Abstracts
Upland cotton (Gossypium hirsutum L.) is an important fiber crop species, which is intensively plagued by a plethora of phytopathogenic fungi such as Fusarium oxysporum f. sp. vasinfectum (Fov) causing severe wilt disease. Resistance gene analogs (RGAs) are the largest class of potential resistance (R) genes depicting highly conserved domains and structures in plants. Additionally, RGAs are pivotal components of breeding projects towards host disease resistance, serving as useful functional markers linked to R genes. In this study, a cloning approach based on conserved RGAs motifs was used in order to amplify 38 RGAs from two upland cotton cultivars differing in their Fov susceptibility. Besides, we assessed the phylogenetic expansion and the evolutionary pressures acting upon 127 RGA homologues, which were previously deposited in GenBank along with the 38 RGAs from this study. A total of 165 RGAs sequences were clustered according to their BLAST(P) similarities in ten paralogous genes groups (PGGs). These RGAs exhibited intensive signs of positive selection as it was revealed by inferring various maximum likelihood analyses. The results showed robust signs of positive selection, acting in almost all PGGs across the phylogeny. The evolutionary analysis revealed the existence of 42 positively selected residue sites across the PGG lineages, putatively affecting their ligand-binding specificities. As RGAs derived markers are in close linkage to R genes, these results could be used in ongoing breeding programs of upland cotton.
Keywords: Fungal diseases, Molecular breeding, Non-synonymous nucleotide substitution
Introduction
Upland cotton (Gossypium hirsutum L.) is one of the most important cultivated fiber crops worldwide. However, upland cotton is susceptible to a wide range of fungal pathogens, which may have detrimental effects in its yield and product quality (Zambounis et al. 2012a). Fusarium oxysporum f. sp. vasinfectum (Fov), an ubiquitous soil inhabiting fungus causing extensive vascular wilt, is among the most destructive fungal diseases of upland cotton (Zambounis et al. 2007). Despite the fact that the genome of upland cotton has been recently sequenced (Li et al. 2015), identification of functional molecular markers, which could be linked to loci related to resistance genes has been hampered due to intraspecific nature of the genome (Tan et al. 2003). Complete resistant upland cotton cultivars against Fov isolates are not reported till date. Nevertheless, numerous efforts have been previously made for assisting disease resistance breeding programs (Fang et al. 2014; Hinchliffe et al. 2005; Niu et al. 2011), mainly by means of cloning segments of putative disease resistance (R) genes known as resistance gene analogues (RGAs).
Employment of molecular breeding approaches allows breeders to meet the challenge of developing disease resistant crops more accurately and faster (Ameline-Torregrosa et al. 2008). Most of these approaches are focused on the family of genes containing NBS (nucleotide-binding site)/LRRs (Leucine-Rich Repeats)-domains which constitute an expanded group of plant receptor genes linked directly to defense reactions (Dangl et al. 2013; Debener and Byrne 2014). In turn, LRR domains evolve novel ligand-binding specificities under strong positive selection with a wealth of different mechanisms, ranging from point mutations, variations in their repeat numbers and tandem gene duplications (Yang et al. 2008; Zambounis et al. 2012b). As a consequence, the modes of evolution of NBS-LRR R genes and of their RGA counterparts are an intricate process in plants (Sekhwal et al. 2015).
The aim of this study, based on the above evidence, was: (1) to amplify novel RGA clones using degenerate primers designed upon conserved motifs of known R genes from two upland cotton cultivars, with differences in their Fov susceptibility, (2) to explore whether it is likely that positive selective signatures are employed as an intensive evolutionary force, acting on a combined RGA dataset comprising these RGA clones, along with the RGAs that were previously deposited in GenBank from upland cotton. The results of this study, allow us to gain insights into the evolutionary profiles of these RGAs in upland cotton, hypothesizing that various episodes of positive selection might contribute in the acquisition of novel pathogen recognition repertoires. In parallel, these results might provide a crucial foundation for the disease resistance breeding programs in upland cotton.
Materials and methods
Upland cotton seeds of the “EMERALD” (Deltapine, Narrabri, NSW, Australia) and the “LACTA” (Bayer Crop Science) cultivars, partially resistant and fully susceptible, respectively to Fov (Zambounis et al. 2012a) were planted in plug trays containing sterile Promix soil and grown in growth chambers with a 16-h photoperiod at 25 °C and 70 μmol m−2 s−1 light. Genomic DNA was extracted from young leaves using the DNeasy Plant Mini Kit (Qiagen Inc., Chatsworth, CA, USA).
A pair of degenerate primers (P-loop_dom:5′-GGTGGTATGGGNAARACNACNYT-3′; GLPL_dom: 5′-CTTGAAAGCVAAWGGNARNCC-3′) was designed with some modifications (Shen et al. 1998) in order to amplify coding regions spanning the most conserved NBS domains (the P-loop and the GLPL) of cotton RGAs. These two degenerate primers were particularly designed based on the above conserved domains of the I2 gene of tomato, which confers resistance to race 2 of Fusarium oxysporum f. sp. lycopersici (Simons et al. 1998) and of the ArabidopsisRPS2 gene, which encodes a protein that specifically recognizes Pseudomonas syringae pv. tomato strains (Axtell et al. 2001).
All PCR amplifications were performed in 50 μl volumes containing 0.2 mΜ of each dNTP, 0.5 μM of each degenerate primer, 1 U of Dy-NAzyme II DNA polymerase (Finnzymes, Espoo, Finland), 2.5 mM of MgCl2 and 100 ng of template genomic DNA. The initial denaturation step was 95 °C for 5 min, followed by 30 cycles of 95 °C for 1 min, 48 °C for 1 min, 72 °C for 2 min, with a final extension step at 72 °C for 10 min. PCR products were electrophoresed on a 1.2 % agarose gel and the 530 bp respective bands were selectively recovered and purified by precipitation with 0.1 volume of 3 M sodium acetate and 2.5 volumes of absolute ethanol to remove excess dNTPs and primers.
The PCR products were cloned into the pGEM-T Easy vector (Promega) and a total of 49 putative recombinant colonies were obtained and sequenced (I.M.B.B., Crete, Greece). The BLAST(hX) program was employed to confirm their homologies with known RGAs and R genes from upland cotton. Sequences with internal stop codons were excluded from the analysis. Thus, there remained 26 sequences from the EMERALD (Gh_EM) and 12 from the LACTA (Gh_LA). These RGA sequence data, 38 in total, were deposited in the Genbank under accession numbers KU680752 through KU680789.
Additionally, based on their NB-ARC domain profiles (PF00931.18/CL0023) and keyword searches, 127 partial RGAs from upland cotton (Gh_GB) previously deposited in GenBank were mined. Using their amino acid sequence IDs, we also retrieved the respective ORF nucleotide sequences using a python script. Therefore, our final RGA dataset consisted of 165 sequences in total. All these sequences had amino acid length up to 100 residues, no internal stop codons, and BLAST hits with known R genes of upland cotton. They shared valid structures and functional domains (LRRs or NB-ARC domain motifs) as it was observed by searches against both the Pfam and InterPro protein databases (Jones et al. 2014).
The phylogenetic relationships among these 165 RGA amino acid sequences were revealed by performing a Muscle alignment (Edgar 2004) and a tree reconstruction employing the RAxML software (Stamatakis 2014) with a gamma model of rate heterogeneity. The above analyses were performed using the Geneious R7 platform (Kearse et al. 2012). The RGAs sequences were consequently clustered using the MCL (Markov Cluster Algorithm) approach with an inflation value of 2.00 (Enright et al. 2002). The clustering process utilized the all-against-all BLAST(P) output as a distance metric and finally ten unique paralogous genes groups (PGGs) were assigned. The validity of these PGGs was confirmed based on the overall similarity throughout the coding sequences, the existence of no or few gaps across the aligned sequences at each PGG, and a 50 % amino acid identity between the sequences in each PGG.
The eight out of the ten PGGs, which contained up to three RGAs, were independently evaluated for positive selective signatures (the remaining two PGGs, which contained only two RGAs each were not evaluated) using the maximum likelihood programs (CODEML and CODEMLSITES) inferring in the PAML software package (Yang 2007). CODEML estimates variable selective pressures among the branches of the phylogeny, whilst CODEMLSITES tests for site-specific codon substitution models permitting the identification of selective signatures among individual amino acids. In order to compare the rates of non-synonymous/synonymous nucleotide substitution mutations (omega values), NJ trees and nucleotide respective alignments were performed (Lynn et al. 2004). Nucleotide coding sequences were initially aligned by a perl script in order to maintain the gaps and to exclude any columns in the alignments that had more than three gaps (Zambounis et al. 2012b). Amino acid alignments were performed using the MUSCLE software (Edgar 2004), whilst the constructions of the respective evolutionary trees were carried out by employing the MEGA 5 software (Tamura et al. 2011). The Poisson correction was employed for all distances calculations along with a gamma distribution among the sites and the rate variations (Zambounis et al. 2012b). In both programs, comparisons among the various selective models were performed by employing likelihood ratio tests. The Bayesian approach allowed further detection of codon residues subjected to positive selection by posterior probabilities.
Results and discussion
Yield of upland cotton can be substantially decreased by harmful fungi, such as by Fov virulent isolates, which might have devastating effects on the vascular system and is a major threat worldwide (Zambounis et al. 2012a). Early recognition is the first crucial step of defense reactions against fungi attacks in plants. It is being commonly mediated by a plethora of rapidly evolving protein receptors, which are containing ligand-binding and signal transduction domains, such as LRRs and NB-ARC domains, respectively (Perazzolli et al. 2014; Sekhwal et al. 2015). The development through molecular breeding of fungal resistant genotypes is crucial towards the substantial establishment of fungal diseases management strategies (Ganopoulos et al. 2011), especially in upland cotton with a complex allotetraploid genome (Fang et al. 2014) being recently available (Li et al. 2015).
In this study, 38 RGAs were cloned and sequenced from two upland cotton cultivars using degenerate primers being designed upon conserved motifs of known R genes. These RGAs were overlapping the coding regions of P-loop and GLPL motifs flanking the RNBS-A, the Kinase-2, the RNBS-B and the RNBS-C motifs of known R genes in upland cotton genome. Since, no introns were present between these conserved motifs, only one expected size, approximately 530 bp in length, was amplified, cloned and sequenced. These experiments have been performed without the upland cotton genome being available at that time and thus, the degenerate primers were designed upon conserved regions of known functional R genes. Besides, in upland cotton there do not exist known R genes whose function is confirmed at the moment. Furthermore, our analysis has been conducted in two upland cotton cultivars, which were different than the one whose whole genome has been sequenced. These two cultivars were also chosen as they are different at their susceptibility against Fov.
BLAST(X) searches of these RGAs revealed that their conserved NB-ARC domains were highly homologous with those of the annotated R genes in the genome of upland cotton. RGAs are in general useful candidates and quite informative components for any disease resistance breeding approach. They are also useful for the accurate characterization of R genes (Chen et al. 2015). These coding sequences, which were identified by PCR-based approaches, were only partial regions of the respective R genes without any solid functional annotations in the genome and thus, mapping them against the genome was technically intricate. However, all these RGA sequences were found to share either Pfam hits with the NB-ARC domain (PF00931.18) or InterProScan hits with the LRR-containing proteins (PTHR23155) when they were compared with the annotated R genes from the upland cotton genome. In the near feature, we have the intention to explore with RNA-seq data and to confirm by RT-PCR approaches their expression levels after challenging both tested cultivars with Fov isolates.
These RGA sequences were also ideal candidates for applying evolutionary approaches in order to gain insights into the positive selective pressures acting upon them. They were sharing conserved domains and features overlapping coding regions and functional domains of R genes. Thus, we mined an ample number of previously deposited RGA sequences of upland cotton from GenBank. For the phylogenetic and evolutionary analyses, our final dataset comprised of the 38 RGAs from this study, in addition to the 127 RGAs, which were previously deposited in GenBank. All-against-all BLAST(P) searches (E value cutoff <10−10) were applied in order to assess the extent to which these 165 RGAs genes were clustered using the MCL approach (similarity cutoff of 50 %), allowing their classification into respective PGGs. They were assigned in 10 PGGs (named as Gh_RGA) in total. Eight of them contained from three up to 62 RGAs and 161 RGAs in total (Table 1). The majority (88 %) of RGAs were clustered in five different PGGs (Gh_RGA-1, Gh_RGA-2, Gh_RGA-5¸ Gh_RGA-6, Gh_RGA-8). In contrast, two Gh_RGA PGGs contained only two RGAs (Table 1); these RGAs were excluded from the further phylogenetic and evolutionary analysis.
Table 1.
Overview of CODEML and CODEMLSITES programs statistics upon the evolutionary analyses in upland cotton RGAs
| RGAs PGGs | Number of clustered RGAs sequenses (composition of clusters) | CODEML analysis | CODEMLSITES analysis | |||||
|---|---|---|---|---|---|---|---|---|
| Proportion of branches under positive selection | Positive selection acting on anchestral (A) and (or) terminal (T) branches | Highest omega values in branches of the evolutionaty trees | Statistical significance (P) | Total number of sitesa | Positively selected sites | Positively selected sitesb | ||
| Gh_RGA-1 | 62 (Gh_GB, Gh_EM, Gh_LA) | 6.8 | A/T | ∞ | <0.01 | 447 | 6 | 2I, 7Y, 9A, 12H, 16R, 17L |
| Gh_RGA-2 | 38 (Gh_GB, Gh_EM, Gh_LA) | 29.7 | A/T | ∞ | < 0.9 | 252 | ||
| Gh_RGA-3 | 5 (Gh_GB) | 12.5 | T | 288.1 | <0.05 | 258 | 2 | 25L, 39Y |
| Gh_RGA-4 | 6 (Gh_GB, Gh_EM) | 10 | A | ∞ | <0.05 | 486 | 3 | 34Q, 42H, 44D |
| Gh_RGA-5 | 13 (Gh_GB) | 12.5 | A/T | ∞ | <0.001 | 510 | 9 | 17S, 31 K, 40 V, 104L, 117A, 121G, 138L, 168F, 169A |
| Gh_RGA-6 | 13 (Gh_GB) | 16.7 | T | 1.88 | <0.01 | 237 | 15 | 2E, 3 K, 8 K, 28D, 30C, 42P, 45E, 50 N, 54E, 55I, 58G, 62 N, 69 V,70A, 72 N |
| Gh_RGA-7 | 5 (Gh_GB) | 42.9 | T | 3.52 | <0.01 | 507 | 6 | 48L, 52D, 99 V, 116E, 152P, 154P |
| Gh_RGA-8 | 19 (Gh_GB) | 13.9 | T | ∞ | <0.9 | 495 | 1 | 8L |
| Gh_RGA-9 | 2 (Gh_EM | NT | ||||||
| Gh_RGA-10 | 2 (Gh_GB) | NT | ||||||
Likelihood ratio test among M8 and M7 models
aLengths are refereing to the alignments feeding the CODEMLSITES program
bPosterior Bayesian probabilities >0.95 are shown in lightface, and >0.99 values are shown in boldface
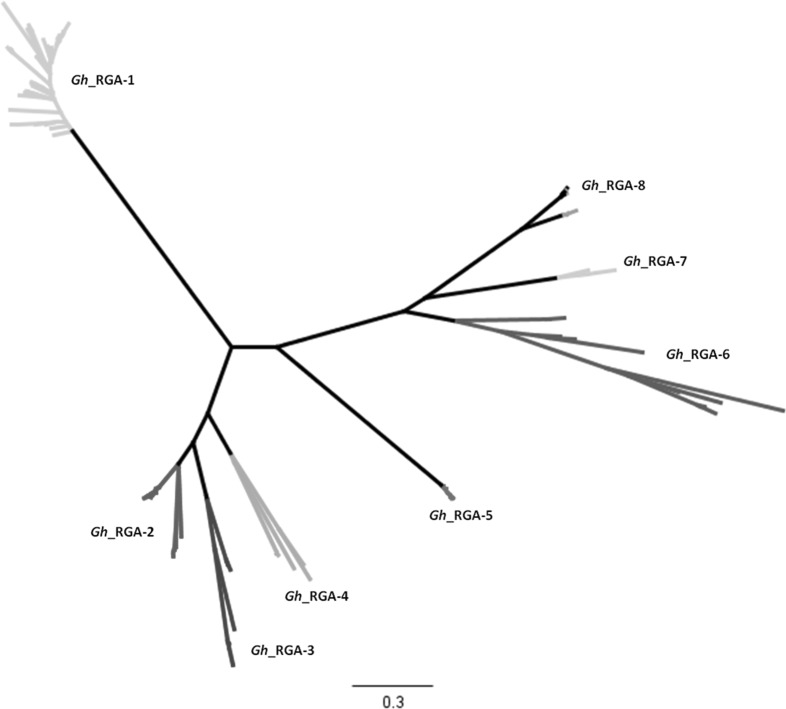
In order to decipher the expansion of the 161 RGAs, which were clustered in the eight PGGs, a RAxML phylogenetic analysis was performed (Fig. 1). The largest portion of the phylogenetic clades contained only a limited number of RGAs, whilst the backbone topology was adequately resolved with up to 320 branches (Fig. 1). The overall pairwise identity among these 161 amino acid sequences was 50.1 %, an indication of a high divergence among them.
Fig. 1.
RAxML phylogenetic reconstruction of the 161 upland cotton RGAs sequences which were aligned using the Muscle software (Edgar 2004). In various colors the eight PGGs which were assigned inferring the MCL-based clustering approch
We also investigated whether accelerated evolution and signatures of positive selection might have also contributed to the divergence of these RGAs. We subjected separately each of the eight PGGs to several successive tests, which could be used for seeking evidence for positive selection (Table 1). Statistically significant evidence of positive selection (omega values higher then 1.00) were detected in all eight PGGs, whereas the highest proportions of branches under positive selection were reported in Gh_RGA-2 (29.7 %) and Gh_RGA-7 (42.9 %) (Table 1). These positive selection signatures were observed rather unequally across the entire tree lineages, acting with a non-general rule. They were evident mostly at terminal branches in seven PGGs, and in four PGGs at their ancient branches (Table 1). We assume that recent episodes of positive selection events have been occurred, overlapping similar more ancient events among these RGAs. Constantly with our results, similar existences of positive selective pressures acting on plant RGAs have been recently reported (Khan et al. 2015; Perazzolli et al. 2014).
Finally, using the CODEMLSITES program and various selection models, we tested whether positive selection was acting at individual amino acid sites. Extensive signs of positive selection, with posterior Bayesian probabilities under the implementation of the most stringent M8 model, were observed in seven PGGs acting mostly towards the N-termini regions (Table 1). Only Gh_RGA-2 did not reflect any evidence of positive selection, perhaps because of the low divergence among the 38 RGAs comprising this PGG. The 42 positively selected sites are depicted in Table 1. Previously, comparative analyses of R genes at Rosaceae species revealed that the solvent-exposed amino acid residues of the LRRs domains were significantly variable with intensive positive selective pressures acting upon them (Perazzolli et al. 2014). Similar results were also observed in a genome wide analysis of the RGA in cherry (Zambounis et al. 2016). Such evidences are interpreted as an additional indication of the active involvement of these regions in fungal pathogens recognition pathways (Zambounis et al. 2012b), which is accurately consistent with the host-pathogen co-evolution processes leading to acquisition of disease resistance specificities (Mondragón-Palomino et al. 2002).
The overall findings of this study might facilitate the development of informative RGA-derived molecular markers being associated with resistant phenotypes and being particularly useful for identifying Fov resistant upland cotton cultivars. In the near feature, we have the intention to perform a more deeply genome wide analysis targeting the annotated R genes, particularly these of the NBS-LRRs family, in older to decipher their expansion and the evolutionary signatures acting upon them in the genome of upland cotton.
Acknowledgments
This research has been funded by the General Secretariat for Research and Technology (GSRT, program PENED 10087), Greece. We thank S. Andriotis S.A. for providing “Emerald” seeds.
Contributor Information
Antonios Zambounis, Phone: +30 2311 257531, Email: antbio@yahoo.gr.
Panagiotis Madesis, Phone: +30 2311 257531, Email: pmadesis@certh.gr.
References
- Ameline-Torregrosa C, Wang B-B, O’Bleness MS, Deshpande S, Zhu H, Roe B, Young ND, Cannon SB. Identification and characterization of nucleotide-binding site-leucine-rich repeat genes in the model plant Medicago truncatula. Plant Physiol. 2008;146(1):5–21. doi: 10.1104/pp.107.104588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axtell MJ, McNellis TW, Mudgett MB, Hsu CS, Staskawicz BJ. Mutational analysis of the ArabidopsisRPS2 disease resistance gene and the corresponding Pseudomonas syringae avrRpt2 avirulence gene. Mol Plant Microbe Interact. 2001;14(2):181–188. doi: 10.1094/MPMI.2001.14.2.181. [DOI] [PubMed] [Google Scholar]
- Chen JY, Huang JQ, Li NY, et al. Genome-wide analysis of the gene families of resistance gene analogues in cotton and their response to Verticillium wilt. BMC Plant Biol. 2015;15:148. doi: 10.1186/s12870-015-0508-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dangl JL, Horvath DM, Staskawicz BJ. Pivoting the plant immune system from dissection to deployment. Science. 2013;341(6147):746–751. doi: 10.1126/science.1236011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debener T, Byrne DH. Disease resistance breeding in rose: current status and potential of biotechnological tools. Plant Sci. 2014;228:107–117. doi: 10.1016/j.plantsci.2014.04.005. [DOI] [PubMed] [Google Scholar]
- Edgar RC. MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinform. 2004;5:1–19. doi: 10.1186/1471-2105-5-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enright AJ, Van Dongen S, Ouzounis CA. An efficient algorithm for large-scale detection of protein families. Nucleic Acids Res. 2002;30:1575–1584. doi: 10.1093/nar/30.7.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang H, Zhou H, Sanogo S, Lipka AE, Fang DD, Percy RG, Hughs SE, Jones DC, Gore MA, Zhang J. Quantitative trait locus analysis of Verticillium wilt resistance in an introgressed recombinant inbred population of Upland cotton. Mol Breed. 2014;33(3):709–720. doi: 10.1007/s11032-013-9987-9. [DOI] [Google Scholar]
- Ganopoulos IV, Kazantzis K, Chatzicharisis I, Karayiannis I, Tsaftaris AS. Genetic diversity, structure and fruit trait associations in Greek sweet cherry cultivars using microsatellite based (SSR/ISSR) and morpho-physiological markers. Euphytica. 2011;181(2):237–251. doi: 10.1007/s10681-011-0416-z. [DOI] [Google Scholar]
- Hinchliffe DJ, Lu Y, Potenza C, Segupta-Gopalan C, Cantrell RG, Zhang JF. Resistance gene analogue markers are mapped to homeologous chromosomes in cultivated tetraploid cotton. Theor Appl Genet. 2005;110:1074–1085. doi: 10.1007/s00122-005-1928-5. [DOI] [PubMed] [Google Scholar]
- Jones P, Binns D, Chang H-Y, Fraser M, Li W, McAnulla C, McWilliam H, Maslen J, Mitchell A, Nuka G, Pesseat S, Quinn AF, Sangrador-Vegas A, Scheremetjew M, Yong SY, Lopez R, Hunter S. InterProScan 5: genome-scale protein function classification. Bioinformatics. 2014;30(9):1236–1240. doi: 10.1093/bioinformatics/btu031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C, Thierer T, Ashton B, Mentjies P, Drummond A. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012;28(12):1647–1649. doi: 10.1093/bioinformatics/bts199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan AM, Khan AA, Azhar MT, Amrao L, Cheema HM. Comparative analysis of resistance gene analogues encoding NBS-LRR domains in cotton. J Sci Food Agr. 2015;96:530–538. doi: 10.1002/jsfa.7120. [DOI] [PubMed] [Google Scholar]
- Li F, Fan G, Lu C, Xiao G, Zou C, Kohel RJ, Ma Z, Shang H, Ma X, Wu J, Liang X, Huang G, Percy RG, Liu K, Yang W, Chen W, Du X, Shi C, Yuan Y, Ye W, Liu X, Zhang X, Liu W, Wei H, Wei S, Huang G, Zhang X, Zhu S, Zhang H, Sun F, Wang X, Liang J, Wang J, He Q, Huang L, Wang J, Cui J, Song G, Wang K, Xu X, Yu JZ, Zhu Y, Yu S. Genome sequence of cultivated Upland cotton (Gossypium hirsutum TM-1) provides insights into genome evolution. Nat Biotechnol. 2015;33(5):524–553. doi: 10.1038/nbt.3208. [DOI] [PubMed] [Google Scholar]
- Lynn DJ, Lloyd AT, Fares MA, O’Farrelly C. Evidence of positively selected sites in mammalian α-defensins. Mol Biol Evol. 2004;21(5):819–827. doi: 10.1093/molbev/msh084. [DOI] [PubMed] [Google Scholar]
- Mondragón-Palomino M, Meyers BC, Michelmore RW, Gaut BS. Patterns of positive selection in the complete NBS-LRR gene family of Arabidopsis thaliana. Genome Res. 2002;12(9):1305–1315. doi: 10.1101/gr.159402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu C, Lu Y, Yuan Y, Percy RG, Ulloa M, Zhang J. Mapping resistance gene analogs (RGAs) in cultivated tetraploid cotton using RGA-AFLP analysis. Euphytica. 2011;181:65–76. doi: 10.1007/s10681-011-0421-2. [DOI] [Google Scholar]
- Perazzolli M, Malacarne G, Baldo A, Righetti L, Bailey A, Fontana P, Velasco R, Malnoy M. Characterization of resistance gene analogues (RGAs) in apple (Malus x domestica Borkh.) and their evolutionary history of the Rosaceae family. PLoS One. 2014;9(2):e83844. doi: 10.1371/journal.pone.0083844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekhwal MK, Li P, Lam I, Wang X, Cloutier S, You FM. Disease resistance gene analogs (RGAs) in plants. Int J Mol Sci. 2015;16(8):19248–19290. doi: 10.3390/ijms160819248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen KA, Meyers BC, Islam-Faidi MN, Chen DB, Stelly DM, Michelmore RW. Resistance gene candidates identified by PCR with degenerate oligonucleoride primers map to clusters of resistance genes in lettuce. Mol Plant Microbe Interact. 1998;11(8):815–823. doi: 10.1094/MPMI.1998.11.8.815. [DOI] [PubMed] [Google Scholar]
- Simons G, Groenendijk J, Wijbrandi J, Reijans M, Groenen J, Diergaarde P, Van der Lee T, Bleeker M, Onstenk J, de Both M, Haring M, Mes J, Cornelissen B, Zabeau M, Vos P. Dissection of the fusarium I2 gene cluster in tomato reveals six homologs and one active gene copy. Plant Cell. 1998;10(6):1055–1068. doi: 10.1105/tpc.10.6.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30(9):1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28(10):2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan H, Callahan FE, Zhang XD, Karaca M, Saha S, Jenkins JN, Creech RG, Ma DP. Identification of resistance gene analogs in cotton (Gossypium hirsutum L.) Euphytica. 2003;134:1–7. doi: 10.1023/A:1026114327168. [DOI] [Google Scholar]
- Yang Z. PAML 4: phylogenetic analysis by maximum likelihood. Mol Biol Evol. 2007;24(8):1586–1591. doi: 10.1093/molbev/msm088. [DOI] [PubMed] [Google Scholar]
- Yang S, Zhang X, Yue J-X, Tian D, Chen J-Q. Recent duplications dominate NBS-encoding gene expansion in two woody species. Mol Genet Genomics. 2008;280(3):187–198. doi: 10.1007/s00438-008-0355-0. [DOI] [PubMed] [Google Scholar]
- Zambounis A, Paplomatas E, Tsaftaris AS. Intergenic spacer-RFLP analysis and direct quantification of Australian Fusarium oxysporum f. sp. vasinfectum isolates from soil and cotton infected tissues. Plant Dis. 2007;91:1564–1573. doi: 10.1094/PDIS-91-12-1564. [DOI] [PubMed] [Google Scholar]
- Zambounis A, Kalamaki M, Tani E, Paplomatas E, Tsaftaris A. Expression analysis of defense-related genes in cotton (Gossypium hirsutum) after Fusarium oxysporum f. sp. vasinfectum infection and following chemical elicitation using a salicylic acid analog and methyl jasmonate. Plant Mol Biol Rep. 2012;30(1):225–234. doi: 10.1007/s11105-011-0335-0. [DOI] [Google Scholar]
- Zambounis A, Elias M, Sterck L, Maumus F, Gachon CMM. Highly dynamic exon shuffling in candidate pathogen receptors…What if brown algae were capable of adaptive immunity? Mol Biol Evol. 2012;29(4):1263–1276. doi: 10.1093/molbev/msr296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambounis A, Ganopoulos I, Avramidou E, Aravanopoulos F, Tsaftaris A, Madesis P (2016) Evidence of extensive positive selection acting on cherry (Prunus avium L.) resistance gene analogs (RGAs) Aust J Crop Sci (in press)