Abstract
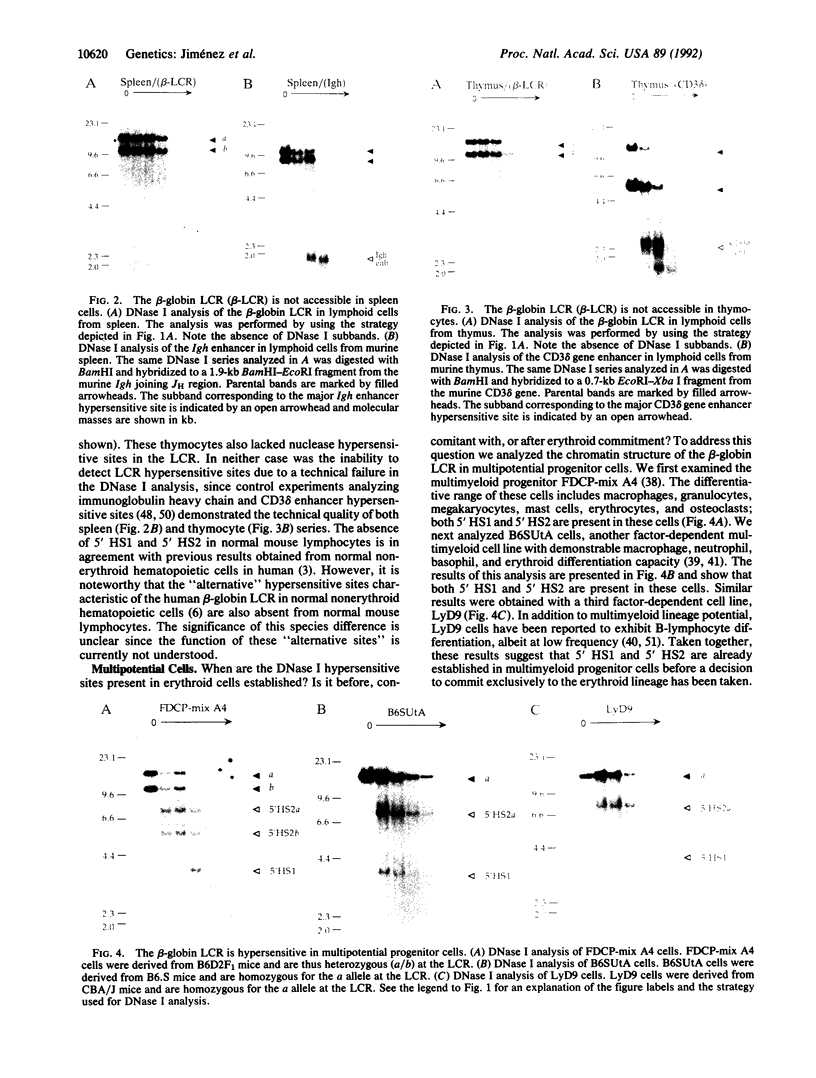
The beta-globin locus control region (LCR) is characterized by erythroid-specific DNase I hypersensitive sites and is involved in the chromatin organization, transcriptional potentiation, developmental regulation, and replication timing of the entire beta-globin gene cluster. When and how the LCR is first activated during erythropoiesis is not known. Here we analyze the chromatin structure of the LCR during early hematopoietic differentiation using nontransformed, multipotential, growth factor-dependent, murine hematopoietic progenitor cells. We show that LCR hypersensitive sites characteristic of erythroid cells are present in three independent multilineage progenitors [FDCP (factor-dependent cell, Paterson)-mix A4, B6SUtA, and LyD9] under conditions of self-renewal. Induction of differentiation down a nonerythroid pathway causes a progressive loss of hypersensitivity in the LCR. These results show that the beta-globin LCR is in an active chromatin configuration prior to erythroid commitment and indicate a significant role for selective gene repression in lineage specification.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alt F. W., Yancopoulos G. D., Blackwell T. K., Wood C., Thomas E., Boss M., Coffman R., Rosenberg N., Tonegawa S., Baltimore D. Ordered rearrangement of immunoglobulin heavy chain variable region segments. EMBO J. 1984 Jun;3(6):1209–1219. doi: 10.1002/j.1460-2075.1984.tb01955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behringer R. R., Ryan T. M., Palmiter R. D., Brinster R. L., Townes T. M. Human gamma- to beta-globin gene switching in transgenic mice. Genes Dev. 1990 Mar;4(3):380–389. doi: 10.1101/gad.4.3.380. [DOI] [PubMed] [Google Scholar]
- Burgess A. W., Camakaris J., Metcalf D. Purification and properties of colony-stimulating factor from mouse lung-conditioned medium. J Biol Chem. 1977 Mar 25;252(6):1998–2003. [PubMed] [Google Scholar]
- Caplan A. I., Ordahl C. P. Irreversible gene repression model for control of development. Science. 1978 Jul 14;201(4351):120–130. doi: 10.1126/science.351805. [DOI] [PubMed] [Google Scholar]
- Collis P., Antoniou M., Grosveld F. Definition of the minimal requirements within the human beta-globin gene and the dominant control region for high level expression. EMBO J. 1990 Jan;9(1):233–240. doi: 10.1002/j.1460-2075.1990.tb08100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crotta S., Nicolis S., Ronchi A., Ottolenghi S., Ruzzi L., Shimada Y., Migliaccio A. R., Migliaccio G. Progressive inactivation of the expression of an erythroid transcriptional factor in GM- and G-CSF-dependent myeloid cell lines. Nucleic Acids Res. 1990 Dec 11;18(23):6863–6869. doi: 10.1093/nar/18.23.6863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin P. T., Kan Y. W. The inactive beta globin gene on a gamma delta beta thalassemia chromosome has a normal structure and functions normally in vitro. Blood. 1988 Mar;71(3):766–770. [PubMed] [Google Scholar]
- Curtin P. T., Liu D. P., Liu W., Chang J. C., Kan Y. W. Human beta-globin gene expression in transgenic mice is enhanced by a distant DNase I hypersensitive site. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7082–7086. doi: 10.1073/pnas.86.18.7082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar V., Nandi A., Schildkraut C. L., Skoultchi A. I. Erythroid-specific nuclease-hypersensitive sites flanking the human beta-globin domain. Mol Cell Biol. 1990 Aug;10(8):4324–4333. doi: 10.1128/mcb.10.8.4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon N., Grosveld F. Human gamma-globin genes silenced independently of other genes in the beta-globin locus. Nature. 1991 Mar 21;350(6315):252–254. doi: 10.1038/350252a0. [DOI] [PubMed] [Google Scholar]
- Driscoll M. C., Dobkin C. S., Alter B. P. Gamma delta beta-thalassemia due to a de novo mutation deleting the 5' beta-globin gene activation-region hypersensitive sites. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7470–7474. doi: 10.1073/pnas.86.19.7470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enver T., Ebens A. J., Forrester W. C., Stamatoyannopoulos G. The human beta-globin locus activation region alters the developmental fate of a human fetal globin gene in transgenic mice. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7033–7037. doi: 10.1073/pnas.86.18.7033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enver T., Greaves D. R. Globin gene switching: a paradigm or what? Curr Opin Biotechnol. 1991 Dec;2(6):787–795. doi: 10.1016/s0958-1669(05)80108-9. [DOI] [PubMed] [Google Scholar]
- Enver T., Nakamoto B., Karlinsey J., Josephson B., Greenberger J., Papayannopoulou T. Erythropoietin changes the globin program of an interleukin 3-dependent multipotential cell line. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9091–9095. doi: 10.1073/pnas.85.23.9091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enver T., Raich N., Ebens A. J., Papayannopoulou T., Costantini F., Stamatoyannopoulos G. Developmental regulation of human fetal-to-adult globin gene switching in transgenic mice. Nature. 1990 Mar 22;344(6264):309–313. doi: 10.1038/344309a0. [DOI] [PubMed] [Google Scholar]
- Enver T., Zhang J. W., Anagnou N. P., Stamatoyannopoulos G., Papayannopoulou T. Developmental programs of human erythroleukemia cells: globin gene expression and methylation. Mol Cell Biol. 1988 Nov;8(11):4917–4926. doi: 10.1128/mcb.8.11.4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans T., Felsenfeld G. The erythroid-specific transcription factor Eryf1: a new finger protein. Cell. 1989 Sep 8;58(5):877–885. doi: 10.1016/0092-8674(89)90940-9. [DOI] [PubMed] [Google Scholar]
- Ford A. M., Bennett C. A., Healy L. E., Navarro E., Spooncer E., Greaves M. F. Immunoglobulin heavy-chain and CD3 delta-chain gene enhancers are DNase I-hypersensitive in hemopoietic progenitor cells. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3424–3428. doi: 10.1073/pnas.89.8.3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester W. C., Epner E., Driscoll M. C., Enver T., Brice M., Papayannopoulou T., Groudine M. A deletion of the human beta-globin locus activation region causes a major alteration in chromatin structure and replication across the entire beta-globin locus. Genes Dev. 1990 Oct;4(10):1637–1649. doi: 10.1101/gad.4.10.1637. [DOI] [PubMed] [Google Scholar]
- Forrester W. C., Novak U., Gelinas R., Groudine M. Molecular analysis of the human beta-globin locus activation region. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5439–5443. doi: 10.1073/pnas.86.14.5439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester W. C., Takegawa S., Papayannopoulou T., Stamatoyannopoulos G., Groudine M. Evidence for a locus activation region: the formation of developmentally stable hypersensitive sites in globin-expressing hybrids. Nucleic Acids Res. 1987 Dec 23;15(24):10159–10177. doi: 10.1093/nar/15.24.10159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester W. C., Thompson C., Elder J. T., Groudine M. A developmentally stable chromatin structure in the human beta-globin gene cluster. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1359–1363. doi: 10.1073/pnas.83.5.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopoulos K., van den Elsen P., Bier E., Maxam A., Terhorst C. A T cell-specific enhancer is located in a DNase I-hypersensitive area at the 3' end of the CD3-delta gene. EMBO J. 1988 Aug;7(8):2401–2407. doi: 10.1002/j.1460-2075.1988.tb03085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaves M. F., Chan L. C., Furley A. J., Watt S. M., Molgaard H. V. Lineage promiscuity in hemopoietic differentiation and leukemia. Blood. 1986 Jan;67(1):1–11. [PubMed] [Google Scholar]
- Greenberger J. S., Sakakeeny M. A., Humphries R. K., Eaves C. J., Eckner R. J. Demonstration of permanent factor-dependent multipotential (erythroid/neutrophil/basophil) hematopoietic progenitor cell lines. Proc Natl Acad Sci U S A. 1983 May;80(10):2931–2935. doi: 10.1073/pnas.80.10.2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosveld F., van Assendelft G. B., Greaves D. R., Kollias G. Position-independent, high-level expression of the human beta-globin gene in transgenic mice. Cell. 1987 Dec 24;51(6):975–985. doi: 10.1016/0092-8674(87)90584-8. [DOI] [PubMed] [Google Scholar]
- Hanscombe O., Whyatt D., Fraser P., Yannoutsos N., Greaves D., Dillon N., Grosveld F. Importance of globin gene order for correct developmental expression. Genes Dev. 1991 Aug;5(8):1387–1394. doi: 10.1101/gad.5.8.1387. [DOI] [PubMed] [Google Scholar]
- Heberlein C., Fischer K. D., Stoffel M., Nowock J., Ford A., Tessmer U., Stocking C. The gene for erythropoietin receptor is expressed in multipotential hematopoietic and embryonal stem cells: evidence for differentiation stage-specific regulation. Mol Cell Biol. 1992 Apr;12(4):1815–1826. doi: 10.1128/mcb.12.4.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyworth C. M., Dexter T. M., Kan O., Whetton A. D. The role of hemopoietic growth factors in self-renewal and differentiation of IL-3-dependent multipotential stem cells. Growth Factors. 1990;2(2-3):197–211. doi: 10.3109/08977199009071506. [DOI] [PubMed] [Google Scholar]
- Kinashi T., Tashiro K., Inaba K., Takeda T., Palacios R., Honjo T. An interleukin-4-dependent precursor clone is an intermediate of the differentiation pathway from an interleukin-3-dependent precursor clone into myeloid cells as well as B lymphocytes. Int Immunol. 1989;1(1):11–19. doi: 10.1093/intimm/1.1.11. [DOI] [PubMed] [Google Scholar]
- Kioussis D., Vanin E., deLange T., Flavell R. A., Grosveld F. G. Beta-globin gene inactivation by DNA translocation in gamma beta-thalassaemia. Nature. 1983 Dec 15;306(5944):662–666. doi: 10.1038/306662a0. [DOI] [PubMed] [Google Scholar]
- Li Q. L., Zhou B., Powers P., Enver T., Stamatoyannopoulos G. Beta-globin locus activation regions: conservation of organization, structure, and function. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8207–8211. doi: 10.1073/pnas.87.21.8207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Zhou B., Powers P., Enver T., Stamatoyannopoulos G. Primary structure of the goat beta-globin locus control region. Genomics. 1991 Mar;9(3):488–499. doi: 10.1016/0888-7543(91)90415-b. [DOI] [PubMed] [Google Scholar]
- Lindenbaum M. H., Grosveld F. An in vitro globin gene switching model based on differentiated embryonic stem cells. Genes Dev. 1990 Dec;4(12A):2075–2085. doi: 10.1101/gad.4.12a.2075. [DOI] [PubMed] [Google Scholar]
- Lloyd J. A., Krakowsky J. M., Crable S. C., Lingrel J. B. Human gamma- to beta-globin gene switching using a mini construct in transgenic mice. Mol Cell Biol. 1992 Apr;12(4):1561–1567. doi: 10.1128/mcb.12.4.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon A. M., Ley T. J. Conservation of the primary structure, organization, and function of the human and mouse beta-globin locus-activating regions. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7693–7697. doi: 10.1073/pnas.87.19.7693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ney P. A., Sorrentino B. P., McDonagh K. T., Nienhuis A. W. Tandem AP-1-binding sites within the human beta-globin dominant control region function as an inducible enhancer in erythroid cells. Genes Dev. 1990 Jun;4(6):993–1006. doi: 10.1101/gad.4.6.993. [DOI] [PubMed] [Google Scholar]
- Orkin S. H. Globin gene regulation and switching: circa 1990. Cell. 1990 Nov 16;63(4):665–672. doi: 10.1016/0092-8674(90)90133-y. [DOI] [PubMed] [Google Scholar]
- Palacios R., Karasuyama H., Rolink A. Ly1+ PRO-B lymphocyte clones. Phenotype, growth requirements and differentiation in vitro and in vivo. EMBO J. 1987 Dec 1;6(12):3687–3693. doi: 10.1002/j.1460-2075.1987.tb02702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pevny L., Simon M. C., Robertson E., Klein W. H., Tsai S. F., D'Agati V., Orkin S. H., Costantini F. Erythroid differentiation in chimaeric mice blocked by a targeted mutation in the gene for transcription factor GATA-1. Nature. 1991 Jan 17;349(6306):257–260. doi: 10.1038/349257a0. [DOI] [PubMed] [Google Scholar]
- Philipsen S., Talbot D., Fraser P., Grosveld F. The beta-globin dominant control region: hypersensitive site 2. EMBO J. 1990 Jul;9(7):2159–2167. doi: 10.1002/j.1460-2075.1990.tb07385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruzina S., Hanscombe O., Whyatt D., Grosveld F., Philipsen S. Hypersensitive site 4 of the human beta globin locus control region. Nucleic Acids Res. 1991 Apr 11;19(7):1413–1419. doi: 10.1093/nar/19.7.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raich N., Enver T., Nakamoto B., Josephson B., Papayannopoulou T., Stamatoyannopoulos G. Autonomous developmental control of human embryonic globin gene switching in transgenic mice. Science. 1990 Nov 23;250(4984):1147–1149. doi: 10.1126/science.2251502. [DOI] [PubMed] [Google Scholar]
- Raich N., Papayannopoulou T., Stamatoyannopoulos G., Enver T. Demonstration of a human epsilon-globin gene silencer with studies in transgenic mice. Blood. 1992 Feb 15;79(4):861–864. [PubMed] [Google Scholar]
- Rifkind R. A., Chui D., Epler H. An ultrastructural study of early morphogenetic events during the establishment of fetal hepatic erythropoiesis. J Cell Biol. 1969 Feb;40(2):343–365. doi: 10.1083/jcb.40.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan T. M., Behringer R. R., Martin N. C., Townes T. M., Palmiter R. D., Brinster R. L. A single erythroid-specific DNase I super-hypersensitive site activates high levels of human beta-globin gene expression in transgenic mice. Genes Dev. 1989 Mar;3(3):314–323. doi: 10.1101/gad.3.3.314. [DOI] [PubMed] [Google Scholar]
- Ryan T. M., Behringer R. R., Townes T. M., Palmiter R. D., Brinster R. L. High-level erythroid expression of human alpha-globin genes in transgenic mice. Proc Natl Acad Sci U S A. 1989 Jan;86(1):37–41. doi: 10.1073/pnas.86.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shehee W. R., Loeb D. D., Adey N. B., Burton F. H., Casavant N. C., Cole P., Davies C. J., McGraw R. A., Schichman S. A., Severynse D. M. Nucleotide sequence of the BALB/c mouse beta-globin complex. J Mol Biol. 1989 Jan 5;205(1):41–62. doi: 10.1016/0022-2836(89)90363-x. [DOI] [PubMed] [Google Scholar]
- Shih D. M., Wall R. J., Shapiro S. G. Developmentally regulated and erythroid-specific expression of the human embryonic beta-globin gene in transgenic mice. Nucleic Acids Res. 1990 Sep 25;18(18):5465–5472. doi: 10.1093/nar/18.18.5465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebenlist U., Hennighausen L., Battey J., Leder P. Chromatin structure and protein binding in the putative regulatory region of the c-myc gene in Burkitt lymphoma. Cell. 1984 Jun;37(2):381–391. doi: 10.1016/0092-8674(84)90368-4. [DOI] [PubMed] [Google Scholar]
- Spangrude G. J., Heimfeld S., Weissman I. L. Purification and characterization of mouse hematopoietic stem cells. Science. 1988 Jul 1;241(4861):58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- Spooncer E., Boettiger D., Dexter T. M. Continuous in vitro generation of multipotential stem cell clones from src-infected cultures. Nature. 1984 Jul 19;310(5974):228–230. doi: 10.1038/310228a0. [DOI] [PubMed] [Google Scholar]
- Storb U., Arp B., Wilson R. The switch region associated with immunoglobulin C mu genes is DNase I hypersensitive in T lymphocytes. Nature. 1981 Nov 5;294(5836):90–92. doi: 10.1038/294090a0. [DOI] [PubMed] [Google Scholar]
- Sutherland H. J., Eaves C. J., Eaves A. C., Dragowska W., Lansdorp P. M. Characterization and partial purification of human marrow cells capable of initiating long-term hematopoiesis in vitro. Blood. 1989 Oct;74(5):1563–1570. [PubMed] [Google Scholar]
- Talbot D., Collis P., Antoniou M., Vidal M., Grosveld F., Greaves D. R. A dominant control region from the human beta-globin locus conferring integration site-independent gene expression. Nature. 1989 Mar 23;338(6213):352–355. doi: 10.1038/338352a0. [DOI] [PubMed] [Google Scholar]
- Talbot D., Philipsen S., Fraser P., Grosveld F. Detailed analysis of the site 3 region of the human beta-globin dominant control region. EMBO J. 1990 Jul;9(7):2169–2177. doi: 10.1002/j.1460-2075.1990.tb07386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taramelli R., Kioussis D., Vanin E., Bartram K., Groffen J., Hurst J., Grosveld F. G. Gamma delta beta-thalassaemias 1 and 2 are the result of a 100 kbp deletion in the human beta-globin cluster. Nucleic Acids Res. 1986 Sep 11;14(17):7017–7029. doi: 10.1093/nar/14.17.7017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai S. F., Martin D. I., Zon L. I., D'Andrea A. D., Wong G. G., Orkin S. H. Cloning of cDNA for the major DNA-binding protein of the erythroid lineage through expression in mammalian cells. Nature. 1989 Jun 8;339(6224):446–451. doi: 10.1038/339446a0. [DOI] [PubMed] [Google Scholar]
- Tuan D., Solomon W., Li Q., London I. M. The "beta-like-globin" gene domain in human erythroid cells. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6384–6388. doi: 10.1073/pnas.82.19.6384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser J. W., Bauman J. G., Mulder A. H., Eliason J. F., de Leeuw A. M. Isolation of murine pluripotent hemopoietic stem cells. J Exp Med. 1984 Jun 1;159(6):1576–1590. doi: 10.1084/jem.159.6.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub H., Davis R., Tapscott S., Thayer M., Krause M., Benezra R., Blackwell T. K., Turner D., Rupp R., Hollenberg S. The myoD gene family: nodal point during specification of the muscle cell lineage. Science. 1991 Feb 15;251(4995):761–766. doi: 10.1126/science.1846704. [DOI] [PubMed] [Google Scholar]