Abstract
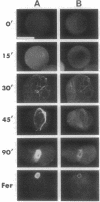
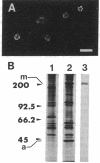
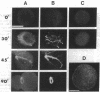
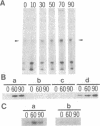
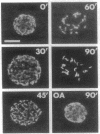
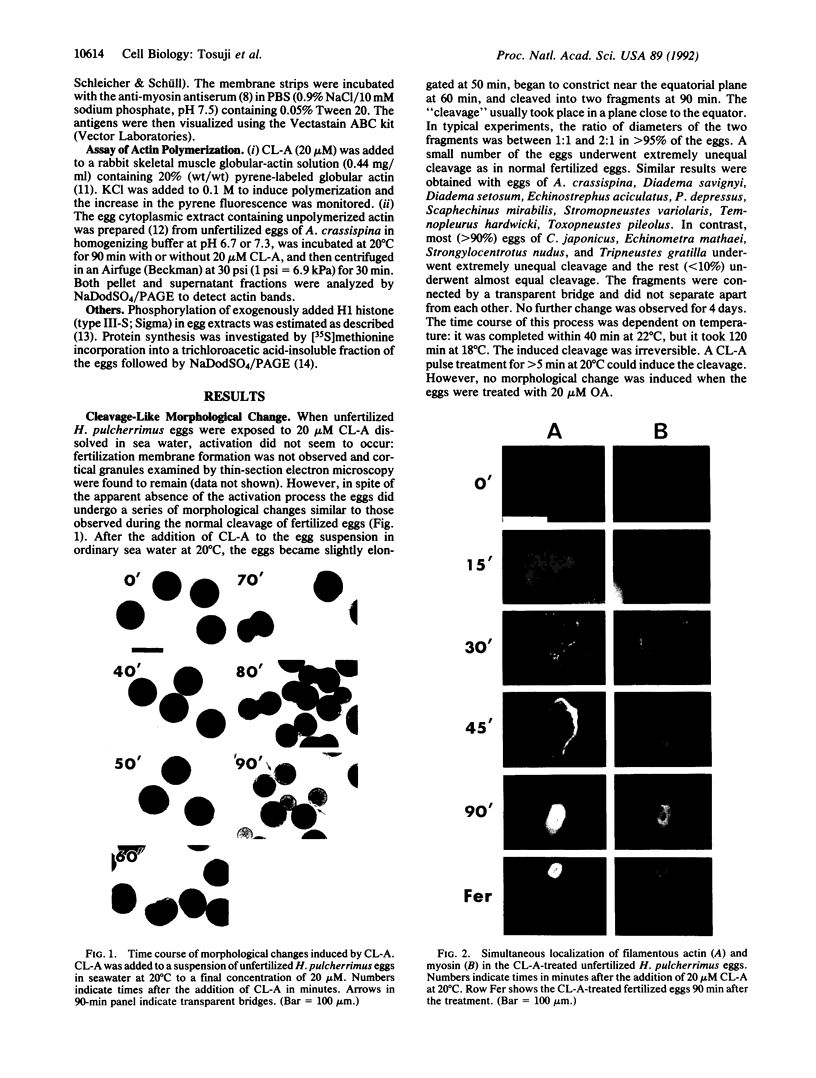
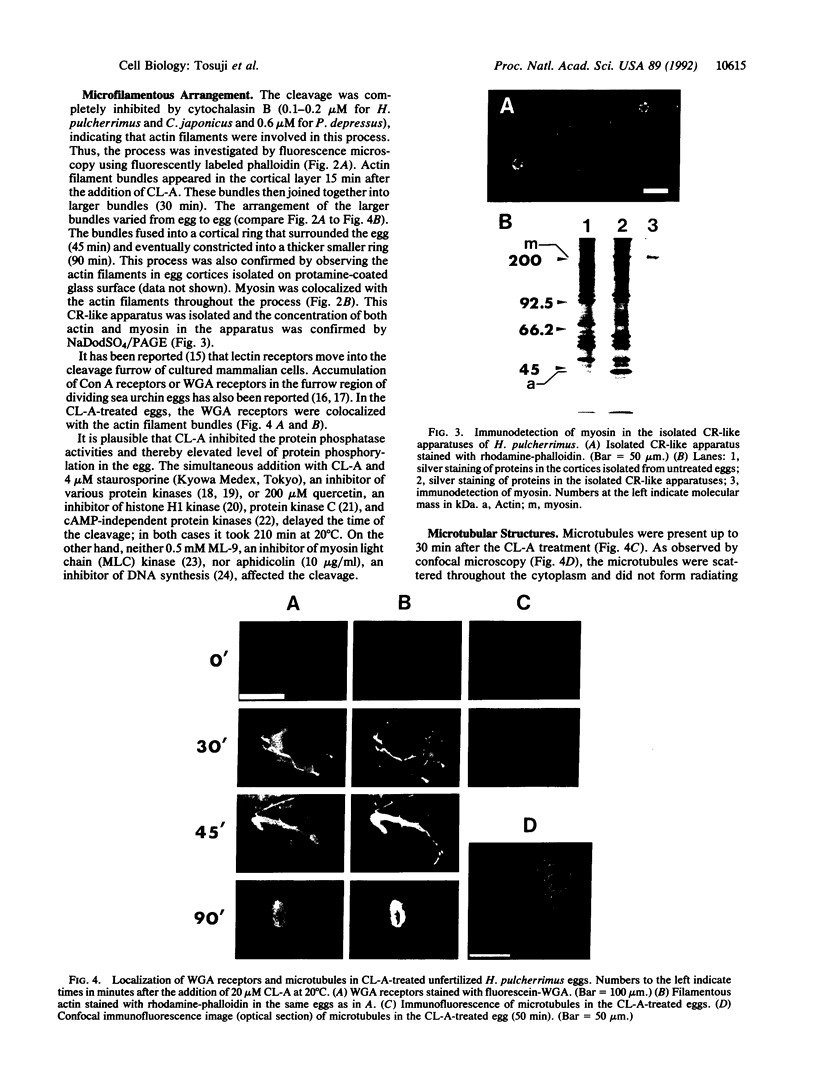
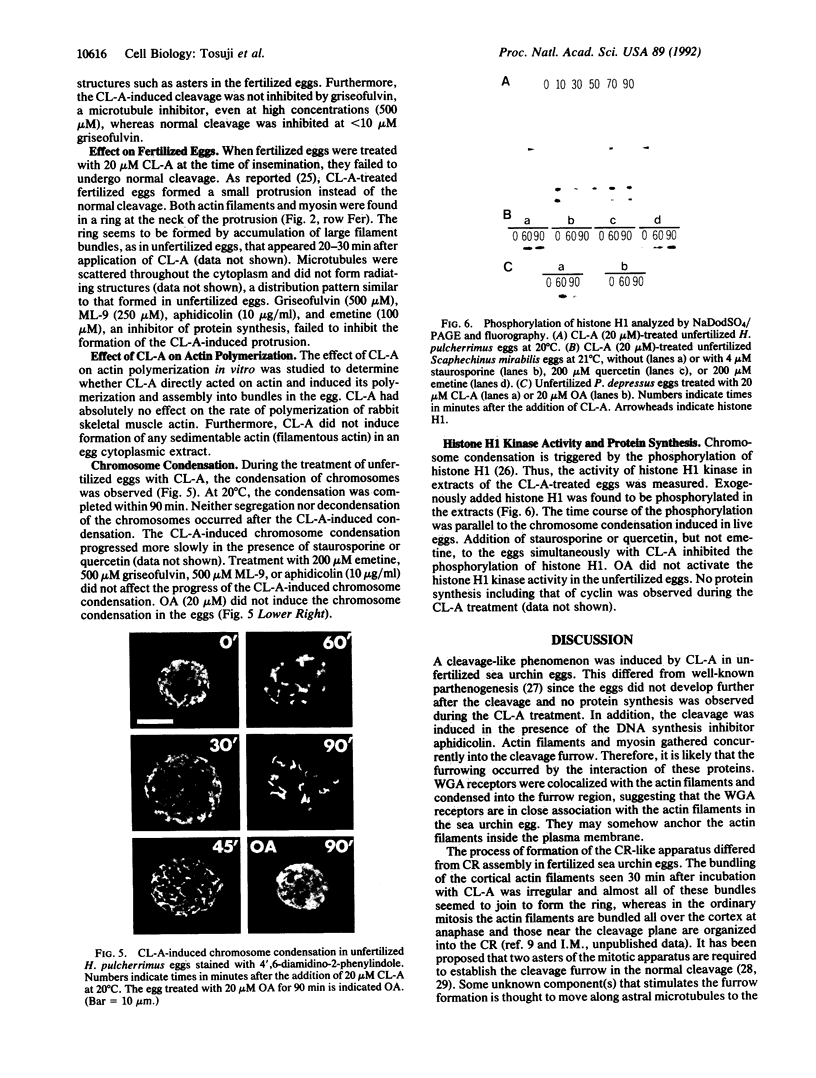
Calyculin A, a protein phosphatase inhibitor, induced cleavage-like morphological change in unfertilized sea urchin eggs. A contractile ring-like apparatus containing both filamentous actin and myosin was formed in the cleavage furrow. Wheat germ agglutinin receptors were also found in the same region. The eggs did not develop further after constriction of the ring. No aster-like microtubular structure was found in the calyculin A-treated eggs. The cleavage was not inhibited by the antimicrotubule drug griseofulvin. Calyculin A also increased histone H1 kinase activity and induced chromosome condensation. These changes also occurred in the presence of emetine (an inhibitor of protein synthesis) and aphidicolin (an inhibitor of DNA synthesis). It is suggested that calyculin A induced these changes in the sea urchin eggs by inhibiting the activity of protein phosphatase 1.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berlin R. D., Oliver J. M., Walter R. J. Surface functions during Mitosis I: phagocytosis, pinocytosis and mobility of surface-bound Con A. Cell. 1978 Oct;15(2):327–341. doi: 10.1016/0092-8674(78)90002-8. [DOI] [PubMed] [Google Scholar]
- Bradbury E. M., Inglis R. J., Matthews H. R. Control of cell division by very lysine rich histone (F1) phosphorylation. Nature. 1974 Feb 1;247(5439):257–261. doi: 10.1038/247257a0. [DOI] [PubMed] [Google Scholar]
- Chartier L., Rankin L. L., Allen R. E., Kato Y., Fusetani N., Karaki H., Watabe S., Hartshorne D. J. Calyculin-A increases the level of protein phosphorylation and changes the shape of 3T3 fibroblasts. Cell Motil Cytoskeleton. 1991;18(1):26–40. doi: 10.1002/cm.970180104. [DOI] [PubMed] [Google Scholar]
- Evans T., Rosenthal E. T., Youngblom J., Distel D., Hunt T. Cyclin: a protein specified by maternal mRNA in sea urchin eggs that is destroyed at each cleavage division. Cell. 1983 Jun;33(2):389–396. doi: 10.1016/0092-8674(83)90420-8. [DOI] [PubMed] [Google Scholar]
- Fernandez A., Brautigan D. L., Mumby M., Lamb N. J. Protein phosphatase type-1, not type-2A, modulates actin microfilament integrity and myosin light chain phosphorylation in living nonmuscle cells. J Cell Biol. 1990 Jul;111(1):103–112. doi: 10.1083/jcb.111.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Félix M. A., Cohen P., Karsenti E. Cdc2 H1 kinase is negatively regulated by a type 2A phosphatase in the Xenopus early embryonic cell cycle: evidence from the effects of okadaic acid. EMBO J. 1990 Mar;9(3):675–683. doi: 10.1002/j.1460-2075.1990.tb08159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziani Y., Chayoth R., Karny N., Feldman B., Levy J. Regulation of protein kinases activity by quercetin in Ehrlich ascites tumor cells. Biochim Biophys Acta. 1982 Feb 25;714(3):415–421. doi: 10.1016/0304-4165(82)90148-9. [DOI] [PubMed] [Google Scholar]
- Gschwendt M., Horn F., Kittstein W., Marks F. Inhibition of the calcium- and phospholipid-dependent protein kinase activity from mouse brain cytosol by quercetin. Biochem Biophys Res Commun. 1983 Dec 16;117(2):444–447. doi: 10.1016/0006-291x(83)91220-2. [DOI] [PubMed] [Google Scholar]
- Hiramoto Y. Analysis of cleavage stimulus by means of micromanipulation of sea urchin eggs. Exp Cell Res. 1971 Oct;68(2):291–298. doi: 10.1016/0014-4827(71)90153-4. [DOI] [PubMed] [Google Scholar]
- Ikegami S., Taguchi T., Ohashi M., Oguro M., Nagano H., Mano Y. Aphidicolin prevents mitotic cell division by interfering with the activity of DNA polymerase-alpha. Nature. 1978 Oct 5;275(5679):458–460. doi: 10.1038/275458a0. [DOI] [PubMed] [Google Scholar]
- Ishihara H., Martin B. L., Brautigan D. L., Karaki H., Ozaki H., Kato Y., Fusetani N., Watabe S., Hashimoto K., Uemura D. Calyculin A and okadaic acid: inhibitors of protein phosphatase activity. Biochem Biophys Res Commun. 1989 Mar 31;159(3):871–877. doi: 10.1016/0006-291x(89)92189-x. [DOI] [PubMed] [Google Scholar]
- Ishihara H., Ozaki H., Sato K., Hori M., Karaki H., Watabe S., Kato Y., Fusetani N., Hashimoto K., Uemura D. Calcium-independent activation of contractile apparatus in smooth muscle by calyculin-A. J Pharmacol Exp Ther. 1989 Jul;250(1):388–396. [PubMed] [Google Scholar]
- Kato Y., Fusetani N., Matsunaga S., Hashimoto K. Calyculins, potent antitumour metabolites from the marine sponge Discodermia calyx: biological activities. Drugs Exp Clin Res. 1988;14(12):723–728. [PubMed] [Google Scholar]
- Kouyama T., Mihashi K. Fluorimetry study of N-(1-pyrenyl)iodoacetamide-labelled F-actin. Local structural change of actin protomer both on polymerization and on binding of heavy meromyosin. Eur J Biochem. 1981;114(1):33–38. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mabuchi I. Biochemical aspects of cytokinesis. Int Rev Cytol. 1986;101:175–213. doi: 10.1016/s0074-7696(08)60249-1. [DOI] [PubMed] [Google Scholar]
- Mabuchi I., Okuno M. The effect of myosin antibody on the division of starfish blastomeres. J Cell Biol. 1977 Jul;74(1):251–263. doi: 10.1083/jcb.74.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabuchi I., Spudich J. A. Purification and properties of soluble actin from sea urchin eggs. J Biochem. 1980 Mar;87(3):785–802. doi: 10.1093/oxfordjournals.jbchem.a132808. [DOI] [PubMed] [Google Scholar]
- Mabuchi I., Tsukita S., Tsukita S., Sawai T. Cleavage furrow isolated from newt eggs: contraction, organization of the actin filaments, and protein components of the furrow. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5966–5970. doi: 10.1073/pnas.85.16.5966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaig C. D., Robinson K. R. The distribution of lectin receptors on the plasma membrane of the fertilized sea urchin egg during first and second cleavage. Dev Biol. 1982 Jul;92(1):197–202. doi: 10.1016/0012-1606(82)90163-4. [DOI] [PubMed] [Google Scholar]
- Meijer L., Arion D., Golsteyn R., Pines J., Brizuela L., Hunt T., Beach D. Cyclin is a component of the sea urchin egg M-phase specific histone H1 kinase. EMBO J. 1989 Aug;8(8):2275–2282. doi: 10.1002/j.1460-2075.1989.tb08353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer L., Pondaven P. Cyclic activation of histone H1 kinase during sea urchin egg mitotic divisions. Exp Cell Res. 1988 Jan;174(1):116–129. doi: 10.1016/0014-4827(88)90147-4. [DOI] [PubMed] [Google Scholar]
- Nakano H., Kobayashi E., Takahashi I., Tamaoki T., Kuzuu Y., Iba H. Staurosporine inhibits tyrosine-specific protein kinase activity of Rous sarcoma virus transforming protein p60. J Antibiot (Tokyo) 1987 May;40(5):706–708. doi: 10.7164/antibiotics.40.706. [DOI] [PubMed] [Google Scholar]
- Patel R., Twigg J., Crossley I., Golsteyn R., Whitaker M. Calcium-induced chromatin condensation and cyclin phosphorylation during chromatin condensation cycles in ammonia-activated sea urchin eggs. J Cell Sci Suppl. 1989;12:129–144. doi: 10.1242/jcs.1989.supplement_12.11. [DOI] [PubMed] [Google Scholar]
- Picard A., Capony J. P., Brautigan D. L., Dorée M. Involvement of protein phosphatases 1 and 2A in the control of M phase-promoting factor activity in starfish. J Cell Biol. 1989 Dec;109(6 Pt 2):3347–3354. doi: 10.1083/jcb.109.6.3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAPPAPORT R. Experiments concerning the cleavage stimulus in sand dollar eggs. J Exp Zool. 1961 Oct;148:81–89. doi: 10.1002/jez.1401480107. [DOI] [PubMed] [Google Scholar]
- Rappaport R. Establishment of the mechanism of cytokinesis in animal cells. Int Rev Cytol. 1986;105:245–281. doi: 10.1016/s0074-7696(08)61065-7. [DOI] [PubMed] [Google Scholar]
- Saitoh M., Ishikawa T., Matsushima S., Naka M., Hidaka H. Selective inhibition of catalytic activity of smooth muscle myosin light chain kinase. J Biol Chem. 1987 Jun 5;262(16):7796–7801. [PubMed] [Google Scholar]
- Schroeder T. E. The contractile ring. II. Determining its brief existence, volumetric changes, and vital role in cleaving Arbacia eggs. J Cell Biol. 1972 May;53(2):419–434. doi: 10.1083/jcb.53.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder T. E. The egg cortex in early development of sea urchins and starfish. Dev Biol (N Y 1985) 1986;2:59–100. doi: 10.1007/978-1-4613-2141-5_3. [DOI] [PubMed] [Google Scholar]
- Tamaoki T., Nomoto H., Takahashi I., Kato Y., Morimoto M., Tomita F. Staurosporine, a potent inhibitor of phospholipid/Ca++dependent protein kinase. Biochem Biophys Res Commun. 1986 Mar 13;135(2):397–402. doi: 10.1016/0006-291x(86)90008-2. [DOI] [PubMed] [Google Scholar]