Abstract
Improvement of the host resistance by using hazard free chemical elicitors is emerging as an alternative approach in the field of plant disease management. In our present work, we have screened the efficacy and possible mechanism of abiogenic elicitors like Dipotassium hydrogen orthophosphate (K2HPO4), Oxalic acid (OA), Isonicotinic acid (INA), Salicylic acid (SA), Acetylsalicylate (AS), Arachidonic acid (AA) and Calcium chloride (CaCl2) to stimulate innate immune responses in Lycopersicum esculentum Mill. Excised tomato leaves, treated with elicitors at three different concentrations, were found to stimulate defense and antioxidative enzymes, total phenol and flavonoid content after 24 h of incubation. CaCl2 (0.5 %) followed by INA (2.5 mM) were found most effective in activation of all such defense molecules in tomato leaves. Furthermore, nitric oxide (NO), a key gaseous mediator in plant defense signaling, was also measured after subsequent elicitor application. Higher doses of elicitors showed an elevated level of reactive oxygen species (ROS) generation, enhanced lipid peroxidation rate and proline content, which indicates the extent of abiotic stress generation on the leaves. However, ROS production, lipid peroxidation rate and proline concentration remain significantly reduced as a result of CaCl2 (0.5 %) and INA (2.5 mM) application. A sharp increase of total chlorophyll content was also recorded due to treatment of CaCl2 (0.5 %). These results demonstrate the effects of different abiogenic elicitors to regulate the production of defense molecules. Results also suggest that among all such chemicals, CaCl2 (0.5 %) and INA (2.5 mM) can be used as a potential elicitor in organic farming of tomato.
Keywords: Antioxidative enzymes, Calcium chloride, Defense molecules, Nitric oxide, Reactive oxygen species, Total chlorophyll
Introduction
Plants are regularly attacked by a broad range of invaders such as viral, bacterial or fungal pathogens which leads to huge reduction of crop yield. In order to protect the crops from the microbial pathogens, farmers generally use various alternatives (Thakur and Sohal 2013). Although the use of chemical pesticides or fungicides remains the general means of control but their application at field level is too expensive and hazardous. Some of the chemical control measures used are also recognized as carcinogens. In the last few decades, considerable research has been completed in understanding the molecular mechanisms leading to development of resistance against various plant pathogens. Consequently, this study could diminish the application of injurious chemicals and provide growers with new alternatives for sustainable agriculture (Hammond-Kosack and Parker 2003; Mejía-Teniente et al. 2010). Detailed exploitation of defense signaling cascades has led to the finding of hazard free compounds known as elicitors which are able to induce defense responses in plants (Gómez-Vásquez et al. 2004). In this scenario, extensive research have been devoted for the detection and expansion of natural and semi-synthetic compounds from all sources to trigger immune responses in plants (Goupil et al. 2012; Chandra et al. 2014a). Till date, diverse group of inducers have been documented and used, including glycopeptides, polymers of carbohydrate, derivatives of lipids, and chemical salts (Acharya et al. 2011a; Chandra et al. 2014a, 2015). In plants, generation of reactive oxygen species (ROS) is one of the earliest indications of elicitor recognition by plants. It further leads to activation of signal transduction pathways, phytoalexin biosynthesis, cell wall strengthening, callose deposition, defense related enzymes synthesis, and the accumulation of pathogenesis-related (PR) proteins (Van Loon and Van Strien 1999; Thakur and Sohal 2013). Development of resistant crop was achieved by the over expressing of various defense related genes encoding defense enzymes and phenol production (Maxson-Stein et al. 2002; Anand et al. 2009; Acharya et al. 2011a, b; Pal et al. 2011). Furthermore, signaling molecule like nitric oxide (NO) beside many of its useful function provide protection of plants in response to abiotic and biotic stressors (Corpas et al. 2011; Leterrier et al. 2012). It was well understood that regulation of plant defense genes by NO signaling depends upon the nature of elicitor used (Laspina et al. 2005; Hasanuzzaman et al. 2012; Chakraborty et al. 2014). In search of potential activators, foliar application of several compounds like salts of calcium, potassium, copper etc. at optimum concentrations has been demonstrated advantageous to improve the superiority of fruits and also provides resistance towards physiological disorders (Alcaraz-López et al. 2005).
In this context, here an attempt has been made to find out efficacy of different abiotic elicitors in production of different plant defense enzymes, total phenol and flavonoid content in a model plant tomato. Furthermore, different abiotic stress markers like production of proline, ascorbate peroxidase and lipid peroxidation rate is also examined to check whether application of those chemicals produce abiotic stress to the plant. Simultaneously, total chlorophyll content was measured in elicitor treated leaves. Finally, production of NO is also monitored to provide possible signaling mechanism of action.
Materials and methods
Plant material
Tomato (Lycopersicum esculentum Mill) is an extremely important crop for the economy of several countries like India and thus chosen as the model plant in this study.
Treatment
To analyze the efficacy of different abiotic elicitor on induction of defense response, healthy leaves of tomato were excised and sprayed with solutions containing abiotic elicitors like Dipotassium hydrogen orthophosphate (K2HPO4), Oxalic acid (OA), Isonicotinic acid (INA), Salicylic acid (SA), Acetylsalicylate (AS), Arachidonic acid (AA) and Calcium chloride (CaCl2). For each elicitor, three different concentrations were used which are listed in Table 1. Leaves sprayed with water served as control. All the sets were incubated in moist chamber for 24 h at room temperature. Each experiment was carried out with three replicates.
Table 1.
List of abiotic elicitors applied on leaves of tomato plants
| Elicitor | Concentration | ||
|---|---|---|---|
| Dipotassium hydrogen orthophosphate (K2HPO4) | 2 mM | 25 mM | 50 mM |
| Oxalic acid (OA) | 1 mM | 2.5 mM | 5 mM |
| Isonicotinic acid (INA) | 2.5 mM | 5 mM | 10 mM |
| Salicylic acid (SA) | 1.25 mM | 2.5 mM | 5 mM |
| Acetylsalicylate (AS) | 1 mM | 2.5 mM | 5 mM |
| Arachidonic acid (AA) | 0.1 mg/ml | 0.25 mg/ml | 0.5 mg/ml |
| Calcium chloride (CaCl2) | 0.5 % | 1 % | 2 % |
Enzyme assays
The leaf tissues were collected from different treated sets after 24 h incubation and homogenized with liquid nitrogen. Five hundred milligrams of powdered sample was extracted with 2 ml of extraction buffer specific for different enzyme, containing 0.1 % polyvinylpyrrolidone (PVP) and 20 ml of 1 mM phenylmethane sulphonyl fluoride (PMSF): 0.1 M of sodium acetate buffer (pH 5.0) for β-1,3 glucanase; 0.1 M sodium borate buffer (pH 8.7) for Phenylalanine Ammonia Lyase (PAL) and 0.1 M of sodium phosphate buffer (pH 7.0) for peroxidase, catalase, ascorbate peroxidase and polyphenol oxidase (Chakraborty et al. 2014). All the extraction procedures were conducted at 4 °C. The homogenate was centrifuged at 11,000×g for 20 min at 4 °C. The supernatants were used as the crude enzyme source for the enzymatic assays. Then it was transferred to a 2 ml Eppendorf tube and stored at −80 °C for further use.
Peroxidase (PO)
PO activity was carried out, following the method of Hemeda and Klein (1990). The substrate was prepared with 5 ml of 1 % guaiacol, 5 ml of 0.3 % H2O2 mixing and 50 ml of 0.05 M sodium phosphate buffer, pH 6.5. The reaction mixture was prepared with 2.95 ml of substrate and 0.05 ml of enzyme extract and the absorption change was measured at 470 nm for 3 min. PO activity was determined by the increase in the absorbance due to guaiacol oxidation and expressed as change in the absorption of the reaction mixture min−1 mg−1 of protein (E = 26.6 mM−1 cm−1).
Polyphenol oxidase (PPO)
PPO activity was estimated using the method of Kumar and Khan (1982). The reaction mixture consisted of 2 ml of 0.1 M sodium phosphate buffer (pH 6.5), 0.5 ml of crude enzyme extract and 1 ml of 0.1 M catechol. The assay mixture was incubated for 10 min at room temperature. Reaction was stopped by adding 1 ml of 2.5 N H2SO4. The absorption of purpurogallin formed was read at 495 nm. The blank was prepared by adding 2.5 N H2SO4 at zero time for the same assay mixture. The PPO activity was expressed in U min−1 mg−1 protein (U = change in 0.1 absorbance min−1 mg−1 protein).
Phenylalanine ammonia-lyase (PAL)
PAL activity was determined as the rate of conversion of L-phenylalanine to transcinnamic acid at 290 nm as mentioned by Dickerson et al. (1984). Assay mixture containing 200 μl of enzyme extract was incubated with 1.3 ml of 0.1 M borate buffer (pH 8.7) and 0.5 ml of 12 mM L-phenyl alanine in the same buffer for 30 min at 30 °C. The amount of transcinnamic acid synthesized was calculated by measuring absorbance at 290 nm after incubation. Enzyme activity was expressed as synthesis of transcinnamic acid (in nmol quantities) min−1 g−1 protein.
β-1,3 glucanase
β-1,3 glucanase activity was assayed according to the method of Pan et al. (1991). The reaction mixture was prepared with crude enzyme extract (50 µl) mixing with equal amount of the substrate 1 % laminarin and was incubated for 1 h at room temperature. Then the reaction was stopped by adding 300 µl of dinitrosalicylic acid reagent followed by boiling for 10 min on a boiling water bath. The resulting colored solution was diluted with the addition of distilled water to make the total volume up to 2 ml, vortexed and the absorption was measured at 520 nm. The blank set was prepared with equal amounts of crude enzyme and laminarin without incubation. The enzyme activity was expressed as μmol of glucose produced min−1 g−1 protein.
Catalase (CAT)
CAT activity was determined spectrophotometrically following the method Cakmak and Horst (1991). The reaction mixture contained: 100 μL of the crude enzyme extract, 50 μL of hydrogen peroxide (0.3 %) and volume was made up to 3 ml by addition of phosphate buffer (50 mM, pH 7.0). The reaction is initiated by the addition of hydrogen peroxide. The decrease in absorbance was recorded for 3 min for a wavelength of 240 nm. The catalase activity is expressed as nmol min−1 g−1 of protein with help of a molar extinction coefficient ε = 39,400 M−1 cm−1.
Ascorbate peroxidase (APX)
APX activity was determined according to Nakano and Asada (1981). The reaction mixture contained 50 mM potassium phosphate (pH 7.0), 0.2 mM EDTA, 0.5 mM ascorbic acid, 2 % H2O2, and 0.1 mL enzyme extract in a final volume of 3 mL. The decrease in absorbance at 290 nm for 1 min was recorded and the amount of ascorbate oxidized was calculated using extinction coefficient (ε = 2.8 mM−1 APX was defined as 1 mmol mL−1 per min at 25 °C, cm−1). Enzyme activity was expressed as µmol min−1 g−1 protein.
Estimation of total protein content
Bradford assay (1976) was employed, to test the protein concentration of each extract using bovine serum albumin as a standard.
Estimation of total phenol
Estimation of total phenol was determined following the method of Zieslin and Ben Zaken (1993). 250 mg of fresh leaf tissue was homogenized in 2 ml of 80 % methanol and the material was kept and maintained in 65 °C for 15 min. The material was then centrifuged at 10,000×g for 10 min at room temperature and the supernatant was collected and was used to estimate the phenol content of the material. The reaction mixture was prepared by adding 1 ml of crude extract to the mixture of 5 ml distilled water and 250 µl of 1 N Folin ciocalteu reagent. The reaction mixture was incubated for 30 min at room temperature. Phenolic content was measured spectrophotometrically at 725 nm using gallic acid as standard. The amount of total phenol was expressed as μg gallic acid produced g−1 tissue.
Estimation of total flavonoid content
Total flavonoid content was determined by following the method of Chang et al. (2002). 150 mg of fresh leaf tissue was ground in 2 ml of 80 % ethanol and the material was kept in dark place for 30 min after that it was then centrifuged at 10,000×g for 5 min at room temperature. The reaction mixture was prepared with 1 ml of crude extract (supernatant) mixed with 4.3 ml of 80 % aqueous ethanol, 0.1 ml of 10 % aluminum nitrate, and 0.1 ml of 1 M aqueous sodium acetate. The reaction mixture was then kept in dark place for 30 min. After incubation, the absorption was measured at 415 nm. The amount of total flavonoid was expressed as mg g−1 of the tissue sample.
Estimation of chlorophyll content
Total chlorophyll was estimated following Arnon’s method (1949). 500 mg of fresh leaf sample was ground in 4 ml of 80 % alkaline acetone (20 ml 0.1 N NaOH) and the extract was centrifuged at 7000×g for 10 min at room temperature. The supernatant was collected and the absorbance of the solution was read at 645 and 663 for total chlorophyll and were calculated by following formula:
where, D = optical density; V = final volume of 80 % acetone (ml); w = dry weight of sample taken (g).
Determination of Lipid Peroxidation rate
Oxidative damage to leaf lipids was estimated by the content of total 2-thiobarbituric acid reactive substances (TBARS) expressed as equivalents of malondialdehyde (MDA). TBARS content was estimated by the method of Cakmak and Horst (1991). Fresh leaf samples (0.2 g) were ground in 5 ml of 0.1 % (w/v) trichloroacetic acid (TCA), at 4 °C. Following the centrifugation at 12,000×g for 5 min, an aliquot of 1 ml from the supernatant was added to 4 mL of 0.5 % (w/v) thiobarbituric acid (TBA) in 20 % (w/v) TCA. Samples were heated at 90 °C for 30 min. Thereafter, the reaction was stopped in ice bath. Centrifugation was performed at 10,000×g for 5 min, and absorbance of the supernatant was recorded at 532 nm on a spectrophotometer and corrected for non-specific turbidity by subtracting the absorbance at 600 nm. The following formula was applied to calculate malondialdehyde content using its absorption coefficient (ε) and expressed as nmol malondialdehyde g−1 fresh mass following the formula:
where ε is the specific extinction coefficient (=155 mM cm−1), V is the volume of crushing medium, W is the fresh weight of leaf, A600 is the absorbance at 600 nm wavelength and A532 is the absorbance at 532 nm wavelength.
Estimation of total proline content
Free proline content in leaves was determined in accordance with the method of Bates et al. (1973). 200 mg of fresh leaf sample was ground in 2 ml of 3 % sulphosalicylic acid in a chilled mortar and pestle. The ground material was centrifuged at 11,000×g for 15 min at 4°C. The supernatant was collected to carry out the experiment. The reaction mixture was prepared with 1 ml of crude extract, 1 ml of 0.5 % ninhydrin reagent and 1 ml of glacial acetic acid. The reaction mixture was boiled for 30 min in a water bath, then after cooling, 3 ml of toluene was added to it. The tubes were shaken and the upper layer of toluene was collected by a separating funnel. The absorption of the colored sample was measured at 520 nm against toluene. The amount of proline was calculated by referring to a standard curve of proline and was expressed as µg of proline g−1 of tissue.
Nitric oxide estimation (NO)
Production of NO was estimated by haemoglobin assay according to the method of Delledonne et al. (2001). Leaf tissues of control and treated set were incubated in a reaction mixture containing 10 mM l-arginine and 10 mM haemoglobin in a total volume of 5 ml of 0.1 M phosphate buffer (pH 7.4). Production of NO was measured spectrophotometrically at 401 nm and NO levels were calculated using an extinction coefficient of 38,600 M−1 cm−1 (Salter and Knowles 1998). After 2 h of incubation, NO content in the reaction mixture was measured as nmol of NO produced g−1 tissue h−1 and compared with appropriate control set.
Real time NO detection
Real time NO production was visualised using membrane permeable fluorochrome 4-5 diaminofluorescein diacetate (DAF-2DA) dye (Bartha et al. 2005). Lower epidermis of leaf was peeled off and placed in a brown bottle containing 1 ml of loading buffer 10 mM KCl, 10 mM Tris HCl (pH 7.2) with DAF-2DA at a final concentration of 10 mM for 20 min in dark. Fluorescence was observed with Leica DMLS microscope at excitation wavelength 480 nm and emission wavelength 500-600 nm.
In vivo detection of H2O2
The in vivo detection of H2O2 in control and treated tomato leaves was carried out using DAB by following the method of Thordal-Christensen et al. (1997). After treatment as mentioned earlier, the cut ends of the leaves were then immersed in a solution containing 1 mg mL−1 diaminobenzidine (DAB) solution (pH 3.8) and incubated for 8 h. After incubation a central 3 cm2 segment of leaves were excised and laid adaxial surface up on filter paper moistened with an ethanol and glacial acetic acid mixture (3:1, v/v) until the chlorophyll had been removed. After bleaching tissues were transferred to water soaked filter paper for at least 4 h to relax and finally to paper soaked with lactoglycerol (1:1:1, lactic acid:glycerol:water, v/v) for another 24 h. The cleared leaf segments were then observed under light microscope.
Statistics
All data presented were mean ± standard deviation (S.D.) of three replicates. Statistical analyses were performed by analysis of variance (ANOVA) using SPSS software version 20 and the significance of difference between the treatments was determined using Duncan’s Multiple Range Test (p < 0.05).
Results
Effects of abiotic elicitors on defense and antioxidative enzyme activity
Application of abiogenic elicitors like K2HPO4, OA, INA, SA, AS, AA and CaCl2 each at three different concentrations were found effective in inducing defense related as well as antioxidative enzymes in tomato leaves. Among all the elicitors, CaCl2 (0.5 %) showed highest inductive ability followed by INA (2.5 mM). After 24 h incubation, CaCl2 (0.5 %) treated leaves showed 2.7, 2.04, 2.11, 2.39, 1.63 and 2.05-fold increased production of PO, PPO, PAL, β-1,3 glucanase, CAT and APX compared to control, respectively (Table 2). Similarly, accumulation of PO, PPO, PAL, β-1,3 glucanase, CAT and APX was noted 2, 1.92, 1.87, 2.16, 1.62 and 1.96-fold higher in INA (2.5 mM) treated leaves compared to control, respectively. Furthermore, highest value for CAT and APX was recorded as 2.22 and 3.01-fold increase over control in the leaves treated with CaCl2 at a concentration 2 %. The least inductive effect was observed in the leaves treated with various concentrations of OA and AA. Enzymes activity was found highest at lower or medium concentrations of the elicitor treatment. However, CAT and APX activity was elevated with the increasing doses of elicitors (Table 2).
Table 2.
Effect of abiotic elicitors on the production of defense and antioxidative enzymes in tomato plants
| Enzymes | Peroxidase (PO) (µmol min−1 mg−1 protein) | Polyphenol oxidase (PPO) (U min−1 mg−1 protein) | Phenylalanine ammonia lyase (PAL) (nmol of transcinnamic acid min−1 g−1 protein) | β-1,3 glucanase (µmol glucose produced min−1 g−1 protein) | Catalase (CAT) (nmol min−1 g−1 protein) | Ascorbate Peroxidase (APX) (µmol min−1 g−1 protein) |
|---|---|---|---|---|---|---|
| Control | 3.76 ± 0.48i | 29.44 ± 1.12k | 94.23 ± 4.39k | 26.38 ± 2.29m | 6.83 ± 0.30l | 0.177 ± 0.021m |
| K2HPO4-2 mM | 6.19 ± 0.06d | 30.07 ± 0.58k | 120.73 ± 7.68hij | 38.66 ± 0.89kl | 9.01 ± 0.42ijk | 0.232 ± 0.026jklm |
| K2HPO4-25 mM | 5.59 ± 0.18e | 33.51 ± 1.43hijk | 137.13 ± 4.90fg | 41.87 ± 1.16hijkl | 11.18 ± 0.48fgh | 0.279 ± 0.01ghijk |
| K2HPO4-50 mM | 5.26 ± 0.36efgh | 38.29 ± 0.99efgh | 131.73 ± 3.74fghi | 42.77 ± 0.88ghijkl | 13.03 ± 0.33de | 0.302 ± 0.024fghij |
| OA-1 mM | 4.90 ± 0.15 h | 31.11 ± 1.16jk | 122.94 ± 4.70hij | 38.14 ± 1.58l | 7.97 ± 0.39kl | 0.206 ± 0.019lm |
| OA-2.5 mM | 5.01 ± 0.15gh | 40.77 ± 1.72ef | 132.45 ± 2.41fgh | 43.37 ± 1.23ghijk | 9.88 ± 0.23hij | 0.237 ± 0.029jklm |
| OA-5 mM | 5.23 ± 0.17efgh | 39.14 ± 1.25efg | 144.13 ± 1.25ef | 42.12 ± 0.67hijkl | 12.19 ± 0.34ef | 0.284 ± 0.037ghijk |
| INA-2.5 mM | 7.52 ± 0.11b | 56.62 ± 0.97ab | 176.33 ± 3.75b | 57.11 ± 2.09b | 11.11 ± 0.55fgh | 0.348 ± 0.028defg |
| INA-5 mM | 6.26 ± 0.21d | 52.66 ± 1.83bcd | 157.04 ± 4.51cd | 49.00 ± 0.74cde | 15.26 ± 0.18b | 0.402 ± 0.025cd |
| INA-10 mM | 5.34 ± 0.22efgh | 42.22 ± 0.61e | 133.43 ± 3.11fgh | 43.83 ± 1.43fghij | 17.54 ± 0.38a | 0.481 ± 0.025ab |
| SA-1.25 mM | 5.09 ± 0.07gh | 32.22 ± 2.00ijk | 120.73 ± 2.73hij | 40.06 ± 0.70jkl | 8.64 ± 0.07jk | 0.241 ± 0.017jklm |
| SA-2.5 mM | 5.27 ± 0.10efgh | 35.62 ± 2.09ghij | 130.40 ± 2.48fghi | 43.85 ± 0.96fghij | 11.38 ± 0.40fg | 0.333 ± 0.009efgh |
| SA-5 mM | 5.55 ± 0.08ef | 41.88 ± 1.28e | 140.89 ± 3.44f | 39.26 ± 1.47jkl | 13.83 ± 0.21cd | 0.366 ± 0.007cdef |
| AS- 1 mM | 5.28 ± 0.05efgh | 29.18 ± 1.19k | 111.67 ± 6.86j | 40.47 ± 2.61ijkl | 9.03 ± 0.44ijk | 0.245 ± 0.009jklm |
| AS-2.5 mM | 5.59 ± 0.21e | 36.37 ± 2.14fghi | 154.37 ± 2.86de | 45.06 ± 1.01efghi | 11.95 ± 0.49ef | 0.272 ± 0.011hijkl |
| AS-5 mM | 5.00 ± 0.05gh | 41.77 ± 1.95e | 134.46 ± 1.04fgh | 43.37 ± 0.62ghijk | 14.14 ± 0.77bcd | 0.314 ± 0.012efghi |
| AA-0.1 mg/ml | 5.12 ± 0.07fgh | 28.92 ± 0.96k | 120.21 ± 5.11hij | 40.72 ± 1.83ijkl | 7.30 ± 0.43l | 0.219 ± 0.019klm |
| AA-0.25 mg/ml | 5.31 ± 0.08efgh | 33.88 ± 0.67hijk | 117.79 ± 3.88ij | 44.16 ± 0.86fghij | 10.14 ± 0.32ghi | 0.252 ± 0.019ijkl |
| AA-0.5 mg/ml | 5.59 ± 0.14e | 37.74 ± 0.95efgh | 133.38 ± 2.59fgh | 41.43 ± 0.61hijkl | 11.95 ± 0.26ef | 0.316 ± 0.025efghi |
| CaCl2-0.5 % | 8.19 ± 0.53a | 60.18 ± 2.26a | 198.97 ± 2.91a | 63.14 ± 2.89a | 10.98 ± 0.29fgh | 0.364 ± 0.011def |
| CaCl2-1 % | 6.65 ± 0.41c | 50.37 ± 1.33cd | 165.38 ± 2.04bcd | 50.63 ± 0.73cd | 12.24 ± 0.26ef | 0.432 ± 0.02bc |
| CaCl2-2 % | 5.41 ± 0.10efg | 42.44 ± 1.30e | 138.42 ± 6.95f | 46.08 ± 0.86defgh | 15.22 ± 0.80b | 0.533 ± 0.019a |
Values represent mean ± SD of three separate experiments, each in triplicate. Different letters within the row indicate significant difference (p < 0.05) from the control set using Duncan’s multiple range test. Same letter within the row denotes no significant difference between the groups
Effects of abiotic elicitor on total phenol and flavonoid content in tomato plant
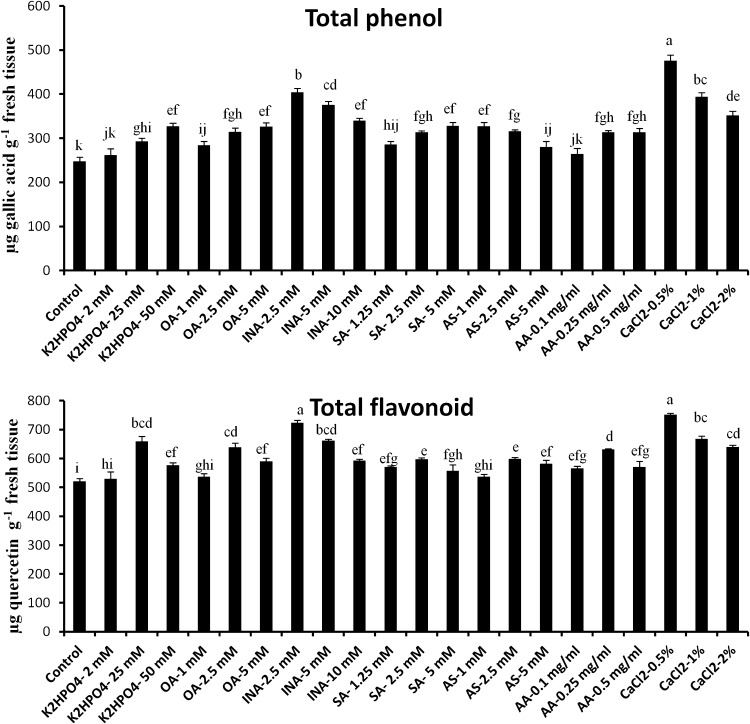
Abiotic elicitors showed a varied degree of influence on the production of total phenol and flavonoid content in tomato leaves. It is clear from the Fig. 1a that among all such abiogenic elicitors, CaCl2 at a concentration 0.5 % and INA (2.5 mM) have greater potential to significantly amplify the production of phenol and flavonoid. 24 h post-elicitation of both the elicitor showed 1.92 and 1.63-fold increase in total phenol content compared to control respectively. However, in case of flavonoid the values were 1.44 and 1.38-fold, respectively. In contrast, compare to other sets of leaves treated with K2HPO4 (2 mM) showed least amount of phenol and total flavonoid production. Phenols are considered as the key component of plant’s natural defense arsenal. So the elevated level of phenol and flavonoid content in excised tomato leaves due to treatment of various abiotic elicitors may confer the enhancement of resistance to the plant.
Fig. 1.
Effect of abiotic elicitors on production of total phenol (a) and total flavonoid content (b) in tomato plants. Values represent mean ± SD of three separate experiments, each in triplicate. Sharing the same letter are not significantly different (p < 0.05) using Duncan’s multiple range test
Effects of abiotic elicitor on NO production in tomato plant
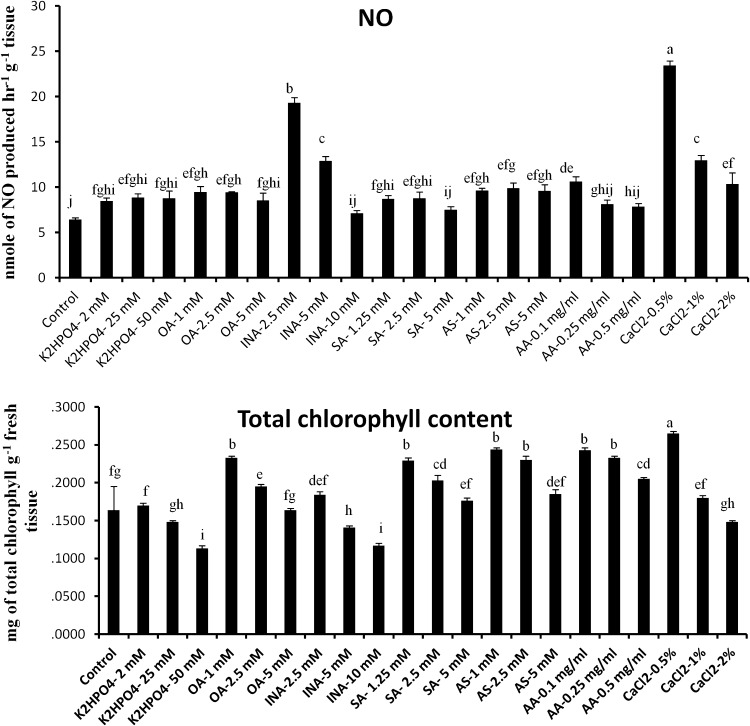
To examine whether defense augmentation in the excised tomato leaves by elicitor treatment is NO mediated, we examined the levels of NO production in all the abiogenic elicitor treated leaves and compared them with the water treated control. Almost 4.5 and 3.3-fold increase of NO production was observed in the CaCl2 (0.5 %) and INA (2.5 mM) treated plants over control, respectively. However, production of NO becomes reduced at the higher concentrations of the same elicitors. Although other elicitors did not show significant change of NO production in the detached tomato leaves compared to control (Fig. 2a).
Fig. 2.
Effect of abiotic elicitors on production of NO (a) and total chlorophyll (b) in tomato plants. Values represent mean ± SD of three separate experiments, each in triplicate. Sharing the same letter are not significantly different (p < 0.05) using Duncan’s multiple range test
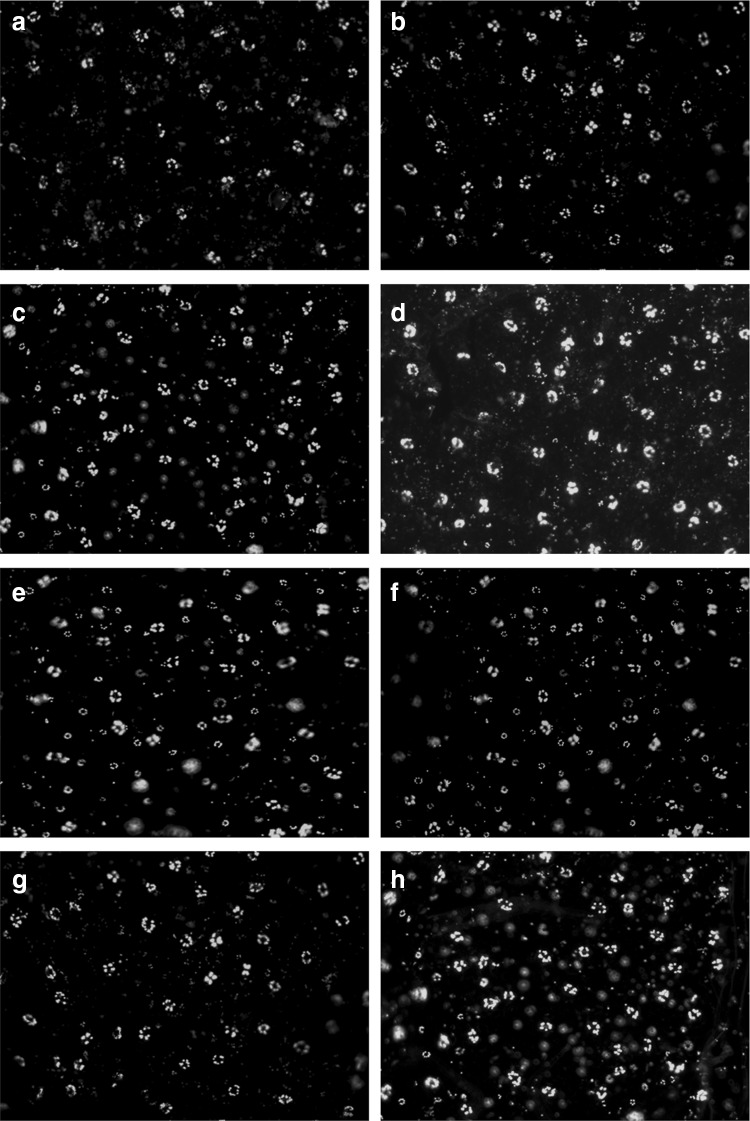
These results were further established by real time visualization by using DAF-2DA, a membrane permeable fluorophore extensively used for imaging of NO. Pattern of change, in NO production showed similar kind of observations as monitored by spectrophotometry (Fig. 3).
Fig. 3.
Real-time determination of NO in leaf epidermal cells by DAF-2DA staining. NO generation was detected by green fluorescence. Control (a), K2HPO4-25 mM (b), OA-1 mM (c), INA-2.5 mM (d), SA-2.5 mM (e), AS-2.5 mM (f), AA-0.1 mg/ml (g), and CaCl2-0.5 % (h), treated set
Effects of abiotic elicitor on chlorophyll content
At higher doses of elicitor treatments, strong inhibition of chlorophyll biosynthesis was recorded (Fig. 2b). However, elicitors at lower concentrations like CaCl2 (0.5 %), AS (1 mM), AA (0.1 mg/ml), OA (1 mM), SA (1.25 mM) and INA (2.5 mM) showed 1.61, 1.48, 1.47, 1.42, 1.23 and 1.12-fold elevation of chlorophyll production than control respectively. In comparison to the control, highest 68 % reduction of total chlorophyll content was observed in tomato leaves treated with K2HPO4 (50 mM).
Effects of abiotic elicitor on ROS production in tomato plant
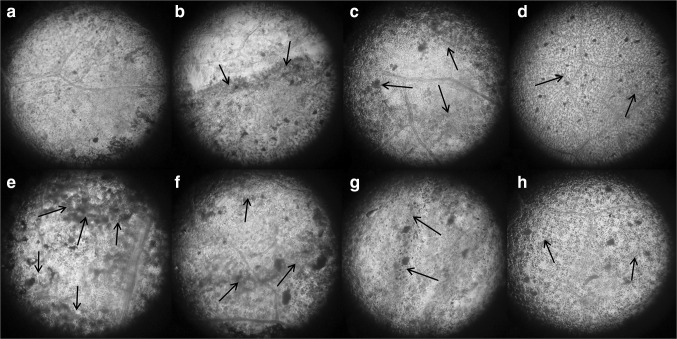
Generation of ROS is a characteristic of elicitor perception by the plants and it is responsible for multifaceted defense responses in plants including signaling. However, excess production of ROS is detrimental to plant tissues as it is involved in programmed cell death. In this connection, generation of ROS in the elicited tomato leaves was monitored by using DAB, a dye widely used for the detection and imaging of H2O2. Formation of H2O2 was detected in the leaves treated with elicitors at optimum concentrations in which it showed greater influence on the accumulation of defense enzymes and NO production. From the Fig. 4 it was clearly indicated that the amount of ROS production varied according to the nature of chemical elicitors used. Relatively higher degree of ROS generation was noticed in all the elicitor treated leaves except those treated with CaCl2 (0.5 %) and INA (2.5 mM). Moderate quantity of H2O2 generation indicates the balanced production of ROS in the leaf tissue.
Fig. 4.
H2O2 detection in tomato leaves by DAB stain, 24 h after abiotic elicitor treatment. Control (a), K2HPO4-25 mM (b), OA-1 mM (c), INA-2.5 mM (d), SA-2.5 mM (e), AS-2.5 mM (f), AA-0.1 mg/ml (g), and CaCl2-0.5 % (h) treated set. Arrows indicate the site of generation of H2O2
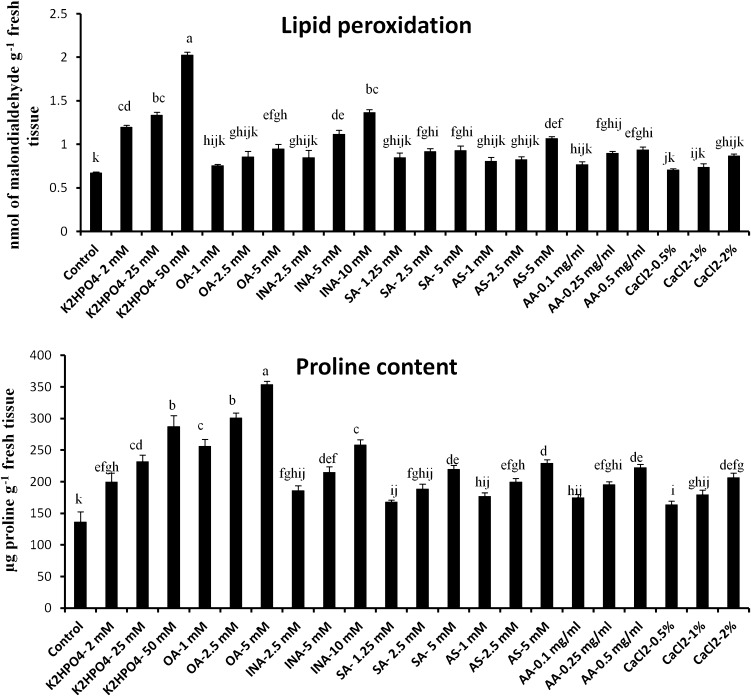
Effects of abiotic elicitor on lipid peroxidation and Proline content in tomato plant
Malondialdehyde (MDA) content was measured in the elicitor treated tomato leaves to justify the extent of oxidative stress. Varied degree of MDA content was observed in tomato leaves according to the nature and concentration of elicitors. Generally, treatment of tomato leaves with the higher dose showed elevated amount of MDA generation. K2HPO4 at a concentration 50 mM caused fourfold increase of MDA content compared to control and was recorded highest (Fig. 5). However, in the leaves treated with CaCl2 (0.5 %), OA (1 mM), SA (1.25 mM), AS (1 mM), AA (0.1 mg/ml) and INA (2.5 mM), MDA content remain as basal level like control (Fig. 5a).
Fig. 5.
Effect of abiotic elicitors on lipid peroxidation (a) and proline content (b) in tomato plants. Values represent mean ± SD of three separate experiments, each in triplicate. Sharing the same letter are not significantly different (p < 0.05) using Duncan’s multiple range test
Like lipid peroxidation rate, proline content in the tomato leaves was also influenced by application of abiotic elicitors. The free proline content was significantly enhanced in the elicitor treated tomato leaves compared to the control (Fig. 5b). In comparison to control, highest 2.5-fold enhanced free proline production was observed in the sets treated with OA at a concentration 5 mM and which was followed by OA (2.5 mM) and K2HPO4 (50 mM). However, least increase in free proline content was observed in the leaves treated with CaCl2 at a concentration 0.5 %. Increased production of free proline content also indicates the generation of oxidative damages to the leaf.
Discussion
In the present scenario of food safety and sustainable agriculture, it is evident to develop a useful strategy for the development of broad-spectrum biotic stress-tolerant crops. Plants need appropriate stimuli or signal to activate their array of defense mechanisms. Elicitors, the small molecules, can able to mimic the pathogen insight by a plant following activation of various modes of complicated defense reactions which includes hypersensitive response, antimicrobial compound synthesis, lignin accumulation in the cell wall, and over-expression of plant defense related genes (Acharya et al. 2011a; Chandra et al. 2014a).
Primary aim of this work was to screen potentiality of selected elicitors to confer broad spectrum disease resistance to the model plant tomato. It was evident from our results that abiotic elicitors like K2HPO4, OA, INA, SA, AS, AA and CaCl2 at a specific concentration significantly induce the plant defense molecules. However, CaCl2 (0.5 %) and INA (2.5 mM) was found to be most efficient among all the tested chemicals. Induced defense enzymes like, PPO and PO participate in a vital role in activation of hypersensitive response. Both of them not only help in the strengthening of cell wall but also act as a transducer of signals to the neighboring unaffected cells (Lamb and Dixon 1997; Acharya et al. 2011a; Chandra et al. 2014a). Another defense enzyme, PAL, being one of the key enzymes of the phenylpropanoid pathway, involved in the biosynthesis of phytoalexins, lignins and related phenolic compounds (Pellegrini et al. 1994). Therefore, augmented PAL activity might be able to reduce the severity of disease occurrence. β-1,3 glucanase, a PR 2 class of protein encoded by the host, breaks the linkage of the key cell wall material of the pathogen (Chandra et al. 2014b). Moreover, activation of all such defense-related molecules (such as defense enzymes, total phenol and flavonoids) is known to play an important role to increase the host resistance against broad range of biotic stresses (Naveen et al. 2013). In this study, excised leaves of tomato when elicited with CaCl2 (0.5 %) and INA (2.5 mM) induced defense enzymes such as PPO, PAL, PO and β-1,3 glucanase on an average twofold and 1.8-fold compared to control, respectively. Although, treatment of tomato leaves with other chemical elicitors such as K2HPO4, OA, SA, AS and AA showed enhancement of those enzymes but values remain, significantly lower than the leaves treated with CaCl2 (0.5 %) or INA (2.5 mM). This observation supports the findings of previous studies where pear fruits, tea plants and chili leaves treated with CaCl2 showed enhanced accumulation of β-1,3 glucanase, PO, PPO and PAL enzymes (Tian et al. 2006; Chandra et al. 2014a; Chakraborty et al. 2015). Results also coincide with Acharya et al. (2011a) where foliar application of INA was found to be the most efficient in the induction of PO and PPO in Raphanus sativus among all the elicitor tested. On the other hand, application of SA (5 mM) and K2HPO4 (50 mM) on detached cashew leaves showed maximum reduction of anthracnose disease caused by Colletotrichum gloeosporioides (Lopez and Lucas 2002). Ajay and Baby (2010) also showed that SA and acibenzolar-S-methyl ester (ASM) application in Camellia sinensis significantly boost defense enzymes production over the control and reduced disease prevalence. However, in our study, SA (5 mM) and K2HPO4 (50 mM) were found less effective to induce defense enzyme activities than CaCl2 (0.5 %) and INA (2.5 mM).
Moreover, healthy plant tissue contains phenols, as preformed antimicrobial compounds, that hamper the growth of fungi and others. Different types of such phenols may include phenolic acids, simple phenols, flavonols, isoflavones and alkaloids (Ashry and Mohamed 2011). Phenol helps in disease resistance in many ways like hypersensitive cell death and by lignifications of cell walls (Biswas et al. 2012). The phenolic compounds may involved in the enhancement of mechanical strength of the host cell walls by which it can restrict the entry of the pathogen (El Modafar et al. 2012). The accumulation of phenolics by application of various elicitors has already been acknowledged (Dong et al. 2010; El Modafar et al. 2012; Gupta et al. 2013). In this work, significantly enhanced accumulation of total phenols and flavonoids was observed in tomato leaves treated with CaCl2 (0.5 %) or INA (2.5 mM), which might be an indication towards the enhancement of defense arsenals in tomato plants.
CAT and APX are the important components of the antioxidant system, played a vital role in eliminating and maintaining the threshold level of H2O2 in a range of cellular organelles (Najami et al. 2008). Our results demonstrated that CAT and APX activities significantly induced at higher concentrations of elicitors which signify the production of ROS due to oxidative stress. Earlier it was reported that application of higher concentration of CuCl2 causes oxidative stress to tomato plant which may be counteracted by the higher accumulation of CAT and APX (Chakraborty et al. 2014). Present study also indicates the same results where highest production of both the antioxidative enzymes was recorded in the leaves treated with CaCl2 (2 %) instead of CaCl2 (0.5 %). Furthermore, earlier reports suggests that formation of H2O2, as a product of oxidative burst, was prerequisite for later gene activation (Repka 2001) but accumulation of H2O2 at higher level turn into detrimental to the cell as it causes peroxidation of membrane lipids (Hasanuzzaman et al. 2012). The extent of lipid peroxidation can be assessed by the amount of MDA production, a derivative of polyunsaturated fatty acid (Lin and Kao 2000). Our results showed that lipid peroxidation was influenced by the exogenous treatment of abiotic elicitors. However, compared to control, lipid peroxidation rate remain at basal level at lower concentration of elicitor treatments. Interestingly, very high amount of MDA content was measured in the leaves treated with K2HPO4 (50 mM) which coincide with the earlier reports, where increased amount of MDA production was observed in wheat, cotton, rice and alfalfa (Sairam and Srivastava 2002; Diego et al. 2003; Tijen and Ìsmail 2005; Wang and Han 2007) under higher concentrations of salts. Although other treatments including CaCl2 (0.5 %) and INA (2.5 mM) did not show significant change of MDA content compared to control. On the other hand, proline accumulation is one of the key implementations of plants towards abiotic stress condition like salinity, draught etc. (Giridara et al. 2000; Ramanjulu and Sudhakar 2001). In the present experiment, free proline content was significantly elevated in the plants treated with higher dose of abiotic elicitors which suggests that those concentrations of elicitors cause abiotic stressful condition to the plants. However, CaCl2 (0.5 %) and INA (2.5 mM) treated leaves showed very less amount of free proline content and values remain very close to control which indicates that plants are not in abiotic stress. Results coincide with the findings of Li et al. (2010) in which proline accumulation became amplified at intermediate salt level (200 mM) in the seedlings of castor bean.
The molecular foundation of complicated signalling cascades of plant defense metabolite production needs further exploration (Xu et al. 2005; Chandra et al. 2014a). NO a small diffusible molecule is nowadays being recognized as an emerging key signaling molecule in response to broad range of stresses in plants (Neill et al. 2003; Romero-Puertas et al. 2004; Khan et al. 2012). It appears to be an early signaling factor that helps in orchestrating a number of downstream signaling pathways (Perchepied et al. 2010). From our laboratory we have reported the correlation between the NO generation and induction of defense-related molecules due to application of elicitor on tea leaves (Chandra et al. 2014b, 2015). Result suggests direct involvement of NO in the signal transduction process leading to induced defense responses in plants. In this present study, elicitor treated leaves of tomato showed superior NO production than the water treated control leaves in dose-dependent manner. Mainly, CaCl2 at a concentration 0.5 % showed the highest amount of NO production in tomato leaves. However, INA (2.5 mM) treated leaves also showed 3.3-fold increase over control. These results coincide with our previous reports where foliar application of CaCl2 and INA in tea and Raphanus sp showed over production of NO with higher accumulation of defense molecules, respectively (Chakraborty et al. 2011a; Chandra et al. 2014b). Furthermore, co-treatment of chitosan-nano particles with NO scavenger or nitric oxide synthase (NOS) inhibitor lower down NO accumulation to basal level in the leaves of tea and indicating a strong positive relationship with plant defense modulators (Chandra et al. 2015). NO signaling is usually related to its cross talk with ROS. Almost all the abiotic stressors response generates free radicals and other oxidants, in different cellular organelles (Mano 2002), which produces oxidative stress in terms of an increased level of ROS in plant cells (Mittler 2002). In the present investigation, DAB staining of tomato leaves revealed that all the treatments except CaCl2 and INA (2.5 mM) induced ROS accumulation over the untreated control. This indicates CaCl2 and INA-treatment produce greater amount of NO in tomato leaves which ultimately scavenge excess amount of ROS and maintain a steady level. Similar reductions of MDA and H2O2 in sodium nitroprusside (SNP, potent NO donor) applied seedlings were documented in various plants (Song et al. 2006; Hasanuzzaman et al. 2011; Kong et al. 2011).
Conclusion
It was observed that the abiotic elicitors mainly CaCl2 (0.5 %) and INA (2.5 mM) have shown a greater induction ability on the production of defense enzymes like PO, PPO, PAL, and β-1,3 glucanase, polyphenols and flavonoids in tomato leaves. Antioxidative enzymes like APX and CAT are also moderately induced. Higher concentrations of elicitors may cause stress condition to the plant as production of MDA and proline become extremely high. The NO accumulation was also elevated in the elicitor treated leaves which suggests that NO might help in balancing ROS level as well as the signal molecule for the stimulatory effect on this model plant system. Moreover, CaCl2 (0.5 %) also increase chlorophyll production in tomato leaves. In conclusion, the overall results suggest that the use of CaCl2 and INA at this low concentration showed strong positive regulation of plant defense. Further work is in progress to understand the detailed mode of action of CaCl2 in in planta level during host-pathogen combination.
Abbreviations
- K2HPO4
Dipotassium hydrogen orthophosphate
- OA
Oxalic acid
- INA
Isonicotinic acid
- SA
Salicylic acid
- AS
Acetylsalicylate
- AA
Arachidonic acid
- CaCl2
Calcium chloride
- PO
Peroxidase
- PPO
Polyphenol oxidase
- PAL
Phenylalanine ammonia-lyase
- CAT
Catalase
- APX
Ascorbate peroxidase
- NO
Nitric oxide
- ROS
Reactive oxygen species
- DAB
Diaminobenzidine
- PVP
Polyvinylpyrrolidone
- PMSF
Phenylmethane sulphonyl fluoride
- TBARS
2-Thiobarbituric acid reactive substances
- MDA
Malondialdehyde
- TCA
Trichloroacetic acid
- TBA
Thiobarbituric acid
- DAF-2DA
4,5-Diaminofluorescein diacetate
- ASM
Acibenzolar-S-methyl ester
- NOS
Nitric oxide synthase
- SNP
Sodium nitroprusside
References
- Acharya K, Chakraborty N, Dutta AK, Sarkar S, Acharya R. Signaling role of nitric oxide in the induction of plant defense by exogenous application of abiotic inducers. Arch Phytopathol Plant Prot. 2011;44:1501–1511. doi: 10.1080/03235408.2010.507943. [DOI] [Google Scholar]
- Acharya K, Chandra S, Chakraborty N, Acharya R. Nitric oxide functions as a signal in induced systemic resistance. Arch Phytopathol Plant Prot. 2011;44:1335–1342. doi: 10.1080/03235408.2010.496552. [DOI] [Google Scholar]
- Ajay D, Baby UI. Induction of systemic resistance to Exobasidium vexans in tea through SAR elicitors. Phytoparasitica. 2010;38:53–60. doi: 10.1007/s12600-009-0068-x. [DOI] [Google Scholar]
- Alcaraz-López C, Botía M, Alcaraz CF, Riquelme F. Induction of fruit calcium assimilation and its influence on the quality of table grapes. Span J Agricult Res. 2005;3(3):335–343. doi: 10.5424/sjar/2005033-156. [DOI] [Google Scholar]
- Anand T, Chandrasekaran A, Raguchander T, Prakasam V, Samiyappan R. Chemical and biological treatments for enhancing resistance in chilli against Colletotrichum capsici and Leveillula taurica. Arch Phytopathol Plant Prot. 2009;42:533–551. doi: 10.1080/03235400701191721. [DOI] [Google Scholar]
- Arnon DI. Copper enzymes in isolated chloroplasts. Polyphenol oxidase in Beta vulgaris. Plant Physiol. 1949;24:1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashry NA, Mohamed HI. Impact of secondary metabolites and related enzymes in flax resistance and or susceptibility to powdery mildew. World J Agric Sci. 2011;7(1):78–85. [Google Scholar]
- Bartha B, Kolbert Z, Erdei L. Nitric oxide production induced by heavy metals in Brassica juncea L. Czern. and Pisum sativum L. Acta Biol Szeged. 2005;49(1–2):9–12. [Google Scholar]
- Bates LS, Waldran RP, Teare ID. Raipid determination of free proline for water studies. Plant Soil. 1973;39:205–208. doi: 10.1007/BF00018060. [DOI] [Google Scholar]
- Biswas SK, Pandey NK, Mohd R. Inductions of defense response in tomato against Fusarium wilt through inorganic chemicals as inducers. Plant Pathol Microbiol. 2012;3(4):1–7. [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem. 1976;72(1–2):248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cakmak I, Horst J. Effect of aluminium on lipid peroxidation, superoxide dismutase, catalase, and peroxidase activities in root tips of soybean (Glycine max) Physiol Plant. 1991;83:463–468. doi: 10.1111/j.1399-3054.1991.tb00121.x. [DOI] [Google Scholar]
- Chakraborty N, Chandra S, Acharya K. Sublethal heavy metal stress stimulates innate immunity in tomato. Sci World J. 2014;208649:1–7. doi: 10.1155/2015/208649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty N, Chandra S, Acharya K. Boosting of innate immunity in Chilli. Res J Pharm Technol. 2015;8(7):885–892. doi: 10.5958/0974-360X.2015.00144.4. [DOI] [Google Scholar]
- Chandra S, Chakraborty N, Chakraborty A, Rai R, Bera B, Acharya K. Induction of defense response against blister blight by calcium chloride in tea. Arch Phytopathol Plant Prot. 2014;47:2400–2409. doi: 10.1080/03235408.2014.880555. [DOI] [Google Scholar]
- Chandra S, Chakraborty N, Chakraborty A, Rai R, Bera B, Acharya K. Abiotic elicitor-mediated improvement of innate immunity in Camellia sinensis. J Plant Growth Regul. 2014;33:849–859. doi: 10.1007/s00344-014-9436-y. [DOI] [Google Scholar]
- Chandra S, Chakraborty N, Dasgupta A, Sarkar J, Panda K, Acharya K. Chitosan nanoparticles: a positive modulator of innate immune responses in plants. Sci Rep. 2015;5(15195):1–13. doi: 10.1038/srep15195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CC, Yang MH, Wen HM, Chern JC. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal. 2002;10(3):178–182. [Google Scholar]
- Corpas FJ, Leterrier M, Valderrama R, Airaki M, Chaki M, Palma JM, Barroso JB. Nitric oxide imbalance provokes a nitrosative response in plants under abiotic stress. Plant Sci. 2011;181:604–611. doi: 10.1016/j.plantsci.2011.04.005. [DOI] [PubMed] [Google Scholar]
- Delledonne M, Zeier J, Marocco A, Lamb C. Signal interactions between nitric oxide and reactive oxygen intermediates in the plant hypersensitive disease resistance response. PNAS, USA. 2001;98(23):13454–13459. doi: 10.1073/pnas.231178298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson DP, Pascholati SF, Hagerman AE, Butler LG, Nicholson RL. Phenylalanine ammonia-lyase and hydroxycinnamate: CoA ligase in maize mesocotyls inoculated with Helminthosporium maydis or Helminthosporium carbonum. Physiol Plant Pathol. 1984;25(2):111–123. doi: 10.1016/0048-4059(84)90050-X. [DOI] [Google Scholar]
- Diego AM, Marco AO, Carlos AM, José C. Photosynthesis and activity of superoxide dismutase peroxidase and glutathione reductase in cotton under salt stress. Environ Exp Bot. 2003;49:69–76. doi: 10.1016/S0098-8472(02)00058-8. [DOI] [Google Scholar]
- Dong J, Wan G, Liang Z. Accumulation of salicylic acidinduced phenolic compounds and raised activities of secondary metabolic and antioxidative enzymes in Salvia miltiorrhiza cell culture. J Biotechnol. 2010;148:99–104. doi: 10.1016/j.jbiotec.2010.05.009. [DOI] [PubMed] [Google Scholar]
- El Modafar C, Elgadda M, El Boutachfaiti R, Abouraicha E, Zehhar N, Petit E, El Alaoui-Talibi Z, Courtois B, Courtois J. Induction of natural defense accompanied by salicylic acid dependant systemic acquired resistance in tomato seedlings in response to bioelicitors isolated from green algae. Sci Hort. 2012;138:55–63. doi: 10.1016/j.scienta.2012.02.011. [DOI] [Google Scholar]
- Giridara KS, Madhusudhan KV, Sreenivasulu N, Sudhakar C. Stress responses in two genotypes of mulberry (Morus alba L.) under NaCl salinity. Ind J Exp Biol. 2000;38:192–195. [PubMed] [Google Scholar]
- Gómez-Vásquez R, Day R, Buschmann H, Randles S, Beeching JR, Cooper RM. Phenylpropanoids, phenylalanine ammonia lyase and peroxidases in elicitor-challenged cassava (Manihot esculenta) suspension cells and leaves. Ann Bot. 2004;94(1):87–97. doi: 10.1093/aob/mch107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goupil P, Benouaret R, Charrier O, ter Halle A, Richard C, Eyheraguibel B, Thiery D, Ledoigt G. Grape marc extract acts as elicitor of plant defense responses. Ecotoxicol. 2012;21:1541–1549. doi: 10.1007/s10646-012-0908-1. [DOI] [PubMed] [Google Scholar]
- Gupta NS, Banerjee M, Basu SK, Acharya K. Involvement of nitric oxide signal in Alternaria alternata toxin induced defense response in Rauvolfia serpentina Benth. ex Kurz calli. Plant Omics J. 2013;6:157–164. [Google Scholar]
- Hammond-Kosack KE, Parker JE. Deciphering plant–pathogen communication: fresh perspectives for molecular resistance breeding. Curr Opin Biotech. 2003;14:177–193. doi: 10.1016/S0958-1669(03)00035-1. [DOI] [PubMed] [Google Scholar]
- Hasanuzzaman M, Hossain MA, Fujita M. Nitric oxide modulates antioxidant defense and the methylglyoxal detoxification system and reduces salinity-induced damage of wheat seedlings. Plant Biotechnol Rep. 2011;5:353–365. doi: 10.1007/s11816-011-0189-9. [DOI] [PubMed] [Google Scholar]
- Hasanuzzaman M, Nahar K, Alam MM, Fujita M. Exogenous nitric oxide alleviates high temperature induced oxidative stress in wheat (Triticum aestivum L.) seedlings by modulating the antioxidant defense and glyoxalase system. AJCS. 2012;6(8):1314–1323. [Google Scholar]
- Hemeda HM, Klein BP. Effects of naturally occurring antioxidants on peroxidase activity of vegetable extracts. J Food Sci. 1990;55(1):184–185. doi: 10.1111/j.1365-2621.1990.tb06048.x. [DOI] [Google Scholar]
- Khan MN, Siddiqui MH, Mohammad F, Naeem M. Interactive role of nitric oxide and calcium chloride in enhancing tolerance to salt stress. Nitric Oxide Biol Chem. 2012;27:210–218. doi: 10.1016/j.niox.2012.07.005. [DOI] [PubMed] [Google Scholar]
- Kong W, Huang C, Chen Q, Zou Y, Zhang J. Nitric oxide alleviates heat stress-induced oxidative damage in Pleurotus eryngii var. tuoliensis. Fung Genet Biol. 2011;49:15–20. doi: 10.1016/j.fgb.2011.12.003. [DOI] [PubMed] [Google Scholar]
- Kumar KB, Khan PA. Peroxidase and polyphenol oxidase in excised ragi (Eleusine corocana cv PR 202) leaves during senescence. Ind J Exp Biol. 1982;20(5):412–416. [PubMed] [Google Scholar]
- Lamb C, Dixon RA. The oxidative burst in plant disease resistance. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:251–275. doi: 10.1146/annurev.arplant.48.1.251. [DOI] [PubMed] [Google Scholar]
- Laspina NV, Groppa MD, Tomaro ML, Benavides MP. Nitric oxide protects sunflower leaves against Cd-induced oxidative stress. Plant Sci. 2005;169:323–330. doi: 10.1016/j.plantsci.2005.02.007. [DOI] [Google Scholar]
- Leterrier M, Valderrama R, Chaki M, Airaki M, Palma JM, Barroso JB, Corpas FJ. Function of nitric oxide under environmental stress conditions. In: Khan NA, Nazar R, Iqbal N, Anjum NA, editors. Phytohormones and abiotic stress tolerance in plants. Berlin: Springer; 2012. pp. 99–113. [Google Scholar]
- Li G, Wan S, Zhou J, Yang Z, Qin P. Leaf chlorophyll fluorescence, hyperspectral reflectance, pigments content, malondialdehyde and proline accumulation responses of castor bean (Ricinus communis L.) seedlings to salt stress levels. Ind Crops Prod. 2010;31:13–19. doi: 10.1016/j.indcrop.2009.07.015. [DOI] [Google Scholar]
- Lin CC, Kao CH. Effect of NaCl stress on H2O2 metabolism in rice leaves. Plant Growth Regul. 2000;30:151–155. doi: 10.1023/A:1006345126589. [DOI] [Google Scholar]
- Lopez AMQ, Lucas JA. Effects of plant defence activators on anthracnose disease of cashew. Eur J Plant Pathol. 2002;108:409–420. doi: 10.1023/A:1016010710703. [DOI] [Google Scholar]
- Mano J. Early events in environmental stresses in plants. Induction mechanisms of oxidative stress. In: Inze D, Van Montagu M, editors. Oxidative stress in plants. London: Taylor and Francis; 2002. pp. 217–246. [Google Scholar]
- Maxson-Stein K, He SY, Hammerschmidt R, Jones AS. Effect of treating apple trees with acibenzolar-S-methyl on fire blight and expression of pathogenesis-related protein genes. Plant Dis. 2002;8:785–790. doi: 10.1094/PDIS.2002.86.7.785. [DOI] [PubMed] [Google Scholar]
- Mejía-Teniente L, Torres-Pacheco I, González-Chavira MM, Ocampo-Velazquez RV, Herrera-Ruiz G, Chapa-Oliver AM, Guevara-González RG. Use of elicitors as an approach for sustainable agriculture. Afr J Biotechnol. 2010;9(54):9155–9162. [Google Scholar]
- Mittler R. Oxidative stress, antioxidants and stress tolerance. Trend Plant Sci. 2002;7:405–410. doi: 10.1016/S1360-1385(02)02312-9. [DOI] [PubMed] [Google Scholar]
- Najami N, Tibor J, Barriah W, Kayam G, Moshe T, Guy M, Volokita M. Ascorbate peroxidase gene family in tomato: its identification and characterization. Mol Genet Genom. 2008;279:171–182. doi: 10.1007/s00438-007-0305-2. [DOI] [PubMed] [Google Scholar]
- Nakano Y, Asada K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981;22(5):867–880. [Google Scholar]
- Naveen J, Hariprasad P, Nayaka SC, Niranjana SR. Cerebroside mediated elicitation of defense response in chilli (Capsicum annuum L.) against Colletotrichum capsici infection. J Plant Interact. 2013;8:65–73. doi: 10.1080/17429145.2012.679704. [DOI] [Google Scholar]
- Neill SJ, Desikan R, Hancock JT. Nitric oxide signaling in plants. New Phytol. 2003;159:11–35. doi: 10.1046/j.1469-8137.2003.00804.x. [DOI] [PubMed] [Google Scholar]
- Pal TK, Bhattacharya S, Chakraborty K. Induction of systemic resistance in rice by leaf extract of Cymbopogan citrus and Ocimum sanctum against seath blight disease. Arch Appl Sci Res. 2011;3:392–400. [Google Scholar]
- Pan SQ, Ye XS, Kúc J. Association of β-1,3-glucanase activity and isoform pattern with systemic resistance to blue mould in tobacco induced by stem injection with Peronospora tabacina or leaf inoculation with tobacco mosaic virus. Physiol Mol Plant Pathol. 1991;39(1):25–39. doi: 10.1016/0885-5765(91)90029-H. [DOI] [Google Scholar]
- Pellegrini L, Rohfritsch O, Fritig B, Legrand M. Phenylalanine ammonia-lyase in tobacco. Molecular cloning and gene expression during the hypersensitive reaction to tobacco mosaic virus and the response to a fungal elicitor. Plant Physiol. 1994;106:877–886. doi: 10.1104/pp.106.3.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perchepied L, Balagué C, Riou C, Claudel-Renard C, Rivière N, Grezes-Besset B, et al. Nitric oxide participates in the complex interplay of defense-related signaling pathways controlling disease resistance to Sclerotinia sclerotiorum in Arabidopsis thaliana. Mol Plant Microbe Interact. 2010;23:846–860. doi: 10.1094/MPMI-23-7-0846. [DOI] [PubMed] [Google Scholar]
- Ramanjulu S, Sudhakar C. Alleviation of NaCl salinity stress by calcium is partly related to the increased proline accumulation in mulberry (Morus alba L.) callus. J Plant Biol. 2001;28:203–206. [Google Scholar]
- Repka V. Elicitor-stimulated induction of defense mechanisms and defense gene activation in grapevine cell suspension cultures. Biol Plant. 2001;44(4):555–565. doi: 10.1023/A:1013742703929. [DOI] [Google Scholar]
- Romero-Puertas MC, Rodríguez-Serrano M, Corpas FJ, Gómez M, del Río LA, Sandalio LM. Cd-induced subcellular accumulation of O2− and H2O2 in pea leaves. Plant Cell Environ. 2004;27:1122–1134. doi: 10.1111/j.1365-3040.2004.01217.x. [DOI] [Google Scholar]
- Sairam RK, Srivastava GC. Changes in antioxidant activity in sub-cellular fractions of tolerant and susceptible wheat genotypes in response to long term salt stress. Plant Sci. 2002;162:897–904. doi: 10.1016/S0168-9452(02)00037-7. [DOI] [Google Scholar]
- Song S, Liu W, Guo S, Shang Q, Zhang Z. Salt resistance and its mechanism of cucumber under effects of exogenous chemical activators. Ying Yong Sheng Tai Xue Bao. 2006;17:1871–1876. [PubMed] [Google Scholar]
- Thakur M, Sohal BS. Role of elicitors in inducing resistance in plants against pathogen infection: a review. ISRN Biochem. 2013;762412:1–10. doi: 10.1155/2013/762412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thordal-Christensen H, Zhang Z, Wei Y, Collinge DB. Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley powdery mildew interaction. Plant J. 1997;11(6):1187–1194. doi: 10.1046/j.1365-313X.1997.11061187.x. [DOI] [Google Scholar]
- Tian S, Wan Y, Qin G, Xu Y. Induction of defense responses against Alternaria rot by different elicitors in harvested pear fruit. Appl Microbiol Biotechnol. 2006;70:729–734. doi: 10.1007/s00253-005-0125-4. [DOI] [PubMed] [Google Scholar]
- Tijen D, Ìsmail T. Comparative lipid peroxidation, antioxidant defense systems and praline content in roots of two rice cultivars differing in salt tolerance. Environ Exp Bot. 2005;53:247–257. doi: 10.1016/j.envexpbot.2004.03.017. [DOI] [Google Scholar]
- Van Loon LC, Van Strien EA. The families of pathogenesis-related proteins, their activities, and comparative analysis of PR-1 type proteins. Physiol Mol Plant Pathol. 1999;55(2):85–97. doi: 10.1006/pmpp.1999.0213. [DOI] [Google Scholar]
- Wang XS, Han JG. Effects of NaCl and silicon on ion distribution in the roots, shoots and leaves of two alfalfa cultivars with different salt tolerance. Soil Sci Plant Nutr. 2007;53(3):278–285. doi: 10.1111/j.1747-0765.2007.00135.x. [DOI] [Google Scholar]
- Xu MJ, Dong JF, Zhu MY. Effect of nitric oxide on catharanthine production and growth of Catharanthus roseus suspension cells. Biotechnol Bioeng. 2005;89:367–371. doi: 10.1002/bit.20334. [DOI] [PubMed] [Google Scholar]
- Zieslin N, Ben Zaken R. Peroxidase activity and presence of phenolic substances in peduncles of rose flowers. Plant Physiol Biochem. 1993;31(3):333–339. [Google Scholar]