Abstract
Collagen scaffolds are often utilized in tissue engineering applications where their performance depends on physical and mechanical properties. This study investigated the effects of collagen source (bovine, porcine, and ovine tendon) on properties of collagen sponge scaffolds cross-linked with 1-ethyl-3-(3-dimethyl aminopropyl) carbodiimide (EDC)/N-hydroxysuccinimide (NHS). Scaffolds were tested for tensile and compressive properties, stability (resistance to enzymatic degradation), pore size, and swelling ratio. No significant differences in tensile modulus were observed, but ovine scaffolds had significantly greater ultimate strain, stress, and toughness relative to bovine and porcine scaffolds. No significant differences in compressive properties, pore size, or swelling ratio were observed as a function of collagen source. Ovine scaffolds were more resistant to collagenase degradation compared to bovine samples, which were more resistant than porcine scaffolds. In comparison to bovine scaffolds, ovine scaffolds performed equivalently or superiorly in all evaluations, and porcine scaffolds were equivalent in all properties except enzymatic stability. These results suggest that collagen sponges derived from bovine, porcine, and ovine tendon have similar physical and mechanical properties, and are all potentially suitable materials for various tissue engineering applications.
Keywords: Collagen, Tissue engineering, Degradation, Mechanical Properties, Pore Size
1. Introduction
Collagen is one of the most widely used biomaterials for tissue engineering. The medical device industry has primarily utilized acid-insoluble collagen as a biomaterial due to its high yield from skin or tendon1. Insoluble collagen can be fabricated into sponges, hydrogels, tubes, powders, and films serving as a scaffolding material for tissue engineering2,3. Specifically, collagen sponge-like scaffolds have demonstrated promise as wound dressing for burns, leg ulcers, pressure sores, and donor sites because of their ability to accelerate wound repair, inhibit wound contraction, and stimulate of epithelium formation3–5. In tissue engineering applications, a collagenous scaffold can serve as a structural template for cells to infiltrate, adhere, and synthesize new tissue.
One of the most common sources of xenogeneic industrial collagen is bovine because of accessibility and abundance3,6. However, these materials carry the slight risk of transmitting bovine spongiform encephalopathy, so closed herds and other precautions must be used to minimize this risk7. Therefore, there has been interest in alternative sources of collagen from non-bovine sources.
Previous studies of insoluble collagen have considered collagen derived from bovine tendon and skin, porcine skin, avian skin and feet, equine tendon, sharkskin, frog skin, jellyfish, ovine skin, fish scale, and rat-tail sources2,8–13. However, direct comparisons between studies cannot be made as a result of critical differences in several confounding variables, including collagen concentration, swelling pH, EDC/NHS concentrations and crosslinking duration, and freezing rates. Although abundant, marine-derived collagens tend to have low denaturation temperatures and enzymatic resistance2,14. Mammalian dermal tissue is another potential source but has many other components including blood vessels, lymph vessels, hair follicles, and sweat glands. Therefore, mammalian tendon appears to be an attractive source for acid-insoluble medical grade collagen.
With beef, pork, and lamb being the three highest consumed mammalian meats per capita in the United States, these species are logical choices as collagen sources15. The tendons can be harvested during the meat harvesting process with minimal waste. However, to our knowledge a direct comparative study on the properties of bovine, porcine, and ovine tendon-derived collagenous scaffolds is lacking. The goal of this study was to assess porcine and ovine derived scaffolds as potential replacements of bovine scaffolds fabricated using a previously developed lyophilization procedure16. We hypothesize that EDC cross-linked collagenous sponge-like scaffolds isolated from porcine and ovine tendons possess comparable mechanical and physical properties as those derived from bovine tendons.
2. Materials and Methods
Several parameters must be considered for the use of collagenous scaffolds in tissue engineering, including the material’s mechanical properties, stability or degradation rate, and the physical properties of porosity and swelling. Specifically, these parameters were characterized in a series of tests including uniaxial tension, unconfined and confined compression, enzymatic stability, average pore size characterization, and swelling ratio.
2.1. Collagen Isolation
Fresh-frozen tendons from bovine (4–8 week old Achilles tendons), porcine (12 week old Achilles tendons), and ovine (32–40 week old extensor tendons) sources were purchased from a local animal tissue supplier (Farm to Pharm, Warren, NJ). The age of the animals from which the tendons were harvested reflects the age of which they are available from slaughter animals in the food industry. Although bovine and porcine Achilles tendons were selected, ovine extensor tendons had to be chosen because harvesting the Achilles tendon would interfere with meat processing.
Tendons were thawed, dissected, transected into 1 cm pieces, frozen, lyophilized, and shredded. Shredded tendon fragments were subjected to 12 consecutive 24 hour treatments: 6 treatments of 0.211 M sodium phosphate, 2 treatments of 0.48 M sodium chloride, 2 treatments of 0.225 M citrate buffer (pH 3.7), and 2 treatments of 0.175 M acetic acid17. After each treatment, samples were centrifuged at 800g for 15 minutes, the supernatant was aspirated, 500 mL of deionized water was added, the solution was placed on mild agitation for 10 minutes, centrifuged once more, the next treatment solution was added, and the sample was placed on mild agitation for 10 minutes then returned to 4°C. The tissue was then subjected to 4 rinses with deionized water, frozen, and lyophilized until completion. The yield of this procedure was defined as the ratio of the final mass of lyophilized collagen to the mass of the initial lyophilized tendon.
2.2. Collagen Scaffold Preparation
The collagen scaffold preparation protocol was adapted from our previous study16. Isolated collagen was ground into a powder with particle size less than 425 µm. Ground collagen (1% w/v) was swollen in a dilute hydrochloric acid solution (pH=2.35), degassed under high vacuum, and injected into a perforated polystyrene mold. The dispersion was frozen via a dry ice-ethanol bath for 30 minutes and lyophilized for about 36 hours or until completion.
Lyophilized scaffolds were cross-linked in a 10 mM EDC and 5 mM NHS, deionized water solution for 5 hours under minimal compression to maintain shape and uniform thickness. The scaffolds were rinsed 3 times in distilled (DI) water for 10 minutes and soaked in a 100 mM sodium phosphate solution for 2 hours to remove any residual byproducts from the reaction. The scaffolds were then rinsed in DI water overnight, frozen in a dry ice-ethanol bath for 30 minutes, and lyophilized for about 24 hours or until completion.
2.3. Tensile Testing and Swelling Ratio
Uniaxial tensile tests were performed on collagen scaffold dogbones with an initial gage length of 56 mm. Samples were weighed before hydration and placed in phosphate buffered solution (PBS) at room temperature overnight. The samples were blotted with filter paper to remove excess water and weighed once more. Swelling ratio was calculated as the mass of the hydrated scaffold to that of the dried scaffold. Average thickness was measured in triplicate using a Z-Mike Model 1202B (Z-Mike, Dayton, OH). The width of the gauge length was measured from images of the samples using MATLAB Version R2013a (The MathWorks, Inc., Natick, MA).
Samples were gripped using cryogenic clamps (Bose ElectroForce, Eden Prairie, MN) and strained at a rate of 14 mm/min until failure with an Instron 5569 (Instron, Norwood, MA). Samples that did not fail midsubstance were omitted. The tensile modulus, ultimate strain, ultimate stress, and toughness were calculated for n=14 samples per collagen source.
2.4. Confined Compression Testing
Four-millimeter diameter plugs by 5 mm height prepared using a biopsy punch were hydrated in PBS and loaded into a custom-made jig described by Armstrong and Mow18. The samples were tested in compressive creep with a 0.1 N load applied for 3600 seconds using an Instron 5542. The aggregate modulus and permeability were calculated, according to Mow’s biphasic theory with a sample size of 15 per collagen source19. Samples that did not generate an accurate fit were omitted.
2.5. Unconfined Compression Testing
Ten-millimeter diameter by nine-millimeter height cylindrical samples prepared using a biopsy punch were tested in uniaxial, unconfined compression, where n=7 for bovine, and n=8 for porcine and ovine sources. The samples were bonded to a metallic disc using a cyanoacrylate to prevent any lateral movement and hydrated in PBS. Samples were compressed at a rate of 0.01 mm/s to a maximum of 75% strain using an Instron 5542. The samples were immersed in PBS throughout the duration of the test. The compressive modulus was defined as the slope of the best linear fit between 2% and 5% strain and compressive strengths at 20%, 40%, and 75% strain were calculated20–22.
2.6. Pore Structure Analysis
Dry scaffolds were sectioned in order to image the interior of the material. The samples were sputter coated in gold (Balzers SCD 004, Oerlikon Balzers, Liechtenstein). 60× magnification images were taken at random locations using an Amray 1830I Scanning Electron Microscope (Amray, Bedford, MA).
Fifteen images per collagen source were converted to binary by applying an adaptive thresholding algorithm. The images were analyzed using a mean linear intercept MATLAB script, adapted from O’Brien et al.23. This determined a best-fit ellipse representing the average pore size of each image. Next, the ellipse major and minor axes were multiplied by 1.5, in order to account for the effect of pores not sectioned through their maximum cross-section24. The mean intercept length was defined as the average of the corrected major and minor axes for each image. The average pore size was calculated from the average of the mean intercept lengths of all images analyzed23.
2.7. Enzymatic Stability
Enzymatic stability of the scaffolds was tested via an in vitro collagenase resistance test. For each source, 5 squares of non-cross-linked collagen scaffolds were cut with a mass of 2.0 ± 0.1 mg. All samples and enzyme solutions were incubated separately at 37°C prior to testing. The samples were evaluated while incubated at 37°C in 2 mL of 500 U/mL bacterial collagenase, from Clostridium histolyticum (n=5 per source). Samples were visually assessed every 5 minutes using a semi-quantitative scale from 0 to 5, with 0 representing complete dissolution and 5 indicating completely intact.
2.8. Statistical Analysis
Data are reported as the mean ± standard deviation. One-way analyses of variance (ANOVA) were performed for all comparisons with Tukey’s post hoc test for multiple comparisons, unless otherwise indicated. Kruskal-Wallis one-way ANOVA on Ranks with Dunn’s post hoc test was performed for ultimate strain, toughness, swelling ratio, and compressive strength comparisons due to failure of normality or equal variance tests. All tests were performed using Sigma Stat Software Version 2.03 (IBM, Armonk, NY) and differences were considered significant at α less than 0.05.
3. Results
3.1. Collagen Isolation
The isolation procedure produced similar yields for each animal source. Bovine, porcine, and ovine collagen were isolated with yields of 74.3%, 75.1%, and 80.0%, respectively. Collagen derived from all sources was white in appearance with no noticeable differences in gross morphology or texture.
3.2. Tensile Testing and Swelling Ratio
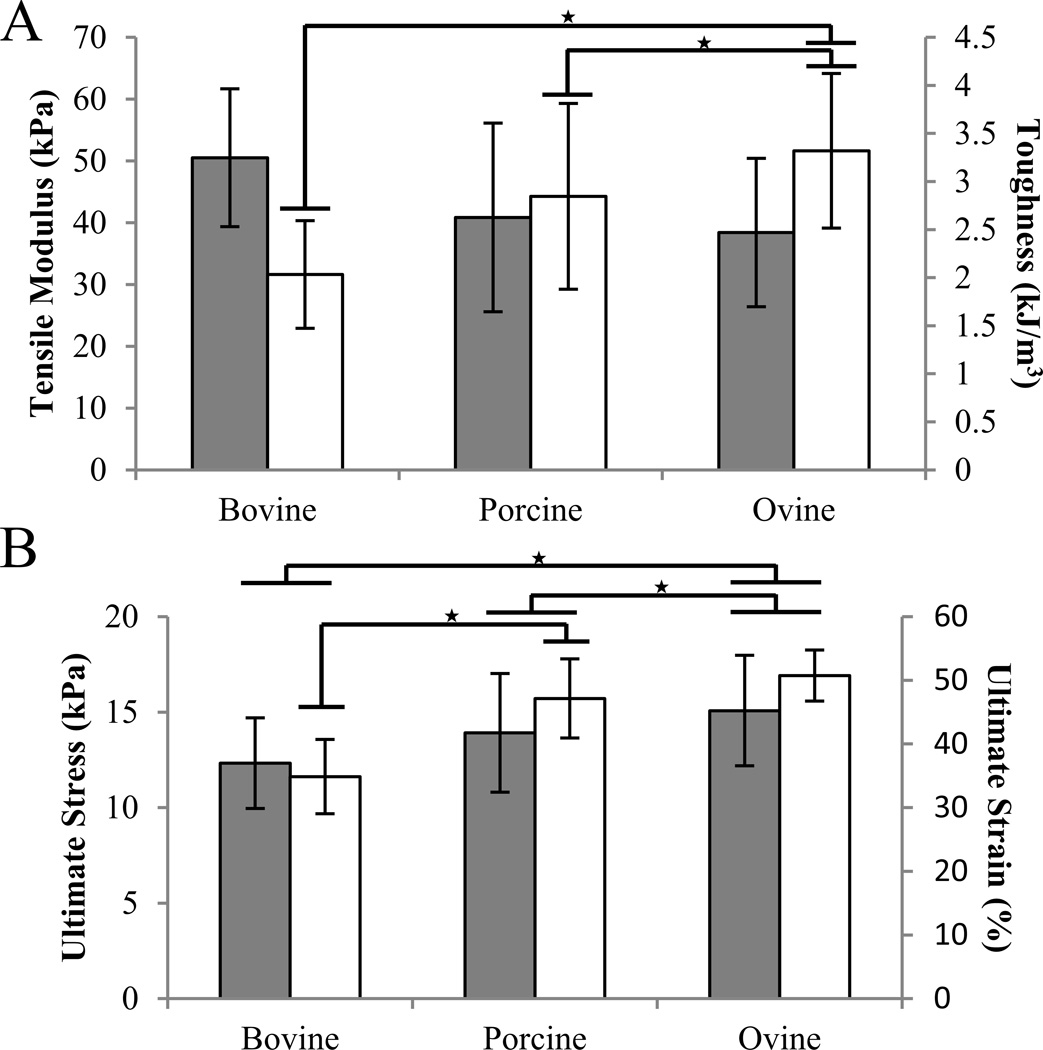
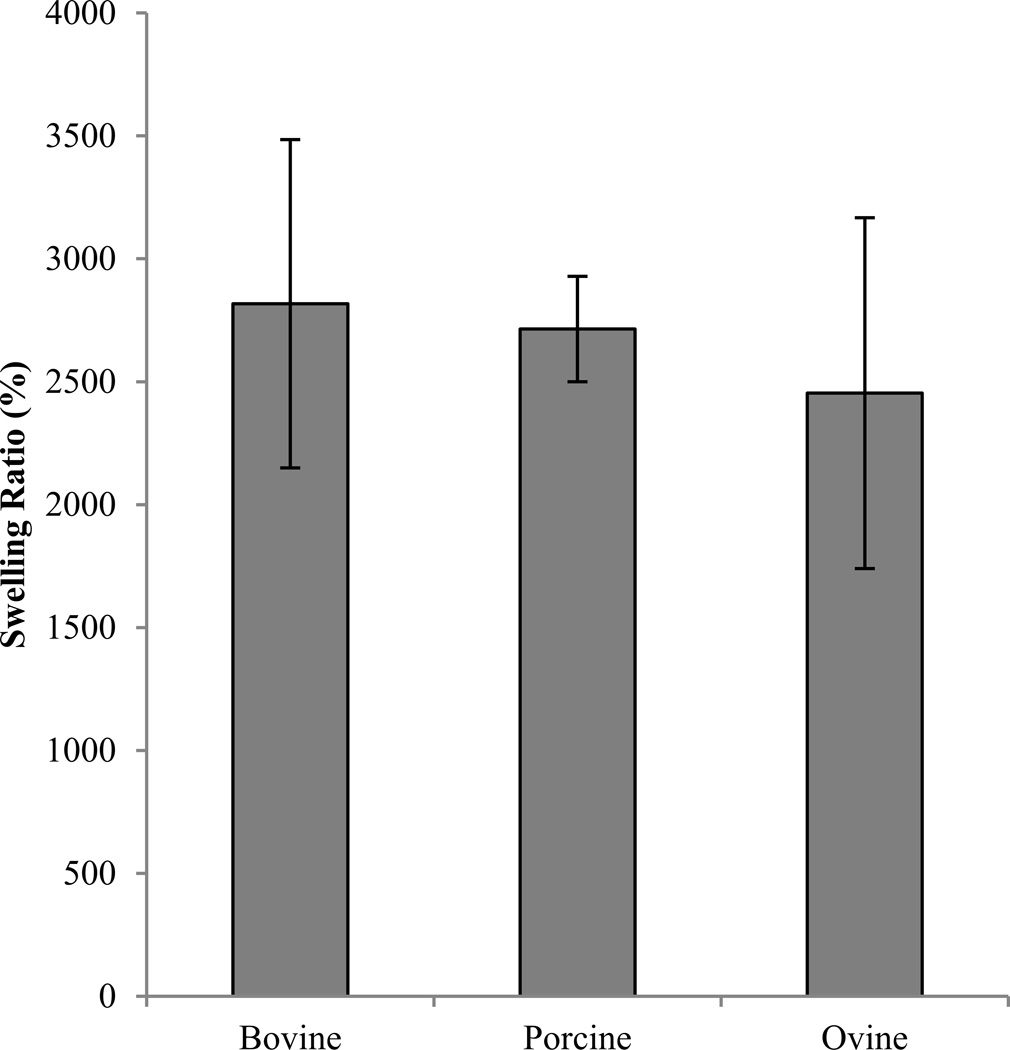
After omission of samples that did not fail midsubstance, sample sizes were n=12 for bovine, n=9 for porcine, and n=14 for ovine sources. No significant differences in the tensile modulus were detected between sources (p=0.105) (Figure 1). Ultimate stress of ovine scaffolds (15.08 ±2.89 kPa) was significantly greater than porcine (13.91 ± 3.11 kPa, p=0.035) and bovine (12.33 ± 2.37 kPa, p=0.034). Ovine ultimate percent strain (50.74 ± 4.02 %) was significantly greater than both porcine (47.15 ± 6.20 %, p<0.001) and bovine scaffolds (34.87 ± 5.83 %, p<0.001), and porcine was greater than bovine scaffolds (ANOVA on Ranks, post hoc Tukey test, p=0.01). Tensile toughness of ovine scaffolds (3.32 ± 0.81 kJ/m3) was significantly greater than porcine (2.85 ± 0.97 kJ/m3, p<0.05) and bovine scaffolds (2.03 ± 0.56 kJ/m3, p<0.05) (ANOVA on Ranks, post hoc Dunn’s Method). No significant differences in swelling ratio were observed between species (ANOVA on Ranks, p=0.270) (Figure 2).
Figure 1.
Tensile properties of collagen scaffolds as affected by species. The values indicated represent mean ± SD. A. Tensile modulus is represented by gray bars and tensile toughness are represented in white. B. Ultimate stress is shown in gray bars and ultimate percent strain in white. ★ indicates p<0.05.
Figure 2.
Swelling ratio of scaffolds of bovine, porcine, and ovine collagen scaffolds.
3.3. Confined Compression
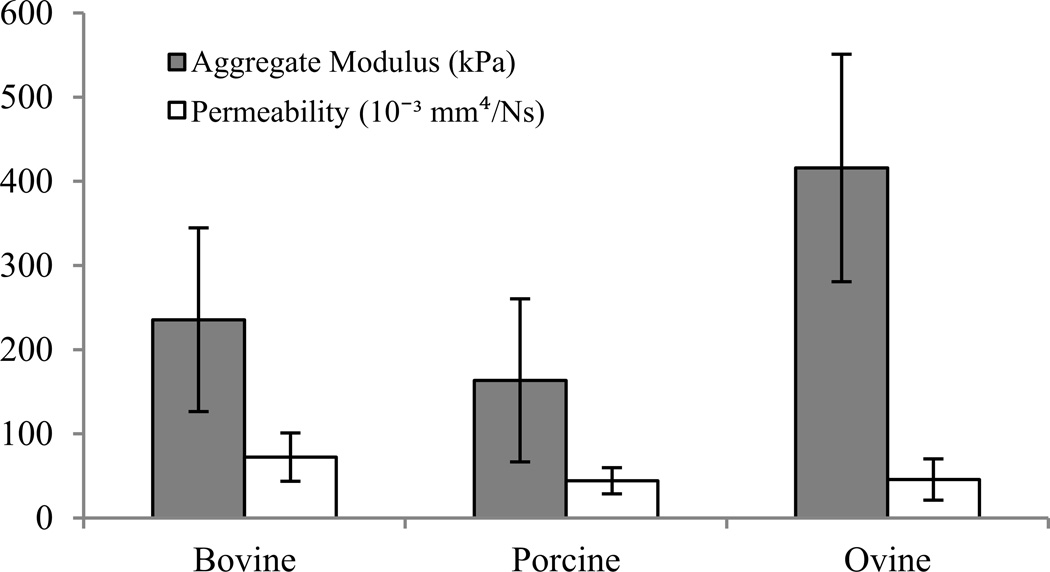
No statistically significant differences were observed in either aggregate modulus or permeability, where n=7 for bovine, and n=8 for porcine and ovine sources after omission of samples with poor fits (Figure 3). However, the bovine aggregate modulus mean was 44% greater than porcine and 43% less than ovine moduli (p=0.230). The bovine permeability mean was 63% and 58% greater than porcine and ovine permeabilities, respectively (p=0.176).
Figure 3.
Confined compressive properties of bovine, porcine, and ovine derived collagenous scaffolds.
3.4. Unconfined Compression
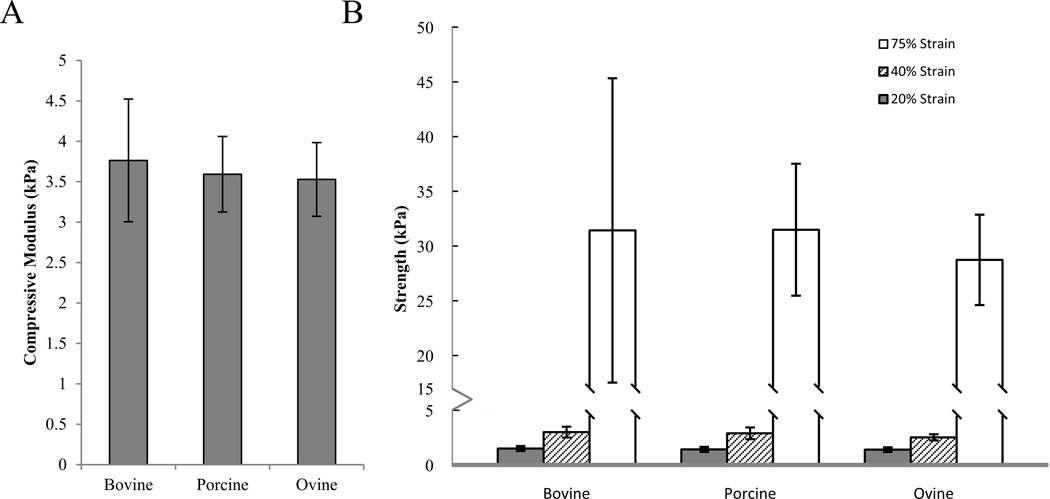
All scaffolds performed in unconfined compression with no significant differences in compressive modulus (p=0.75) and compressive strengths at 20%, 40%, and 75% strain (p=0.325, p=0.153, and p=0.696, respectively) (Figure 4).
Figure 4.
Unconfined compressive properties of collagen scaffolds as affected by species. A. Compressive moduli and B. Compressive Strength at 20% (gray), 40% (stripes), and 75% (white) strain.
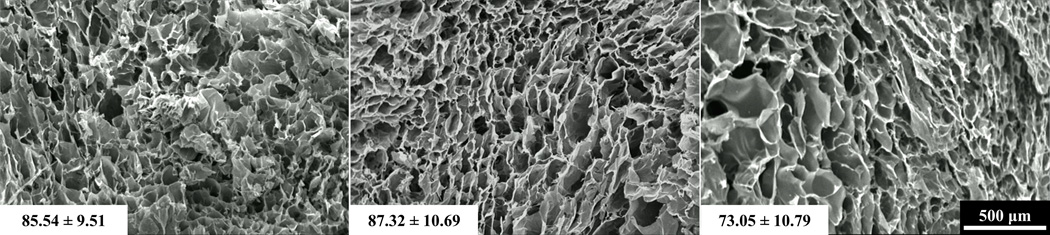
3.5. Pore Structure Analysis
Visual inspection revealed no discernable differences in pore structure between species (Figure 5). All groups displayed heterogeneous scaffold strut architecture with no apparent alignment in any direction. The mean pore size of scaffolds derived from bovine, porcine, and ovine tendons were not significantly different (p=0.425) (Figure 5). The ovine mean pore size (73.05 ± 10.79 µm) was slightly less than bovine (85.84 ± 9.51 µm) and porcine (87.32 ± 10.69 µm) mean pore sizes.
Figure 5.
Representative SEM micrographs of collagen scaffolds from bovine (left), porcine (middle), and ovine (right) sources. Average pore diameter is shown in the bottom left corner of each image.
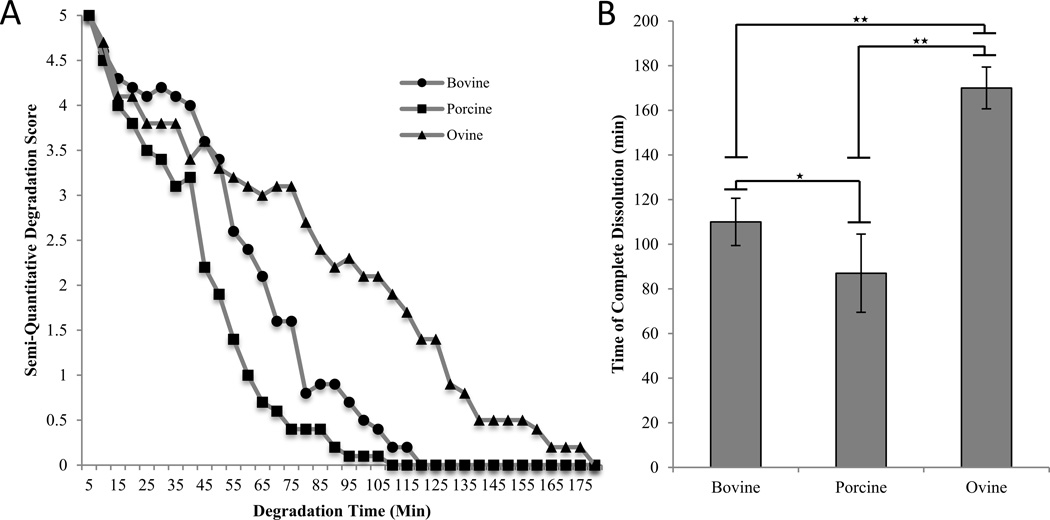
3.6. Enzymatic Stability
The semi-quantitative assessment of the samples revealed an initial steady rate of degradation with a diminishing rate as the samples approach complete dissolution (Figure 6a). Significant differences between all three groups were observed (p<0.001). For experimental samples, ovine scaffolds (170 ± 9 min) resisted complete degradation significantly longer than bovine samples (110 ± 11 min) and bovine samples endured significantly longer than porcine samples (87 ± 17 min) (Figure 6b).
Figure 6.
Enzymatic stability of collagen samples from bovine, porcine, and ovine scaffolds. A. Semi-quantitative degradation score of bovine, porcine, and ovine collagen scaffold samples as a function of degradation time. Circles, squares, and triangles represent bovine, porcine, and ovine samples, respectively. B. Time of complete dissolution for scaffold samples. ★ indicates p<0.05 and ★★ p<0.01.
4. Discussion
Collagenous biomaterials are frequently utilized in tissue engineering, serving as a porous structural template for tissue regeneration. Mammalian tendons were utilized because they can be easily collected at slaughterhouses without interfering with the meat harvesting process and have superior properties over collagens derived from other tissues or animals2,14,25. With cattle representing one of the primary sources of these materials, there is a need to explore other sources in order to reduce the potential risks associated with the transmission of prion based bovine diseases7. This study directly demonstrates that bovine, porcine, and ovine tendon derived collagen harvested from slaughter-aged animals can produce scaffolds with similar overall physical and mechanical properties.
A non-enzymatic isolation procedure was employed for the preparation of insoluble collagen through successive treatments of tendinous tissue with alkali and acids3. This method is advantageous because the process is inexpensive and preserves the fibrillar structure of collagen formed in vivo, which imparts polymeric collagen its mechanical strength and resilience. Roughly 70–80% of the dry weight of normal tendon is collagen and insoluble collagen constitutes 98% of the total collagen composition26,27. The yield of this procedure produced an expected value ranging from 74% to 80%, verifying the efficiency of the collagen isolation protocol.
Once isolated, ground fibrillar collagen was swollen in an acidic solution, subsequently frozen, and freeze-dried. The freezing process forms a continuous interpenetrating network of ice crystals, which are removed through sublimation during the freeze-drying process, resulting in a highly porous sponge-like scaffold. The removal of water causes irreversible intermolecular cross-linking between collagen aggregates28. EDC is employed to directly crosslink glutamic acid or aspartic acid to amide groups of neighboring collagen molecules29. NHS is used in conjunction with EDC in order to improve the efficacy of the crosslinking reaction by suppressing side reactions29,30.
Swelling ratio is an important parameter because it indicates how the scaffold will respond in contact with blood and other body fluids and can affect the cell differentiation process31,32. The results of the scaffolds used in this study, composed of 1% collagen and cross-linked with 10 mM EDC for 5 hours, differed from those found by Francesko et al. They reported a swelling ratio of approximately 1700% for scaffolds fabricated with the same concentrations but the crosslinking reaction duration was 24 hours33. The increased swelling we observed relative to those results is expected, due to the reduction in crosslinking time9.
Compressive properties and pore structure are important parameters for biomaterials to be used in cartilaginous and fibrocartilaginous tissues. For these applications, scaffolds must be sufficiently permeable with interconnecting pores to facilitate cell growth, migration, and nutrition, and possess adequate compressive properties to support the large loads applied to these tissues34. All scaffolds evaluated in this study possessed a porous ultrastructure with an average pore diameter of about 80 µm. This is consistent with previous studies where matrix morphology of lyophilized collagenous scaffolds was more dependent on freezing rate than on the collagen source9,23,35. For example, Angele et al. found pore sizes ranging from 50 to 350 µm for 0.1% to 3% collagen bovine and equine scaffolds with no differences between non-cross-linked and cross-linked scaffolds9.
The optimal pore size for cell infiltration and adhesion varies with cell type23,36. Pore sizes as small as 20 µm have been shown to promote the chondrogenic phenotype, sizes greater than 80 µm are necessary for fibrogenic ingrowth, and pores in the range of 150–200 µm are necessary for fibrochondrogenic ingrowth3,37,38. Therefore, the scaffolds in this study possess pore sizes within the range suitable for various tissue engineering applications, including cartilage and fibrocartilage repair or regeneration.
On the other hand, the permeabilities of the scaffolds were in the range 0.044–0.072 mm4/Ns, which are larger than those of cartilage (approximately 10−2 mm4/Ns) and menisci (10−3 mm4/Ns)39,40. With aggregate moduli of 400 kPa and 790 kPa for human menisci and articular cartilage, respectively, a purely collagenous scaffold is well below the range of a suitable biomaterial for cartilaginous tissue replacement27,41. However, collagen scaffolds can be augmented with glycosaminoglycans in order to improve the compressive stiffness towards that of native cartilaginous tissues42. Additionally, the large pore size and permeability should allow for robust cell infiltration and tissue ingrowth and subsequent improvement in mechanical performance.
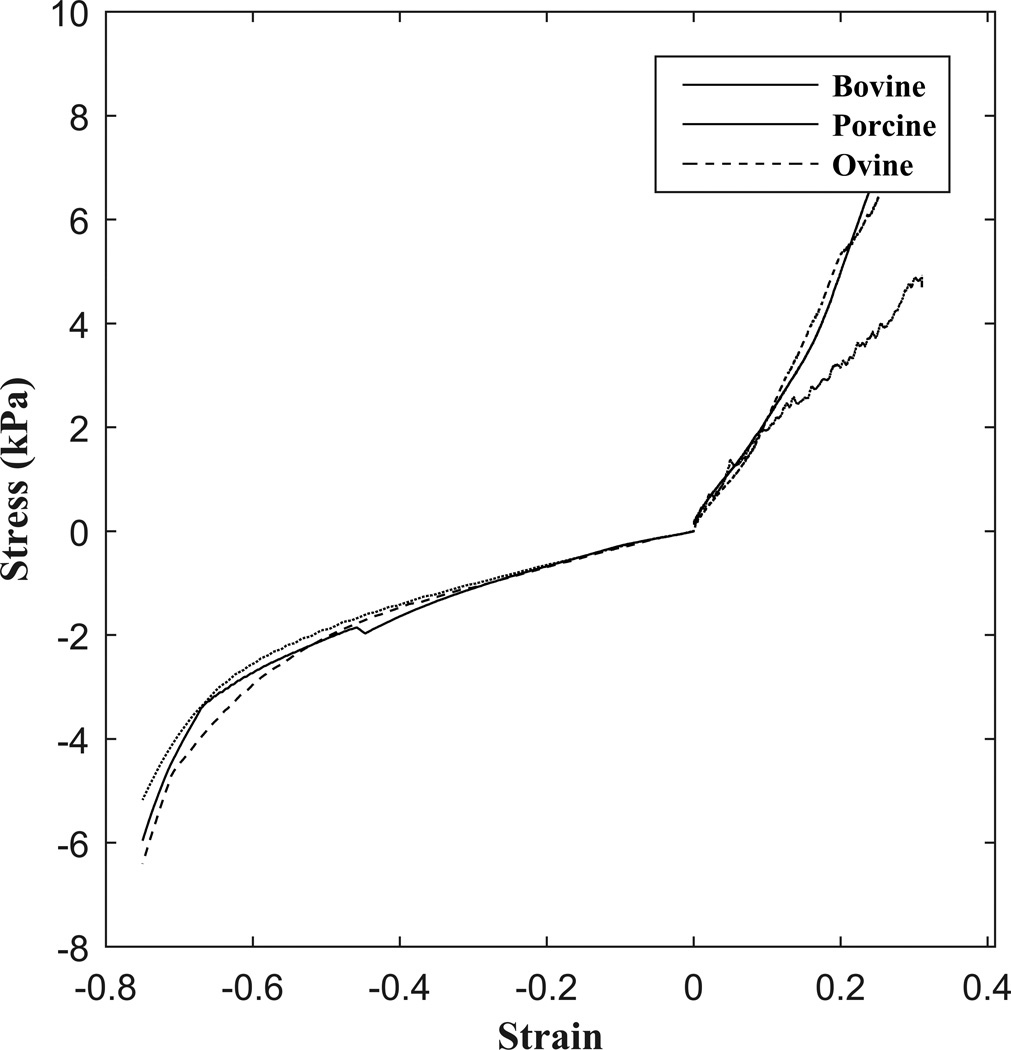
Collagen scaffolds are typically tested in unconfined, uniaxial tension when utilized as bone substitutes. These properties are vital to ensure long-term structural and functional success in vivo42. Our protocol used similar gauge lengths and strain rates and produced values within the range of those previously reported22,42,43. The compressive modulus demonstrated similar trends to that of the tensile modulus between collagen sources. Scaffolds exhibited compressive characteristics of elastomeric cellular solids with 3 distinct loading phases, including a linear elastic region, an elastic collapse region, and densification, consistent with other findings in the literature (Figure 7)22,35.
Figure 7.
Characteristic stress-strain curve in unconfined compression and tension for bovine, porcine, and ovine collagenous scaffolds.
The significant differences in tensile properties and enzymatic stability found in this study were unexpected; however, this is not the first evidence of functional differences between collagen species26. Angele et al. found superior tensile mechanical properties and enzymatic stability for equine tendon collagen scaffolds compared to those of bovine tendon scaffolds9. These differences were most likely a result of differences in the amino acid composition of collagens between species given that EDC crosslinking is heavily dependent on the primary structure of collagen2,9,44. The crosslinking reaction is particularly affected by differences in lysine and hydroxylysine composition. EDC cross-links between the carboxylic group of glutamic and aspartic acid to the amino group of lysine29. The tensile properties determined in this study were on the same order of magnitude as those in the literature, but were substantially different9,33. Similar strain rates were used in previous studies on collagenous scaffolds; however, those studies were performed on samples with inadequate length to cross-sectional area ratios, failing to satisfy the minimum requirements for a true tensile test9,33,45.
Enzymatic stability of collagen biomaterials to proteases is an essential parameter to success. The rate of degradation of biomaterials must be controlled in tissue engineering to facilitate a continuous tissue reconstruction. Ovine scaffolds resisted degradation almost twice as long as porcine scaffolds and more than 50% longer than bovine scaffolds. This may be because collagenase cleavage sites are more effectively masked by the endogenous crosslinks in ovine scaffolds than in other sources9. Bacterial collagenase catalyzes hydrolytic cleavage of collagen in non-polar regions, either in a single alpha-chain or across the entire triple helix in a lateral fashion9. In vitro bacterial collagenase digestion rates are well correlated with in vitro macrophage-mediated degradation and in vivo degradation rates46,47.
There were limitations with this study. It is well known that endogenous cross-linking of collagenous tissues increases with age and there is an associated effect on in vivo remodeling characteristics48. The animals from which the tendons were harvested were at variable points in their lifecycles, which reflects the age of which the tissues become available from the waste of the meat industry. The bovine and porcine tendons were harvested from very immature animals; however, ovine tendons were from nearly skeletally mature animals. In addition, ovine extensor tendons were evaluated in this study whereas Achilles tendons were assessed from the other sources. There has been some evidence that collagen from tendons of different functions can possess differences in mechanical properties49. Furthermore, in order to truly discern the small differences observed in the material properties found in this study would require enormous sample sizes; therefore a few of the results were insufficiently powered. Finally, 1% collagen sponges were utilized; however, this is a relatively small concentration compared to those typically utilized in vivo for tissue engineering.
This work demonstrates that there are subtle mechanical and enzymatic differences in scaffolds composed of these materials. Ovine scaffolds performed equivalent or better in comparison to bovine scaffolds in all mechanical and enzymatic evaluations. Porcine scaffolds acted similarly or better than bovine scaffolds for all metrics except enzymatic stability. However, these differences in enzymatic stability can be overcome with the utilization of the proper crosslinking protocol. With a reduced risk of transmissible prion diseases, ovine- and porcine-derived collagen scaffolds provide an alternative to bovine-derived scaffolds for various tissue engineering applications.
Acknowledgments
This work was supported, in part, by Novopedics Inc. (New Brunswick, NJ). S.G. was supported, in part, by the National Institutes of Health under Ruth L. Kirschstein National Research Service Award T32 GM8339 from the National Institute of General Medical Sciences.
References
- 1.Deyl ZA, M . Preparation of insoluble collagen. 1st. Oxford: Joynson-Bruvvers; 1976. The methodology of connective tissue research; pp. 1–7. [Google Scholar]
- 2.Lin YK, Liu DC. Comparison of physical–chemical properties of type I collagen from different species. Food Chemistry. 2006;99(2):244–251. [Google Scholar]
- 3.Chvapil M. Collagen sponge: theory and practice of medical applications. J Biomed Mater Res. 1977;11(5):721–741. doi: 10.1002/jbm.820110508. [DOI] [PubMed] [Google Scholar]
- 4.Doillon CJ, Whyne CF, Berg RA, Olson RM, Silver FH. Fibroblast-collagen sponge interactions and the spatial deposition of newly synthesized collagen fibers in vitro and in vivo. Scan Electron Microsc. 1984;(Pt 3):1313–1320. [PubMed] [Google Scholar]
- 5.Yannas IV, Burke JF. Design of an artificial skin. I. Basic design principles. J Biomed Mater Res. 1980;14(1):65–81. doi: 10.1002/jbm.820140108. [DOI] [PubMed] [Google Scholar]
- 6.Badylak SF, Freytes DO, Gilbert TW. Extracellular matrix as a biological scaffold material: Structure and function. Acta Biomaterialia. 2009;5(1):1–13. doi: 10.1016/j.actbio.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 7.Kim Y, Nowzari H, Rich SK. Risk of Prion Disease Transmission through Bovine-Derived Bone Substitutes: A Systematic Review. Clinical Implant Dentistry and Related Research. 2013;15(5):645–653. doi: 10.1111/j.1708-8208.2011.00407.x. [DOI] [PubMed] [Google Scholar]
- 8.Parenteau-Bareil R, Gauvin R, Cliche S, Gariepy C, Germain L, Berthod F. Comparative study of bovine, porcine and avian collagens for the production of a tissue engineered dermis. Acta Biomater. 2011;7(10):3757–3765. doi: 10.1016/j.actbio.2011.06.020. [DOI] [PubMed] [Google Scholar]
- 9.Angele P, Abke J, Kujat R, Faltermeier H, Schumann D, Nerlich M, Kinner B, Englert C, Ruszczak Z, Mehrl R, et al. Influence of different collagen species on physico-chemical properties of crosslinked collagen matrices. Biomaterials. 2004;25(14):2831–2841. doi: 10.1016/j.biomaterials.2003.09.066. [DOI] [PubMed] [Google Scholar]
- 10.Song E, Kim SY, Chun T, Byun HJ, Lee YM. Collagen scaffolds derived from a marine source and their biocompatibility. Biomaterials. 2006;27(15):2951–2961. doi: 10.1016/j.biomaterials.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 11.Olde Damink LH, Dijkstra PJ, Van Luyn MJ, Van Wachem PB, Nieuwenhuis P, Feijen J. Changes in the mechanical properties of dermal sheep collagen during in vitro degradation. J Biomed Mater Res. 1995;29(2):139–147. doi: 10.1002/jbm.820290202. [DOI] [PubMed] [Google Scholar]
- 12.Nomura Y, Sakai H, Ishii Y, Shirai K. Preparation and some properties of type I collagen from fish scales. Bioscience Biotechnology and Biochemistry. 1996;60(12):2092–2094. doi: 10.1271/bbb.60.2092. [DOI] [PubMed] [Google Scholar]
- 13.Robinson JJ. Comparative biochemical analysis of sea urchin peristome and rat tail tendon collagen. Comp Biochem Physiol B Biochem Mol Biol. 1997;117(2):307–313. doi: 10.1016/s0305-0491(97)00092-8. [DOI] [PubMed] [Google Scholar]
- 14.Li H, Liu BL, Gao LZ, Chen HL. Studies on bullfrog skin collagen. Food Chemistry. 2004;84(1):65–69. [Google Scholar]
- 15.USDA Agricultural Projections to 2025. Office of the Chief Economist, World Agricultural Outlook Board, U.S. Department of Agriculture. Prepared by the Interagency Agricultural Projections Committee. Long-term Projections Report OCE-2016-1. :99. [Google Scholar]
- 16.Merriam AR, Patel JM, Culp BM, Gatt CJ, Dunn MG. Successful Total Meniscus Reconstruction Using a Novel Fiber-Reinforced Scaffold: A 16-and 32-Week Study in an Ovine Model. American Journal of Sports Medicine. 2015;43(10):2528–2537. doi: 10.1177/0363546515595065. [DOI] [PubMed] [Google Scholar]
- 17.Deyl Z, Miksik I, Eckhardt A. Preparative procedures and purity assessment of collagen proteins. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;790(1–2):245–275. doi: 10.1016/s1570-0232(03)00158-2. [DOI] [PubMed] [Google Scholar]
- 18.Armstrong CG, Mow VC. Variations in the intrinsic mechanical properties of human articular cartilage with age, degeneration, and water content. J Bone Joint Surg Am. 1982;64(1):88–94. [PubMed] [Google Scholar]
- 19.Mow VC, Kuei SC, Lai WM, Armstrong CG. Biphasic creep and stress relaxation of articular cartilage in compression?. Theory and experiments. J Biomech Eng. 1980;102(1):73–84. doi: 10.1115/1.3138202. [DOI] [PubMed] [Google Scholar]
- 20.Haugh MG, Jaasma MJ, O'Brien FJ. The effect of dehydrothermal treatment on the mechanical and structural properties of collagen-GAG scaffolds. J Biomed Mater Res A. 2009;89(2):363–369. doi: 10.1002/jbm.a.31955. [DOI] [PubMed] [Google Scholar]
- 21.Gelinsky M, Welzel PB, Simon P, Bernhardt A, Konig U. Porous three-dimensional scaffolds made of mineralised collagen: Preparation and properties of a biomimetic nanocomposite material for tissue engineering of bone. Chemical Engineering Journal. 2008;137(1):84–96. [Google Scholar]
- 22.Kane RJ, Roeder RK. Effects of hydroxyapatite reinforcement on the architecture and mechanical properties of freeze-dried collagen scaffolds. J Mech Behav Biomed Mater. 2012;7:41–49. doi: 10.1016/j.jmbbm.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 23.O'Brien FJ, Harley BA, Yannas IV, Gibson L. Influence of freezing rate on pore structure in freeze-dried collagen-GAG scaffolds. Biomaterials. 2004;25(6):1077–1086. doi: 10.1016/s0142-9612(03)00630-6. [DOI] [PubMed] [Google Scholar]
- 24.Gibson LJ, Ashby MF. Cellular solids: structure and properties. Cambridge: Cambridge university press; 1997. [Google Scholar]
- 25.Merriam A. Development of a Hybrid Polymer Fiber/Collagen Sponge Meniscus Scaffold. Rutgers: The State University of New Jersey; 2013. [Google Scholar]
- 26.Friess W. Collagen--biomaterial for drug delivery. Eur J Pharm Biopharm. 1998;45(2):113–136. doi: 10.1016/s0939-6411(98)00017-4. [DOI] [PubMed] [Google Scholar]
- 27.Mow VC, Gu WY, Chen FH. Structure and Function of Articular Cartilage and Meniscus. In: Mow VC, Huiskes Rik, editors. Basic orthopaedic biomechanics & mechano-biology. Lippincott Williams & Wilkins: 2005. pp. 181–257. [Google Scholar]
- 28.Schoof H, Apel J, Heschel I, Rau G. Control of pore structure and size in freeze-dried collagen sponges. J Biomed Mater Res. 2001;58(4):352–357. doi: 10.1002/jbm.1028. [DOI] [PubMed] [Google Scholar]
- 29.Sung HW, Chang WH, Ma CY, Lee MH. Crosslinking of biological tissues using genipin and/or carbodiimide. Journal of Biomedical Materials Research Part A. 2003;64(3):427–438. doi: 10.1002/jbm.a.10346. [DOI] [PubMed] [Google Scholar]
- 30.Damink LO, Dijkstra P, Van Luyn M, Van Wachem P, Nieuwenhuis P, Feijen J. Cross-linking of dermal sheep collagen using a water-soluble carbodiimide. Biomaterials. 1996;17(8):765–773. doi: 10.1016/0142-9612(96)81413-x. [DOI] [PubMed] [Google Scholar]
- 31.Lu HK, Lee SY, Lin FP. Elastic modulus, permeation time and swelling ratio of a new porcine dermal collagen membrane. J Periodontal Res. 1998;33(5):243–248. doi: 10.1111/j.1600-0765.1998.tb02196.x. [DOI] [PubMed] [Google Scholar]
- 32.Park H, Guo X, Temenoff JS, Tabata Y, Caplan AI, Kasper FK, Mikos AG. Effect of swelling ratio of injectable hydrogel composites on chondrogenic differentiation of encapsulated rabbit marrow mesenchymal stem cells in vitro. Biomacromolecules. 2009;10(3):541–546. doi: 10.1021/bm801197m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Francesko A, Soares da Costa D, Reis RL, Pashkuleva I, Tzanov T. Functional biopolymer-based matrices for modulation of chronic wound enzyme activities. Acta Biomater. 2013;9(2):5216–5225. doi: 10.1016/j.actbio.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 34.Murphy CM, O'Brien FJ. Understanding the effect of mean pore size on cell activity in collagen-glycosaminoglycan scaffolds. Cell Adh Migr. 2010;4(3):377–381. doi: 10.4161/cam.4.3.11747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harley BA, Leung JH, Silva EC, Gibson LJ. Mechanical characterization of collagen-glycosaminoglycan scaffolds. Acta Biomater. 2007;3(4):463–474. doi: 10.1016/j.actbio.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 36.Li S-T. Biologic biomaterials: tissue-derived biomaterials (collagen) The biomedical engineering handbook. 1995;2:42–41. [Google Scholar]
- 37.Nehrer S, Breinan HA, Ramappa A, Young G, Shortkroff S, Louie LK, Sledge CB, Yannas IV, Spector M. Matrix collagen type and pore size influence behaviour of seeded canine chondrocytes. Biomaterials. 1997;18(11):769–776. doi: 10.1016/s0142-9612(97)00001-x. [DOI] [PubMed] [Google Scholar]
- 38.Tsuruga E, Takita H, Itoh H, Wakisaka Y, Kuboki Y. Pore size of porous hydroxyapatite as the cell-substratum controls BMP-induced osteogenesis. Journal of Biochemistry. 1997;121(2):317–324. doi: 10.1093/oxfordjournals.jbchem.a021589. [DOI] [PubMed] [Google Scholar]
- 39.Sweigart MA, Zhu CF, Burt DM, DeHoll PD, Agrawal CM, Clanton TO, Athanasiou KA. Intraspecies and interspecies comparison of the compressive properties of the medial meniscus. Ann Biomed Eng. 2004;32(11):1569–1579. doi: 10.1114/b:abme.0000049040.70767.5c. [DOI] [PubMed] [Google Scholar]
- 40.Athanasiou KA, Rosenwasser MP, Buckwalter JA, Malinin TI, Mow VC. Interspecies comparisons of in situ intrinsic mechanical properties of distal femoral cartilage. J Orthop Res. 1991;9(3):330–340. doi: 10.1002/jor.1100090304. [DOI] [PubMed] [Google Scholar]
- 41.Proctor CS, Schmidt MB, Whipple RR, Kelly MA, Mow VC. Material properties of the normal medial bovine meniscus. J Orthop Res. 1989;7(6):771–782. doi: 10.1002/jor.1100070602. [DOI] [PubMed] [Google Scholar]
- 42.Gleeson JP, Plunkett NA, O'Brien FJ. Addition of hydroxyapatite improves stiffness, interconnectivity and osteogenic potential of a highly porous collagen-based scaffold for bone tissue regeneration. Eur Cell Mater. 2010;20:218–230. doi: 10.22203/ecm.v020a18. [DOI] [PubMed] [Google Scholar]
- 43.Cunniffe GM, Dickson GR, Partap S, Stanton KT, O'Brien FJ. Development and characterisation of a collagen nano-hydroxyapatite composite scaffold for bone tissue engineering. J Mater Sci Mater Med. 2010;21(8):2293–2298. doi: 10.1007/s10856-009-3964-1. [DOI] [PubMed] [Google Scholar]
- 44.Zeeman R, Dijkstra PJ, van Wachem PB, van Luyn MJA, Hendriks M, Cahalan PT, Feijen J. Crosslinking and modification of dermal sheep collagen using 1,4-butanediol diglycidyl ether. Journal of Biomedical Materials Research. 1999;46(3):424–433. doi: 10.1002/(sici)1097-4636(19990905)46:3<424::aid-jbm16>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 45.Standard A. Standard test method for tensile properties of plastics. ASTM International. Designation: D. 2003 [Google Scholar]
- 46.Alberti KA, Xu Q. Biocompatibility and degradation of tendon-derived scaffolds. Regen Biomater. 2016;3(1):1–11. doi: 10.1093/rb/rbv023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yahyouche A, Zhidao X, Czernuszka JT, Clover AJ. Macrophage-mediated degradation of crosslinked collagen scaffolds. Acta Biomater. 2011;7(1):278–286. doi: 10.1016/j.actbio.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 48.Badylak SF. Decellularized allogeneic and xenogeneic tissue as a bioscaffold for regenerative medicine: factors that influence the host response. Annals of biomedical engineering. 2014;42(7):1517–1527. doi: 10.1007/s10439-013-0963-7. [DOI] [PubMed] [Google Scholar]
- 49.Thorpe CT, Udeze CP, Birch HL, Clegg PD, Screen HR. Specialization of tendon mechanical properties results from interfascicular differences. J R Soc Interface. 2012;9(76):3108–3117. doi: 10.1098/rsif.2012.0362. [DOI] [PMC free article] [PubMed] [Google Scholar]