackstone EH. Duration of red-cell storage and complications after cardiac surgery. N Engl J Med. 2008;358:1229–1239. doi: 10.1056/NEJMoa070403. [DOI] [PubMed] [Google Scholar]
- 13.Lacroix J, Hebert PC, Fergusson DA, Tinmouth A, Cook DJ, Marshall JC, Clayton L, McIntyre L, Callum J, Turgeon AF, Blajchman MA, Walsh TS, Stanworth SJ, Campbell H, Capellier G, Tiberghien P, Bardiaux L, van de Watering L, van der Meer NJ, Sabri E, Vo D Investigators A and the Canadian Critical Care Trials G. Age of transfused blood in critically ill adults. N Engl J Med. 2015;372:1410–1418. doi: 10.1056/NEJMoa1500704. [DOI] [PubMed] [Google Scholar]
- 14.Steiner ME, Ness PM, Assmann SF, Triulzi DJ, Sloan SR, Delaney M, Granger S, Bennett-Guerrero E, Blajchman MA, Scavo V, Carson JL, Levy JH, Whitman G, D’Andrea P, Pulkrabek S, Ortel TL, Bornikova L, Raife T, Puca KE, Kaufman RM, Nuttall GA, Young PP, Youssef S, Engelman R, Greilich PE, Miles R, Josephson CD, Bracey A, Cooke R, McCullough J, Hunsaker R, Uhl L, McFarland JG, Park Y, Cushing MM, Klodell CT, Karanam R, Roberts PR, Dyke C, Hod EA, Stowell CP. Effects of red-cell storage duration on patients undergoing cardiac surgery. N Engl J Med. 2015;372:1419–1429. doi: 10.1056/NEJMoa1414219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berra L, Coppadoro A, Yu B, Lei C, Spagnolli E, Steinbicker AU, Bloch KD, Lin T, Sammy FY, Warren HS, Fernandez BO, Feelisch M, Dzik WH, Stowell CP, Zapol WM. Transfusion of stored autologous blood does not alter reactive hyperemia index in healthy volunteers. Anesthesiology. 2012;117:56–63. doi: 10.1097/ALN.0b013e31825575e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berra L, Pinciroli R, Stowell CP, Wang L, Yu B, Fernandez BO, Feelisch M, Mietto C, Hod EA, Chipman D, Scherrer-Crosbie M, Bloch KD, Zapol WM. Autologous transfusion of stored red blood cells increases pulmonary artery pressure. Am J Respir Crit Care Med. 2014;190:800–807. doi: 10.1164/rccm.201405-0850OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lei C, Yu B, Shahid M, Beloiartsev A, Bloch KD, Zapol WM. Inhaled nitric oxide attenuates the adverse effects of transfusing stored syngeneic erythrocytes in mice with endothelial dysfunction after hemorrhagic shock. Anesthesiology. 2012;117:1190–1202. doi: 10.1097/ALN.0b013e318272d866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baron DM, Yu B, Lei C, Bagchi A, Beloiartsev A, Stowell CP, Steinbicker AU, Malhotra R, Bloch KD, Zapol WM. Pulmonary hypertension in lambs transfused with stored blood is prevented by breathing nitric oxide. Anesthesiology. 2012;116:637–647. doi: 10.1097/ALN.0b013e318246ef77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baek JH, D’Agnillo F, Vallelian F, Pereira CP, Williams MC, Jia Y, Schaer DJ, Buehler PW. Hemoglobin-driven pathophysiology is an in vivo consequence of the red blood cell storage lesion that can be attenuated in guinea pigs by haptoglobin therapy. J Clin Invest. 2012;122:1444–1458. doi: 10.1172/JCI59770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Solomon SB, Wang D, Sun J, Kanias T, Feng J, Helms CC, Solomon MA, Alimchandani M, Quezado M, Gladwin MT, Kim-Shapiro DB, Klein HG, Natanson C. Mortality increases after massive exchange transfusion with older stored blood in canines with experimental pneumonia. Blood. 2013;121:1663–1672. doi: 10.1182/blood-2012-10-462945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cortes-Puch I, Wang D, Sun J, Solomon SB, Remy KE, Fernandez M, Feng J, Kanias T, Bellavia L, Sinchar D, Perlegas A, Solomon MA, Kelley WE, Popovsky MA, Gladwin MT, Kim-Shapiro DB, Klein HG, Natanson C. Washing older blood units before transfusion reduces plasma iron and improves outcomes in experimental canine pneumonia. Blood. 2014;123:1403–1411. doi: 10.1182/blood-2013-11-539353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Donadee CL, Gladwin MT. Hemodialysis hyperhemolysis. A novel mechanism of endothelial dysfunction and cardiovascular risk? J Am Coll Cardiol. 2010;55:460–462. doi: 10.1016/j.jacc.2009.08.061. [DOI] [PubMed] [Google Scholar]
- 23.Villagra J, Shiva S, Hunter LA, Machado RF, Gladwin MT, Kato GJ. Platelet activation in patients with sickle disease, hemolysis-associated pulmonary hypertension, and nitric oxide scavenging by cell-free hemoglobin. Blood. 2007;110:2166–2172. doi: 10.1182/blood-2006-12-061697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Balla J, Vercellotti GM, Jeney V, Yachie A, Varga Z, Jacob HS, Eaton JW, Balla G. Heme, heme oxygenase, and ferritin: how the vascular endothelium survives (and dies) in an iron-rich environment. Antioxid Redox Signal. 2007;9:2119–2137. doi: 10.1089/ars.2007.1787. [DOI] [PubMed] [Google Scholar]
- 25.Schaer DJ, Buehler PW, Alayash AI, Belcher JD, Vercellotti GM. Hemolysis and free hemoglobin revisited: exploring hemoglobin and hemin scavengers as a novel class of therapeutic proteins. Blood. 2013;121:1276–1284. doi: 10.1182/blood-2012-11-451229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tolosano E, Fagoonee S, Morello N, Vinchi F, Fiorito V. Heme scavenging and the other facets of hemopexin. Antioxid Redox Signal. 2010;12:305–320. doi: 10.1089/ars.2009.2787. [DOI] [PubMed] [Google Scholar]
- 27.Kristiansen M, Graversen JH, Jacobsen C, Sonne O, Hoffman HJ, Law SK, Moestrup SK. Identification of the haemoglobin scavenger receptor. Nature. 2001;409:198–201. doi: 10.1038/35051594. [DOI] [PubMed] [Google Scholar]
- 28.Vermeulen Windsant IC, Snoeijs MG, Hanssen SJ, Altintas S, Heijmans JH, Koeppel TA, Schurink GW, Buurman WA, Jacobs MJ. Hemolysis is associated with acute kidney injury during major aortic surgery. Kidney Int. 2010;77:913–920. doi: 10.1038/ki.2010.24. [DOI] [PubMed] [Google Scholar]
- 29.Yu B, Lei C, Baron DM, Steinbicker AU, Bloch KD, Zapol WM. Diabetes augments and inhaled nitric oxide prevents the adverse hemodynamic effects of transfusing syngeneic stored blood in mice. Transfusion. 2012;52:1410–1422. doi: 10.1111/j.1537-2995.2011.03473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gilson CR, Kraus TS, Hod EA, Hendrickson JE, Spitalnik SL, Hillyer CD, Shaz BH, Zimring JC. A novel mouse model of red blood cell storage and posttransfusion in vivo survival. Transfusion. 2009;49:1546–1553. doi: 10.1111/j.1537-2995.2009.02173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Melnikov VY, Faubel S, Siegmund B, Lucia MS, Ljubanovic D, Edelstein CL. Neutrophil-independent mechanisms of caspase-1- and IL-18-mediated ischemic acute tubular necrosis in mice. J Clin Invest. 2002;110:1083–1091. doi: 10.1172/JCI15623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hod EA, Zhang N, Sokol SA, Wojczyk BS, Francis RO, Ansaldi D, Francis KP, Della-Latta P, Whittier S, Sheth S, Hendrickson JE, Zimring JC, Brittenham GM, Spitalnik SL. Transfusion of red blood cells after prolonged storage produces harmful effects that are mediated by iron and inflammation. Blood. 2010;115:4284–4292. doi: 10.1182/blood-2009-10-245001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin T, Kwak YH, Sammy F, He P, Thundivalappil S, Sun G, Chao W, Warren HS. Synergistic inflammation is induced by blood degradation products with microbial Toll-like receptor agonists and is blocked by hemopexin. J Infect Dis. 2010;202:624–632. doi: 10.1086/654929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hod EA, Brittenham GM, Billote GB, Francis RO, Ginzburg YZ, Hendrickson JE, Jhang J, Schwartz J, Sharma S, Sheth S, Sireci AN, Stephens HL, Stotler BA, Wojczyk BS, Zimring JC, Spitalnik SL. Transfusion of human volunteers with older, stored red blood cells produces extravascular hemolysis and circulating non-transferrin-bound iron. Blood. 2011;118:6675–6682. doi: 10.1182/blood-2011-08-371849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vermeulen Windsant IC, de Wit NC, Sertorio JT, van Bijnen AA, Ganushchak YM, Heijmans JH, Tanus-Santos JE, Jacobs MJ, Maessen JG, Buurman WA. Hemolysis during cardiac surgery is associated with increased intravascular nitric oxide consumption and perioperative kidney and intestinal tissue damage. Front Physiol. 2014;5:340. doi: 10.3389/fphys.2014.00340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mayeur N, Minville V, Jaafar A, Allard J, Al Saati T, Guilbeau-Frugier C, Fourcade O, Girolami JP, Schaak S, Tack I. Morphologic and functional renal impact of acute kidney injury after prolonged hemorrhagic shock in mice. Crit Care Med. 2011;39:2131–2138. doi: 10.1097/CCM.0b013e31821f04f0. [DOI] [PubMed] [Google Scholar]
- 37.Yu B, Raher MJ, Volpato GP, Bloch KD, Ichinose F, Zapol WM. Inhaled nitric oxide enables artificial blood transfusion without hypertension. Circulation. 2008;117:1982–1990. doi: 10.1161/CIRCULATIONAHA.107.729137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Solomon SB, Cortes-Puch I, Sun J, Remy KE, Wang D, Feng J, Khan SS, Sinchar D, Kim-Shapiro DB, Klein HG, Natanson C. Transfused older stored red blood cells improve the clinical course and outcome in a canine lethal hemorrhage and reperfusion model. Transfusion. 2015;55:2552–2563. doi: 10.1111/trf.13213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Deal CP, Green HD. Effects of pH on blood flow and peripheral resistance in muscular and cutaneous vascular beds in the hind limb of the pentobarbitalized dog. Circ Res. 1954;2:148–154. doi: 10.1161/01.res.2.2.148. [DOI] [PubMed] [Google Scholar]
- 40.Baron DM, Beloiartsev A, Nakagawa A, Martyn T, Stowell CP, Malhotra R, Mayeur C, Bloch KD, Zapol WM. Adverse effects of hemorrhagic shock resuscitation with stored blood are ameliorated by inhaled nitric oxide in lambs. Crit Care Med. 2013;41:2492–2501. doi: 10.1097/CCM.0b013e31828cf456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Larsen R, Gozzelino R, Jeney V, Tokaji L, Bozza FA, Japiassu AM, Bonaparte D, Cavalcante MM, Chora A, Ferreira A, Marguti I, Cardoso S, Sepulveda N, Smith A, Soares MP. A central role for free heme in the pathogenesis of severe sepsis. Sci Transl Med. 2010;2:51ra71. doi: 10.1126/scitranslmed.3001118. [DOI] [PubMed] [Google Scholar]
- 42.Satoh T, Satoh H, Iwahara S, Hrkal Z, Peyton DH, Muller-Eberhard U. Roles of heme iron-coordinating histidine residues of human hemopexin expressed in baculovirus-infected insect cells. Proc Natl Acad Sci U S A. 1994;91:8423–8427. doi: 10.1073/pnas.91.18.8423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boretti FS, Buehler PW, D’Agnillo F, Kluge K, Glaus T, Butt OI, Jia Y, Goede J, Pereira CP, Maggiorini M, Schoedon G, Alayash AI, Schaer DJ. Sequestration of extracellular hemoglobin within a haptoglobin complex decreases its hypertensive and oxidative effects in dogs and guinea pigs. J Clin Invest. 2009;119:2271–2280. doi: 10.1172/JCI39115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Andersen CB, Torvund-Jensen M, Nielsen MJ, de Oliveira CL, Hersleth HP, Andersen NH, Pedersen JS, Andersen GR, Moestrup SK. Structure of the haptoglobin-haemoglobin complex. Nature. 2012;489:456–549. doi: 10.1038/nature11369. [DOI] [PubMed] [Google Scholar]
- 45.Lalich JJ. The Influence of Injections of Homologous Hemoglobin on the Kidneys of Normal and Dehydrated Animals. J Exp Med. 1947;86:153–158. doi: 10.1084/jem.86.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zager RA, Gamelin LM. Pathogenetic mechanisms in experimental hemoglobinuric acute renal failure. Am J Physiol. 1989;256:446–455. doi: 10.1152/ajprenal.1989.256.3.F446. [DOI] [PubMed] [Google Scholar]
- 47.Liang X, Lin T, Sun G, Beasley-Topliffe L, Cavaillon JM, Warren HS. Hemopexin down-regulates LPS-induced proinflammatory cytokines from macrophages. J Leukoc Biol. 2009;86:229–235. doi: 10.1189/jlb.1208742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smith A, Morgan WT. Haem transport to the liver by haemopexin. Receptor-mediated uptake with recycling of the protein. Biochem J. 1979;182:47–54. doi: 10.1042/bj1820047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mollan TL, Jia Y, Banerjee S, Wu G, Kreulen RT, Tsai AL, Olson JS, Crumbliss AL, Alayash AI. Redox properties of human hemoglobin in complex with fractionated dimeric and polymeric human haptoglobin. Free Radic Biol Med. 2014;69:265–277. doi: 10.1016/j.freeradbiomed.2014.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tolosano E, Fagoonee S, Hirsch E, Berger FG, Baumann H, Silengo L, Altruda F. Enhanced splenomegaly and severe liver inflammation in haptoglobin/hemopexin double-null mice after acute hemolysis. Blood. 2002;100:4201–4208. doi: 10.1182/blood-2002-04-1270. [DOI] [PubMed] [Google Scholar]
- 51.Etzerodt A, Kjolby M, Nielsen MJ, Maniecki M, Svendsen P, Moestrup SK. Plasma clearance of hemoglobin and haptoglobin in mice and effect of CD163 gene targeting disruption. Antioxid Redox Signal. 2013;18:2254–2263. doi: 10.1089/ars.2012.4605. [DOI] [PubMed] [Google Scholar]
- 52.Prestia K, Bandyopadhyay S, Slate A, Francis RO, Francis KP, Spitalnik SL, Fidock DA, Brittenham GM, Hod EA. Transfusion of stored blood impairs host defenses against Gram-negative pathogens in mice. Transfusion. 2014;54:2842–2851. doi: 10.1111/trf.12712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cortes-Puch I, Remy KE, Solomon SB, Sun J, Wang D, Al-Hamad M, Kelly SM, Sinchar D, Bellavia L, Kanias T, Popovsky MA, Kim-Shapiro DB, Klein HG, Natanson C. In a canine pneumonia model of exchange transfusion, altering the age but not the volume of older red blood cells markedly alters outcome. Transfusion. 2015;55:2564–2575. doi: 10.1111/trf.13275. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.