Abstract
Objective
Assisted reproductive technology has been associated with an increase in multiple pregnancies. The most effective strategy for reducing multiple pregnancies is single embryo transfer. Beginning in October 2015, the National Supporting Program for Infertility in South Korea has limited the number of embryos that can be transferred per in vitro fertilization (IVF) cycle depending on the patient's age. However, little is known regarding the effect of age and number of transferred embryos on the clinical outcomes of Korean patients. Thus, this study was performed to evaluate the effect of the number of transferred blastocysts on clinical outcomes.
Methods
This study was carried out in the Fertility Center of CHA Gangnam Medical Center from January 2013 to December 2014. The clinical outcomes of 514 women who underwent the transfer of one or two blastocysts on day 5 after IVF and of 721 women who underwent the transfer of one or two vitrified-warmed blastocysts were analyzed retrospectively.
Results
For both fresh and vitrified-warmed cycles, the clinical pregnancy rate and live birth or ongoing pregnancy rate were not significantly different between patients who underwent elective single blastocyst transfer (eSBT) and patients who underwent double blastocyst transfer (DBT), regardless of age. However, the multiple pregnancy rate was significantly lower in the eSBT group than in the DBT group.
Conclusion
The clinical outcomes of eSBT and DBT were equivalent, but eSBT had a lower risk of multiple pregnancy and is, therefore, the best option.
Keywords: Blastocyst transfer, In vitro fertilization, Live birth pregnancy rate, Multiple pregnancy, Single embryo transfer
Introduction
Since the first baby conceived using in vitro fertilization (IVF) was born in South Korea in 1985, IVF-embryo transfer (ET) has become a routine procedure for the treatment of infertility in many IVF clinics. It has been well documented that IVF-ET involves a high probability of multiple pregnancies due to the transfer of multiple embryos after controlled ovarian stimulation. The most effective way to reduce multiple pregnancies is to transfer a single embryo. In 2008, the Korean Ministry of Health and Welfare developed guidelines for determining the number of transferred embryos based on the patient's age and the quality of the embryo. Consequently, the rate of multiple births in IVF patients has decreased from 34.2% of IVF pregnancies in 2006 to 27.1% in 2011 [1]. However, this rate is still much higher than the rate of multiple births that occur in normal pregnancies [2]. According to Statistics Korea [3], 3.5% of total live births in 2014 were multiple births, whereas in 2005, before the National Supporting Program for Infertility (NSPI) began, only 2.2% of births were multiple births.
Multiple pregnancies, which have become increasingly common due to the support of the NSPI and the use of assisted reproductive technology (ART), are associated with a higher risk of low birth weight and premature birth than single pregnancies [1]. The increase in premature births and low birth weight that has been associated with the use of ART has also been associated with the higher rate of multiple births [4,5,6]. In fact, in 2014, 57.3% of multiple births were born prematurely (before 37 weeks) [3]. Since premature babies with low birth weight require care in a neonatal intensive care unit, the social and financial aspects of this phenomenon must be considered [7]. To address this problem, starting in October 2015, the NSPI has limited the number of transferred embryos depending on the patient's age, regardless of embryo quality. However, many IVF clinics are concerned that limiting the number of embryos that can be transferred may have an adverse effect on the pregnancy rate.
Therefore, the objective of this study was to evaluate the clinical outcomes of patients depending on the number of blastocysts transferred in fresh and vitrified-warmed cycles.
Methods
1. Patients and study design
This study was performed from January 2013 to December 2014 in the Fertility Center of CHA Gangnam Medical Center. In this study, we analyzed the clinical outcomes of 514 women who underwent a transfer of one or two blastocysts on day 5 after IVF, as well as the outcomes of 721 women who underwent a transfer of one or two vitrified-warmed blastocysts. The single blastocyst transfer (SBT) group was divided into subgroups corresponding to elective single blastocyst transfer (eSBT) and compulsory single blastocyst transfer (cSBT) depending on the presence of surplus blastocysts. The number of transferred blastocysts was decided based on a patient-physician consultation. The outcomes were also analyzed according to age, using a cutoff of 35 years.
2. Ovarian stimulation
Controlled ovarian stimulation was performed through pituitary down-regulation with gonadotropin-releasing hormone analogues or antagonists, as well as recombinant follicle-stimulating hormone (FSH). When at least two follicles reached 18 mm in diameter, recombinant human chorionic gonadotropin (hCG) was injected for final oocyte maturation. After 34 to 36 hours, oocyte retrieval was performed under transvaginal ultrasound guidance. The retrieved oocytes were cultured in an environment of 6% CO2, 5% O2, and 89% N2. Fertilization was performed by IVF or intracytoplasmic sperm injection and verified by presence of two pronuclei after 16 to 18 hours.
3. Embryo culture and transfer
Sequential media (Cleavage Medium and Blastocyst Medium, Cook Medical, Brisbane, Australia) or single media (Continuous Single Culture, Irvine Scientific, Santa Ana, CA, USA) was used to support the development of the embryos to the blastocyst stage. Transabdominal ultrasound-guided ET was performed on day 5 after oocyte retrieval, using a soft catheter (Cook Medical, Bloomington, IN, USA). The highest-quality blastocysts were selected for transfer. Surplus blastocysts after transfer were vitrified on days 5 and 6. A serum β-hCG test was performed 14 days after ET. Clinical pregnancy was defined as the presence of a gestational sac on ultrasonography and the presence of a fetal heartbeat. Miscarriage was defined as the loss of a pregnancy before week 12 of gestation.
4. Vitrification and warming
For vitrification, artificial shrinkage was performed on all blastocysts and assisted hatching was carried out with a laser. After artificial shrinkage, the blastocysts were preequilibrated in HEPES medium (Quinn's-HEPES; SAGE, In Vitro Fertilization, Trumbull, CT, USA) supplemented with 7.5% ethylene glycol (EG; St. Louis, MO, USA) and 7.5% dimethyl sulfoxide (DMSO; Sigma-Aldrich, St. Louis, MO, USA) for 2.5 minutes and then placed for the final equilibration in 15% EG, 15% DMSO, and 0.5 M sucrose. The blastocysts were then loaded onto an electron microscopic (EM) gold grid (EM Grid, SPI Supplies, West Chester, PA, USA) using a fine glass pipette. The EM grids containing the oocytes were immediately plunged into slush liquid nitrogen using Vit-Master (IMT, Ness Ziona, Israel). For warming, the EM grids containing the vitrified blastocysts were sequentially transferred to culture dishes containing HEPES medium supplemented with 0.5 M, 0.25 M, 0.125 M, and 0.0 M sucrose at intervals of 2.5 minutes and 20% human serum albumin (SAGE BioPharma, Bedminster, NJ, USA). The vitrified-warmed blastocysts were washed with blastocyst medium (Cook Medical) at 37℃ in an atmosphere of 6% CO2, 5% O2, and 89% N2, and then cultured until the next day.
5. Statistics
Qualitative variables were expressed as frequencies (number) and percentages, and compared using the chi-square test. Quantitative variables were expressed as mean±standard deviation and were analyzed for statistical significance using one-way analysis of variance in Graphpad Prism 5 (Graphpad Software, La Jolla, CA, USA). The p-values <0.05 were considered to indicate statistical significance.
Results
1. Fresh cycles
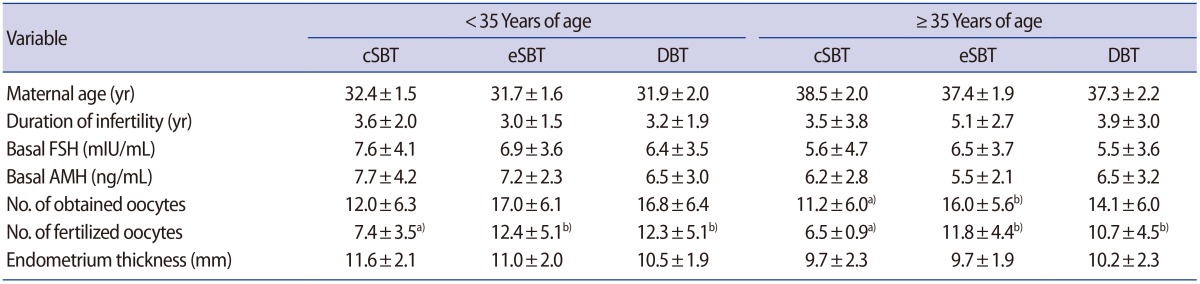
The women in the cSBT, eSBT, and double blastocyst transfer (DBT) groups were compared with respect to age, duration of infertility, basal FSH and anti-Müllerian hormone levels, and endometrium thickness (Table 1). The number of fertilized oocytes in the cSBT group was lower than in the eSBT and DBT groups, regardless of age, and only one blastocyst was available for transfer in the cSBT group. In women under 35 years of age, the cSBT group included seven cycles, the eSBT group included 22 cycles, and the DBT group included 238 cycles. In women 35 years of age and older, the cSBT group included 16 cycles, the eSBT group comprised 24 cycles, and the DBT group included 207 cycles (Table 2). In women less than 35 years of age, a significant difference was found between the cSBT and DBT groups in the clinical pregnancy rate (14.3% vs. 60.1%, p=0.02) and the live birth or ongoing pregnancy rate (0% vs. 52.9%, p=0.006). However, no significant differences were found between the eSBT and DBT groups in either the clinical pregnancy rate (68.1% vs. 60.1%, p=0.45) or the live birth or ongoing pregnancy rate (63.6% vs. 52.9%, p=0.34). In women 35 years of age and older, no outcomes displayed statistically significant variation among the three groups. In women less than 35 years of age, the multiple pregnancy rate was significantly lower in the eSBT group than in the DBT group (p=0.006), although the reason for the absence of a significant difference in women 35 years and over may have been the small number of cycles resulting in pregnancies in the two SBT groups.
Table 1. Characteristics of the patients who underwent fresh blastocyst transfer cycles.
Values are presented as mean±standard deviation.
cSBT, compulsory single blastocyst transfer; eSBT, elective single blastocyst transfer; DBT, double blastocyst transfer; FSH, follicle-stimulating hormone; AMH, anti-Müllerian hormone.
Different superscripts a) vs. b) in the same row indicate significant differences (p<0.05).
Table 2. Clinical outcomes of fresh blastocyst transfer cycles.
Values are presented as number (%).
cSBT, compulsory single blastocyst transfer; eSBT, elective single blastocyst transfer; DBT, double blastocyst transfer.
Different superscripts a) vs. b) in the same row indicate significant differences (p<0.05).
2. Vitrified-warmed cycles
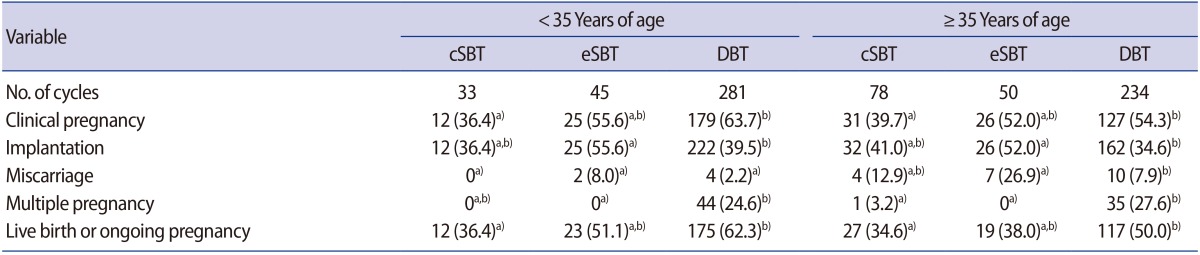
Of the patients who underwent vitrified-warmed blastocyst transfer cycles and were under 35 years of age, the cSBT group included 33 cycles, the eSBT group included 45 cycles, and the DBT group included 281 cycles. In women 35 years of age and older, the cSBT group consisted of 78 cycles, the eSBT group included 50 cycles, and the DBT group comprised 234 cycles (Table 3). For women less than 35 years of age, the clinical pregnancy rates in the cSBT group were significantly lower than in the DBT group (36.4% vs. 63.7%, p=0.002) and the live birth or ongoing pregnancy rate was also significantly lower (36.4% vs. 62.3%, p=0.004). However, as in the fresh cycles, no significant differences were found between the eSBT and DBT groups in the clinical pregnancy rate (55.6% vs. 63.7%, p=0.30) and the live birth or ongoing pregnancy rate (51.1% vs. 62.3%, p=0.16). In women 35 years and over, no significant difference was found between the eSBT and DBT groups in the pregnancy rate (52.0% vs. 54.3%, p=0.77) or in the live birth or ongoing pregnancy rate (38.0% vs. 50.0%, p=0.12). The multiple pregnancy rate was significantly higher in the DBT groups than in the eSBT groups regardless of age (p=0.005 and p=0.002, respectively).
Table 3. Clinical outcomes of vitrified-warmed blastocyst transfer cycles.
Values are presented as number (%).
cSBT, compulsory single blastocyst transfer; eSBT, elective single blastocyst transfer; DBT, double blastocyst transfer.
Different superscripts a) vs. b) in the same row indicate significant differences (p<0.05).
Discussion
This study analyzed the outcomes of blastocyst transfer in patients undergoing IVF-ET and vitrified-warmed ET. In our study, fewer patients underwent SBT than underwent DBT. For the patients in the fresh cSBT group (4.5%), only one blastocyst was present, meaning that the embryos could not be specifically chosen for transfer. Patients in the fresh eSBT group (8.9%) presumably did not want to have a twin birth. The remainder of the women (86.6%) undergoing the transfer of fresh blastocysts chose to transfer two embryos. The most likely reason for this is that many people want to have twins due to the influence of the media. Although extended embryo culture techniques have been enhanced by improving the culture medium and selecting the highest-quality embryos, multiple transfers still take place in ART in Korea, leading to the possibility of multiple pregnancies.
According to the NSPI, ART patients have a much higher possibility of multiple births than the general population. In 2011, multiple births in pregnancies resulting from ART were more than nine times more common than in pregnancies overall (27.7% vs. 2.9%) [1]. Both the women carrying multiple babies and the babies themselves may have more health problems than in single pregnancies, and, therefore, such women may have to spend more time and money on health care. Multiple pregnancies are also more likely to result in preterm birth (before 37 weeks of pregnancy), and to have complications including preeclampsia, gestational diabetes, anemia, cholestasis, dermatoses, and hyperemesis gravidarum [8,9,10]. Preterm babies may also have more health complications, including cerebral palsy, retinopathy, bronchopulmonary dysplasia, and growth problems [8]. Multiple births are also more likely to be associated with physical, emotional, and financial stress. Thus, it is desirable to reduce the incidence of multiple pregnancies in ART by limiting the number of transferred embryos.
In some studies, DBT was found to show better clinical outcomes than SBT. Berin et al. [11] reported that the transfer of two blastocysts during a frozen cycle produced higher live birth rates and twin live birth rates than a single frozen blastocyst transfer. A 2014 meta-analysis reported that elective single ET in fresh IVF cycles yielded a lower live birth rate than double ET [12]. In contrast, in a recent study, Alansari and Akande [13] reported that increasing the number of blastocysts transferred regardless of age did not result in any significant increase in the clinical pregnancy rate, whereas it did lead to a significant increase in the multiple pregnancy rate. In a preimplantation genetic screening study, the ongoing pregnancy rate per randomized patient was similar for single euploid blastocyst transfer and double euploid blastocyst transfer [14]. However, little is known about the clinical outcomes of Korean patients depending on the age and number of transferred blastocysts.
In this study, we looked at the clinical outcomes associated with the number of blastocysts transferred in patients who underwent cSBT, eSBT, and DBT. With the exception of women aged 35 years and over in the fresh transfer cycles, the live birth or ongoing pregnancy rate was significantly higher in the DBT group than in the cSBT group. No significant differences were found between the eSBT and DBT groups in either the fresh or vitrified-warmed cycles. In fresh cycles performed in women less than 35 years of age, the pregnancy rate and live birth or ongoing pregnancy rate were significantly higher in the eSBT group than in the cSBT group (68.1% vs. 14.3%, p=0.01 and 63.6% vs. 0%, p=0.01, respectively). This discrepancy most likely occurred because the transfer of good-quality blastocysts was more common in the eSBT group than in the cSBT group (95.5% vs. 42.9%, data not shown). Therefore, we believe that producing more high-quality blastocysts through the improvement of culture conditions is necessary. For women aged 35 years and over in vitrified-warmed cycles, although the live birth or ongoing pregnancy rate was not significantly different between the eSBT and DBT groups (38.0% vs. 50.0%, p=0.12), the miscarriage rate was significantly higher in the eSBT group than in the DBT group (26.9% vs. 7.9%, p=0.005). Therefore, for women aged 35 years and over undergoing vitrified-warmed cycles, we found that DBT resulted in a higher live birth or ongoing pregnancy rate than SBT.
For patients with at least two blastocysts, SBT and DBT yielded comparable outcomes in terms of the pregnancy rate and the live birth or ongoing pregnancy rate, although the multiple pregnancy rate was higher for all DBT groups. This study showed that eSBT led to comparable outcomes to DBT, but a lower multiple pregnancy rate. Therefore, in order to reduce the multiple pregnancy rate and to optimize the health of the mother and the baby, we suggest that, after consultation with the patient, the highest-quality embryo should be selected and a single blastocyst should be transferred.
Footnotes
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, Republic of Korea (HI12C0055).
Conflict of interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Ministry of Health and Welfare. A study for the improvement of sub-fertility couple supporting program [Internet] Sejong: Ministry of Health and Welfare; 2013. [cited 2013 Nov 29]. Available from: http://www.prism.go.kr/ [Google Scholar]
- 2.Van Voorhis BJ. Clinical practice: in vitro fertilization. N Engl J Med. 2007;356:379–386. doi: 10.1056/NEJMcp065743. [DOI] [PubMed] [Google Scholar]
- 3.Statistics Korea. Final results of birth statistics in 2014 [Internet] Daejeon: Statistics Korea; 2015. [cited 2015 August 25]. Available from: http://kostat.go.kr/portal/korea/ [Google Scholar]
- 4.Schieve LA, Meikle SF, Ferre C, Peterson HB, Jeng G, Wilcox LS. Low and very low birth weight in infants conceived with use of assisted reproductive technology. N Engl J Med. 2002;346:731–737. doi: 10.1056/NEJMoa010806. [DOI] [PubMed] [Google Scholar]
- 5.D'Angelo DV, Whitehead N, Helms K, Barfield W, Ahluwalia IB. Birth outcomes of intended pregnancies among women who used assisted reproductive technology, ovulation stimulation, or no treatment. Fertil Steril. 2011;96:314–320.e2. doi: 10.1016/j.fertnstert.2011.05.073. [DOI] [PubMed] [Google Scholar]
- 6.Messerlian C, Maclagan L, Basso O. Infertility and the risk of adverse pregnancy outcomes: a systematic review and meta-analysis. Hum Reprod. 2013;28:125–137. doi: 10.1093/humrep/des347. [DOI] [PubMed] [Google Scholar]
- 7.Janvier A, Spelke B, Barrington KJ. The epidemic of multiple gestations and neonatal intensive care unit use: the cost of irresponsibility. J Pediatr. 2011;159:409–413. doi: 10.1016/j.jpeds.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 8.Practice Committee of American Society for Reproductive Medicine. Multiple gestation associated with infertility therapy: an American Society for Reproductive Medicine Practice Committee opinion. Fertil Steril. 2012;97:825–834. doi: 10.1016/j.fertnstert.2011.11.048. [DOI] [PubMed] [Google Scholar]
- 9.Ombelet W, De Sutter P, Van der Elst J, Martens G. Multiple gestation and infertility treatment: registration, reflection and reaction–the Belgian project. Hum Reprod Update. 2005;11:3–14. doi: 10.1093/humupd/dmh048. [DOI] [PubMed] [Google Scholar]
- 10.Henderson CE, Scarpelli S, LaRosa D, Divon MY. Assessing the risk of gestational diabetes in twin gestation. J Natl Med Assoc. 1995;87:757–758. [PMC free article] [PubMed] [Google Scholar]
- 11.Berin I, McLellan ST, Macklin EA, Toth TL, Wright DL. Frozen-thawed embryo transfer cycles: clinical outcomes of single and double blastocyst transfers. J Assist Reprod Genet. 2011;28:575–581. doi: 10.1007/s10815-011-9551-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McLernon DJ, Harrild K, Bergh C, Davies MJ, de Neubourg D, Dumoulin JC, et al. Clinical effectiveness of elective single versus double embryo transfer: meta-analysis of individual patient data from randomised trials. BMJ. 2010;341:c6945. doi: 10.1136/bmj.c6945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alansari L, Akande V. How to maximize the pregnancy rate with no increase in multiple pregnancy rates following blastocyst embryo transfer? Is blastocyst transfer time the missing ingredient? Middle East Fertil Soc J. 2015;20:241–245. [Google Scholar]
- 14.Forman EJ, Hong KH, Ferry KM, Tao X, Taylor D, Levy B, et al. In vitro fertilization with single euploid blastocyst transfer: a randomized controlled trial. Fertil Steril. 2013;100:100–107.e1. doi: 10.1016/j.fertnstert.2013.02.056. [DOI] [PubMed] [Google Scholar]