Abstract
Background and purpose: Silymarin, a standardized extract of the milk thistle seeds, has been widely used to treat chronic hepatitis, cirrhosis, and other types of toxic liver damage. Despite increasing studies on the action of silymarin and its major active constituent, silybin in their therapeutic properties against insulin resistance, diabetes and hyperlipidaemia in vitro and in vivo, the mechanism underlying silymarin action remains unclear.
Experimental approach: C57BL/6 mice were fed high-fat diet (HFD) for 3 months to induce obesity, insulin resistance, hyperlipidaemia, and fatty liver. These mice were then continuously treated with HFD alone or mixed with silymarin at 40 mg/100 g for additional 6 weeks. Biochemical analysis was used to test the serum lipid and bile acid profiles. Farnesyl X receptor (FXR) and nuclear factor kappa B (NF-κB) transactivities were analyzed in liver using a gene reporter assay based on quantitative RT-PCR.
Key results: Silymarin treatment ameliorated insulin resistance, dyslipidaemia and inflammation, and reconstituted the bile acid pool in liver of diet-induced obesity. Associated with this, silybin and silymarin enhanced FXR transactivity. Consistently, in HepG2 cells, silybin inhibited NF-κB signaling, which was enhanced by FXR activation.
Conclusion and implications: Our results suggest that silybin is an effective component of silymarin for treating metabolic syndrome by stimulating FXR signaling.
Keywords: silymarin, silybin, metabolic syndrome, non-alcoholic fatty liver disease, farnesyl X receptor
Introduction
Metabolic syndrome encompasses a cluster of conditions including obesity, type 2 diabetes, dyslipidaemia, atherosclerosis, cardiovascular disease, NAFLD and hepatobiliary diseases (Bonora, 2006; Rui, 2014). Because of rapid transition to over-nutritious environment and concealed disease progression, MS has become an epidemic and one leading killer worldwide (Finucane et al., 2011; Wahi et al., 2011). Maintaining a lifestyle with balanced energy metabolism is necessary to prevent but is not sufficient to treat MS, suggesting a critical importance for pharmacotherapy in treating and reversing MS. However, due to its complex pathogenesis, there are few approved prescription drugs that are able to effectively cure MS (Heal et al., 2009; Douglas et al., 2013).
The farnesyl X receptor (FXR, Nr1h4) serves as an intracellular BA-responsive ligand-activated NR (Makishima et al., 1999). It is ubiquitous in all tissues but highly expressed in liver, ileum, and adrenal gland (Matsubara et al., 2013). FXR has been shown to play an essential role in controlling BA homeostasis and protecting against liver injury resulting from cholestasis or drug induction, as well as maintaining normal lipid and glucose metabolism by regulating the expression of a series of downstream target genes (Westin et al., 2005; Cariou et al., 2006; Lee et al., 2006; Zhang et al., 2006, 2012; Kim et al., 2007; Matsubara et al., 2013). FXR-/- mice have an array of metabolic derangements including impaired glucose tolerance and insulin sensitivity, increased plasma lipids levels, serious NAFLD and liver damage, etc. (Kong et al., 2008; Lefebvre and Staels, 2014). FXR is also reported to protect against toxic liver damage, hepatitis, and cirrhosis (Liu et al., 2003; Yang et al., 2007; Matsubara et al., 2013; Beuers et al., 2015). Targeting FXR is considered as a promising avenue in the treatment of liver diseases.
Inflammation is a complex biological response that is involved in various diseases. It has been reported that the FXR signal is involved in regulation of the inflammation response (Arrese and Karpen, 2010; Gadaleta et al., 2011). Antagonizing FXR can interact with NF-κB signaling leading to exacerbation of inflammation (Wang et al., 2008). NF-κB as a nuclear transcription factor directs the inflammatory response by controlling the expression of genes involved in inflammation (Hayden and Ghosh, 2008). In addition, numerous studies have implicated the NF-κB inflammatory program in the development of multiple metabolic diseases, and almost all liver diseases accompany hepatic inflammation (Elsharkawy and Mann, 2007). Interfering with NF-κB-driven inflammation could decrease hyperglycaemia and insulin resistance in patients (Baker et al., 2011).
In the US and Europe, about 65% of patients with liver metabolic disorders take herbal medicine (Tindle et al., 2005). Due to their apparent beneficial effects to human health, treatments with natural herbal medicine for diverse chronic MSs have become more and more popular (Stickel et al., 2002; Corns, 2003; Adewusi and Afolayan, 2010; Salomone et al., 2016). Silymarin is a natural herbal extraction from the fruit and seeds of the Silybum marianum (Milk thistle), consisting of seven flavonolignans (silybin A, silybin B, isosilybin A, isosilybin B, silychristin, isosilychristin, and silydianin; Kvasnička et al., 2003; Abenavoli et al., 2010; Biedermann et al., 2014). Milk thistle fruits and seeds have been used to treat liver and biliary disorders for more than 2000 years (Saller et al., 2001; Hoofnagle, 2005; Tamayo and Diamond, 2007; Abenavoli et al., 2010). Previous studies have proved that silymarin have antioxidant, anti-inflammation and anti-cancer effect properties, and improve homeostatic maintenance of glycaemia (Angulo et al., 2000; Kidd and Head, 2005; Gazák et al., 2007; Loguercio et al., 2012; Polyak et al., 2013). The flavonolignan silybin (a 1:1 mixture of silybin A and silybin B, which accounts for about 50–70% of the silymarin extract) is identified as the major active ingredient in silymarin (Kvasnička et al., 2003; Lee and Liu, 2003). Silymarin is used in the treatment of toxic liver damage, chronic hepatitis, and cirrhosis in some EU countries and China (Saller et al., 2001). Although clinical practices and animal experiments have repeatedly proven the effective therapeutic roles of silymarin and silybin in vivo (Tamayo and Diamond, 2007), the underlying molecular mechanism remains largely unknown. In the past 10 years, the mechanistic studies of silymarin and silybin were concentrated on reducing the mitochondrial ROS generation, inhibiting glycogenolysis and gluconeogenesis or blocking the activation of intrahepatic NF-κB signal pathway (Velussi et al., 1997; Abenavoli et al., 2010; Wu et al., 2011; Raina et al., 2013). No clear molecular target of silymarin or silybin has been reported. Here we identified silymarin and silybin as novel FXR agonists, and found that silybin inhibited the NF-κB signal pathway via negative crosstalk with the FXR in hepatocytes. In animal experiments, we found silymarin attenuated hyperlipidaemia, insulin resistance, and altered the composition of liver BAs pool in HF diet-fed C57BL/6 mice. These therapeutic effects of silymarin in vivo may be linked to the regulation of FXR and NF-κB signaling pathways by silybin, the major active constituent in silymarin.
Materials and Methods
Chemicals and Diets
Silymarin (Legalon, Madaus, German) powder was dissolved in dimethylsulfoxide to the final concentration of 40 mg/ml for cell culture. Silybin, GW4064, T090173, Rosiglitazone, int-777, WY14643, rifampicin, TNFα, and all of the BAs standard references mentioned in this article were purchased from Sigma–Aldrich (St. Louis, MO, USA). HF diets (60% of calories derived from fat), and low-calorie diets (10% of calories derived from fat) were purchased from Research Diet (D12492, D12450B, New Brunswick, NJ, USA).
Cell Culture and Treatment
HepG2 (ATCC) cells were seeded on six-well plates (1 × 106 cells/well) and grown to 80% confluence with high-glucose DMEM containing 10% FBS at 37°C in 5% CO2. The following day, cells were treated with vehicle control, silymarin (40 μg/ml) or silybin (25 μM). After 18-h treatment, the cells were treated with or without TNFα (10 ng/mL) and then collected for RNA isolation after 6-h incubation.
Transient Transfection of Cultured Cells and Reporter Assays
The reporter assay was performed using the Dual-Luciferase Reporter Assay System (Promega, USA) as previously described (Huang et al., 2006). For NR transcription activity assay, the expression plasmids for phFXR, phRXR and FXR-dependent reporter (EcRE-LUC), pCMXGal-hPPARα, γ, LXRα, β, and PXR-LBD and the Gal4 reporter vector MH100 × 4-TK-Luc were co-transfected with a reporter construct so that 1 μg of the relevant plasmid combined with 1 μg of reporter plasmids and 0.1 μg of pREP7 (Renilla luciferase) reporter could be used to normalize transfection efficiencies. The transfection mixture, which contained 10 μg of total plasmids and 15 μl FuGENE-HD (Roche) per ml of DMEM, was added to HEK293T cells (ATCC) for 24 h and then removed. The FXR, PPARα, PPARγ, LXRs, and PXR agonists (GW4064, WY14643, Rosiglitazone, T090173, and Rifampicin, respectively), silymarin or silybin were added to fresh media and the cells were incubated for another 24 h to determine luciferase activity.
To characterize NF-κB activity, HepG2 cells were co-transfected with p65 expression vector, NF-κBx3-LUC, control plasmid pREP7, and/or FXR/RXR expression plasmids using FuGENE-HD. After 24 h, cells were pre-treated with the control, silymarin, or silybin for 18 h before treatment with TNFα (10 ng/mL) for 6 h. Subsequently, cells were collected and subjected to luciferase activity analysis. To determine the level of hTGR5 activation, hTGR5 expression plasmid and luciferase reporter pCRE-Luc and pREP7 were transiently transfected into HEK293T cells for 24 h. Then cells were incubated with the control, silymarin (40 μg/ml), or silybin (50 μM) for another day before being used for reporter assay. The renilla luciferase activity was assayed to normalize transfection efficiencies. All of the transfection experiments were performed in triplicate and repeated at least three times independently.
Animal Experiment
All animal protocols used in this study were approved by Shanghai University of Traditional Chinese Medicine (Approval Number: SZY20150524). Female C57BL/6 mice were purchased from the SLAC Laboratory (Shanghai, China). Animals were housed and bred according to standardized procedures, under controlled temperature (22–23°C) and on a 12-h light, 12-h dark cycle. Six-week-old female mice were fed with HF diet for 12 weeks to induce obesity and these mice were then randomly divided into three groups according to body weight: Chow group (10% of calories derived from fat), HF group (HF, 60% of calories derived from fat), and Silymarin group (HF diet supplemented with silymarin powder, Legalon from MADAUS GmbH, Germany, at dose of 40 mg/100 g diet). Mice were treated for additional 6 weeks. Food intake amount was measured by recording food weight every 2 days throughout the experiment. The amount of food intake over a 24-h period was calculated.
Intraperitoneal Glucose Tolerance and Insulin Tolerance
At the end of the treatment, mice were fasted overnight (12 h). The baseline glucose values (0 min), prior to the injection of glucose (1 g/kg body weight), were measured through tail vein. Additional blood samples were collected at regular intervals (15, 30, 60, and 90 min) during glucose tolerance tests. For the IPITT, non-fasted glucose levels were determined from the tail vein (0 min). Then insulin (Sigma, St. Louis, MO, USA) was injected intraperitoneally (0.75 U/kg body weight). Subsequent blood samples were taken at 15, 30, 60, 90, and 120 min after insulin administration for glucose measurement.
Serum Chemistry Analysis
At the end of animal experiment study, mice were anesthetized 20% urethane (Sigma, St. Louis, MO, USA) and cardiac blood was taken. Levels of serum alanine aminotransferase, AST, TG, TC, HDL-c, and LDL-c were measured using a Hitachi 7020 Automatic Analyzer (Hitachi, Limited, Tokyo, Japan) with 100 μl of heart blood serum.
Histochemistry
Liver tissues were fixed in formalin, and paraffin-embedded sections were cut at 5 μm. Sections were stained with haematoxylin and eosin according to a standard procedure.
Hepatic Lipid Content Analysis
Lipid content was measured as described (Folch et al., 1957). Briefly, liver tissues (100 mg) were homogenized with 2 ml chloroform-methanol and then agitated overnight on an orbital shaker at 4°C. The homogenate was then centrifuged (5 min at 5,000 rpm), 0.9% NaCl solution was subsequently added to the liquid phase before the samples were vortexed. Phase separation was induced by centrifugation (2,000 rpm for 10 min), and the bottom phase was removed to a new tube and evaporated to dryness, Samples were then resuspended in 500 μl chloroform-1% Triton X-100, evaporated to dryness, and finally resuspended in 500 μl of water. The quantities of total cholesterol and triglycerides (KINGHA WK, China) in liver lipid extracts were then assayed by using enzymatic kits according to the manufacturers’ protocols.
RNA Extraction and Quantitative PCR
Total RNA from HepG2 cells or mouse livers was isolated using the TRIzol method (Invitrogen, Carlsbad, CA, USA). The first-strand cDNA was synthesized with a cDNA synthesis kit (Fermentas, Madison, WI, USA). Quantitative real-time polymerase chain reaction (PCR) was carried out using SYBR green PCR Mastermix. The results were analyzed on an ABI StepOnePlus real-time PCR system (Applied Biosystems, USA). Values were normalized to Beta-actin. Sequences for primers are listed in Tables 1 and 2.
Table 1.
Sequences of the human primers used in real time polymerase chain reaction (PCR).
| Gene | Sense primer | Anti-sense primer |
|---|---|---|
| NR1H4 | ATGGGAATGTTGGCTGAATG | CCTGCATGACTTTGTTGTCG |
| NR0B2 | AGGCCTCCAAGCCGCCTCCCACATTGGGC | GCAGGCTGGTCGGAAACTTGAGGGT |
| LRH1 | CTAGAAGCTGTAAGGGCCGACCG | TCCATTGGCTCGGATGAGGGCT |
| SULT2A1 | AACAGGACACAGGAAGAACCAT | CAGTCCCCAGATACACCTTTTC |
| ABCC2 | TCGGAATGTGAATAGCCTGAAG | CGCAAGGATGATGAAGAATATCG |
| ABCB4 | GCAGACGGTGGCCCTGGTTGG | TGGAAAACAGCACCGGCTCCTG |
| APOC2 | GAGATGCCTAGCCCGACCTTCCTCAC | GCTCAGTCTGAACCTGGGGGATCAGG |
| CYP7A1 | GAGAAGGCAAACGGGTGAAC | AGCACAGCCCAGGTATGGA |
| CYP8B1 | CCCTCTTTCCCTACCTCTCAGT | AAGTGTGTGACCATAAGCAGGA |
| PPARα | GAAATGGGAAACATCCAAGAGA | CACAGGATAAGTCACCGAGGA |
| ACOX1 | CCAAGCTTTCCTGCTCAGTGTT | CCCCCAGTCCCTTTTCTTCA |
| CPT1α | TGGCGTCTGAGAAGCATCAGCATA | ACACCACGTAAAGGCAGAAGAGGT |
| ACC | GGATGGTGTTCACTCGGTAATAGA | GGGTGATATGTGCTGCGTCAT |
| β-ACTIN | AATCTGGCACCACACCTTCTA | ATAGCACAGCCTGGATAGCAAC |
Table 2.
Sequences of the mouse primers used in real time PCR.
| Gene | Sense primer | Anti-sense primer |
|---|---|---|
| β-actin | TGTCCACCTTCCAGCAGATGT | AGCTCAGTAACAGTCCGCCTAGA |
| Nr1h4 | TTCCTCAAGTTCAGCCACAG | TCGCCTGAGTTCATAGATGC |
| Nr0b2 | GGAGTCTTTCTGGAGCCTTG | ATCTGGGTTGAAGAGGATCG |
| Lrh1 | TCAGTTCGATCAGCGGGAGTTTGT | TGCAGGTTCTCCAGGTTCTTCACA |
| Hnf4α | GTGCTTCCGGGCTGGCATGAA | AGGTGATCTGCTGGGACAGAACC |
| Pparα | AGGCTGTAAGGGCTTCTTTCG | GGCATTTGTTCCGGTTCTTC |
| Pparβ | AGTGACCTGGCGCTCTTCAT | CGCAGAATGGTGTCCTGGAT |
| Pparγ | CGCTGATGCACTGCCTATGA | AGAGGTCCACAGAGCTGATTCC |
| Pgc1α | TGTTCCCGATCACCATATTCC | GGTGTCTGTAGTGGCTTGATTC |
| Pgc1β | GGGTGCGCCTCCAAGTG | TCTACAGACAGAAGATGTTATGTGAACAC |
| G6pc | GTGGCAGTGGTCGGAGACT | ACGGGCGTTGTCCAAAC |
| Pck1 | CACCATCACCTCCTGGAAGA | GGGTGCAGAATCTCGAGTTG |
| Apoc2 | TGATGTTGGGAAATGAGG | ATCGGGTATGTCTTCTGGTA |
| Apoai | ACTCGGGACTTCTGGGATA | AGTGTCTTCAGGTGGGTTTT |
| Acc | GAATCTCCTGGTGACAATGCTTATT | GGTCTTGCTGAGTTGGGTTAGCT |
| Scd1 | CTTATCATTGCCAACACCA | CTTCTCGGCTTTCAGGTC |
| Tnfα | ATGGATCTCAAAGACAACCAACTAG | ACGGCAGAGAGGAGGTTGACTT |
| Mcp-1 | AGGTCCCTGTCATGCTTC | GTGCTTGAGGTGGTTGTG |
| Il1β | TCGTGCTGTCGGACCCATAT | GGTTCTCCTTGTACAAAGCTCATG |
| Cox2 | TGAGCAACTATTCCAAACCAGC | GCACGTAGTCTTCGATCACTATC |
| Cyp7a1 | TGATCCTCTGGGCATCTCAAGCAA | AGCTCTTGGCCAGCACTCTGTAAT |
| Cyp8b1 | GGACAGCCTATCCTTGGTGA | GACGGAACTTCCTGAACAGC |
| Cyp27a1 | GAGAGTGAATCAGGGGACCA | CCATTTGGGAAGGAAAGTGA |
| Ntcp | TATCAGCCCCCTTCAATTTC | GTGAGCCTTGATCTTGCTGA |
| Baat | CCATTGAAAAAGCTCATGGA | ATCAGCTGTGCTATGGCTTG |
| Bacs | GATGCCCTTGCTACACTCT | AGGACAAGCCCTATCGTAT |
| Abcb11 | CGGACCTGTATTGTCATTGC | CCCTTCTGGTCCATCAGTTT |
Quantification of BAs in Mice
Measurement of TBAs in mouse livers and feces was performed as the following. Livers were weighed and homogenized in 10X volume water. TBA level determination was performed on homogenate samples using the TBA Measurement Kit (KeHua, China) after quantification of intracellular protein concentration. The TBA levels were normalized to intracellular protein content. Protein concentrations were determined by BCA protein assay kit (Sigma, St. Louis, MO, USA). The fecal TBA analysis was performed as the same method described above and calculated by normalizing the feces weight.
To measure hepatic BA pool size and composition, liver-tissue samples were weighed, and TBA was extracted in 5X volumes of acetonitrile followed by centrifugation at 14,300 rpm for 10 min. The supernatant was dried under nitrogen steam before re-dissolved in methanol solution (methanol:water:formic acid = 50:50:0.01) and then subject to centrifugation at 14,300 rpm for 10 min. Supernatant bile salt species were analyzed by ultra-performance liquid chromatography (UPLC) triple time of flight/MS analysis (UPLC-MS, Waters Co., MA, USA). The relevant parameters were described in a previous publication (Yang et al., 2012).
Molecular Docking
The crystal structure of NR FXR (PDB code 1OT7) was retrieved from the Research Collaboratory for Structural Bioinformatics (RCSB) protein data bank. Silybin A and silybin B were constructed using the sketcher module in Sybyl and their minimum energy conformations were calculated using the Minimize module of Sybyl. The force field was Tripos with an 8 Å cutoff for non-bonded interactions, and the atomic point charges were also calculated with Gasteiger-Huckel. Minimizations were achieved using the steepest descent method for the first 100 steps, followed by the Broyden-Fletcher-Goldfarb-Shanno (BFGS) method until the root-mean-square (RMS) of the gradient became <0.005 kcal/(mol⋅Å). The Surflex-Dock module implemented in the Sybyl program was used for the docking studies. The obeticholic acid binding pocket of FXR was used for the docking simulation. Both silybin A and silybin B were docked into the binding site by an empirical scoring function and a patented search engine in Surflex-Dock, applied with the automatic docking. Other parameters were established by default in the software.
Statistical Analysis
All values were expressed as means ± SEM and analyzed using the statistical package for the social science (SPSS, version 15.0). Paired or unpaired two tailed t-tests were used to detect difference in the mean values of treatment group and control and analysis of variance (ANOVA) for the difference among more than two groups. Differences with P values <0.05 were considered to be statistically significant.
Results
Silymarin and Silybin Stimulate FXR Transactivity In vitro
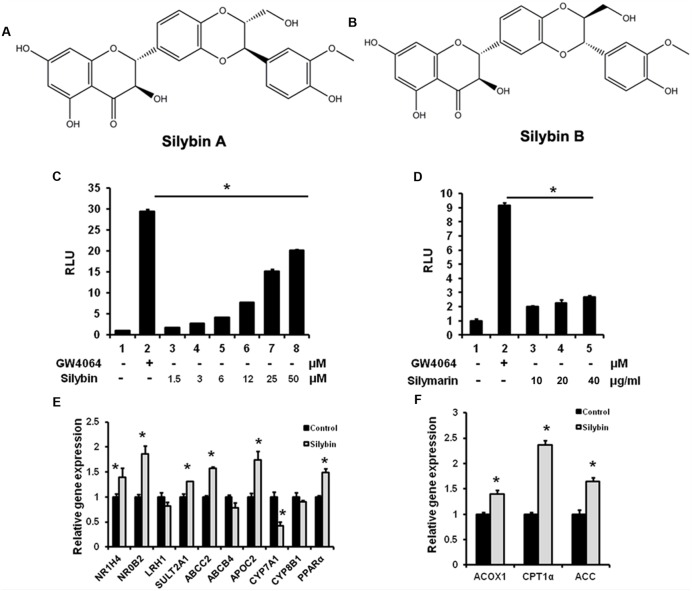
To test whether silymarin and its major constituent silybin (silybin A:B = 1:1, Figures 1A,B) are able to activate FXR, we performed a gene reporter assay by co-transfected hFXR, hRXR expression vectors, and FXR-dependent reporter (EcRE-LUC) into HEK293T cells. The results demonstrated that silybin activated the FXR transactivity in a dose-dependent manner (Figure 1C). Silymarin showed similar effects but with lower activity in (Figure 1D). We also analyzed the effects of silybin and silymarin on other important NR transactivity and the results showed that there were no effects of silybin or silymarin on PXR, PPARγ, α, β/δ, and LXRα, β transactivities (data not shown), suggesting that the silybin activates FXR specifically.
FIGURE 1.
Silybin and silymarin activate FXR transactivity in vitro. (A,B) Structure of Silybin A and Silybin B. Silybin (C) and silymarin (D) activate FXR transactivity. HEK293T cells were co-transfected with phFXR, phRXR expression plasmids, and FXR-dependent reporter (EcRE-LUC) for 24 h and treated with the FXR agonist GW4064 (10 μM), control (DMSO), silybin (1.5, 3, 6, 12, 25, 50 μM) and silymarin (10, 20, and 40 μg/ml) for another 24 h. The relative luciferase activities (RLU) were measured by comparison to renilla luciferase activities. (E,F) The relative gene expression levels in silybin-treated (25 μM) and control (DMSO) HepG2 cells. Beta-ACTIN was used as an internal control for normalizing the mRNA levels. The results represent three independent experiments, and data are presented as means ± SEM. (n = 3). ∗P < 0.05 vs. HEK293T or HepG2 control.
Farnesyl X receptor agonists have been shown to activate a series of target genes. We therefore examined whether silybin could regulate known target gene expression in HepG2 cells. As expected, silybin treatment induced FXR target gene expression when compared with mock control groups (Figures 1E,F), suggesting an increased FXR transactivity by silybin.
TGR5, abile acid sensitive G-protein coupled receptor, has been reported to bind with diverse FXR agonists (Chen et al., 2011; Keitel and Häussinger, 2012). To clarify whether silybin and silymarin also activate TGR5-cAMP signaling, we transiently transfected hTGR5 expression plasmid and pCRE-Luc reporter into HEK293T cells over a period of 24 h. Even with a high dose (Silybin at 50 μM and silymarin at 40 μg/ml), neither silybin nor silymarin stimulated the pCRE-Luc reporter signaling (data not shown), indicating that both drugs have limited influence on activating hTGR5.
Silybin Interacts with FXR
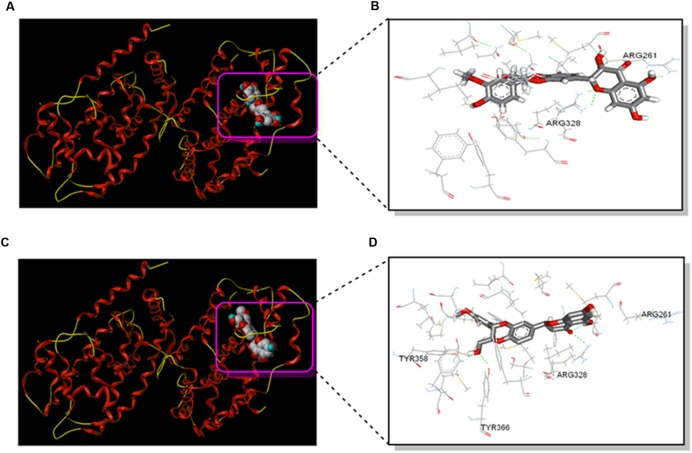
To further test potential direct interaction between silybin and NR FXR, the structure of the complex of FXR and silybin was analyzed by molecular docking. The obeticholic acid binding site of FXR has been validated as the binding pocket of silybin. Both silybin A and silybin B were docked into the binding pocket, the pose that ranked first complex with NR FXR were shown in Figures 2A,C. Silybin skeleton was surrounded by a hydrophobic pocket composed of Arg261, Met262, Leu284, Met287, Ala288, His291, Ile294, Met325, Arg328, Ser329, Ile332, Phe333, Leu345, Ile349, Phe363, and Tyr366, where van der Waals forces play a dominant role in the interactions. These interactions were postulated to be the primary reason for silybin activity. The specific interaction of silybin A and silybin B with FXR is also displayed in Figures 2B,D. Hydrogen-bonding interactions showed that silybin A was bound to Arg261 and Arg328 of subunit A of FXR, while silybin B is bound to Arg328 and Tyr366 of subunit A of FXR. These H-bonds help to enhance the binding stability.
FIGURE 2.
Silybin interacts with FXR. (A) The structure of the complex of the FXR and Silybin A by molecular docking. (B) The interaction map of the complex of the FXR and silybin A in the binding site. (C) The structure of the complex of the FXR and silybin B by molecular docking. (D) The interaction map of the complex of the FXR and silybin B in the binding site.
Silymarin Exerts Hypoglycaemic Effects in DIO Mice
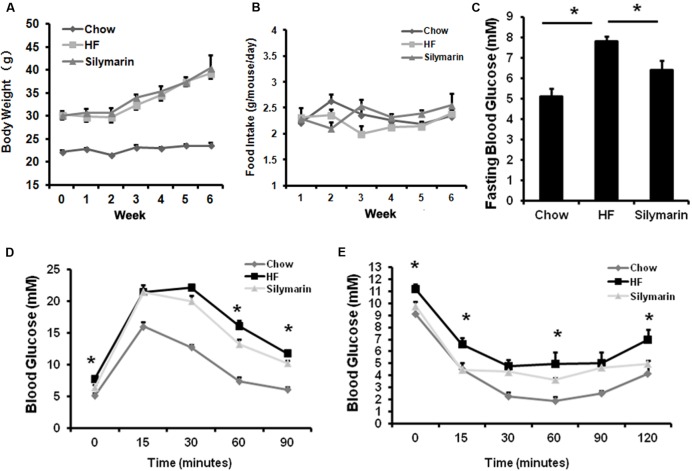
To test whether silymarin affects glucose homeostasis, obese C57BL/6 mice fed HFD were divided in two groups, one continued to feed on a HF diet as control and the other fed HF diet mixed with silymarin (100 mg Legalon or 40 mg silymarin/100 g diet) for additional 6 weeks. Silymarin treatment significantly ameliorated fasting glucose (Figure 3C), glucose intolerance at 30 and 60 min (Figure 3D), and insulin tolerance in obese mice at 15, 60, and 120 min (Figure 3E) without changes in body weight gain or food intake (Figures 3A,B), suggesting that silymarin improves glucose homeostasis in DIO mice.
FIGURE 3.
Silymarin improves blood glucose homeostasis in DIO C57BL/6 mice. (A) Body weight during the 6-week treatment. (B) Food intake amount. (C) Fasting blood glucose level. (D) Glucose tolerance test (GTT). The mice were fasted overnight, and glucose were intraperitoneally injected (1 g of/kg body weight) and blood glucose levels were determined at the indicated time points. (E) Insulin tolerance test (ITT). The non-fasted mice were intraperitoneally injected with insulin (0.75 U/kg body weight) and blood glucose levels were determined. Data are presented as means ± SEM (n = 7). ∗P < 0.05, vs. Chow or HF group, NS, No significance.
Silymarin Attenuates Dyslipidaemia in DIO Mice
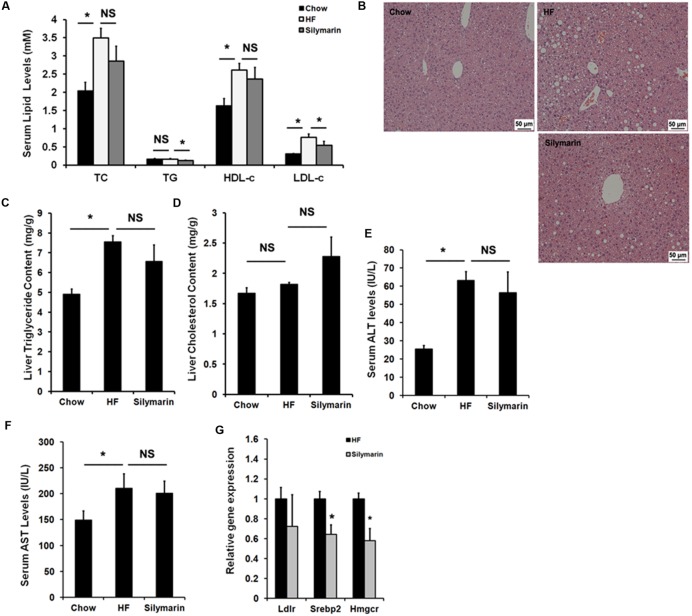
We next assayed the lipid profile in the diet-induced obese mice. As expected, HF diet feeding elevated serum TC, HDL-c, and LDL-c levels in HF-fed control mice compared to Chow controls (Figure 4A), and notably, 6-week silymarin treatment decreased serum LDL-c levels in DIO mice (Figure 4A), but not TG, TC, and HDL-c. OCA and other FXR agonists have been identified to have therapeutic effects against NFALD and NASH. We therefore determined whether silymarin could improve hepatic steatosis. The HE staining results showed that silymarin treatment did not significantly reduce hepatic steatosis (Figure 4B), and this was confirmed by lipid content measurement, which showed no notably reduction when compared to HF-fed controls (Figures 4C,D). Serum ALT and AST levels were markedly higher in DIO mice compared to control mice, but no difference in ALT or AST levels were found between DIO controls and silymarin-treated mice (Figures 4E,F). Subsequent gene expression analysis (Figure 4G) indicated that silymarin markedly decreased mRNAs of Srebp2 and Hmgcr, two genes involved in cholesterol metabolism in DIO mice. Collectively, these results suggest that silymarin corrects the serum dyslipidaemia in DIO mice.
FIGURE 4.
Silymarin ameliorates dyslipidemia in DIO mice. (A) Serum TC, TG, LDL-c, HDL-c. (B) H&E staining of liver sections (×200). (C) Liver TG level. (D) Liver TC level. (E) Serum ALT level. (F) Serum AST level. (G) The relative gene expression levels in the livers. Beta-Actin was used as an internal control for normalizing the mRNA levels. Data are presented as means ± SEM (n = 7). ∗P < 0.05, vs. Chow or HF group, NS, No significance.
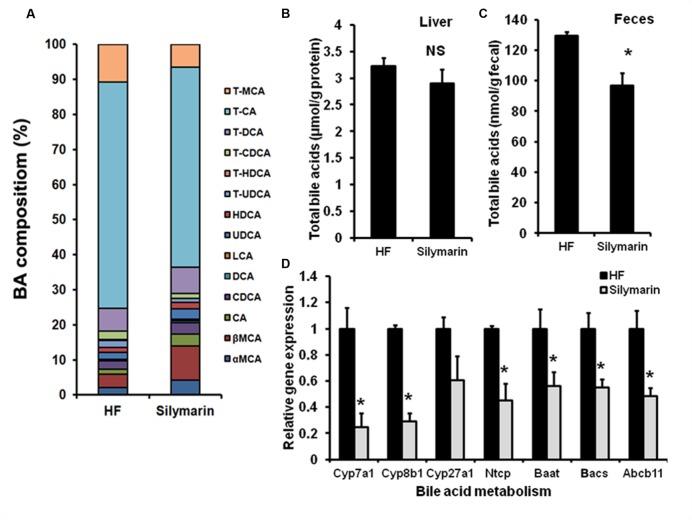
Silymarin Alters BA Composition In vivo
The central function of FXR is to regulate BA homeostasis. Therefore, TBAs in livers and feces were determined following analysis of hepatic BA composition. After silymarin treatment, DIO mice showed markedly decreased TBA levels in feces and a tendency, although not statistically significant, toward a decreased level in liver, compared with HF controls (Figures 5B,C). Meanwhile, the composition of hepatic BA was also altered by silymarin treating. In contrast to high fat diet fed mice, silymarin treatment strikingly decreased fraction of TCA, murine taurocholic acid (mTCA), TCDCA, and TUDCA, which was accompanied with an increasing fraction of the α,β murine taurocholic acid (α,β mTCA), and CA in silymarin-treated DIO mice (Figure 5A; Table 3). Moreover, gene expression experiments showed that a series of genes such as Cyp7a1, Cyp8b1, Ntcp, Baat, Bacs, and Abcb11 were markedly decreased in the silymarin treatment group as compared to HF controls (Figure 5D). These data suggested silymarin contributes to the changes of BA metabolism via influencing the BA signal pathway.
FIGURE 5.
Silymarin alters BA composition in DIO mice. (A) Hepatic TBA level. (B) Fecal TBA level. (C) Hepatic BA composition. (D) The relative gene expression levels in the livers. Beta-Actin was used as an internal control for normalizing the mRNA levels. Data are presented as means ± SEM (n = 7). ∗P < 0.05, vs. HF group, NS, No significance.
Table 3.
Hepatic BAs composition (%) in DIO mice.
| BA | HF | Silymarin |
|---|---|---|
| T-MCA | 11.24 | 6.22 |
| T-CA | 67.32 | 54.91 |
| T-DCA | 6.74 | 7.25 |
| T-CDCA | 2.54 | 1.31 |
| T-HDCA | 0.38 | 0.09 |
| T-UDCA | 2.02 | 1.07 |
| HDCA | 1.39 | 1.75 |
| UDCA | 2.18 | 2.87 |
| LCA | 0.05 | 0.35 |
| DCA | 0.35 | 0.53 |
| CDCA | 2.44 | 3.22 |
| CA | 1.46 | 3.13 |
| βMCA | 4.04 | 9.43 |
| αMCA | 2.17 | 4.07 |
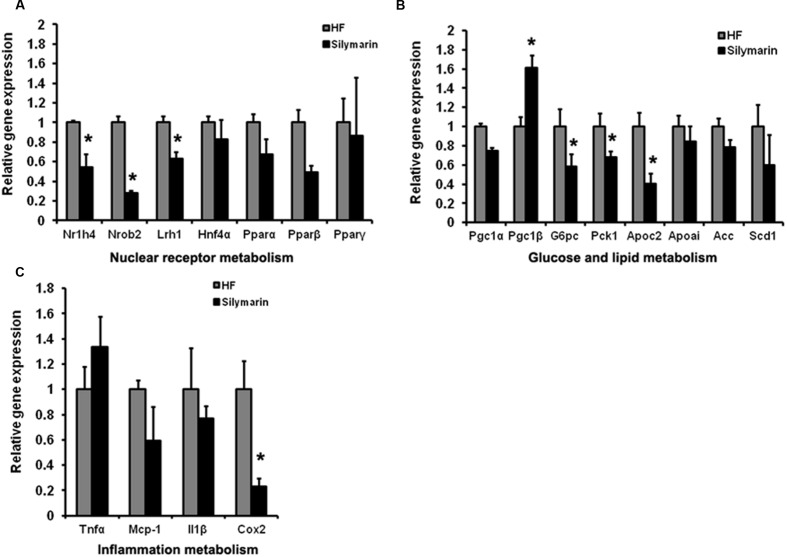
Silymarin Mediates the Hepatic FXR Signaling and Alleviates Inflammation
As a major organ tissue delivering FXR signals, the liver is involved in diverse metabolic physiological functions as well as the regulation of inflammation. We further address whether silymarin mediates signaling downstream of FXR in the liver. We compared the expression levels of a cluster of FXR target genes in the liver tissues between HF control mice and HF supplemented silymarin group mice using quantitative RT-PCR. Liver samples from silymarin-treated mice displayed significantly lower expression levels of Nr1h4, Nr0b2, Lrh1, G6pc, Pck1, and Apoc2 as well as inflammatory cytokine Cox2 while Pgc1β levels were significantly higher (Figures 6A–C). These results suggest that silymarin is able to alter hepatic FXR signaling and therefore modulates its downstream targets related to gluconeogenesis, lipogenesis, and inflammation in DIO mouse livers.
FIGURE 6.
Silymarin regulates FXR signal and alleviates the inflammation in DIO mice liver. The mRNA levels of NRs (A), glucose and lipid metabolism related genes (B), and inflammation metabolism related genes (C) in the livers. Beta-actin was used as an internal control for normalizing the mRNA levels. Data are presented as means ± SEM (n = 7). ∗P < 0.05, vs. HF group.
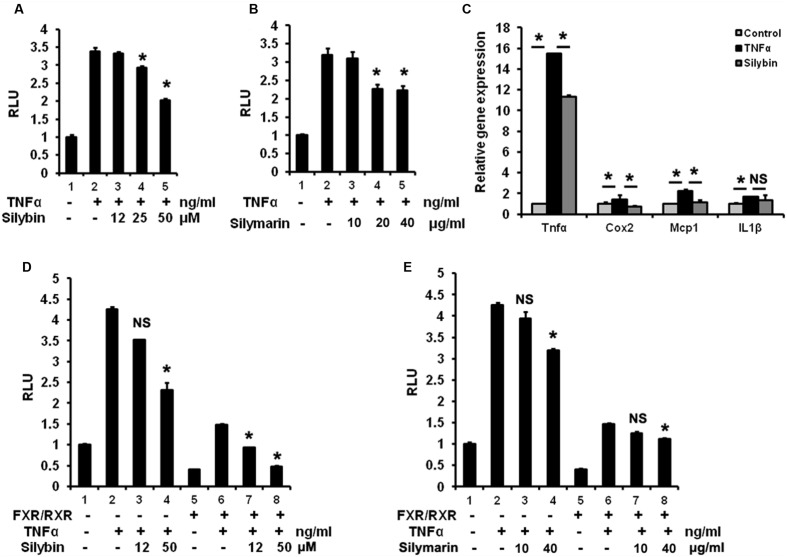
Silymarin and Silybin Inhibit NF-κB Signaling Associated with FXR Activation
To test potential inhibitory effect on NF-κB pathway by silybin and silymarin in vitro, HepG2 cells over-expressing p65 and NF-κBx3-Luc were pre-treated with silybin and silymarin at the indicated different concentrates, in a condition with an enhanced NF-κB reporter activity by TNFα stimulation. Our results (Figures 7A,B) showed that silybin (25 and 50 μM) and silymarin (20 and 40 μg/ml) suppressed the NF-κB transactivity induced by TNFα in HepG2 cells. Silybin (25 μM) significantly reduced the levels of TNFα-induced expression of TNFα, COX2, and MCP-1 mRNA as compared to vehicle (Figure 7C). FXR agonists such as GW4064 and 6ECDCA were reported to inhibit NF-κB activity via FXR activation (Wang et al., 2008). To test whether silybin and silymarin have similar effects, we pre-treated HepG2 cells with silybin and silymarin prior to TNFα treatments. Silybin (50 μM) and silymarin (40 μg/ml) weakened NF-κB signaling in the condition with p65/NF-κBx3-Luc transfection alone. Meanwhile, the transfection of these cells with p65/NF-κBx3-Luc plus FXR/RXR inhibited NF-κB activity in the absence of exogenous drug (Figures 7D,E). Furthermore, addition of silybin to HepG2 cells with FXR/RXR overexpression showed an augmented suppressing effects, resulting in significant depression on NF-κB activity (Figures 7D,E). These results indicate that silybin and silymarin may strengthen interference with NF-κB activity correlated with the activation of FXR.
FIGURE 7.
Silymarin and silybin inhibit NF-κB signaling via activating FXR. Silybin (A) and silymarin (B) repressed NF-κB transactivity in HepG2 cells. HepG2 cells were co-transfected with the NF-κB reporter plasmids and P65 expression plasmids for 24 h and pretreated with the control (DMSO), silybin (12, 25, 50 μM)or silymarin (10, 20, and 40 μg/ml) for 18 h before treatment with TNFα (10 ng/mL) for another 6 h. The RLU were measured by comparison to renilla luciferase activities. ∗P < 0.05 compared to the groups of TNFα (n = 3). (C) The relative gene expression levels in silybin-treated (25 μM) and control (DMSO) HepG2 cells incubated with TNFα (10 ng/mL). Beta-actin was used as an internal control for normalizing the mRNA levels. ∗P < 0.05 compared to the groups of TNFα or controls (n = 3). Silybin (D) and silymarin (E) enhanced the inhibition of NF-κB signaling via activating FXR. HepG2 cells were co-transfected with the NF-κB reporter plasmids and P65 expression plasmids with or without FXR/RXR expression plasmids before treatment with control (DMSO), TNFα (10 ng/mL), silybin (12 and 50 μM), or silymarin (10 and 40 μg/ml) according to (A,B). RLU were measured by comparison to renilla luciferase activities. ∗P < 0.05 vs. the groups of TNFα (n = 3). The results represent three independent experiments, and data are presented as means ± SEM. NS, No significance.
Discussion
In the present study, we provided evidence showing that silybin and silymarin activated NR FXR and triggered a negatively crosstalk with NF-κB. We also confirmed that silymarin can be used to treat MS, especially to ameliorate hyperglycaemia, dyslipidaemia, and regulate BA homeostasis in DIO mice. These effects were probably achieved via the stimulation of FXR signaling and suppression of NF-κB responses. Our data support the possibility to use silybin as a novel natural FXR agonist that can effectively treat and cure MD.
Previous studies have confirmed that silybin represents about 50–70% bioactive compounds in the silymarin extract (Kim et al., 2003; Loguercio and Festi, 2011). FXR is an important molecular target that can be used to control hepatobiliary diseases of enterohepatic cycling, which has prompted the idea that silybin might be correlated with FXR (Matsubara et al., 2013). As expected, FXR gene reporter assay revealed that silybin and silymarin apparently activated FXR reporter signal in a dose dependent manner, which was proved by the change of mRNA levels of FXR downstream target genes in HepG2 cells, including increased expressions of NR0B2, SULT2A1, ABCC2, APOC2, and PPARα, but decreased expression of CYP7A1. The molecular docking assay further supported a direct binding between silybin and FXR. These data support that silybin and silymarin are capable of enhancing the FXR transactivity. These results suggest that silybin is a potential agonist of FXR.
Farnesyl X receptor activation leading to improving lipid and glucose metabolism has received a particularly intense attention. Fasted FXR-null mice exhibit impaired glucose intolerance, insulin insensitivity, and increased levels of plasma triglyceride, free fatty acids, and cholesterol (Sinal et al., 2000; Zhang et al., 2006; Cipriani et al., 2010; Prawitt et al., 2011). Treatment with FXR agonists, GW4064, or INT-747, has been shown to reduce plasma triglyceride, glucose levels, and improve insulin sensitivity in several models of obesity and diabetes (Zhang et al., 2006; Mason et al., 2010). These phenotypes have been attributed to the ability of FXR to control the expression of several genes involved in glycolipid metabolism. In our study, we also found that silymarin treatment effectively lowered HF diet-induced hyperglycaemia and insulin resistance in DIO mice, similarly to the previous reports (Kazazis et al., 2014; Voroneanu et al., 2016). Furthermore, gene expression analysis has demonstrated down-regulation in the expression of certain genes, including Pgc1α, G6pc, and Pck1, in silymarin-treated mice, suggesting effective inhibition of hepatic gluconeogenesis, which could explain the anti-diabetic efficacy of silymarin. Interestingly, these gene changes were reminiscent of recent findings that FXR activation downregulates the gluconeogenic program.
Previous reports showed that silymarin may be able to alleviate hepatic steatosis (Ni and Wang, 2016). However, we did not observe an improvement of the morphology in the liver of DIO mice. This probably caused by the shorter phase of treatment in our study. Extension of the treatment may result in better result in morphology of the liver tissues.
It is well established that the inflammation response is positively correlated with IR and diabetes (Baker et al., 2011). Recently, silybin and silymarin were repeatedly reported to be able to block inflammatory reactions in multifarious cells via antagonizing transcription factor NF-κB (Bremner and Heinrich, 2002; Dhanalakshmi et al., 2002; Salamone et al., 2012). Consistently, we obtained similar results showing that silybin and silymarin inhibited the TNFα-induced NF-κB signal in HepG2 cells as well as reducing TNFα, COX2, and MCP1 expression levels stimulated by TNFα in human hepatocytes. These inflammatory cytokines are all shown to be directly regulated by NF-κB (Baker et al., 2011). Notably, it is unknown whether silybin interacts with NF-κB, i.e., the mechanism underlying silybin control of NF-κB remains to be elucidated. The anti-inflammatory effects of FXR have been well documented (Shaik et al., 2015). An FXR agonist has been reported to activate anti-inflammation responses via negatively regulation of NF-κB (Wang et al., 2008). Deficiency of FXR was also found to increase the development of liver and intestine cancer via the upregulation of inflammation (Yang et al., 2007). In the present study, we found that NF-κB activity was largely inhibited in HepG2 cells transfected with FXR/RXR in the absence of xenobiotics. We found that silybin treatment, significantly enhanced the inhibitory effect on NF-κB activitys, compared to the treatment condition without FXR/RXR. Silybin suppressed NF-κB transactivity without FXR/RXR transfection. Furthermore, the inhibitory efficiency of silybin on the NF-κB reporter was increased by nearly 20% in HepG2 cells with FXR/RXR overexpression. This suggests that suppression of NF-κB transactivity by silybin may be correlated with the activation level of FXR signaling. That silymarin treatment notably lowered Cox2 gene expression in the liver of DIO mice also supported the anti-inflammation action of silymarin in vivo. Our data suggested that reduced hepatic inflammation signal in DIO mice by silymarin may contribute to the antidiabetic effect.
Farnesyl X receptor is a key modulator of BA metabolism in the enterohepatic system that acts via various feedforward and feedback loops. FXR deficiency impairs BA homeostasis (Matsubara et al., 2013). After a 6-week treatment, the hepatic and fecal TBA levels in silymarin-treated mice were lower than in HF controls, indicating a decreased BA pool size. In addition, decreased proportions of TCA, mTCA, TCDCA, and TUDCA with increased fraction of (α, β mTCA) and CA in silymarin-treated mice compared to HF controls showed an altered hepatic BA composition. Hepatic Lrh1, Ntcp, Cyp7a1, and Cyp8b1 are essential genes in enterohepatic BA metabolism and negatively regulated by FXR activation, which respectively, promotes BA gene expression, uptakes of conjugated BAs, controls BA synthesis and BA hydroxylation. Consistent with the characteristics of reported FXR agonists, silymarin markedly reduced Lrh1, Ntcp, Cyp7a1, and Cyp8b1 gene expression levels in DIO mice, indicating that FXR is able to activate the feedback loop of FXR signaling in liver tissue of the mice. This finding further supports that silymarin is a natural FXR agonist modulating BA homeostasis.
One complicating factor in the study of FXR biology is that BAs are also ligands for multiple other receptors controlling MS (Sinal et al., 2000). Here we excluded the agonist activity of silybin on activating several important BA-related receptors such as PPARα, γ, LXRα, β, PXR, and TGR5 in vitro via reporter assays. However, we found that silymarin elevated the proportions of LCA, (α, β mTCA), and CA in vivo. All of these compounds are endogenous ligands for TGR5, which indicates that endogenous TGR5 activation by several BAs might mediate partial silymarin therapeutic effects.
Conclusion
we found that both silybin and silymarin stimulated FXR transactivity and inhibited NF-κB signaling in vitro. Silmarin supplementation ameliorated insulin resistance, hyperlipidaemia and inflammation progress, and reconstituted the BA pool in DIO mice. Our data suggest that the effects of silymarin on metabolic disorders may be related to FXR activation and NF-κB inhibition and that the majority of these effects are mediated by its main active compound, silybin. Whether FXR is involved in the treatment of silymarin for toxic liver damage, hepatitis, and cirrhosis warrants further studies.
Author Contributions
MG, Y-MM, LY, GJ, QT, and CH conceived the experiments. MG, PZ, YZ, YW, SF, YnL, and YfL. performed the experiments, JH performed molecular docking assay, MG and CH analyzed the data. All authors discussed the results and commented on the manuscript. MG, QT, and CH wrote the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
- ALT
alanine aminotransferase
- AST
aspartate transaminase
- BA
bile acid
- CA
cholic acid
- DIO
diet-induced obesity
- DMSO
dimethylsulfoxide
- FXR
farnesyl X receptor
- HDL-c
high density lipoprotein cholesterol
- HF
high-fat
- IPITT
intraperitoneal insulin tolerance test
- LDL-c
low density lipoprotein cholesterol
- NAFLD
non-alcoholic fatty liver disease
- NF-κB
nuclear factor kappa B
- NR
nuclear receptor
- MS
metabolic syndrome
- Rosi
rosiglitazone
- TBA
total bile acid
- TC
total cholesterol
- TCA
taurocholic acid
- TCDCA
tauro-chenodeoxycholic acid
- TG
triglyceride
- TUDCA
tauro-ursodeoxycholic acid
References
- Abenavoli L., Capasso R., Milic N., Capasso F. (2010). Milk thistle in liver diseases: past, present, future. Phytother. Res. 24 1423–1432. 10.1002/ptr.3207 [DOI] [PubMed] [Google Scholar]
- Adewusi E. A., Afolayan A. J. (2010). A review of natural products with hepatoprotective activity. J. Med. Plants Res. 4 1318–1334. 10.5897/jmpr09.472 [DOI] [Google Scholar]
- Angulo P., Patel T., Jorgensen R. A., Therneau T. M., Lindor K. D. (2000). Silymarin in the treatment of patients with primary biliary cirrhosis with a suboptimal response to ursodeoxycholic acid. Hepatology 32 897–900. 10.1053/jhep.2000.18663 [DOI] [PubMed] [Google Scholar]
- Arrese M., Karpen S. J. (2010). Nuclear receptors, inflammation, and liver disease: insights for cholestatic and fatty liver diseases. Clin. Pharmacol. Ther. 87 473–478. 10.1038/clpt.2010.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker R. G., Hayden M. S., Ghosh S. (2011). NF-κB, inflammation, and metabolic disease. Cell Metab. 13 11–22. 10.1016/j.cmet [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beuers U., Trauner M., Jansen P., Poupon R. (2015). New paradigms in the treatment of hepatic cholestasis: from UDCA to FXR, PXR and beyond. J. Hepatol. 62 S25–S37. 10.1016/j.jhep.2015.02.023 [DOI] [PubMed] [Google Scholar]
- Biedermann D., Vavříková E., Cvak L., Křen V. (2014). Chemistry of silybin. Nat. Prod. Rep. 31 1138–1157. 10.1039/c3np70122k [DOI] [PubMed] [Google Scholar]
- Bonora E. (2006). The metabolic syndrome and cardiovascular disease. Ann. Med. 38 64–80. 10.1080/07853890500401234 [DOI] [PubMed] [Google Scholar]
- Bremner P., Heinrich M. (2002). Natural products as targeted modulators of the nuclear factor- K B pathway. J. Pharm. Pharmacol. 54 453–472. 10.1211/0022357021778637 [DOI] [PubMed] [Google Scholar]
- Cariou B., Van H. K., Duransandoval D., van Dijk T. H., Grefhorst A., Abdelkarim M., et al. (2006). The farnesoid X receptor modulates adiposity and peripheral insulin sensitivity in mice. J. Biol. Chem. 281 11039–11049. 10.1074/jbc.m510258200 [DOI] [PubMed] [Google Scholar]
- Chen X., Lou G., Meng Z., Huang W. (2011). TGR5: a novel target for weight maintenance and glucose metabolism. Exp. Diabetes Res. 2011:853501 10.1155/2011/853501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipriani S., Mencarelli A., Palladino G., Fiorucci S. (2010). FXR activation reverses insulin resistance and lipid abnormalities and protects against liver steatosis in Zucker (fa/fa) obese rats. J. Lipid Res. 51 771–784. 10.1194/jlr.M001602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corns C. M. (2003). Herbal remedies and clinical biochemistry. Ann. Clin. Biochem. 40 489–507. 10.1258/000456303322326407 [DOI] [PubMed] [Google Scholar]
- Dhanalakshmi S., Singh R. P., Agarwal C., Agarwal R. (2002). Silibinin inhibits constitutive and TNFalpha-induced activation of NF-kappaB and sensitizes human prostate carcinoma DU145 cells to TNFalpha-induced apoptosis. Oncogene 21 1759–1767. 10.1038/sj.onc.1205240 [DOI] [PubMed] [Google Scholar]
- Douglas I. J., Langham J., Bhaskaran K., Brauer R. (2013). Orlistat and the risk of acute liver injury: self controlled case series study in UK Clinical Practice Research Datalink. BMJ 346 270–270. 10.1136/bmj.f1936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsharkawy A. M., Mann D. A. (2007). Nuclear factor-κB and the hepatic inflammation-fibrosis- cancer axis. Hepatology. 46 590–597. 10.1002/hep.21802 [DOI] [PubMed] [Google Scholar]
- Finucane M. M., Stevens G. A., Cowan M. J., Danaei G., Lin J. K., Paciorek C. J., et al. (2011). National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9⋅1 million participants. Lancet 377 557–567. 10.1016/S0140-6736(10)62037-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folch J., Lees M., Stanley G. H. S. (1957). A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 226 497–509. [PubMed] [Google Scholar]
- Gadaleta R. M., van Erpecum K. J., Oldenburg B., Willemsen E. C., Renooij W., Murzilli S., et al. (2011). Farnesoid X receptor activation inhibits inflammation and preserves the intestinal barrier in inflammatory bowel disease. Gut 60 463–472. 10.1136/gut.2010.233304 [DOI] [PubMed] [Google Scholar]
- Gazák R., Walterová D., Kren V. (2007). Silybin and silymarin–new and emerging applications in medicine. Curr. Med. Chem. 14 315–338. 10.2174/092986707779941159 [DOI] [PubMed] [Google Scholar]
- Hayden M. S., Ghosh S. (2008). Shared principles in NF-kappaB signaling. Cell 132 344–362. 10.1016/j.cell.2008.01.020 [DOI] [PubMed] [Google Scholar]
- Heal D. J., Gosden J., Smith S. L. (2009). Regulatory challenges for new drugs to treat obesity and comorbid metabolic disorders. Br. J. Clin. Pharmacol. 68 861–874. 10.1111/j.1365-2125.2009.03549.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoofnagle J. H. (2005). Milk thistle and chronic liver disease. Hepatology 42 4 10.1002/hep.20787 [DOI] [PubMed] [Google Scholar]
- Huang C., Zhang Y., Gong Z., Sheng X., Li Z., Zhang W., et al. (2006). Berberine inhibits 3T3-L1 adipocyte differentiation through the PPARgamma pathway. Biochem. Biophys. Res. Commun. 348 571–578. 10.1016/j.bbrc.2006.07.095 [DOI] [PubMed] [Google Scholar]
- Kazazis C. E., Evangelopoulos A. A., Kollas A., Vallianou N. G. (2014). The therapeutic potential of milk thistle in diabetes. Rev. Diabet. Stud. 11 167–174. 10.1900/RDS.2014.11.167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keitel V., Häussinger D. (2012). Perspective: TGR5 (Gpbar-1) in liver physiology and disease. Clin. Res. Hepatol. Gastroenterol. 36 412–419. 10.1016/j.clinre.2012.03.008 [DOI] [PubMed] [Google Scholar]
- Kidd P., Head K. (2005). A review of the bioavailability and clinical efficacy of milk thistle phytosome: a silybin-phosphatidylcholine complex (Siliphos®). Altern. Med. Rev. 10 193–203. [PubMed] [Google Scholar]
- Kim I., Ahn S. H., Inagaki T., Choi M., Ito S., Guo G. L., et al. (2007). Differential regulation of bile acid homeostasis by the farnesoid X receptor in liver and intestine. J. Lipid Res. 48 2664–2672. 10.1194/jlr.m700330-jlr200 [DOI] [PubMed] [Google Scholar]
- Kim N. C., Graf T. N., Sparacino C. M., Wani M. C., Wall M. E. (2003). Complete isolation and characterization of silybins and isosilybins from milk thistle (Silybum marianum). Org. Biomol. Chem. 1 1684–1689. 10.1039/b300099k [DOI] [PubMed] [Google Scholar]
- Kong B., Luyendyk J. P., Tawfik O., Guo G. L. (2008). Farnesoid X receptor deficiency induces nonalcoholic steatohepatitis in low-density lipoprotein receptor-knockout mice fed a high-fat diet. J. Pharmacol. Exp. Ther. 328 116–122. 10.1124/jpet.108.144600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvasnička F., BìBa B., ŠevčìK R., Voldřich M., Krátká J. (2003). Analysis of the active components of silymarin. J. Chromatogr. A 990 239–245. 10.1016/S0021-9673(02)01971-4 [DOI] [PubMed] [Google Scholar]
- Lee D. Y., Liu Y. (2003). Molecular structure and stereochemistry of silybin A, silybin B, isosilybin A, and isosilybin B, Isolated from Silybum marianum (milk thistle). J. Nat. Prod. 66 1171–1174. 10.1021/np030163b [DOI] [PubMed] [Google Scholar]
- Lee F. Y., Lee H., Hubbert M. L., Edwards P. A., Zhang Y. (2006). FXR, a multipurpose nuclear receptor. Trends Biochem. Sci. 31 572–580. 10.1016/j.tibs.2006.08.002 [DOI] [PubMed] [Google Scholar]
- Lefebvre P., Staels B. (2014). Failing FXR expression in the liver links aging to hepatic steatosis. J. Hepatol. 60 689–690. 10.1016/j.jhep.2014.01.001 [DOI] [PubMed] [Google Scholar]
- Liu Y., Binz J., Numerick M. J., Dennis S., Luo G., Desai B., et al. (2003). Hepatoprotection by the farnesoid X receptor agonist GW4064 in rat models of intra- and extrahepatic cholestasis. J. Clin. Invest. 112 1678–1687. 10.1172/jci18945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loguercio C., Andreone P., Brisc C., Brisc M. C., Bugianesi E., Chiaramonte M., et al. (2012). Silybin combined with phosphatidylcholine and vitamin E in patients with nonalcoholic fatty liver disease: a randomized controlled trial. Free. Radic. Biol. Med. 52 1658–1665. 10.1016/j.freeradbiomed.2012.02.008 [DOI] [PubMed] [Google Scholar]
- Loguercio C., Festi D. (2011). Silybin and the liver: from basic research to clinical practice. World J. Gastroenterol. 17 2288–2301. 10.3748/wjg.v17.i18.2288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makishima M., Okamoto A. Y., Repa J. J., Tu H., Learned R. M., Luk A., et al. (1999). Identification of a nuclear receptor for bile acids. Science 284 1362–1365. 10.1126/science.284.5418.1362 [DOI] [PubMed] [Google Scholar]
- Mason A., Luketic V., Lindor K., Hirschfield G., Gordon S., Mayo M., et al. (2010). Farnesoid-x receptor agonists: a new class of for the treatment of pbc? an international study evaluating the addition of int-747 to ursodeoxycholic acid. J. Hepatol. 52 S1–S2. 10.1016/S0168-8278(10)60004-9 [DOI] [Google Scholar]
- Matsubara T., Fei L., Gonzalez F. J. (2013). FXR signaling in the enterohepatic system. Mol. Cell. Endocrinol. 368 17–29. 10.1016/j.mce.2012.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni X., Wang H. (2016). Silymarin attenuated hepatic steatosis through regulation of lipid metabolism and oxidative stress in a mouse model of nonalcoholic fatty liver disease (NAFLD). Am. J. Trans. Res. 8 1073–1081. [PMC free article] [PubMed] [Google Scholar]
- Polyak S. J., Ferenci P., Pawlotsky J. M. (2013). Hepatoprotective and antiviral functions of silymarin components in hepatitis C virus infection. Hepatology 57 1262–1271. 10.1002/hep.26179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prawitt J., Abdelkarim M., Stroeve J. H. M., Popescu I., Duez H., Velagapudi V. R., et al. (2011). Farnesoid X receptor deficiency improves glucose homeostasis in mouse models of obesity. Diabetes Metab. Res. Rev. 60 1861–1871. 10.2337/db11-0030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raina K., Agarwal C., Agarwal R. (2013). Effect of silibinin in human colorectal cancer cells: targeting the activation of NF-κB signaling. Mol. Carcinog. 52 195–206. 10.1002/mc.21843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rui L. (2014). Energy metabolism in the liver. Compr. Physiol. 4 177–197. 10.1002/cphy.c130024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone F., Galvano F., Cappello F., Mangiameli A., Barbagallo I., Li Volti G. (2012). Silibinin modulates lipid homeostasis and inhibits nuclear factor kappa B activation in experimental nonalcoholic steatohepatitis. Transl. Res. 159 477–486. 10.1016/j.trsl.2011.12.003 [DOI] [PubMed] [Google Scholar]
- Saller R., Meier R., Brignoli R. (2001). The use of silymarin in the treatment of liver diseases. Drugs 61 2035–2063. 10.2165/00003495-200161140-00003 [DOI] [PubMed] [Google Scholar]
- Salomone F., Godos J., Zelber-Sagi S. (2016). Natural antioxidants for non-alcoholic fatty liver disease: molecular targets and clinical perspectives. Liver Int. 36 5–20. 10.1111/liv.12975 [DOI] [PubMed] [Google Scholar]
- Shaik F. B., Prasad D. V., Narala V. R. (2015). Role of farnesoid X receptor in inflammation and resolution. Inflamm. Res. 64 9–20. 10.1007/s00011-014-0780-y [DOI] [PubMed] [Google Scholar]
- Sinal C. J., Tohkin M., Miyata M., Ward J. M., Lambert G., Gonzalez F. J. (2000). Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell 102 731–744. 10.1016/S0092-8674(00)00062-3 [DOI] [PubMed] [Google Scholar]
- Stickel F., Brinkhaus B., Krähmer N., Seitz H. K., Hahn E. G., Schuppan D. (2002). Antifibrotic properties of botanicals in chronic liver disease. Hepatogastroenterology 49 1102–1108. [PubMed] [Google Scholar]
- Tamayo C., Diamond S. (2007). Review of clinical trials evaluating safety and efficacy of milk thistle (Silybum marianum [L.] Gaertn.). Integr. Cancer Ther. 6 146–157. 10.1177/1534735407301942 [DOI] [PubMed] [Google Scholar]
- Tindle H. A., Davis R. B., Phillips R. S., Eisenberg D. M. (2005). Trends in use of complementary and alternative medicine by US adults: 1997–2002. Altern. Ther. Health Med. 11 42–49. [PubMed] [Google Scholar]
- Velussi M., Cernigoi A. M., Ariella D. M., Dapas F., Caffau C., Zilli M. (1997). Long-term (23 months) treatment with an anti-oxidant drug (silymarin) is effective on hyperinsulinemia, exogenous insulin need and malondialdehyde levels in cirrhotic diabetic patients. J. Hepatol. 26 871–879. 10.1016/S0168-8278(97)80255-3 [DOI] [PubMed] [Google Scholar]
- Voroneanu L., Nistor I., Dumea R., Apetrii M., Covic A. (2016). Silymarin in Type 2 diabetes mellitus: a systematic review and meta-analysis of randomized controlled trials. J. Diabetes Res. 2016 5147468 10.1155/2016/5147468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahi G., LeBlanc P. J., Hay J. A., Faught B. E., O’Leary D., Cairney J. (2011). Metabolic syndrome in children with and without developmental coordination disorder. Res. Dev. Disabil. 32 2785–2789. 10.1016/j.ridd.2011.05.030 [DOI] [PubMed] [Google Scholar]
- Wang Y. D., Chen W. D., Wang M., Yu D., Forman B. M., Huang W. (2008). Farnesoid X receptor antagonizes nuclear factor κB in hepatic inflammatory response. Hepatology 48 1632–1643. 10.1002/hep.22519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westin S., Heyman R. A., Martin R. (2005). FXR, a therapeutic target for bile acid and lipid disorders. Mini Rev. Med. Chem. 5 719–727. 10.2174/1389557054553802 [DOI] [PubMed] [Google Scholar]
- Wu C. H., Huang S. M., Yen G. C. (2011). Silymarin: a novel antioxidant with antiglycation and antiinflammatory properties in vitro and in vivo. Antioxid. Redox Signal. 14 353–366. 10.1089/ars.2010.3134 [DOI] [PubMed] [Google Scholar]
- Yang F., Huang X., Yi T., Yen Y., Moore D. D., Huang W. (2007). Spontaneous development of liver tumors in the absence of the bile acid receptor farnesoid X receptor. Cancer Res. 67 863–867. 10.1158/0008-5472.CAN-06-1078 [DOI] [PubMed] [Google Scholar]
- Yang F., Xu Y., Xiong A., He Y., Yang L., Wan Y. J., et al. (2012). Evaluation of the protective effect of Rhei Radix et Rhizoma against α-naphthylisothiocyanate induced liver injury based on metabolic profile of bile acids. J. Ethnopharmacol. 144 599–604. 10.1016/j.jep.2012.09.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Wang Y. D., Chen W. D., Wang X., Lou G., Liu N., et al. (2012). Promotion of liver regeneration/repair by farnesoid X receptor in both liver and intestine in mice. Hepatology 56 2336–2343. 10.1002/hep.25905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Lee F. Y., Barrera G., Lee H., Vales C., Gonzalez F. J., et al. (2006). Activation of the nuclear receptor FXR improves hyperglycemia and hyperlipidemia in diabetic mice. Proc. Natl. Acad. Sci. U.S.A. 103 1006–1011. 10.1073/pnas.0506982103 [DOI] [PMC free article] [PubMed] [Google Scholar]