Abstract
Background
Low levels of physical activity can cause various physical symptoms or illness. However, few studies on this association have been conducted in young adults. The aim of this study was to investigate the association between physical activity levels and physical symptoms or illness in young adults.
Methods
Subjects were university students who participated in a web-based self-administered questionnaire in a university in Seoul in 2013. We obtained information on physical activities and physical symptoms or illness in the past year. Independent variables were defined as symptoms or illness which were associated with decreased academic performance. Logistic regression was performed to calculate odds ratios (ORs) and 95% confidence intervals (CIs) of each physical symptom or illness with adjustment for covariables.
Results
A total of 2,201 participants were included in the study. The main physical symptoms or illness among participants were severe fatigue (64.2%), muscle or joint pain (46.3%), gastrointestinal problems (43.1%), headache or dizziness (38.6%), frequent colds (35.1%), and sleep problems (33.3%). Low physical activity levels were significantly associated with high ORs of physical symptoms or illness. Multivariable-adjusted ORs (95% CIs) in the lowest vs. highest tertile of physical activity were 1.45 (1.14–1.83) for severe fatigue, 1.35 (1.07–1.70) for frequent colds, and 1.29 (1.02–1.63) for headaches or dizziness. We also found that lower levels of physical activity were associated with more physical symptoms or bouts of illness.
Conclusion
Low physical activity levels were significantly associated with various physical symptoms or illness among university students. Also, individuals in the lower levels of physical activity were more likely to experience more physical symptoms or bouts of illness than those in the highest tertile of physical activity.
Keywords: Physical Activity, Young Adult, Students, Fatigue, Headache, Dizziness, Myalgia, Arthralgia, Gastrointestinal Diseases, Sleep, Common Cold
INTRODUCTION
Ensuring health in young adults is critical for their health in their later adulthood, future generations' health, and social prospects.1) Nonetheless, a considerable number of young adults, especially university students, has been reported to suffer various physical symptoms or illness, such as headache, sleep disorder, and digestive symptoms.2,3,4) One of the main causes is decreased physical activity levels, which can lead to such physical symptoms or illness, whereas regular exercise and increased levels of physical activity can decrease the risk of those health problems.4,5,6,7,8,9,10,11,12,13,14) In addition, regular moderate exercise can decrease the risks of cardiovascular disease, hypertension, type 2 diabetes, osteoporosis, obesity, breast or colorectal cancer, and mortality.15,16,17,18,19) Based on this evidence, the American Heart Association recommends moderate levels of cardio-exercise for 30 minutes at least 5 times per week or strenuous exercise for 20 minutes at least 3 times per week for 18–65 year old adults.20) Similarly, regular physical exercise for 30 minutes at least 5 times per week is recommended in Korea as part of the tertiary national health promotion project.21)
Although the benefits of physical exercise are well-known, only 40% of young adults worldwide in 201022) and 45.5% of university students in the US23) met the recommendation. The physical activity levels in Korea are far lower than the global statistics; furthermore, recent trends in physical activities, including walking, have decreased since 2005 among all Korean adults, including young adults.24) The Korea National Health and Nutritional Examination Survey 2013 reported that only 26% of young adults aged 19–29 years practiced moderate or higher levels of exercise.24) Another study conducted among university students in Korea reported that only 20.8% of students regularly performed moderate levels of exercise.3) Since adolescence, most university students in Korea are sedentary in their lifestyle as they prepare for university entry examinations and later, employment applications. In addition, many students spend their leisure time watching TV or playing video games.3)
To present, only a few studies have been conducted on the association between physical activity levels and physical symptoms or illness in young adulthood. We aimed to examine the association between physical activity levels and various physical symptoms or illness among university students in Korea to determine the importance of physical activities in young adults.
METHODS
1. Study Participants
Subjects of this study were students of Seoul National University (SNU), who voluntarily participated in a student health survey conducted over 4 weeks in 2013. A total of 2,501 students participated in the survey, which included 8.9% of all students of the university. We excluded 300 students who did not answer the questions on physical activity, resulting in a total study population of 2,201 students. This study was approved by the institutional review board of Seoul National University (IRB number, SNUIRB 1301/001-007).
2. Variables
The student health survey used in this study was modified from the Global School-based Student Health Survey developed by the World Health Organization (WHO) and US Centers for Disease Control and Prevention,25) the National College Health Assessment developed by the American College Health Association,26) and the health statistics annual report in 2012 of the university at which this study was conducted.27)
Participants completed a web-based self-administered questionnaire on demographics (sex, age, academic status, residence, parents' education levels, and household income), height, weight, health-related lifestyle in the past year (smoking, alcohol use, dietary habits, and physical activity), and self-rated health status.
To assess physical symptoms or illness experienced in the past year, the following questions were asked: "Have you experienced any of the following health problems? (yes/no) (1) severe fatigue, (2) frequent colds, (3) gastrointestinal problems (indigestion, abdominal pain, diarrhea, or constipation), (4) muscle or joint pain (neck, shoulders, lower back, knees, or other), (5) headache or dizziness, (6) sleep problems (insomnia, snoring, or sleep insufficiency), (7) visual or hearing problems, and (8) any injuries or their sequelae." For those who answered that they had experienced any of the above problems, further questions were asked: "How did your health problems affect your school life? (1) no effect, (2) decreased academic performance, (3) absence from school, (4) leave of absence, (5) failure or holdover, or (6) interpersonal problems." For the current analysis, we defined physical symptoms or illness which were associated with decreased academic performance as the independent variable. In addition, the number of physical symptoms or illness reported by each participant was calculated and categorized into 4 groups (0, 1–2, 3–4, and 5–6 symptoms).
Physical activity levels were evaluated using the International Physical Activity Questionnaire,28,29) and total physical activity levels (metabolic equivalent [MET]-min/wk) were categorized into tertiles for analyses.
As covariates, age, sex, academic status (undergraduate, master's course, or doctoral course), hometown (Seoul, urban, rural, or foreign country), residence (living with parents, living alone, dormitory, or other), household income, smoking (non-smoker, ex-smoker, or current smoker), alcohol use (non-drinker, moderate, or heavy), weight status (underweight, normal weight, overweight, or obese), self-rated mental health (good, fair, or poor), and presence of chronic disease (hepatitis, hypertension, diabetes, or dyslipidemia) were included. Moderate drinking was defined as consuming 14 standard drinks or less per week in men and 7 or less standard drinks per week in women, and heavy drinking was defined as more than these amounts. Body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meters (kg/m2). Standard BMI cutoff points for the Korean population30) were used to categorize weight status: BMI less than 18.5 kg/m2 was defined as underweight, 18.5–22.9 kg/m2 as normal, 23–24.9 kg/m2 as overweight, and 25 kg/m2 or above as obese. Self-rated mental health was asked as "In the past year, how do you describe your general mental health?: (1) excellent, (2) good, (3) fair, (4) poor, or (5) very poor" We combined those who answered "excellent" and "good" into one group and those who answered "poor" and "very poor" into another group, resulting in 3 groups (good, fair, poor).
3. Statistical Analysis
To describe characteristics of study participants, frequencies, means, and standard deviations were calculated by physical activity levels. Frequencies of each physical symptom or illness among all participants and numbers of major symptoms per person were described. We conducted chi-square tests for categorical variables and variance analysis for continuous variables.
Logistic regression was performed to calculate odds ratios (ORs) and 95% confidence intervals (CIs) of each physical symptom or illness by physical activity tertiles, and was adjusted for covariates. We examined whether the associations between physical activity levels and physical symptoms or illness varied by sex. Tests for interaction were performed using the Wald test, using cross-product terms of physical activity and sex variables.
Because serious physical symptoms or illness could have lowered physical activity levels, we considered the possibility of reverse causality and performed sensitivity analyses after excluding the individuals who reported absence from school, leave of absence, failure or holdover, or interpersonal problems due to their physical symptoms or illness due to their symptoms or illness.
To assess the association between physical activity levels and the number of physical symptoms or illness, multinomial logistic regression was performed with adjustment for the covariates. After combining the lowest and median tertiles of physical activity into one group, we calculated multivariable-adjusted ORs for each number of physical symptoms or illness using binary logistic regression, with the highest tertile of physical activity as the reference.
All P-values were two-sided, and P<0.05 was considered statistically significant. Statistical analyses were performed using IBM SPSS ver. 20.0 (IBM Co., Armonk, NY, USA) software.
RESULTS
1. General Characteristics of Participants
A total of 2,201 participants were included in this study (Table 1). Male participants comprised 58.1%, and the mean age was 24.3 years. The median physical activity level was 765 MET-min/wk. Physical activity levels were higher in younger participants and were higher in men compared to women (P≤0.001).
Table 1. Characteristics of study participants by physical activity tertile.
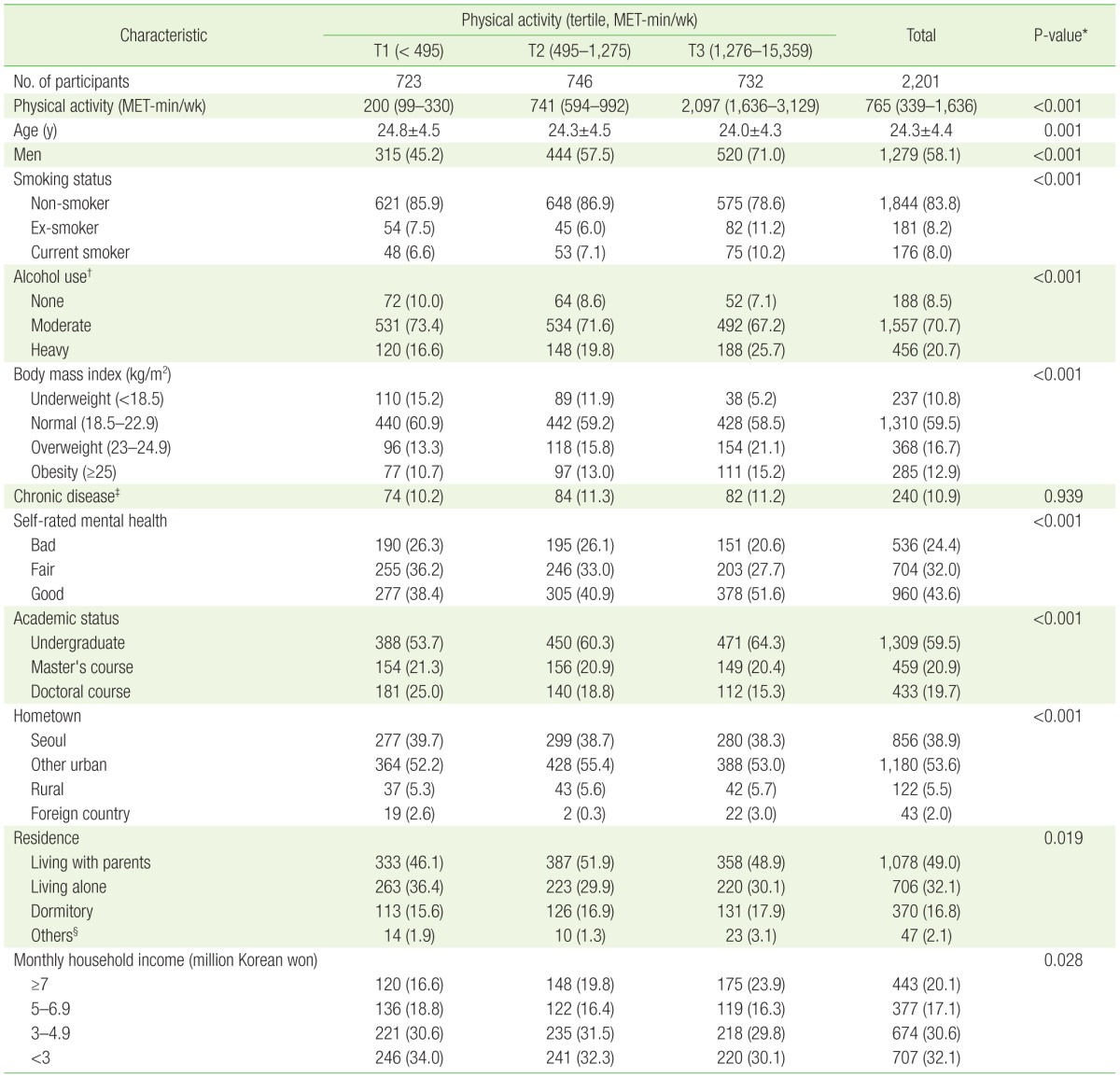
Values are presented as median (interquartile range), mean±standard deviation, or number (%).
MET, metabolic equivalent of task.
*From analysis of variance for continuous variable and chi-square test for categorical variables, respectively. †Moderate ≤14 drinks/wk in men, ≤7 drinks/wk in women; heavy >14 drinks/wk in men, >7 drinks/wk in women. ‡Included hypertension, diabetes mellitus, dyslipidemia, and hepatitis. §Living in a lodging house or with relatives or married, etc.
2. Frequencies of Physical Symptoms or Illness
Among the physical symptoms or illness exprienced in the past year, participants reported that severe fatigue (64.2%), muscle or joint pain (46.3%), gastrointestinal problems (43.1%), headache or dizziness (38.6%), frequent colds (35.1%), sleep problems (33.3%), hearing or visual problems (15.8%), and injuries or their sequelae (5.0%) affected their school life and decreased academic performance (Table 2). Based on the 6 main physical symptoms or illness reported by more than 30% of the partcipants, the mean number of physical symptoms or illness in each individual was 2.6 (2.4 in men and 2.9 in women), showing a greater number in women (P-value <0.001). Up to 21.2% of participants responded that they had experienced 5–6 physical symptoms or bouts of illness which decreased their academic performance in the past year.
Table 2. Frequency of physical symptoms or bouts of illness experienced in the past year.
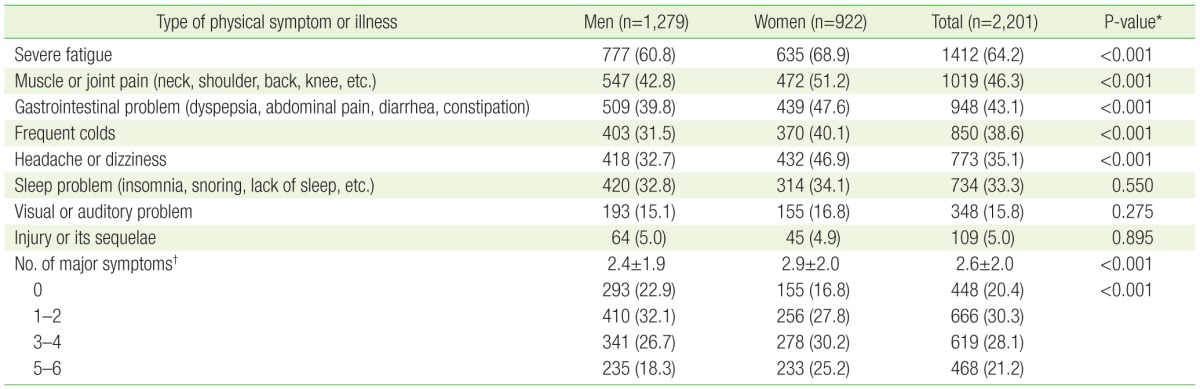
Values are presented as number (%) or mean±standard deviation. Participants were asked to choose as many symptoms or illnesses as they experienced in the past year.
*From chi-square test for categorical variables and independent t-test for the mean of number of major symptoms. †Included severe fatigue, muscle or joint pain, gastrointestinal problems, frequent colds, headache or dizziness, and sleep problems.
3. Physical Activity Levels and Physical Symptoms or Illness
After multivariable logistic regression, lower physical activity levels were associated with higher ORs of physical symptoms or illness that affected participants' academic performance (Table 3). Statistically significant associations were found for severe fatigue (P for trend=0.002), frequent colds (P for trend=0.012), and headache or dizziness (P for trend=0.034). Compared to the highest tertile of physical activity, multivariable-adjusted ORs (95% CIs) in the lowest tertile were 1.45 (1.14–1.83) for severe fatigue, 1.35 (1.07–1.70) for frequent colds, and 1.29 (1.02–1.63) for headaches or dizziness. Sleep problems and gastrointestinal symptoms were marginally associated with physical activity levels (P for trend=0.086, 0.069, respectively), although values were not statistically significant. No significant association was found between physical activity levels and muscle or joint pain (P for trend=0.408).
Table 3. Odds ratios* and 95% confidence intervals of physical symptom or illness by physical activity tertile.
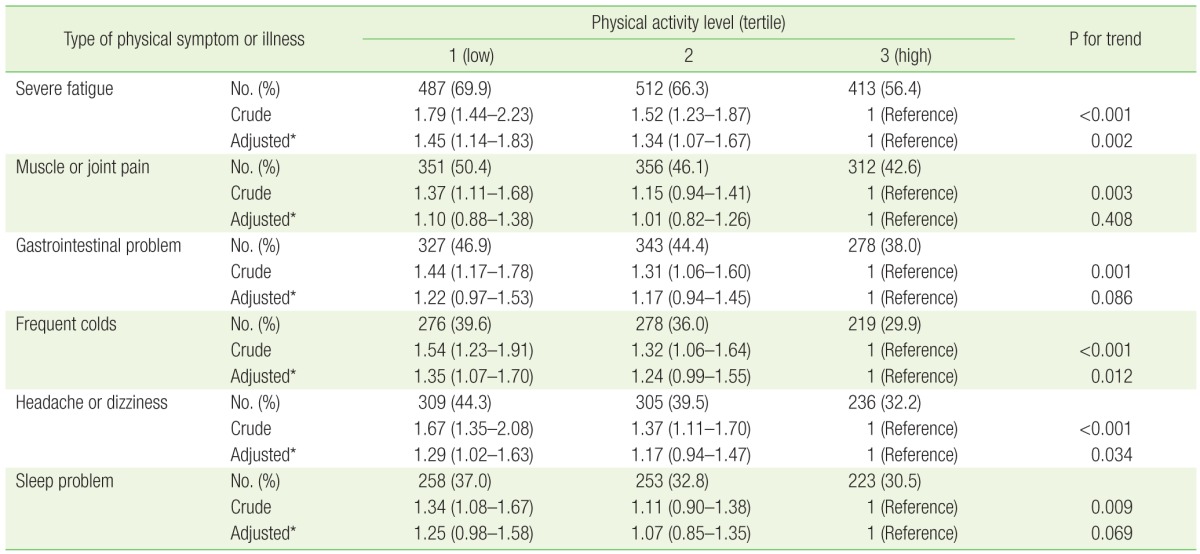
Values are presented as number (%) or odds ratio (95% confidence interval).
*Adjusted for age (continuous), sex, smoking status (none/past/current), alcohol use (none/moderate/heavy), body mass index (underweight/normal/overweight/obese), chronic disease (no/yes), self-rated mental health (bad/fair/good), academic status (undergraduate/master's course/doctoral course), hometown (Seoul/other urban/rural/foreign country), residence (living with parents/living alone/dormitory/others), and income (≥7, 5–6.9, 3–4.9, <3 million Korean won).
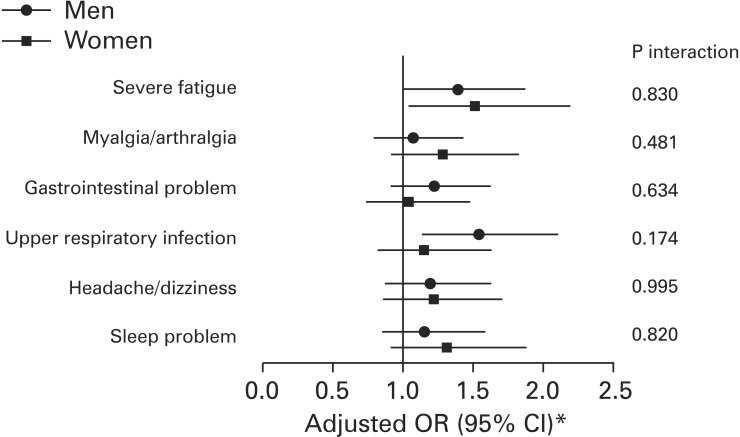
In the subgroup analysis, we found no significant interactions by sex for the associations between physical activity levels and physical symptoms or illness (P interaction: ≥0.292 for the median tertile vs. highest tertile, ≥0.174 for the lowest tertile vs. highest tertile) (Figure 1).
Figure 1. Multivariable ORs* and 95% CIs of physical symptoms or illness between lowest and highest tertiles of physical activity by sex. OR, odds ratio; CI, confidence interval. *Multivariable ORs for the lowest tertile of physical activity were calculated using logistic regression with the highest tertile as the reference and adjusted for age (continuous), sex, smoking status (none/past/current), alcohol use (none/moderate/heavy), body mass index (underweight/normal/overweight/obese), chronic disease (no/yes), self-rated mental health (bad/fair/good), academic status (undergraduate/master's course/doctoral course), hometown (Seoul/other urban/rural/foreign country), residence (living with parents/living alone/dormitory/others), and income (≥7, 5–6.9, 3–4.9, <3 million Korean won).
Individuals who reported serious limitations to their school life, such as absence from school, leave of absence, failure or holdover, or interpersonal problems, due to their physical symptoms or illness were likely to have experienced more severe symptoms or illness, which in turn, could have decreased their physical activity levels. In sensitivity analyses, we excluded individuals who reported serious limitations to school life due to symptoms or illness (427 for severe fatigue, 116 for muscle or joint pain, 200 for gastrointestinal symptoms, 158 for frequent colds, 134 for headache or dizziness, and 187 for sleep problems). After these exclusions, the results from remaining participants were similar to that of the main analyses: compared to the highest tertile, the ORs (95% CIs) in the lowest tertile were 1.42 (1.09–1.85) for severe fatigue, 1.09 (0.87–1.38) for muscle or joint pain, 1.33 (1.04–1.70) for headache or dizziness, 1.30 (1.03–1.65) for gastrointestinal symptoms, 1.38 (1.08–1.77) for frequent colds, and 1.29 (1.00–1.68) for sleep problems.
We further performed sensitivity analyses using different categorizations of physical activity levels. Using the WHO criteria,25) those who (1) performed strenuous exercise of 1,500 MET-min at least 3 times per week or (2) a total of at least 3,000 MET-min of exercise per week were defined as highly active; those who (1) performed strenuous activity for at least 20 minutes per day at least 3 times per week or (2) a moderate level of exercise or walked for at least 30 minutes per day at least 5 days per week, with total activity levels lower than those of highly active individuals, were defined as moderately active; and those who did not meet the above criteria were defined as having a low level of physical activity. As with the main results us ing the WHO physical activity criteria, we found similar associations (P for trend: severe fatigue 0.032, frequent colds 0.01, headache or dizziness 0.037). In addition, using quartiles of physical activity, the results were consistent (P for trend: severe fatigue 0.011, frequent colds 0.006, headache or dizziness 0.045).
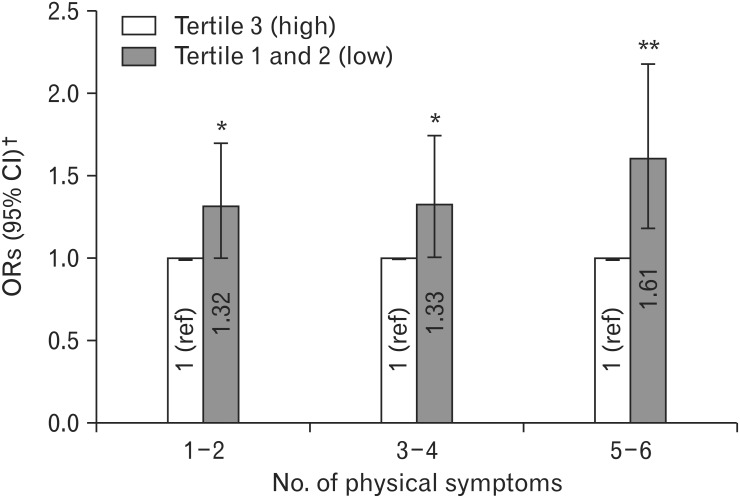
We found that lower levels of physical activity were associated with greater numbers of physical symptoms or bouts of illness, especially with 5–6 kinds of symptoms (Table 4). After multivariable-adjusted multinomial logistic regression, individuals in the median and lowest tertiles of physical activity were significantly more likely to experience 5–6 kinds of physical symptoms or illness compared to those in the highest tertile; the ORs (95% CIs) were 1.52 (1.08–2.14) in the median tertile and 1.73 (1.21–2.47) in the lowest tertile of physical activity. Since the ORs in the median tertile and the lowest tertile were similar, we combined the two tertiles into one group; the number of physical symptoms or bouts of illness in the combined group were subsequently compared to that in the highest tertile of physical activity (Figure 2). Individuals with lower levels of physical activity significantly tended to experience one or more physical symptoms or bouts of illness compared to those in the highest tertile of physical activity. The adjusted ORs (95% CIs) were 1.32 (1.01–1.71) for experiencing 1–2 different kinds of physical symptoms or illness, 1.33 (1.01–1.75) for experiencing 3–4 kinds, and 1.61 (1.19–2.19) for experiencing 5–6 kinds.
Table 4. Multivariable odds ratios* and 95% confidence intervals of number of physical symptoms by physical activity tertile.
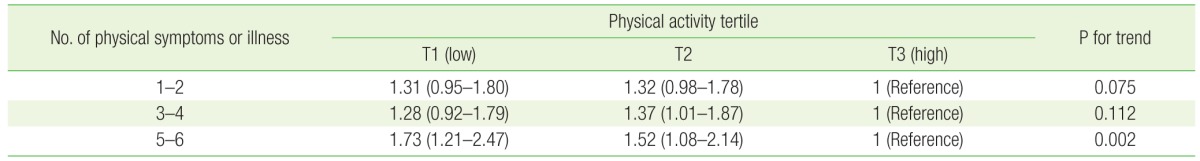
Values are presented as odds ratio (95% confidence interval).
*Multivariable odds ratio were calculated using multinomial logistic regression and adjusted for age (continuous), sex, smoking status (none/past/current), alcohol use (none/moderate/heavy), body mass index (underweight/normal/overweight/obese), chronic disease (no/yes), self-rated mental health (bad/fair/good), academic status (undergraduate/master's course/doctoral course), hometown (Seoul/other urban/rural/foreign country), residence (living with parents/living alone/dormitory/others), and income (≥7, 5–6.9, 3–4.9, <3 million Korean won).
Figure 2. Multivariable ORs† and 95% CIs of number of physical symptoms by physical activity levels. OR, odds ratio; CI, confidence interval; ref, reference. *P-value <0.05. **P-value <0.01. †Multivariable ORs were calculated using binary logistic regression and adjusted for age (continuous), sex, smoking status (none/past/current), alcohol use (none/moderate/heavy), body mass index (underweight/normal/overweight/obese), chronic disease (no/yes), self-rated mental health (bad/fair/good), academic status (undergraduate/master's course/doctoral course), hometown (Seoul/other urban/rural/foreign country), residence (living with parents/living alone/dormitory/others), and income (≥7, 5–6.9, 3–4.9, <3 million Korean won).
DISCUSSION
In this study among university students, low levels of physical activity were significantly associated with various physical symptoms or illness that led to decreased academic performance. Severe fatigue, frequent colds, and headache or dizziness were significantly associated with low levels of physical activity, and sleep problems and gastrointestinal symptoms were marginally associated. In addition, individuals with lower levels of physical activity were more likely to experience greater numbers of physical symptoms or bouts of illness compared to those in the highest tertile of physical activity.
Similar to our results, a meta-analysis of 12 cohorts reported that adults who were physically active tended to have lower levels of fatigue than inactive adults.31) Another study among patients with chronic fatigue syndrome reported a negative association between fatigue and the level of exercise.8) Several studies reported that increasing physical activity can improve fatigue and reduce weight gain, depression, and anxiety among cancer survivors or patients with multiple sclerosis or Parkinson's disease.32,33,34,35) Although the mechanisms are unclear, some evidence supports that physical activity modifies the levels of neurotransmitters, such as monoamines, histamine, acetylcholine, and γ-aminobutyric acid, which affects the levels of fatigue.36)
In our study, a linear negative association between physical activity levels and the common cold was observed. A prior cross-sectional study reported a U- or J-curve association between physical activity levels and the common cold. The authors hypothesized that moderate levels of physical activity may improve the immune system to help prevent the common cold, while strenuous exercise can produce excess free radicals and increase the risk of the common cold.37) Another cross-sectional study found no association between physical activity levels and the common cold.38) To the best of our knowledge, no longitudinal study has been conducted on this association, and the association between physical activity and the risk of common cold remains unclear.
It is well-known that physical activity can worsen migraine headaches, and migraine headaches were associated with a lower level of physical activity in a cross-sectional study.8) In contrast, low levels of physical activity were significantly associated with an increased risk of non-migraine headaches, including tension-type headache in a prospective study designed to exclude reverse causality.6) In the current study, we also found significant associations between low physical activity levels and headaches in the main and sensitivity analyses.
Several previous studies among athletes who practiced strenuous exercise reported a J-curve association between physical activity levels and gastrointestinal problems, such as heartburn, indigestion, and diarrhea, with the fewest symptoms occurring with moderate levels of physical activity.7,10) We found a borderline linear negative association between digestive symptoms and physical activity levels, although it was not statistically significant. Because the levels of physical activity in our participants (mean of 2,643 MET-min/wk) were relatively lower than those in the previous studies among athletes, the association found in our study cannot be applied to those with higher levels of physical activity.
Previous studies found that regular moderate exercise can increase slow-wave sleep, total sleep duration, and improved sleep quality.33) We found a borderline association between physical activity levels and sleep problems, although the association was not statistically significant. The non-significance may be due to the broad definition of sleep problems in our study, including insomnia, snoring, or insufficient sleep, whereas prior studies on sleep were more specific to insomnia. In addition, our study population consisted of university students, who were more likely to suffer involuntarily from sleep insufficiency due to their academic activities.
Strenuous exercise can cause musculoskeletal pain from muscle damage, sprain, or post-exercise syndrome.23,39) Whereas, low levels of physical activity are a risk factor for low back pain.40) Thus, both low and very high levels of physical activity can cause different types of musculoskeletal pain. In our study, no significant associations between physical activity levels and muscle or joint pain were found among university students. One of the reasons for the null association in our study may be that we did not specify the types of muscle or joint pain, which can include both musculoskeletal pains from vigorous exercise and pains due to sedentary lifestyle.
A prior cohort study in Australia reported that increasing physical activity decreased the numbers of various physical symptoms in women.41) Consistent with these prior results, we also found that low physical activity levels were associated with greater numbers of physical symptoms or bouts of illness among young university students.
Our study had several strengths. This study included a relatively large number of young adults; this age group has been far less investigated for the factors associated with health problems and illness compared to other age groups. We collected a broad range of information on demographic, socioeconomic, anthropometric, and lifestyle factors; thereby, we were able to adjust for potential confounders in analyses. We conducted various sensitivity and subgroup analyses, and the results were almost similar to those of the main analyses, thus providing evidence regarding the robustness of our results.
This study has the following limitations. First, the cross-sectional nature of this study limits causal and temporal inference. To overcome this limitation to some degree, we performed sensitivity analyses, and the results were consistent with the main results. Second, because our study was based on the participants' memories, we cannot exclude the possibility of incompleteness of memory. Lastly, our study population consisted of Korean university students at one university; therefore, the results cannot be generalized to the entire population, but it may reduce the possibility of confounding by other demographic variables, such as age or ethnicity.
In conclusion, reduced levels of physical activity were significantly associated with various physical symptoms or illness that limited students' academic performance. Severe fatigue, frequent colds, and headache or dizziness were significantly associated with low physical activity levels, and sleep problems and gastrointestinal symptoms were marginally associated. In addition, individuals with lower levels of physical activity were more likely to experience a greater number of physical symptoms or bouts of illness compared to those with higher levels of physical activity. To confirm these associations, further prospective and interventional studies on the relationship between physical activity and the development of physical symptoms or health problems are warranted.
Footnotes
CONFLICT OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
- 1.World Health Organization. Health for the world's adolescents: a second chance in the second decade. Geneva: World Health Organization; 2014. [Google Scholar]
- 2.Seoul National University Health Service Center. Student health checkup report 2013. Seoul: Seoul National University; 2013. [Google Scholar]
- 3.Kim YB, Park CM, Kim HH, Han CH. Health behavior and utilization of university health clinics. J Korean Soc Sch Health Educ. 2010;11:79–91. [Google Scholar]
- 4.Park JY, Kim NH. Relationships between physical activity, health status, and quality of life of university students. J Korean Public Health Nurs. 2013;30:153–165. [Google Scholar]
- 5.Driver HS, Taylor SR. Exercise and sleep. Sleep Med Rev. 2000;4:387–402. doi: 10.1053/smrv.2000.0110. [DOI] [PubMed] [Google Scholar]
- 6.Varkey E, Hagen K, Zwart JA, Linde M. Physical activity and headache: results from the Nord-Trondelag Health Study (HUNT) Cephalalgia. 2008;28:1292–1297. doi: 10.1111/j.1468-2982.2008.01678.x. [DOI] [PubMed] [Google Scholar]
- 7.Simrén M. Physical activity and the gastrointestinal tract. Eur J Gastroenterol Hepatol. 2002;14:1053–1056. doi: 10.1097/00042737-200210000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Vercoulen JH, Bazelmans E, Swanink CM, Fennis JF, Galama JM, Jongen PJ, et al. Physical activity in chronic fatigue syndrome: assessment and its role in fatigue. J Psychiatr Res. 1997;31:661–673. doi: 10.1016/s0022-3956(97)00039-3. [DOI] [PubMed] [Google Scholar]
- 9.Bize R, Johnson JA, Plotnikoff RC. Physical activity level and health-related quality of life in the general adult population: a systematic review. Prev Med. 2007;45:401–415. doi: 10.1016/j.ypmed.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 10.Peters HP, De Vries WR, Vanberge-Henegouwen GP, Akkermans LM. Potential benefits and hazards of physical activity and exercise on the gastrointestinal tract. Gut. 2001;48:435–439. doi: 10.1136/gut.48.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spierings EL, Ranke AH, Honkoop PC. Precipitating and aggravating factors of migraine versus tension-type headache. Headache. 2001;41:554–558. doi: 10.1046/j.1526-4610.2001.041006554.x. [DOI] [PubMed] [Google Scholar]
- 12.Atkinson G, Davenne D. Relationships between sleep, physical activity and human health. Physiol Behav. 2007;90:229–235. doi: 10.1016/j.physbeh.2006.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kikuchi H, Yoshiuchi K, Ohashi K, Yamamoto Y, Akabayashi A. Tension-type headache and physical activity: an actigraphic study. Cephalalgia. 2007;27:1236–1243. doi: 10.1111/j.1468-2982.2007.01436.x. [DOI] [PubMed] [Google Scholar]
- 14.Kujala UM, Taimela S, Viljanen T. Leisure physical activity and various pain symptoms among adolescents. Br J Sports Med. 1999;33:325–328. doi: 10.1136/bjsm.33.5.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee IM, Shiroma EJ, Lobelo F, Puska P, Blair SN, Katzmarzyk PT, et al. Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. Lancet. 2012;380:219–229. doi: 10.1016/S0140-6736(12)61031-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sigal RJ, Kenny GP, Wasserman DH, Castaneda-Sceppa C, White RD. Physical activity/exercise and type 2 diabetes: a consensus statement from the American Diabetes Association. Diabetes Care. 2006;29:1433–1438. doi: 10.2337/dc06-9910. [DOI] [PubMed] [Google Scholar]
- 17.Berlin JA, Colditz GA. A meta-analysis of physical activity in the prevention of coronary heart disease. Am J Epidemiol. 1990;132:612–628. doi: 10.1093/oxfordjournals.aje.a115704. [DOI] [PubMed] [Google Scholar]
- 18.Vuori IM. Dose-response of physical activity and low back pain, osteoarthritis, and osteoporosis. Med Sci Sports Exerc. 2001;33(6 Suppl):S551–S586. doi: 10.1097/00005768-200106001-00026. [DOI] [PubMed] [Google Scholar]
- 19.McCann BA, Ewing RH Smart Growth America; Surface Transportation Policy Project. Measuring the health effects of sprawl: a national analysis of physical activity, obesity and chronic disease. Washington (DC): Smart Growth America; 2003. [Google Scholar]
- 20.Haskell WL, Lee IM, Pate RR, Powell KE, Blair SN, Franklin BA, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Circulation. 2007;116:1081–1093. doi: 10.1161/CIRCULATIONAHA.107.185649. [DOI] [PubMed] [Google Scholar]
- 21.Government of the Republic of Korea. National health plan 2020. Seoul: Government of the Republic of Korea; 2011. [Google Scholar]
- 22.World Health Organization. Physical activity [Internet] Geneva: World Health Organization [cited 2015 Oct 1]; Available from: http://www.who.int/mediacentre/factsheets/fs385/en/ [Google Scholar]
- 23.Jones BH, Cowan DN, Tomlinson JP, Robinson JR, Polly DW, Frykman PN. Epidemiology of injuries associated with physical training among young men in the army. Med Sci Sports Exerc. 1993;25:197–203. [PubMed] [Google Scholar]
- 24.Ministry of Health and Welfare, Korea Centers for Disease Control and Prevention. Korea health statistics 2013: Korea National Health and Nutrition Examination Survey (KNHANESVI-1) Cheongju: Korea Centers for Disease Control and Prevention; 2014. [Google Scholar]
- 25.Centers for Disease Control and Prevention. CDC global school-based student health survey (GSHS) [Internet] Atlanta (GA): Centers for Disease Control and Prevention; [cited 2015 Sep 4]. Available from: http://www.cdc.gov/GSHS/ [Google Scholar]
- 26.American College Health Association-National College Health Assessment. ACHA-NCHA data [Internet] Hanover (MD): American College Health Association-National College Health Assessment; [cited 2015 Sep 4]. Available from: http://www.acha-ncha.org/ [Google Scholar]
- 27.Seoul National University Health Service Center. Health statistics annual report 2012. Seoul: Seoul National University; 2012. [Google Scholar]
- 28.Oh JY, Yang YJ, Kim BS, Kang JH. Validity and reliability of Korean version of International Physical Activity Questionnaire (IPAQ) short form. J Korean Acad Fam Med. 2007;28:532–541. [Google Scholar]
- 29.International Physical Activity Questionnaire Research Committee. Guidelines for data processing and analysis of the International Physical Activity Questionnaire (IPAQ)-short and long forms [Internet] 2005. [cited 2015 Jun 29]. Available from: http://www.academia.edu/5346814/Guidelines.
- 30.Inoue S. The Asia-Pacific perspective: redefining obesity and its treatment. Sydney: Health Communications Australia; 2000. [cited 2015 Sep 2]. Available from: http://www.vepachedu.org/TSJ/BMI-Guidelines.pdf. [Google Scholar]
- 31.Puetz TW. Physical activity and feelings of energy and fatigue: epidemiological evidence. Sports Med. 2006;36:767–780. doi: 10.2165/00007256-200636090-00004. [DOI] [PubMed] [Google Scholar]
- 32.Speck RM, Courneya KS, Masse LC, Duval S, Schmitz KH. An update of controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. J Cancer Surviv. 2010;4:87–100. doi: 10.1007/s11764-009-0110-5. [DOI] [PubMed] [Google Scholar]
- 33.Mostert S, Kesselring J. Effects of a short-term exercise training program on aerobic fitness, fatigue, health perception and activity level of subjects with multiple sclerosis. Mult Scler. 2002;8:161–168. doi: 10.1191/1352458502ms779oa. [DOI] [PubMed] [Google Scholar]
- 34.Garber CE, Friedman JH. Effects of fatigue on physical activity and function in patients with Parkinson's disease. Neurology. 2003;60:1119–1124. doi: 10.1212/01.wnl.0000055868.06222.ab. [DOI] [PubMed] [Google Scholar]
- 35.Dimeo FC, Stieglitz RD, Novelli-Fischer U, Fetscher S, Keul J. Effects of physical activity on the fatigue and psychologic status of cancer patients during chemotherapy. Cancer. 1999;85:2273–2277. [PubMed] [Google Scholar]
- 36.Stahl SM. The psychopharmacology of energy and fatigue. J Clin Psychiatry. 2002;63:7–8. doi: 10.4088/jcp.v63n0102. [DOI] [PubMed] [Google Scholar]
- 37.Matthews CE, Ockene IS, Freedson PS, Rosal MC, Merriam PA, Hebert JR. Moderate to vigorous physical activity and risk of upper-respiratory tract infection. Med Sci Sports Exerc. 2002;34:1242–1248. doi: 10.1097/00005768-200208000-00003. [DOI] [PubMed] [Google Scholar]
- 38.Hemilä H, Virtamo J, Albanes D, Kaprio J. Physical activity and the common cold in men administered vitamin E and beta-carotene. Med Sci Sports Exerc. 2003;35:1815–1820. doi: 10.1249/01.MSS.0000093616.60899.92. [DOI] [PubMed] [Google Scholar]
- 39.Cimmino MA, Parisi M, Moggiana GL, Maio T, Mela GS. Prevalence of self-reported peripheral joint pain and swelling in an Italian population: the Chiavari study. Clin Exp Rheumatol. 2001;19:35–40. [PubMed] [Google Scholar]
- 40.Heneweer H, Vanhees L, Picavet HS. Physical activity and low back pain: a U-shaped relation? Pain. 2009;143:21–25. doi: 10.1016/j.pain.2008.12.033. [DOI] [PubMed] [Google Scholar]
- 41.Brown WJ, Mishra G, Lee C, Bauman A. Leisure time physical activity in Australian women: relationship with well being and symptoms. Res Q Exerc Sport. 2000;71:206–216. doi: 10.1080/02701367.2000.10608901. [DOI] [PubMed] [Google Scholar]