Abstract
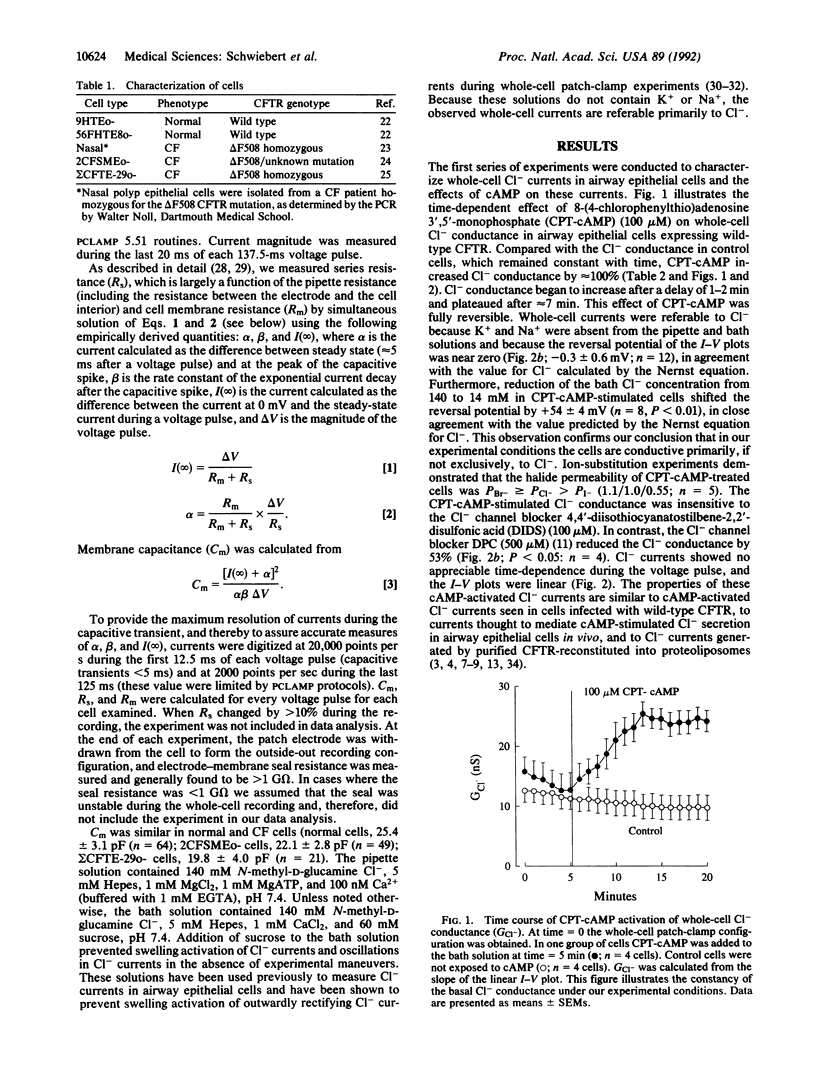
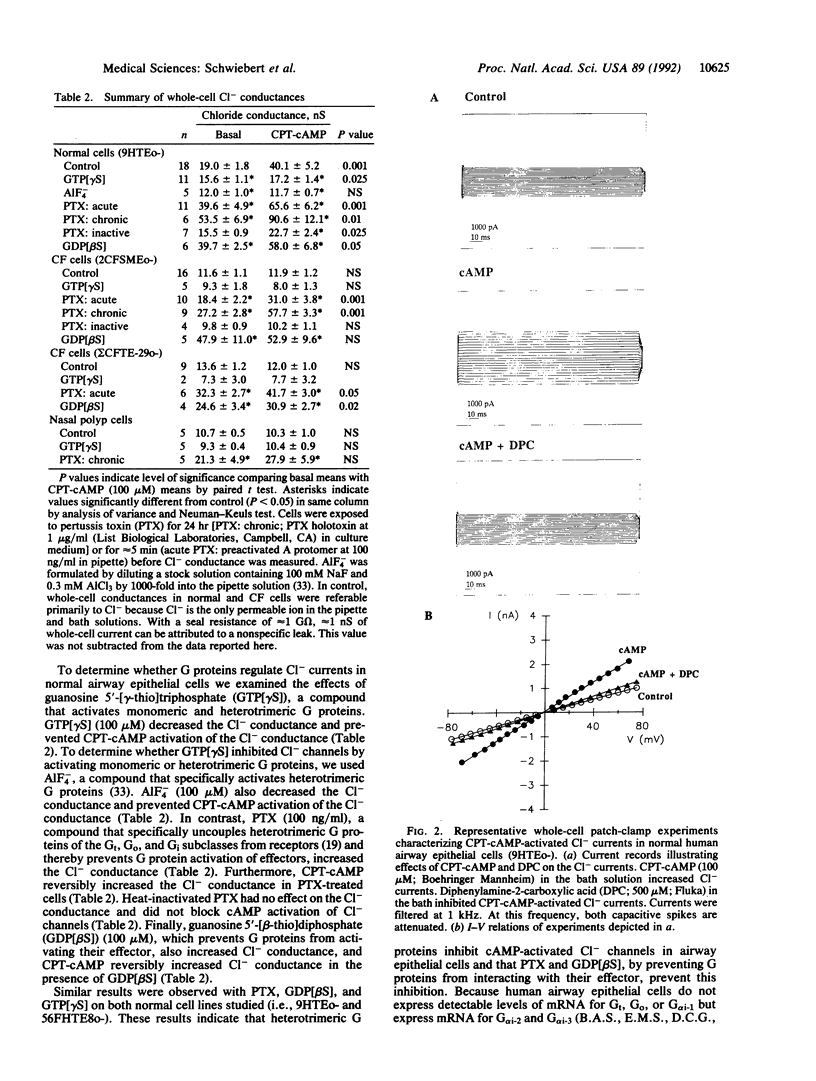
Cystic fibrosis (CF) is a genetic disease characterized, in part, by defective regulation of Cl- secretion by airway epithelial cells. In CF, cAMP does not activate Cl- channels in the apical membrane of airway epithelial cells. We report here whole-cell patch-clamp studies demonstrating that pertussis toxin, which uncouples heterotrimeric GTP-binding proteins (G proteins) from their receptors, and guanosine 5'-[beta-thio]diphosphate, which prevents G proteins from interacting with their effectors, increase Cl- currents and restore cAMP-activated Cl- currents in airway epithelial cells isolated from CF patients. In contrast, the G protein activators guanosine 5'-[gamma-thio]triphosphate and AlF4- reduce Cl- currents and inhibit cAMP from activating Cl- currents in normal airway epithelial cells. In CF cells treated with pertussis toxin or guanosine 5'-[beta-thio]diphosphate and in normal cells, cAMP activates a Cl- conductance that has properties similar to CF transmembrane-conductance regulator Cl- channels. We conclude that heterotrimeric G proteins inhibit cAMP-activated Cl- currents in airway epithelial cells and that modulation of the inhibitory G protein signaling pathway may have the therapeutic potential for improving cAMP-activated Cl- secretion in CF.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson M. P., Rich D. P., Gregory R. J., Smith A. E., Welsh M. J. Generation of cAMP-activated chloride currents by expression of CFTR. Science. 1991 Feb 8;251(4994):679–682. doi: 10.1126/science.1704151. [DOI] [PubMed] [Google Scholar]
- Anderson M. P., Welsh M. J. Calcium and cAMP activate different chloride channels in the apical membrane of normal and cystic fibrosis epithelia. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6003–6007. doi: 10.1073/pnas.88.14.6003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barasch J., Kiss B., Prince A., Saiman L., Gruenert D., al-Awqati Q. Defective acidification of intracellular organelles in cystic fibrosis. Nature. 1991 Jul 4;352(6330):70–73. doi: 10.1038/352070a0. [DOI] [PubMed] [Google Scholar]
- Bear C. E., Li C. H., Kartner N., Bridges R. J., Jensen T. J., Ramjeesingh M., Riordan J. R. Purification and functional reconstitution of the cystic fibrosis transmembrane conductance regulator (CFTR). Cell. 1992 Feb 21;68(4):809–818. doi: 10.1016/0092-8674(92)90155-6. [DOI] [PubMed] [Google Scholar]
- Berger H. A., Anderson M. P., Gregory R. J., Thompson S., Howard P. W., Maurer R. A., Mulligan R., Smith A. E., Welsh M. J. Identification and regulation of the cystic fibrosis transmembrane conductance regulator-generated chloride channel. J Clin Invest. 1991 Oct;88(4):1422–1431. doi: 10.1172/JCI115450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne H. R., Sanders D. A., McCormick F. The GTPase superfamily: conserved structure and molecular mechanism. Nature. 1991 Jan 10;349(6305):117–127. doi: 10.1038/349117a0. [DOI] [PubMed] [Google Scholar]
- Brown A. M. A cellular logic for G protein-coupled ion channel pathways. FASEB J. 1991 May;5(8):2175–2179. doi: 10.1096/fasebj.5.8.1708737. [DOI] [PubMed] [Google Scholar]
- Brown A. M., Birnbaumer L. Ionic channels and their regulation by G protein subunits. Annu Rev Physiol. 1990;52:197–213. doi: 10.1146/annurev.ph.52.030190.001213. [DOI] [PubMed] [Google Scholar]
- Chabre M. Aluminofluoride and beryllofluoride complexes: a new phosphate analogs in enzymology. Trends Biochem Sci. 1990 Jan;15(1):6–10. doi: 10.1016/0968-0004(90)90117-t. [DOI] [PubMed] [Google Scholar]
- Cheng S. H., Gregory R. J., Marshall J., Paul S., Souza D. W., White G. A., O'Riordan C. R., Smith A. E. Defective intracellular transport and processing of CFTR is the molecular basis of most cystic fibrosis. Cell. 1990 Nov 16;63(4):827–834. doi: 10.1016/0092-8674(90)90148-8. [DOI] [PubMed] [Google Scholar]
- Cliff W. H., Frizzell R. A. Separate Cl- conductances activated by cAMP and Ca2+ in Cl(-)-secreting epithelial cells. Proc Natl Acad Sci U S A. 1990 Jul;87(13):4956–4960. doi: 10.1073/pnas.87.13.4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cliff W. H., Frizzell R. A. Separate Cl- conductances activated by cAMP and Ca2+ in Cl(-)-secreting epithelial cells. Proc Natl Acad Sci U S A. 1990 Jul;87(13):4956–4960. doi: 10.1073/pnas.87.13.4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozens A. L., Yezzi M. J., Chin L., Simon E. M., Finkbeiner W. E., Wagner J. A., Gruenert D. C. Characterization of immortal cystic fibrosis tracheobronchial gland epithelial cells. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):5171–5175. doi: 10.1073/pnas.89.11.5171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalemans W., Barbry P., Champigny G., Jallat S., Dott K., Dreyer D., Crystal R. G., Pavirani A., Lecocq J. P., Lazdunski M. Altered chloride ion channel kinetics associated with the delta F508 cystic fibrosis mutation. Nature. 1991 Dec 19;354(6354):526–528. doi: 10.1038/354526a0. [DOI] [PubMed] [Google Scholar]
- Dietl P., Kizer N., Stanton B. A. Conductive properties of a rabbit cortical collecting duct cell line: regulation by isoproterenol. Am J Physiol. 1992 Apr;262(4 Pt 2):F578–F582. doi: 10.1152/ajprenal.1992.262.4.F578. [DOI] [PubMed] [Google Scholar]
- Drumm M. L., Pope H. A., Cliff W. H., Rommens J. M., Marvin S. A., Tsui L. C., Collins F. S., Frizzell R. A., Wilson J. M. Correction of the cystic fibrosis defect in vitro by retrovirus-mediated gene transfer. Cell. 1990 Sep 21;62(6):1227–1233. doi: 10.1016/0092-8674(90)90398-x. [DOI] [PubMed] [Google Scholar]
- Drumm M. L., Wilkinson D. J., Smit L. S., Worrell R. T., Strong T. V., Frizzell R. A., Dawson D. C., Collins F. S. Chloride conductance expressed by delta F508 and other mutant CFTRs in Xenopus oocytes. Science. 1991 Dec 20;254(5039):1797–1799. doi: 10.1126/science.1722350. [DOI] [PubMed] [Google Scholar]
- Duszyk M., French A. S., Man S. F. Cystic fibrosis affects chloride and sodium channels in human airway epithelia. Can J Physiol Pharmacol. 1989 Oct;67(10):1362–1365. doi: 10.1139/y89-217. [DOI] [PubMed] [Google Scholar]
- Duszyk M., French A. S., Man S. F. The 20-pS chloride channel of the human airway epithelium. Biophys J. 1990 Feb;57(2):223–230. doi: 10.1016/S0006-3495(90)82525-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frindt G., Sackin H., Palmer L. G. Whole-cell currents in rat cortical collecting tubule: low-Na diet increases amiloride-sensitive conductance. Am J Physiol. 1990 Mar;258(3 Pt 2):F562–F567. doi: 10.1152/ajprenal.1990.258.3.F562. [DOI] [PubMed] [Google Scholar]
- Frizzell R. A., Halm D. R., Rechkemmer G., Shoemaker R. L. Chloride channel regulation in secretory epithelia. Fed Proc. 1986 Nov;45(12):2727–2731. [PubMed] [Google Scholar]
- Gregory R. J., Cheng S. H., Rich D. P., Marshall J., Paul S., Hehir K., Ostedgaard L., Klinger K. W., Welsh M. J., Smith A. E. Expression and characterization of the cystic fibrosis transmembrane conductance regulator. Nature. 1990 Sep 27;347(6291):382–386. doi: 10.1038/347382a0. [DOI] [PubMed] [Google Scholar]
- Gruenert D. C., Basbaum C. B., Welsh M. J., Li M., Finkbeiner W. E., Nadel J. A. Characterization of human tracheal epithelial cells transformed by an origin-defective simian virus 40. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5951–5955. doi: 10.1073/pnas.85.16.5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenert D. C., Basbaum C. B., Widdicombe J. H. Long-term culture of normal and cystic fibrosis epithelial cells grown under serum-free conditions. In Vitro Cell Dev Biol. 1990 Apr;26(4):411–418. doi: 10.1007/BF02623833. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hamilton S. L., Codina J., Hawkes M. J., Yatani A., Sawada T., Strickland F. M., Froehner S. C., Spiegel A. M., Toro L., Stefani E. Evidence for direct interaction of Gs alpha with the Ca2+ channel of skeletal muscle. J Biol Chem. 1991 Oct 15;266(29):19528–19535. [PubMed] [Google Scholar]
- Hescheler J., Rosenthal W., Trautwein W., Schultz G. The GTP-binding protein, Go, regulates neuronal calcium channels. 1987 Jan 29-Feb 4Nature. 325(6103):445–447. doi: 10.1038/325445a0. [DOI] [PubMed] [Google Scholar]
- Kartner N., Hanrahan J. W., Jensen T. J., Naismith A. L., Sun S. Z., Ackerley C. A., Reyes E. F., Tsui L. C., Rommens J. M., Bear C. E. Expression of the cystic fibrosis gene in non-epithelial invertebrate cells produces a regulated anion conductance. Cell. 1991 Feb 22;64(4):681–691. doi: 10.1016/0092-8674(91)90498-n. [DOI] [PubMed] [Google Scholar]
- Kasai H. Tonic inhibition and rebound facilitation of a neuronal calcium channel by a GTP-binding protein. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8855–8859. doi: 10.1073/pnas.88.19.8855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley G. G., Aassar O. S., Forrest J. N., Jr Endogenous adenosine is an autacoid feedback inhibitor of chloride transport in the shark rectal gland. J Clin Invest. 1991 Dec;88(6):1933–1939. doi: 10.1172/JCI115517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerem B., Rommens J. M., Buchanan J. A., Markiewicz D., Cox T. K., Chakravarti A., Buchwald M., Tsui L. C. Identification of the cystic fibrosis gene: genetic analysis. Science. 1989 Sep 8;245(4922):1073–1080. doi: 10.1126/science.2570460. [DOI] [PubMed] [Google Scholar]
- Lemna W. K., Feldman G. L., Kerem B., Fernbach S. D., Zevkovich E. P., O'Brien W. E., Riordan J. R., Collins F. S., Tsui L. C., Beaudet A. L. Mutation analysis for heterozygote detection and the prenatal diagnosis of cystic fibrosis. N Engl J Med. 1990 Feb 1;322(5):291–296. doi: 10.1056/NEJM199002013220503. [DOI] [PubMed] [Google Scholar]
- McCann J. D., Li M., Welsh M. J. Identification and regulation of whole-cell chloride currents in airway epithelium. J Gen Physiol. 1989 Dec;94(6):1015–1036. doi: 10.1085/jgp.94.6.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padnos E. Treatment of the pulmonary aspects of mucoviscidosis by injection of pertussis vaccine. Rev Allergy. 1965 Jul-Aug;19(4):408–414. [PubMed] [Google Scholar]
- Rich D. P., Anderson M. P., Gregory R. J., Cheng S. H., Paul S., Jefferson D. M., McCann J. D., Klinger K. W., Smith A. E., Welsh M. J. Expression of cystic fibrosis transmembrane conductance regulator corrects defective chloride channel regulation in cystic fibrosis airway epithelial cells. Nature. 1990 Sep 27;347(6291):358–363. doi: 10.1038/347358a0. [DOI] [PubMed] [Google Scholar]
- Rich D. P., Anderson M. P., Gregory R. J., Cheng S. H., Paul S., Jefferson D. M., McCann J. D., Klinger K. W., Smith A. E., Welsh M. J. Expression of cystic fibrosis transmembrane conductance regulator corrects defective chloride channel regulation in cystic fibrosis airway epithelial cells. Nature. 1990 Sep 27;347(6291):358–363. doi: 10.1038/347358a0. [DOI] [PubMed] [Google Scholar]
- Schwiebert E. M., Light D. B., Fejes-Toth G., Naray-Fejes-Toth A., Stanton B. A. A GTP-binding protein activates chloride channels in a renal epithelium. J Biol Chem. 1990 May 15;265(14):7725–7728. [PubMed] [Google Scholar]
- Shoemaker R. L., Frizzell R. A., Dwyer T. M., Farley J. M. Single chloride channel currents from canine tracheal epithelial cells. Biochim Biophys Acta. 1986 Jun 26;858(2):235–242. doi: 10.1016/0005-2736(86)90328-7. [DOI] [PubMed] [Google Scholar]
- Solc C. K., Wine J. J. Swelling-induced and depolarization-induced C1-channels in normal and cystic fibrosis epithelial cells. Am J Physiol. 1991 Oct;261(4 Pt 1):C658–C674. doi: 10.1152/ajpcell.1991.261.4.C658. [DOI] [PubMed] [Google Scholar]
- Tabcharani J. A., Chang X. B., Riordan J. R., Hanrahan J. W. Phosphorylation-regulated Cl- channel in CHO cells stably expressing the cystic fibrosis gene. Nature. 1991 Aug 15;352(6336):628–631. doi: 10.1038/352628a0. [DOI] [PubMed] [Google Scholar]
- Tilly B. C., Kansen M., van Gageldonk P. G., van den Berghe N., Galjaard H., Bijman J., de Jonge H. R. G-proteins mediate intestinal chloride channel activation. J Biol Chem. 1991 Feb 5;266(4):2036–2040. [PubMed] [Google Scholar]
- Welsh M. J. An apical-membrane chloride channel in human tracheal epithelium. Science. 1986 Jun 27;232(4758):1648–1650. doi: 10.1126/science.2424085. [DOI] [PubMed] [Google Scholar]
- Welsh M. J. Single apical membrane anion channels in primary cultures of canine tracheal epithelium. Pflugers Arch. 1986;407 (Suppl 2):S116–S122. doi: 10.1007/BF00584940. [DOI] [PubMed] [Google Scholar]
- Widdicombe J. H., Wine J. J. The basic defect in cystic fibrosis. Trends Biochem Sci. 1991 Dec;16(12):474–477. doi: 10.1016/0968-0004(91)90183-v. [DOI] [PubMed] [Google Scholar]
- Wilk-Blaszczak M. A., French A. S., Man S. F. Halide permeation through 10 pS and 20 pS anion channels in human airway epithelial cells. Biochim Biophys Acta. 1992 Feb 17;1104(1):160–166. doi: 10.1016/0005-2736(92)90145-c. [DOI] [PubMed] [Google Scholar]
- Worrell R. T., Frizzell R. A. CaMKII mediates stimulation of chloride conductance by calcium in T84 cells. Am J Physiol. 1991 Apr;260(4 Pt 1):C877–C882. doi: 10.1152/ajpcell.1991.260.4.C877. [DOI] [PubMed] [Google Scholar]
- Zeitlin P. L., Lu L., Rhim J., Cutting G., Stetten G., Kieffer K. A., Craig R., Guggino W. B. A cystic fibrosis bronchial epithelial cell line: immortalization by adeno-12-SV40 infection. Am J Respir Cell Mol Biol. 1991 Apr;4(4):313–319. doi: 10.1165/ajrcmb/4.4.313. [DOI] [PubMed] [Google Scholar]




