Abstract
Empathy deficit is a core feature of schizophrenia which may lead to social dysfunction. The present study was carried out to investigate functional and structural abnormalities associated with empathy in patients with schizophrenia using functional magnetic resonance imaging (fMRI) and voxel-based morphometry (VBM). A sample of 14 schizophrenia patients and 14 healthy control subjects matched for age, sex and education were examined with structural high-resolution T1-weighted MRI; fMRI images were obtained during empathy task in the same session. The analysis was carried out using SPM8 software. On behavioural assessment, schizophrenic patients (83.00±29.04) showed less scores for sadness compared to healthy controls (128.70±22.26) (p<0.001). fMRI results also showed reduced clusters of activation in the bilateral fusiform gyrus, left lingual gyrus, left middle and inferior occipital gyrus in schizophrenic subjects as compared to controls during empathy task. In the same brain areas, VBM results also showed reduced grey and white matter volumes. The present study provides an evidence for an association between structural alterations and disturbed functional brain activation during empathy task in persons affected with schizophrenia. These findings suggest a biological basis for social cognition deficits in schizophrenics.
Keywords: BOLD, empathy, fMRI, Interpersonal Reactivity Index, schizophrenia, VBM
1. Introduction
It is generally accepted that empathy is one of the key elements of social cognition and important for successful social relationships (Eisenberg and Miller 1987). Neurodevelopmental disorders like schizophrenia and autism are associated with difficulties in responding empathically leading to social dysfunction associated with these disorders (Henry et al. 2008).
There are several neuropsychological studies which have reported impaired empathic abilities in schizophrenia (Montag et al. 2007; Bora et al. 2008; Derntl et al. 2009; Achim et al. 2011; Lee et al. 2011; Haker et al. 2012; Smith et al. 2012). Most of the studies have shown that schizophrenic patients showed lower Interpersonal Reactivity Index (IRI) scores in cognitive empathy (for perspective-taking) and emotional empathy (for empathy concern) and higher IRI score for personal distress (Montag et al. 2007; Derntl et al. 2009; Achim et al. 2011; Lee et al. 2011; Smith et al. 2012). Schizophrenia patients have shown empathy dysfunction based on their relative empathy quotient ratings, which are further found to be associated with impairment in other social cognitive tasks (Bora et al. 2008). Another study has shown that schizophrenia patients have lower empathic accuracy than controls, and their empathic accuracy was less influenced by the emotional expressivity of the target (Lee et al. 2011).
There are few functional neuroimaging studies which have investigated neural correlates of empathy deficits in schizophrenia (Benedetti et al. 2009; Lee et al. 2010; Derntl et al. 2012; Harvey et al. 2013). These studies investigated the neural correlates of empathy in schizophrenia patients by using a cartoon task measuring theory of mind/cognitive empathy as well as affective/emotional empathy (Benedetti et al. 2009; Lee et al. 2010; Derntl et al. 2012) and video clips of empathy accuracy task (Harvey et al. 2013). Benedetti et al. (2009) have shown greater response of the right superior temporal gyrus to affective empathy cartoons and stronger response of the right transverse as well as right posterior superior temporal gyrus to cognitive empathy cartoons in patients, while controls showed the opposite pattern. Lee et al. (2010) reported stronger response of the right superior temporal gyrus to the cognitive empathy cartoons in controls as compared to patient group. Additionally, Lee et al. also observed that the patients showed greater activation in the left insula and the right middle/inferior frontal cortex in the emotional and inhibitory conditions in patients compared to controls. Derntl et al. (2012) have shown hypoactivation in a fronto-temporo-parietal network including the amygdala in schizophrenic patients as compared to controls which according to author revealed deficit in the core component (i.e. emotion recognition, perspective taking and affective responsiveness) of empathy. An fMRI study by Harvey et al. (2013) further confirmed that schizophrenics demonstrate an impaired ability to understand others’ internal states.
Although several neuroimaging studies have reported empathy deficits in schizophrenia, the structural and functional correlates of empathy deficits is very few. In the present study, we used both fMRI and VBM techniques to investigate whether functional deficits in schizophrenia are also associated with structural brain abnormalities in related brain regions during an empathy task. Earlier neuroimaging studies on empathy were restricted to stimuli having mimicked facial expression of an individual. In the present study, we presented subjects with photographs taken after natural disasters or calamities, which represent collective and natural emotions of the affected population (Modi et al. 2008).
2. Materials and methods
The study was conducted at the NMR Research Centre, Institute of Nuclear Medicine and Allied Sciences (INMAS), DRDO, Delhi, India and was approved by the Institutional ethics committee of Post Graduate Institute of Medical Education and Research (PGIMER), Dr. Ram Manohar Lohia Hospital (RMLH), New Delhi, India and the Institutional Review Board, INMAS, DRDO, Delhi, India. After complete description of the study to the participants, written informed consent was obtained from all the participants.
2.1 Subjects
Fourteen schizophrenia patients and fourteen healthy controls, matched for age, sex and socio-economic status, underwent MRI scans. Table 1 shows the demographic and clinical characteristics of both patients and control subjects. Schizophrenia subjects were recruited from outpatients at Department of Psychiatry, PGIMER, RMLH, New Delhi, India.
Table 1.
Demographic and clinical characteristics of schizophrenia patients and healthy controls
| Schizophrenic patients (N=14) (mean±SD) | Healthy controls (N=14) (mean±SD) | p-Value | |
|---|---|---|---|
| Age (years) | 31.50±9.40 | 27.21±4.78 | 0.140 |
| Gender (Male/Female) | 11/3 | 10/4 | |
| Handedness (R/L) | 14/0 | 14/0 | |
| Years of education | 9.6±3.6 | 11.3±2.1 | 0.133 |
| Age of onset (years) | 23.7±8.1 | ||
| Duration of illness (weeks) | 481.4±334.3 | ||
| SANS total score | 12.6±5.4 | ||
| SAPS total score | 8.6±4.2 | ||
| Antipsychotic equivalent dosage of CPZ, mg/day | 389.3±222.03 | ||
| Duration of antipsychotic drug taken (in weeks) | 574.5±312.6 | ||
| Interpersonal Reactivity Index | |||
| Fantasy (FS) | 11.2±4.9 | 11.8±6.4 | 0.968 |
| Perspective taking (PT)** | 14.1±3.0 | 19.4±3.3 | 0.002 |
| Empathic concern (EC)* | 16.8±7.1 | 23.1±3.2 | 0.026 |
| Personal distress (PD) | 15.2±5.6 | 15.9±4.2 | 0.778 |
SANS: Scale for the Assessment of Negative Symptoms; SAPS: Scale for the Assessment of Positive Symptoms; CPZ: chlorpromazine. Asterisk (*) indicates significant difference between groups (*p<0.05 and **p<0.001).
We included patients aged between 22–47 years with a DSM-IV diagnosis of schizophrenia, interviewed using the Hindi version of the Diagnostic Interview for Genetic Studies (DIGS) (Nurnberger et al. 1994; Deshpande et al. 1998). The diagnosis was confirmed after a diagnostic review by a board certified psychiatrist. Patients with a history of alcohol/illicit substances or individuals with any neurological disorders that interfered with the diagnosis or cognitive evaluations were excluded. All patients were on antipsychotic medications at the time of MRI acquisition.
The controls were recruited from the local community. Only those subjects who had no prior psychiatric/psychological disorders, no history of major head injury (loss of consciousness), no mental retardation or any contra-indications on MRI were enrolled. Both patients and controls did not reveal any abnormalities on physical and neurologic examinations. A neuroradiologist examined the MRI scans to rule out any other coexisting pathology.
2.2 Interpersonal Reactivity Index
All participants completed the Interpersonal Reactivity Index (IRI) to assess self-reported empathy (Davis 1983). The IRI is a reliable and valid way of measuring one’s belief in one’s own empathic tendencies (Davis et al. 1994). According to Davis’ model, empathy is a stable personality trait that comprises of four components: personal distress (PD), empathic concern (EC), perspective taking (PT), and fantasy scale (FS). Davis’ model led to the development of the Interpersonal Reactivity Index (IRI) (Davis 1983), a measure comprised four subscales that include two measures for the cognitive components of empathy (PT and FS) and two measures for the emotional components of empathy (EC and PD) (Davis 1983). PD refers to ‘self-oriented feelings of personal anxiety and unease in intense interpersonal settings’. EC reflects the tendency to experience feelings of sympathy and concern for unfortunate others. PT is the ability to understand the viewpoint of others, which contributes to the development of healthy relationships. FS is the tendency to imagine the feelings and actions of fictitious characters. Participants responded using a five-point Likert scale, with 1 being ‘does not describe me well’ and 5 being ‘describes me very well’.
2.3 Data acquisition
The functional and structural data were collected in the same session on a 3 tesla whole-body MRI system (Magnetom Skyra, Siemens, Germany) with a 20-channel head-and-neck coil. Subjects lay in the supine position with their heads supported and immobilized within the head coil using foam-pads (vendor provided), to minimize head movement and gradient noise. Thirty-six axial slices parallel to the bicommissural plane through the fronto-parietal cortex covering the whole brain volume using an echo-planar imaging sequence (matrix=64×64, field of view=240 mm, TE=36 ms, TR=3 s, flip angle=90°, slice thickness=3 mm) were obtained. Block paradigm (BABABABA….) with alternating phases of activation (A) and baseline (B) was chosen. 132 sequential image volumes (belonging to five cycles + one baseline for eliminating T1 saturation effects and acclimatization of the patient to the gradient noise) were taken.
High-resolution MR images were obtained with a T1-weighted 3D gradient echo sequence (MPRAGE: Magnetization Prepared Rapid Acquisition Gradient Echo, 160 sagittal slices, slice thickness=1 mm, field of view=256 mm, matrix size=256×256, TR=1900 ms, TE=2.07 ms, TI=900 ms). In addition, a T2-weighted turbo spin echo (TE=100 ms, flip angle=150°, field of view=220 mm, matrix size=312×512) scan with 25 axial 4 mm slices and 1.2 mm gap of the whole brain was acquired for neuro-diagnostic evaluation to rule out any other neurological problem.
2.4 fMRI task paradigm
In the fMRI experiment, the active phase consisted of natural photographs depicting the misery, suffering and human loss at the aftermath of any calamity or disaster. These photographs were downloaded from Google search engine which invokes negative emotions such as sadness, fear, anger or disgust. For stimulus optimisation, 75 such photographs were rated by a group of 25 psychologists. They rated each photograph under differential rating scale for their emotional content (sadness, fear, anger and disgust) using a five point rating scale – from 0 (least emotional content) up to 4 (maximum emotional content). Out of 75 photographs, 35 photographs that got highest ratings for sadness were selected for the fMRI experiment. None of the photographs chosen for the study had a mean rating below 3.5 in their sadness content. Each block consists of 7 pictures and none of the pictures were repeated. The baseline consisted of mosaic photographs so as to maintain the cognitive load in the subjects and avoid the effect of emotional content, if any.
The fMRI task paradigm comprised two conditions (active and baseline phases) run in a blocked design. Each block consisted of 12 brain volume measurements. A total of 132 sequential image volumes (belonging to 5 cycles plus one additional baseline for eliminating T1 saturation effects and acclimatization of the patient to the gradient noise) were taken. Participants viewed the photographs through a fMRI Hardware system by Nordic NeuroLab (http://www.nordicneurolab.com/Products_and_Solutions/nordic_fMRI_solution/index.aspx) mounted on the head coil. During the scan, the subjects were only asked to feel the emotions invoked in them while viewing those photographs. After completion of the scan (outside the scanner), they were asked to rate the photographs in a five point rating scale about the intensity of emotions invoked in them while viewing those photographs when inside the scanner. The subject’s response was noted in the response sheet, ranging from 0 (absence of any emotional reaction) to 4 (the strongest emotion felt during the viewing of photographs), which was used for behavioural data analysis.
2.5 Analysis of IRI and behavioural data
An independent sample t-test was performed for behavioural data and all 4 sub-scales of IRI index [personal distress (PD), empathic concern (EC), perspective taking (PT), and fantasy (FS)] to evaluate the difference between empathy in healthy controls and schizophrenia patients. A p value of less than 0.05 was considered to be statistically significant.
2.6 Functional data analysis
fMRI data were processed with Statistical Parametric Mapping (SPM8, Wellcome Department of Cognitive Neurology, London, UK, http://www.fil.ion.ucl.ac.uk/spm) software package implemented in MATLAB R2008a (Version 7.6.0, Math works, Sherbon, MA).The first 12 brain volumes (one baseline) of fMRI data of each subject were discarded to remove the initial transit signal fluctuations and subsequent images were re-aligned within the session to remove any minor movements. Translational or rotational movement of more than ±1.5 mm or ±1.5° respectively was not considered for analysis. The T1-weighted high-resolution anatomical images were co-registered to the mean functional images and spatially normalized to the Montreal Neurological Institute (MNI305) brain template. The time-course images were normalized using the same normalization parameters and then smoothed with a 6×6×6 mm3 (full width at half maximum) Gaussian smoothing kernel. The EPI-images were high-pass filtered (128 s) to remove low frequency signal drift. A statistic parametric map (SPM) was generated for each subject under each condition by fitting the stimulation paradigm of the functional data, convolved with a hemodynamic response function. Condition-specific effects at each voxel were estimated using the general linear model (Friston and Worksley, 2005). Individual first level contrast images were generated for the empathy task versus baseline contrast (FWE corrected, p<0.05). One-sample t-test in both the groups was performed to generate an average activation map using the contrast images from the single-subject analyses. For the between-group analyses, two-sample t-test was performed. The resulting statistical map was set at a combined threshold of p<0.001 for each voxel and a minimum cluster size >59 voxels for one sample t-test and >25 voxels for two sample t-test, which resulted in a corrected threshold of p<0.05 as determined by AlphaSim in REST software (www.restfmri.net). For a given voxel-level threshold, the required minimum cluster size for both within and between group analysis was determined by Alphasim via Monte Carlo simulation.
The anatomical representation of the clusters was related to cytoarchitechtonic maps as implemented in SPM Anatomy Toolbox (Eickhoff et al. 2005). This toolbox provides a routine, standardized application of probabilistic cytoarchitectonic maps as an anatomical reference for functional activations.
2.7 Structural data analysis
2.7.1 Image processing
Images were processed using Statistical Parametric Mapping (SPM8, Wellcome Department of Cognitive Neurology) implemented in MATLAB R2008a (version 7.6.0 (Mathworks, Sherborn, MA)). All the steps for data processing were followed as described in detail by Ashburner (2010) In order to improve the registration of the MRI images, Diffeomorphic Anatomic Registration Through Exponentiated Lie algebra algorithm (DARTEL) toolbox for SPM8 was used. Briefly, the anatomical images were firstly reoriented so that the mm coordinate of the anterior commissure matched the origin (0, 0, 0), and the orientation approximated Montreal Neurological Institute space. Then, T1-weighted images were segmented and classified into grey matter, white matter and cerebrospinal fluid (CSF) probability maps using the ‘new-segment’ option implemented in SPM8, which gives both the native space versions and DARTEL imported versions of the tissues. All the grey matter and white matter images were spatially non-linearly normalized to the population template which was created by grey matter and white matter maps and DARTEL, and then Jacobian modulated. These images were affine transformed to MNI space, and finally, were smoothed with a 10 mm full-width at half-maximum (FWHM) Gaussian kernel.
Total grey matter (GM) volume, white matter (WM) volume, CSF volume and Total Intracranial Volume (TIV) were calculated using a MATLAB script downloaded from the SPM email list (http://www.jiscmail.ac.uk/cgi-bin/wa.exe?A2=ind0807&L=SPM&P=R7075&1).
2.7.2 Statistical analysis
The grey matter, white matter and CSF volumes of all the subjects were normalized by dividing the individual value by total intracranial volume (TIV) of the respective subjects (table 3). Normalized GM (nGM) volume, normalized WM (nWM) volume, normalized CSF (nCSF) volume and TIV were compared among the two groups using the Student’s independent t-test.
Table 3.
Normalized grey matter volume (nGM), normalized white matter volume (nWM), normalized cerebrospinal fluid volume (nCSF) and total intracranial volume (TIV) for two groups (mean±SD)
| TIV (litres)* | nGM* | nWM* | nCSF* | |
|---|---|---|---|---|
| Controls | 1.5491±0.0981 | .4678±.0075 | .3239±0.0113 | .2083±.0081 |
| Schizophrenia | 1.4402±0.1475 | .4557±.0119 | .3152±0.0108 | .2291±.0135 |
TIV, nGM, nWM and nCSF showed significant difference between two groups (p>0.05).
The normalized, segmented, smoothed and modulated grey matter and white matter data were analyzed using a voxel-wise statistical parametric mapping. ‘Two-sample t-test’ analysis with normalized grey matter volume or normalized white matter volume of the individual subjects was used to find the regional changes in the grey or white matter volumes respectively. TIV was entered as a global variable to correct for the global brain volume of different subjects. A whole brain analysis was performed, with a significance level of p<0.001, uncorrected for multiple comparisons. Since this threshold might have led to false positive results, in those areas which passed this threshold a small volume correction (SVC) was further applied, setting the cut off value for significance at FWE corrected p<0.05 (at cluster level) and using a 8 mm radius. The anatomical representation of the clusters was related to cytoarchitectonic maps as implemented in the SPM Anatomy Toolbox (Eickhoff et al. 2005).
3. Results
3.1 Psychological and behavioural performance
3.1.1 IRI Scores
A significant difference in perspective taking (PT) (a component of cognitive empathy) and empathy concerns (EC) (a component of emotional empathy) (p<0.05) were obtained in schizophrenia patients as compared to controls (table 1).
3.1.2 Behavioural performance
Among all the negative emotions, sadness was primarily invoked in all the subjects while viewing the photographs taken at the aftermath of some calamity, as reported by them in the interview following the fMRI session. Schizophrenic patients (83.00±29.04) showed lower scores for sadness as compared to healthy controls (128.70±22.26) (p<0.001).
Regarding any impact of medication or duration of illness, no significant correlation was found between the duration of antipsychotic drug taken or CPZ-equivalents and any of the IRI scores in the patient group.
3.2 fMRI results
3.2.1 Within-group analysis
Control group
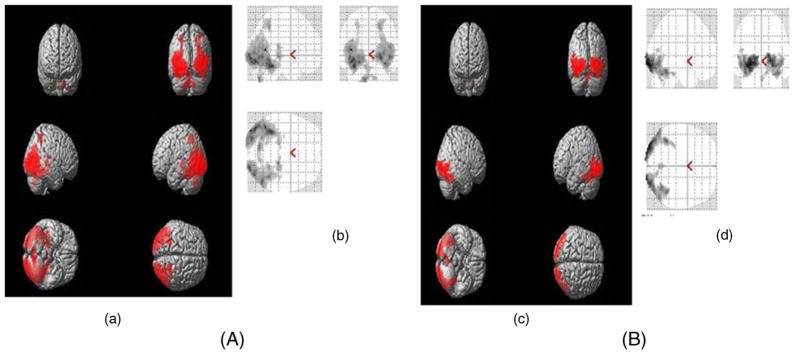
In control subjects, significant clusters of activation were found in the middle temporal gyrus, occipital gyrus, calcarine gyrus, lingual gyrus, fusiform gyrus regions, superior parietal lobule, thalamus and cerebellum using one sample t-test (table 2; figure 1A).
Table 2.
Brain Regions involved in empathy task in healthy subjects and patients with schizophrenia
| Hemisphere | Localization of peak voxels | MNI coordinates | t-Value | Cluster size (voxels) |
|---|---|---|---|---|
| Controls | ||||
| L | Middle temporal gyrus | −42 −58 10 | 20.11 | 2137 |
| L | Inferior occipital gyrus | −42 −82 −11 | 14.95 | |
| L | Middle occipital gyrus | −36 −91 −5 | 14.37 | |
| L | Fusiform gyrus | −36 −46 −20 | 14.07 | |
| R | Inferior occipital gyrus | 27 −94 −5 | 13.46 | 1628 |
| R | Calcarine gyrus | 24 −97 1 | 13.01 | |
| R | Middle temporal gyrus | 48 −64 −2 | 12.50 | |
| R | Middle occipital gyrus | 33 −82 4 | 10.73 | |
| R | Fusiform gyrus | 39 −46 −20 | 8.78 | |
| R | Lingual gyrus | 21 −85 −5 | 8.51 | |
| L | Cerebellum | −9 −73 −41 | 10.39 | 177 |
| R | Cerebellum | 15 −70 −38 | 6.90 | |
| R | Thalamus | 18 −28 −2 | 11.16 | 109 |
| R | Superior parietal lobule | 24 −49 49 | 9.41 | 98 |
| Patients | ||||
| R | Calcarine gyrus | 21 −100 1 | 8.53 | 662 |
| R | Inferior occipital gyrus | 27 −91 −5 | 7.54 | |
| R | Fusiform gyrus | 42 −49 −17 | 6.37 | |
| L | Middle occipital gyrus | −27 −97 −5 | 9.75 | 608 |
| L | Inferior occipital gyrus | −42 −79 −11 | 6.65 | |
| L | Fusiform Gyrus | −39 −49 −17 | 6.74 | |
| Controls > Patients | ||||
| L | Lingual gyrus | −24 −91 −14 | 5.01 | 112 |
| L | Inferior occipital gyrus (BA18) | −36 −85 −11 | 4.55 | |
| L | Middle occipital gyrus | −48 −76 10 | 4.22 | |
| Patients > Controls | ||||
| No significant activation | ||||
L, Left; R, Right; BA, Brodmann’s Area.
Figure 1.
(a, c) Three-dimensional rendered view and (b, d) Glass-Brain view showing group analysis of BOLD activation pattern in the brain of (A) healthy controls and (B) schizophrenic patients.
Patient group
In the patient group, significant activations were found in calcarine gyrus, fusiform gyrus and middle and inferior occipital gyrus (table 2; figure 1B).
3.2.2 Between-group analysis
Control > Patients
The patient group showed reduced activations in the left lingual gyrus, left middle and inferior occipital gyrus as compared to controls (table 2).
Patients > Control
No significant cluster of activation was found in this contrast (table 2).
3.3 VBM results
The subject groups did not show any significant differences with respect to age, gender and education. Student’s t-test reveals statistically significant inter-group differences in all the volumetric parameters, i.e. normalized grey matter volume (nGM), normalized white matter volume (nWM), normalized cerebrospinal fluid volume (nCSF) and total intracranial volume (TIV) shown in table 3.
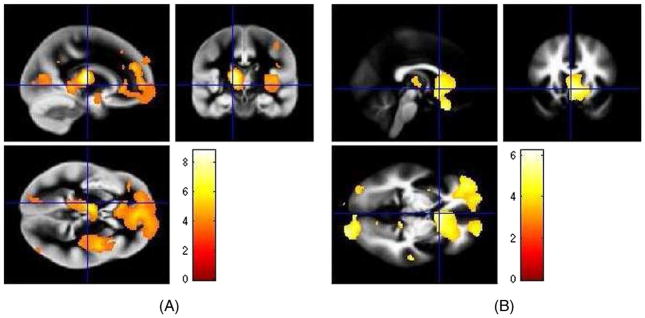
VBM results showed that schizophrenic patients had significantly reduced grey and white matter volumes in the anterior cingulated cortex, superior medial gyrus, frontal gyrus, occipital gyrus, temporal gyrus, thalamus, lingual gyrus, putamen, cuneus, precuneus and calcarine gyrus as compared to healthy controls (tables 4 and 5; figure 2).
Table 4.
Regions of GM volume changes
| Hemisphere | Localization of peak voxels | MNI coordinates | t-Value | Cluster size (voxels) |
|---|---|---|---|---|
| Controls versus Schizophrenic Subjects Contrast | ||||
| L | Anterior cingulated cortex | 0 50 3 | 5.60 | 12069 |
| R | Anterior cingulated cortex | 5 47 6 | 5.58 | |
| L | Inferior frontal gyrus | −36 39 −6 | 4.98 | |
| R | Putamen | 33 −1 6 | 5.90 | 6164 |
| R | Insula lobe | 44 −3 4 | 5.71 | |
| R | Rolandic operculum | 47 −4 19 | 3.64 | |
| L | Thalamus | −15 −18 12 | 8.80 | 3894 |
| L | Lingual gyrus | −15 −37 −3 | 4.88 | |
| R | Precentral gyrus | 51 −6 40 | 5.89 | 3517 |
| R | Postcentral gyrus | 59 −3 36 | 5.64 | |
| R | Middle frontal gyrus | 51 3 52 | 4.77 | |
| L | Calcarine gyrus | −17 −75 12 | 4.31 | 1864 |
| L | Cuneus | −11 −81 15 | 4.13 | |
| L | Middle occipital gyrus | −20 −91 −3 | 3.85 | |
| R | Inferior frontal gyrus | 36 35 −18 | 4.38 | 734 |
| R | Superior orbital gyrus | 23 18 −14 | 3.68 | |
| R | Precuneus | 20 −61 30 | 4.69 | 717 |
| R | Inferior occipital gyrus | 47 −61 −14 | 4.14 | |
| R | Fusiform gyrus | 41 −60 −12 | 4.13 | 680 |
| R | Inferior temporal gyrus | 50 −73 −11 | 3.87 | |
| R | Middle frontal gyrus | 32 57 15 | 3.92 | 430 |
| R | Inferior temporal gyrus | −51 −57 −5 | 4.44 | 155 |
MNI: Montreal Neurological Institute; BA: Brodmann area; L: Left; R: Right.
Table 5.
Regions of WM volume changes
| Hemisphere | Localization of peak voxels | MNI coordinates | t-Value | Cluster size (voxels) |
|---|---|---|---|---|
| Controls versus Schizophrenic Subjects Contrast | ||||
| R | Superior orbital gyrus | 20 30 −17 | 5.25 | 14144 |
| R | Superior frontal gyrus | 21 62 22 | 4.69 | |
| R | Anterior cingulated cortex | 5 47 6 | 4.68 | |
| L | Middle orbital gyrus | −36 54 −11 | 5.87 | 8844 |
| L | Middle frontal gyrus | −30 56 9 | 5.05 | |
| L | Superior frontal gyrus | −17 44 42 | 4.88 | |
| L | Inferior frontal gyrus | −48 36 6 | 4.77 | |
| R | Calcarine gyrus | 14 −93 −2 | 5.12 | 3604 |
| L | Calcarine gyrus | 5 −96 −3 | 4.87 | |
| R | Cuneus | 15 −91 15 | 4.48 | |
| L | Middle occipital gyrus | −41 −82 31 | 6.23 | 1438 |
| R | Superior temporal gyrus | 59 −28 10 | 4.74 | 1070 |
| R | Lingual gyrus | 14 −34 −2 | 4.16 | 747 |
| R | Precuneus | 9 −49 7 | 3.83 | |
| L | Middle temporal gyrus | −42 −48 12 | 4.07 | 244 |
| L | Cerebellum | −50 −52 −45 | 3.83 | 131 |
MNI: Montreal Neurological Institute; BA: Brodmann area; L: Left; R: Right.
Figure 2.
Overlay of the peak cluster on the grey and white matter template provided in SPM8, of (A) grey matter volume loss in and (B) white matter volume loss in schizophrenic subjects as compared to controls in VBM Analysis.
4. Discussion
This study aimed to investigate whether the functional alterations are associated with structural brain abnormalities in related brain regions or in anatomically closely connected areas during empathy task using fMRI and VBM techniques. For the fMRI task, actual photographs taken after natural disasters or calamities were used, which represent collective and natural emotions of the affected population. Our fMRI results revealed that schizophrenia patients showed reduced cluster of activations in left middle occipital gyrus and bilaterally in the inferior occipital gyrus, fusiform gyrus, calcarine gyrus and cuneus as compared to controls. Middle temporal gyrus, thalamus, superior parietal lobule and cerebellum were found to be activated in controls only. VBM results also showed grey and white matter volume loss in these brain areas in patient group as compared to controls (tables 4 and 5).
There are few functional studies which have reported functional abnormalities in various brain areas in schizophrenic patients which are associated with empathy task (Benedetti et al. 2009; Lee et al. 2010; Derntl et al. 2012). These studies have suggested the dysfunction in fronto-temporo-parietal network, which is known to be crucial for social cognition during empathy or theory of mind tasks in schizophrenia. In line with these studies, our results also showed functional and structural alterations in these brain regions in schizophrenic patients. Besides this, few brain regions such as thalamus, superior parietal lobule and cerebellum did not show any significant cluster of activations in schizophrenic patients. VBM results also showed significantly reduced grey and white matter volumes in these brain regions. These findings suggest that grey and white matter volume reduction in these brain areas might be a cause of functional deficits in empathy in schizophrenia.
It is reported in the literature that there are different brain areas which are involved in empathy (Ochsner et al. 2004; Völlm et al. 2006; Decety and Moriguchi 2007). In line with these studies, we have also found functional activation in brain areas in healthy controls which are associated with empathy. Control group showed activations in the middle temporal gyrus, occipital gyrus, calcarine gyrus, lingual gyrus, fusiform gyrus regions, superior parietal lobule, thalamus and cerebellum. It is reported that a significant bilateral activations in middle occipital gyrus and calcarine, spreading into inferior occipital and fusiform gyrus and the cerebellum is most likely due to the different need for visual perception of emotions (Ochsner et al. 2004; Adolphs, 2002). Brain regions such as middle temporal gyrus, thalamus, superior parietal lobule and cerebellum are known to be associated with emotion processing (Ochsner et al. 2004).
In the present study, schizophrenia patients showed reduced functional activations in calcarine gyrus, fusiform gyrus and middle and inferior occipital gyrus. Schizophrenic patients also showed significantly reduced grey and white matter volumes in these brain regions as compared to healthy controls. The functional and structural abnormalities in these brain areas are suggestive of biological basis for cognitive deficits in schizophrenia.
Besides these results, the present VBM study also revealed grey and white matter volume loss in anterior cingulated cortex and frontal gyrus. It is well known that the frontal lobe is mainly involved in attention and memory functions, whereas anterior cingulated cortex is associated with emotion and executive functions. Any grey or white matter reduction in these areas might be responsible for deficits in attention, memory, emotion and executive functions in schizophrenia. We have also found grey matter volume loss in the precentral gyrus, postcentral gyrus, putamen, rolandic operculum and insula lobe in persons affected with schizophrenia as compared to healthy controls. These brain regions are mainly involved in motor functions and reduced grey matter volume in these regions might be associated with motor function impairment in these patients. Previous studies reported grey and white matter volume loss in the anterior cingulated cortex, frontal gyrus and motor areas (Ananth et al. 2002; Sigmundsson et al. 2001; Shapleske et al. 2002; Hulshoff et al. 2002), which are in consistence with our findings.
There are certain limitations of the present exploratory study. Our patient sample is relatively small; a greater number of subjects would yield more statistically significant results. In addition, all of the patients were taking antipsychotic medications. Previous neuroimaging studies on empathy in schizophrenia have reported that results remained significant when controlling for medication as covariate (Benedetti et al. 2009). Another study by Harvey et al. (2006) also observed no significant effect of drug treatment on emotion deficits in schizophrenia patients. Hence, deficits in socio-emotional abilities appear to be independent of pharmacological treatment. We also did not found any significant correlation between doses of chlorpromazine equivalent antipsychotic and any IRI scores and symptoms severity (i.e., SANS and SAPS scores) in our patient group.
In conclusion, by combining the analysis of fMRI and VBM data, our results showed impaired functional and structural brain networks underlying empathy deficits in schizophrenia patients during empathy task which might be associated with reduced empathy in them as earlier suggested by psychological evaluations. Our findings suggested the functional and structural alterations in brain regions, which include occipital lobe, fusiform gyrus, calcarine gyrus, cuneus, thalamus, superior parietal lobule and cerebellum along with temporo-parietal lobe. These findings suggest a biological basis of cognitive deficits in social attributes in schizophrenia.
Acknowledgments
This work was supported by DRDO R&D Project No. INM-311(4.1), and funded in part by grant from the Fogarty International Centre, NIH The Impact of Yoga Supplementation on Cognitive Function Among Indian Outpatients Grant #1R01TW008289 to TB. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or other funding agencies.
References
- Achim AM, Ouellet R, Roy MA, Jackson PL. Assessment of empathy in first-episode psychosis and meta-analytic comparison with previous studies in schizophrenia. Psychiatry Res. 2011;190:3–8. doi: 10.1016/j.psychres.2010.10.030. [DOI] [PubMed] [Google Scholar]
- Adolphs R. Neural systems for recognizing emotion. Curr Opin Neurobiol. 2002;212:169–177. doi: 10.1016/s0959-4388(02)00301-x. [DOI] [PubMed] [Google Scholar]
- Ananth H, Popescu I, Critchley HD, Good CD, Frackowiak RS, Dolan RJ. Cortical and subcortical gray matter abnormalities in schizophrenia determined through structural magnetic resonance imaging with optimized volumetric voxelbased morphometry. Am J Psychiatry. 2002;159:1497–1505. doi: 10.1176/appi.ajp.159.9.1497. [DOI] [PubMed] [Google Scholar]
- Ashburner J. VBM Tutorial. 2010 http://www.fil.ion.ucl.ac.uk/~john/misc/VBMclass10.Pdf.
- Ashburner J, Friston KJ. Voxel-based morphometry-the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Bernasconi A, Bosia M, Cavallaro R, Dallaspezia S, Falini A, Poletti S, Radaelli D, et al. Functional and structural brain correlates of theory of mind and empathy deficits in schizophrenia. Schizophr Res. 2009;114:154–160. doi: 10.1016/j.schres.2009.06.021. [DOI] [PubMed] [Google Scholar]
- Bora E, Gökçen S, Veznedaroglu B. Empathic abilities in people with schizophrenia. Psychiatry Res. 2008;160:23–29. doi: 10.1016/j.psychres.2007.05.017. [DOI] [PubMed] [Google Scholar]
- Davis M, Luce C, Kraus S. The heritability of characteristics associated with dispositional empathy. J Pers. 1994;62:369–391. doi: 10.1111/j.1467-6494.1994.tb00302.x. [DOI] [PubMed] [Google Scholar]
- Davis M. Measuring individual differences in empathy: Evidence for a multidimensional approach. J Pers Soc Psychol. 1983;44:113–126. [Google Scholar]
- Decety J, Moriguchi Y. The empathic brain and its dysfunction in psychiatric populations: implications for intervention across different clinical conditions. BioPsychoSocial Med. 2007:1–22. doi: 10.1186/1751-0759-1-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derntl B, Finkelmeyer A, Toygar TK, Hulsmann A, Schneider F, Falkenberg DI, Habel U. Generalized deficit in all core components of empathy in schizophrenia. Schizophr Res. 2009;108:197–206. doi: 10.1016/j.schres.2008.11.009. [DOI] [PubMed] [Google Scholar]
- Derntl B, Finkelmeyer A, Voss B, Eickhoff SB, Kellermann T, Schneider F, Habel U. Neural correlates of the core facets of empathy in schizophrenia. Schizophr Res. 2012;136:70–81. doi: 10.1016/j.schres.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande SN, Mathur MN, Das SK, Bhatia T, Sharma S, Nimgaonkar VL. A Hindi version of the diagnostic interview for genetic studies. Schizophr Bull. 1998;24:489–493. doi: 10.1093/oxfordjournals.schbul.a033343. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. NeuroImage. 2005;25:1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Eisenberg N, Miller PA. The relation of empathy to prosocial and related behaviors. Psychol Bull. 1987;101:91–119. [PubMed] [Google Scholar]
- Friston KJ, Worksley KJ. Analysis of fMRI time series revisited again. Neuroimage. 2005;2:173–181. doi: 10.1006/nimg.1995.1023. [DOI] [PubMed] [Google Scholar]
- Haker H, Schimansky J, Jann S, Rossler W. Self-reported empathic abilities in schizophrenia: A longitudinal perspective. Psychiatry Res. 2012;200:1028–1031. doi: 10.1016/j.psychres.2012.04.004. [DOI] [PubMed] [Google Scholar]
- Harvey PD, Patterson TL, Potter LS, Zhong K, Brecher M. Improvement in social competence with short-term atypical antipsychotic treatment: a randomized, double-blind comparison of quetiapine versus risperidone for social competence, social cognition, and neuropsychological functioning. Am J Psychiatry. 2006;163:1918–1925. doi: 10.1176/ajp.2006.163.11.1918. [DOI] [PubMed] [Google Scholar]
- Harvey PO, Zaki J, Lee J, Ochsner K, Green MF. Neural Substrates of Empathic Accuracy in People with Schizophrenia. Schizophr Bull. 2013;39:617–628. doi: 10.1093/schbul/sbs042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry JD, Bailey PE, Rendell PG. Empathy, social functioning and schizotypy. Psychiatry Res. 2008;160:15–22. doi: 10.1016/j.psychres.2007.04.014. [DOI] [PubMed] [Google Scholar]
- Hulshoff Pol HE, Schnack HG, Bertens MGBC, van Haren NEM, van der Tweel I, Staal WG, Baaré WFC, Kahn RS. Volume changes in gray matter in patients with schizophrenia. Am J Psychiatry. 2002;159:244–250. doi: 10.1176/appi.ajp.159.2.244. [DOI] [PubMed] [Google Scholar]
- Lee J, Zaki J, Harvey PO, Ochsner K, Green MF. Schizophrenia patients are impaired in empathic accuracy. Psychol Med. 2011;41:2297–2304. doi: 10.1017/S0033291711000614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SJ, Kang DH, Kim C, Gu BM, Park J, Choi C, Shin NY, Lee J-M, et al. Multi-level comparison of empathy in schizophrenia: an fMRI study of a cartoon task. Psychiatry Res. 2010;181:121–129. doi: 10.1016/j.pscychresns.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Modi S, Khushu S, Bhattacharya M, Raj SB, Varshney A, Mandal MK. European Congress of Radiology. Vienna, Austria: ECR; 2008. Neurocognitive processing of socially relevant negative emotions – an fMRI study. [Google Scholar]
- Montag C, Heinz A, Kunz D, Gallinat J. Self-reported empathic abilities in schizophrenia. Schizophr Res. 2007;92:85–89. doi: 10.1016/j.schres.2007.01.024. [DOI] [PubMed] [Google Scholar]
- Nurnberger JI, Jr, Blehar MC, Kaufmann CA. Diagnostic interview for genetic studies. Rationale, unique features and training NIHM Genetics Initiative. Arch Gen Psychiatry. 1994;51:849–859. doi: 10.1001/archpsyc.1994.03950110009002. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Knierim K, Ludlow DH, Hanelin J, Ramachandran T, Glover G, Mackey SC. Reflecting upon feelings: an fMRI study of neural systems supporting the attribution of emotion to self and other. J Cogn Neurosci. 2004;16:1746–1772. doi: 10.1162/0898929042947829. [DOI] [PubMed] [Google Scholar]
- Shapleske J, Rossell SL, Chitnis XA, Suckling J, Simmons A, Bullmore ET, Woodruff PW, David AS. A computational morphometric MRI study of schizophrenia: effects of hallucinations. Cereb Cortex. 2002;12:1331–1341. doi: 10.1093/cercor/12.12.1331. [DOI] [PubMed] [Google Scholar]
- Sigmundsson T, Suckling J, Maier M, Williams S, Bullmore E, Greenwood K, Fukuda R, Ron M, et al. Structural abnormalities in frontal, temporal, and limbic regions and interconnecting white matter tracts in schizophrenic patients with prominent negative symptoms. Am J Psychiatry. 2001;158:234–243. doi: 10.1176/appi.ajp.158.2.234. [DOI] [PubMed] [Google Scholar]
- Smith MJ, Horan WP, Karpouzian TM, Abram SV, Cobia DJ, Csernansky JG. Self-reported empathy deficits are uniquely associated with poor functioning in schizophrenia. Schizophr Res. 2012;137:196–202. doi: 10.1016/j.schres.2012.01.012. [DOI] [PubMed] [Google Scholar]
- Völlm BA, Taylor AN, Richardson P, Corcoran R, Stirling J, McKie S, Deakin JF, Elliott R. Neuronal correlates of theory of mind and empathy: a functional magnetic resonance imaging study in a nonverbal task. Neuroimage. 2006;29:90–98. doi: 10.1016/j.neuroimage.2005.07.022. [DOI] [PubMed] [Google Scholar]