Abstract
Little is known of the capacity that marine metazoans have to evolve under rapid p CO 2 changes. Consequently, we reared a marine polychaete, Ophryotrocha labronica, previously cultured for approximately 33 generations under a low/variable pH regime, under elevated and low p CO 2 for six generations. The strain used was found to be tolerant to elevated p CO 2 conditions. In generations F1 and F2 females’ fecundity was significantly lower in the low p CO 2 treatment. However, from generation F3 onwards there were no differences between p CO 2 treatments, indicating that trans‐generational effects enabled the restoration and maintenance of reproductive output. Whilst the initial fitness recovery was likely driven by trans‐generational plasticity (TGP), the results from reciprocal transplant assays, performed using F7 individuals, made it difficult to disentangle between whether TGP had persisted across multiple generations, or if evolutionary adaptation had occurred. Nonetheless, both are important mechanisms for persistence under climate change. Overall, our study highlights the importance of multi‐generational experiments in more accurately determining marine metazoans’ responses to changes in p CO 2, and strengthens the case for exploring their use in conservation, by creating specific p CO 2 tolerant strains of keystone ecosystem species.
Keywords: adaptive potential, climate change, evolutionary adaptation, fecundity, multi‐generational experiment, ocean acidification, parental effects, trans‐generational plasticity
Introduction
Marine metazoans will face a series of significant changes in oceanic pCO2 levels over the coming centuries. Firstly, increases in oceanic pCO2 levels are predicted to occur at an unprecedented rate, leading to a subsequent reduction in pH; a phenomenon commonly termed ocean acidification (OA) (Doney et al. 2009). Secondly, the successful implementation of low‐emissions policies (Frölicher and Joos 2010) and other climate change mitigation measures, such as carbon capture and storage (Bickle 2009; Szulczewski et al. 2012), should result in a decrease of oceanic pCO2 levels, and thus a rise in seawater pH. Coastal environments will be particularly affected by acidification/‘de‐acidification’ processes due to the unstable equilibrium of coastal water carbonate systems, which are affected by multiple human activities operating at various spatial and temporal scales (Strong et al. 2014). Thus, one of the major challenges in marine global change biology, as well as marine coastal conservation, is to accurately predict how marine metazoans will respond to rapid changes in pCO2. Whilst the large body of existing literature documents the potential negative impacts of elevated pCO2 conditions on a wide range of marine species (Kroeker et al. 2013; Wittmann and Pörtner 2013), the majority of studies have focussed on short‐term, within‐generation, effects of modern‐day populations (Kroeker et al. 2013). Consequently, our understanding on the multi‐generational responses of marine metazoans to rapid changes in pCO2 is limited (Munday et al. 2013; Reusch 2014; Sunday et al. 2014). This lack of knowledge is restricting our ability to accurately predict whether, and how, marine biodiversity will cope with expected environmental pCO2 changes.
For most marine metazoan species, exposure to changing pCO2 conditions will occur over multiple generations. Future populations may, therefore, be able to maintain current‐day performance through the process of evolutionary adaptation. Evolutionary adaptation occurs when selection on existing genetic variation shifts the average phenotype of a population towards the fitness peak that matches its present environment (Sunday et al. 2014). A handful of studies have investigated marine metazoan populations naturally exposed to elevated pCO2 in situ, and show that evolutionary adaptation to OA conditions is possible (Maas et al. 2012; Calosi et al. 2013; Lewis et al. 2013; Pespeni et al. 2013). The rate at which such adaptation occurred, however, is unknown. During rapid climate change, evolutionary adaptation will most likely depend on the extent of existing phenotypic/genotypic variation within populations (Lande and Shannon 1996). Several breeding experiments, focussing on early life stages, have shown that sufficient phenotypic/genotypic variation exists within natural populations of marine metazoans, potentially enabling rapid evolutionary adaptation to changes in pCO2 conditions (e.g. Sunday et al. 2011; Foo et al. 2012; Kelly et al. 2013; Malvezzi et al. 2015). Many marine species, however, have complex life histories, and each life stage can respond to different selection pressures (Miller et al. 2013; Cripps et al. 2014). Therefore, focussing solely on early life stages may over‐ or under‐estimate the potential for any adaptive response to a rapid change in pCO2 (Sunday et al. 2014).
Nongenetic inheritance mechanisms may also influence the potential for evolutionary adaptation to rapid climatic changes (Chevin et al. 2010; Bonduriansky et al. 2012; Klironomos et al. 2012; Gomez‐Mestre and Jovani 2013). Trans‐generational plasticity (TGP) is the process whereby the environment experienced by parents significantly alters the reactions norms (i.e. phenotypes), and thus fitness, of their offspring (Mousseau and Fox 1998). TGP has the potential to be adaptive, but may also have deleterious effects (Marshall and Uller 2007). Either way, TGP can be an important source of variation in performances between individuals, ultimately influencing short‐term selection and the evolutionary trajectories of populations (Mousseau and Fox 1998; Badyaev and Uller 2009; Bonduriansky et al. 2012). TGP has largely been shown to increase offspring fitness of marine metazoans in response to changes in pCO2. For example, Miller et al. (2012) showed that preconditioning adult anemone‐fish, Amphiprion melanopus, to elevated pCO2 mediated the negative impacts on juvenile growth, survival and metabolic rates (see also Allan et al. 2014; Murray et al. 2014; Pedersen et al. 2014; Parker et al. 2015). However, the extent to which TGP influences the next generation can depend on whether the parental population has experienced within‐generation acclimation; the process by which an individual alters its physiological, behavioural or morphological characteristics through phenotypic plasticity to better suit an environment (Munday et al. 2013). Recently, Dupont et al. (2013) showed that the pre‐exposure of adult sea urchins to elevated pCO2 for 4 months had a negative effect on larval survival. In contrast, after 16 months of pre‐exposure, during which adults had acclimated, the negative effect on larval survival was no longer detected (see also Donelson et al. 2012; Suckling et al. 2014). Furthermore, the majority of trans‐generational experiments have been restricted across one, or maximum two, generations leaving it unclear whether or not adaptive TGP can extend into future generations allowing time for evolutionary adaptation to catch up (Chevin et al. 2010), or even facilitate the process (Pigliucci et al. 2006; Crispo 2007; Bonduriansky et al. 2012; Gomez‐Mestre and Jovani 2013).
Breeding and trans‐generational studies to‐date have provided valuable insights into the evolutionary potential of marine metazoans to rapid changes in pCO2 conditions. However, there is still an urgent need for longer term experiments that encompass all life‐history stages, across multiple generations, to more accurately predict how populations of marine metazoans might respond to the rapid changes in pCO2 expected to occur (Munday et al. 2013; Sunday et al. 2014). Here, we firstly report the results of a multi‐generational exposure experiment in which we reared a laboratory strain of a marine polychaete, Ophryotrocha labronica La Greca and Bacci 1962; for six generations under elevated (1000 μatm) and low (400 μatm) pCO2 conditions. The O. labronica strain used was found to perform better under elevated pCO2 conditions (generation F1). Secondly, to disentangle whether our observed multi‐generational responses were driven by phenotypic plasticity or by evolutionary adaptation (Calosi et al. 2013; Sunday et al. 2014; Thor and Dupont 2015) we report the results of a reciprocal transplant assay experiment between pCO2 treatments with F7 individuals. Throughout the experiment we measured a range of fitness‐related life‐history traits, as well as metabolic rates as a proxy for physiological performance. Ophryotrocha labronica is a globally distributed benthic polychaete typically found in heterogeneous, organically enriched environments (e.g. fouling communities in harbours, Prevedelli et al. 2005; Simonini et al. 2009). Additionally, it is an excellent species for multi‐generational studies as it can be easily cultured under laboratory conditions and has a short generation time (~17 days at 27°C; Åkesson 1976). It was hypothesized that the multi‐generational exposure to low pCO2 conditions would enable worms to initially increase, and then maintain, performance levels similar to those observed under elevated pCO2 conditions. Although we were specifically investigating the response of an elevated pCO2 tolerant population to low pCO2 levels, something that is not an immediate conservation issue as compared with OA studies, the results from this study will give valuable insight into how marine metazoans may respond to rapid pCO2 changes in general.
Materials and methods
Animal collection and husbandry
The laboratory strain of O. labronica used originated from >40 individuals collected in June 2008 in the harbour of Porto Empedocle (Sicily, Italy; 37°17′4″N, 13°31′3″E). The laboratory strain was housed at the Marine Biology and Ecology laboratory of the University of Modena and Reggio Emilia (Modena, Italy) in culture for approximately 30 generations at relatively constant salinity (mean ± SD: 35 ± 2) (obtained by dissolving an artificial sea salt – Reef Crystals, Instant Ocean – in distilled water) and photoperiod (L:D of 12:12 h), but at variable temperature (min/max = 12/30°C) to mimic natural seasonal variation (Massamba‐N'Siala et al. 2012). In Nov 2012, 120 individuals from a population of approx. 1200 were transported to the Marine Biology and Ecology Research Centre (MBERC) of Plymouth University (Plymouth, UK) and kept for a further three generations at conditions as close as possible to those experienced by the worms just before collection in the laboratory of Modena (T = 20 ± 1°C, S = 34 ± 1 (0.22 μm filtered natural sea water), L:D of 12:12 h).
Pre‐experimental phase: pH regimes of the worm cultures
Initially, we did not have details of the pH regime experienced by the worms in culture for the ~33 generations prior to the start of our experiment. However, our discovery that the strain of O. labronica had greater fitness under elevated pCO2 conditions in generation F1 prompted us to monitor the pH of the culture. Daily measurements for 3 months at MBERC revealed that the worms in culture experienced on average low, but highly variable, pH conditions (Fig. 1; 7.71 ± 0.20) most likely resulting from the feeding and maintenance protocol employed. Briefly, worm cultures were water changed every 7–10 days (the high peaks) and fed on the same day by adding 0.75–1 mL of spinach minced in sea water (300 g L−1). The fermentation of spinach during the periods between water changes caused recurrent and persistent reductions in pH (Fig. 1). Although pH data were not collected from the cultures of O. labronica at the University of Modena and Reggio Emilia, as the feeding and maintenance protocol of these cultures were identical in the two laboratories we are confident that they too experienced a similar pH regime for the 30 generations in which they were in culture there. In fact, pH measurements of the cultures subsequently made at the University of Modena and Reggio Emilia revealed that pH levels varied depending on how frequently water changes were performed, and ranged from 8.2 to 7.4 (G. Massamba‐N'Siala, personal communication).
Figure 1.
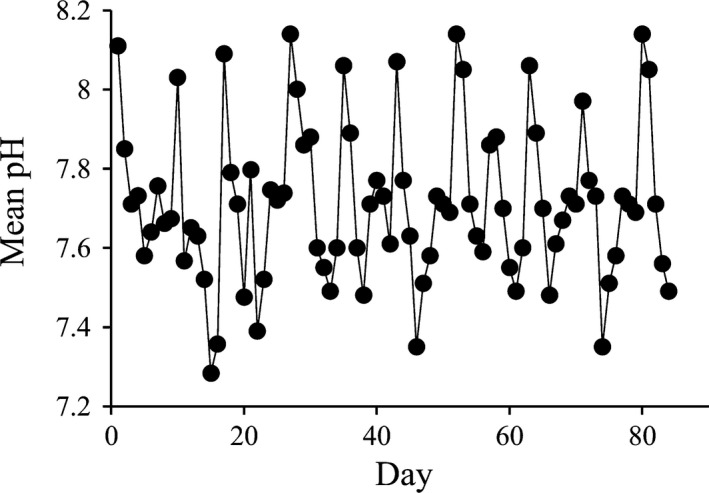
Mean pH of the Ophryotrocha labronica worm laboratory cultures measured daily for a 3‐month period.
Experimental setup and physico‐chemical parameters
The experimental CO2 and temperature manipulation system was a modified version of that described in Pistevos et al. (2011). Briefly, the system comprised of two trays (60 cm × 30 cm × 15 cm, vol. 13 L), half‐filled with de‐ionized water. Each tray housed eight air‐tight experimental chambers (four per pCO2 treatment). Three chambers per pCO2 treatment contained a six‐well culture plate (Corning Ltd, Sunderland, UK), filled with filtered natural sea water (0.22 μm, S = 33), which contained the worms (three broods of offspring and three adult pairs per chamber). The fourth chamber in each treatment contained a glass dish with filtered natural sea water (0.22 μm, S = 30) at the same temperature and pH of the wells, which was used for daily partial water changes of the culturing wells. Culture plates were covered with a breathable sealing film (Aeraseal; Alpha Laboratories Ltd, Eastleigh, UK), which allowed gas exchange whilst reducing evaporation, and thus avoiding large salinity and temperature fluctuations.
Elevated pCO2 conditions were achieved by mixing ambient air, supplied by an aquarium air pump (Mistral 4000; Aqua Medic, Loveland, CO, USA), with CO2 gas using adjustable airline gang vales (Algarde; Armitage Pet Care, Nottingham, UK) to produce a nominal pCO2 concentration of 1000 μatm (year 2100 predicted levels ‐ IPCC, 2013). The CO2 content of the resultant gas mixture was measured using a CO2 analyser (LI‐840A; Li‐Cor, Lincoln, NE, USA), and then supplied to each chamber via an airline connected to a de‐capped micro centrifuge tube (Eppendorf) inserted through the top of the chamber. Low pCO2 conditions were achieved via supplying air with an aquarium air pump (Mistral 4000; Aqua Medic) to each experimental chamber, and represented present‐day levels. The experimental system was maintained at 27°C, as this temperature produced fast generation times (Åkesson 1976) whilst still being within the thermal range naturally experienced in the summer months (Massamba‐N'Siala et al. 2012). The temperature in the water in each tray was controlled by a re‐circulating water bath (R5; Grant Instruments Cambridge Ltd, Herts, UK). Additionally, each tray contained two circulation pumps (Koralia nano 900; Hydor, Sacramento, CA, USA) to ensure an even temperature distribution within the tray (max temperature fluctuation recorded during the experiment was 1°C).
Throughout the experiment, all wells were observed daily using a custom‐built bio‐imaging system for aquatic animals (Tills et al. 2013). Polychaetes were fed daily ad libitum on minced spinach (Massamba‐N'Siala et al. 2011, 2012). Uneaten spinach was removed with daily partial water changes to maintain good water quality. Additionally, temperature, salinity and pH were measured daily in one randomly chosen well per experimental chamber. Up to twice a week, seawater samples from each treatment were collected to determine total alkalinity. This was done by transferring the water from the glass dish in the fourth experimental chamber of each treatment to a borosilicate bottles (vol. = 150 mL), immediately poisoned with mercuric chloride solution (30 μm, conc. = 0.02%) and kept in the dark prior to analysis. Total alkalinity was measured using an automated acid‐base alkalinity titrating system (AS‐ALK2; Apollo SciTech Inc, Bogart, GA, USA). Carbonate system parameters not measured directly were calculated using CO2SYS (Pierrot et al. 2006) and the Mehrbach constants (Mehrbach et al. 1973) refitted by Dickson and Millero (1987). Seawater parameters are presented in Table 1.
Table 1.
Physico‐chemical parameters of seawater for the elevated and low p CO 2 treatments. Values are means ±1 SD for pH (NBS scale), salinity, temperature, total alkalinity (TA), carbon dioxide partial pressure (p CO 2), bicarbonate and carbonate ion concentration ([]) and ([]), and calcite and aragonite saturation states (Ωcal and Ωara)
| Parameter | Elevated | Low |
|---|---|---|
| pH | 7.68 ± 0.06A | 7.99 ± 0.06B |
| Salinity | 34.05 ± 1.04A | 33.76 ± 1.24A |
| Temperature (°C) | 27.11 ± 0.46A | 27.16 ± 0.53A |
| TA (μequiv kg−1) | 2178.77 ± 178.12A | 2201.66 ± 70.05A |
| pCO2 (μatm) | 1137.28 ± 193.05A | 461.91 ± 36.21B |
| [] (μmol kg−1) | 1955.76 ± 167.23A | 1759.04 ± 58.48B |
| [] (μmol kg−1) | 92.58 ± 10.76A | 182.47 ± 14.52B |
| Ω cal | 2.23 ± 0.26A | 4.39 ± 0.35B |
| Ω ara | 1.47 ± 0.17A | 2.89 ± 0.23B |
Superscript capital letters indicate a significant difference between treatments by way of GLM's (P < 0.05).
Experimental design
Experiment one: multi‐generational exposure
To obtain a large enough population of juveniles to conduct the multi‐generational experiment (Fig. 2), 16 breeding pairs were formed from individuals of the MBERC laboratory culture, acclimated (1°C h−1) to 27°C. Seventy‐two hours after hatching, 20 juveniles from each pair were haphazardly assigned to either the elevated or low pCO2 treatment. Juveniles were not moved on the day of hatching as preliminary observation showed that handling of juvenile in the first 72 h from hatching could result in high mortality levels independent of pCO2 concentrations. Juvenile growth rates and survival were determined 7 days posthatching (see below). When the first reproductive event was observed new pairs were formed by crossing individuals from different parents to avoid inbreeding, and placed into a new well within the same multi‐well plate. Spare worms were kept in their well until offspring from the pairs made were ready to be transferred to a new well and then removed. The first egg mass spawned by a pair was used to produce offspring for the next generation. The second egg mass was used to determine fecundity and egg volume. If a male died before the second egg mass was produced, it was replaced so that the reproductive performance of females could still be determined (as in Massamba‐N'Siala et al. 2011, 2012). For this purpose, spare males of the current generation were kept in the Boveri glass bowl in the fourth experimental chamber of the corresponding treatment. Adult size was determined the day that a female produced its second egg mass. The day after a pair had produced their second egg mass, metabolic rates of the female were measured and the male was removed. The above procedure was repeated up until the stage where generation F6 reached maturity. Adult life‐history traits, as well as metabolic rates, were only measured in females because their contribution to life‐history depiction is more relevant than that of males (Stearns 1992; Massamba‐N'Siala et al. 2011).
Figure 2.
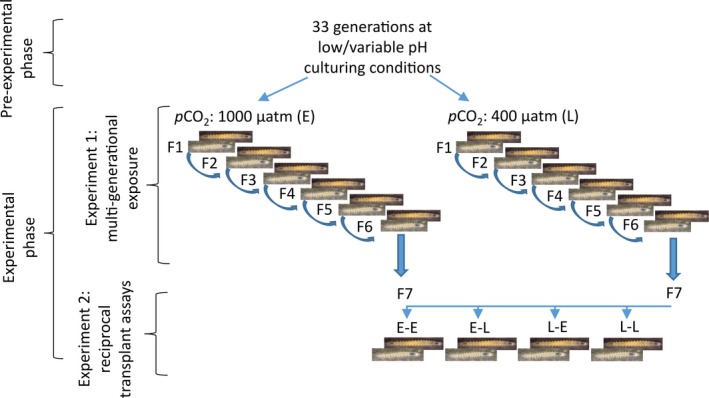
Diagram of the experimental design. E: elevated p CO 2; L: low p CO 2.
Experiment two: reciprocal transplant assays
Once F6 juveniles reached maturity, five females per brood were moved into a separate well until they reached a sufficiently large size to ensure enough offspring could be produced in their first brood to perform reciprocal transplants between pCO2 treatments. Consequently, data were not collected for generation F6 adults. Females were then paired up with males from different parents as in all previous generations. From each pair, 20 juveniles were moved to a different well in the same pCO2 treatment as their parents (control assay) and 20 juveniles were moved to a well in the other pCO2 treatment (reciprocal assay) 72 h posthatching (Fig. 2). The experimental procedure followed was identical to that described for generations F2–F5, except that the first three egg masses spawned by a pair were used to determine fecundity, and that metabolic rates of females were measured the day after they spawned their third egg mass and were only carried out haphazardly on half of the females.
Determination of life‐history traits
Juvenile growth rates were determined by counting the number of chaetigers (i.e. segments bearing bristles) of five randomly selected individuals per brood using the bio‐imaging system. Juvenile growth rates were standardized to the number of chaetigers added per day (number of chaetigers day−1).
Juvenile survival per well was measured by counting the number of individuals in a well under low power magnification (×10) (MZ12; Leica, Solms, Germany), and was expressed as the percentage of the total number of individuals at time zero.
Female fecundity was measured as the number of eggs produced per chaetiger (number of eggs chaetiger−1) to account for any effect due to differences in body size (Massamba‐N'Siala et al. 2011, 2012). Counts were made under low‐medium magnification (×50) (MZ12; Leica).
Adult size was determined using the bio‐imaging system by counting the number of chaetigers a female had on the day it produced its second egg mass.
Egg volume, a proxy for egg quality (Allen and Marshall 2014), was determined by taking a picture of the second egg mass using the bio‐imaging system. The longest and shortest axes of 10 eggs were then measured using imageJ (http://rsb.info.nih.gov/ij/) and egg volume (expressed as × 10−3 mm3) calculated using the formula:
where A is the short radius and B the long radius (Simonini and Prevedelli 2003).
Determination of metabolic rates
Oxygen uptake was measured as a proxy for metabolic rate (MO2) using closed system respirometry. The partial pressure of oxygen (pO2) in sea water within the respirometry chambers was determined using a modified version of the method described by Calosi et al. (2013) for small‐size worms. Briefly, polychaetes were placed individually into a respirometry chamber (vol. = 0.12 mL), filled with filtered sea water (0.22 μm, S = 33–34) of the original pCO2 treatment. Once sealed, the chambers were placed inside a water bath to maintain constant temperature (T = 27 ± 0.5°C). All individuals were allowed to settle for 30 min before measurements began. The decline in pO2 within each respirometer was determined using an optical oxygen analyser system (GEN III 5000 series; OxySense, Dallas, TX, USA). Measurements of pO2 were made at regular intervals (20–30 min) for every respirometer for a period of approx. 1.5 h, and the pO2 in the respirometer never fell below 70% O2 saturation to avoid polychaetes experiencing hypoxia. MO2 was calculated as the change in pO2 h−1 from the linear least‐squares regression of pO2 (mbar) plotted against time (min). This was multiplied by the solubility coefficient for oxygen, adjusted for salinity and temperature (Green and Carrit 1967), and the volume of water within each respirometer. Blanks were also run to correct for any microbial respiration in the sea water. MO2 values were expressed as μmol O2 h−1 STPD.
Statistical analysis
The effects of ‘pCO2 treatment’, ‘generation’ and their interaction (experiment one) on all traits was tested using general linear models (GLM's), with ‘tray’ as a random factor nested within ‘pCO2 treatment’, ‘tub’ as a random factor nested within ‘pCO2 treatment’ × ‘tray’ and ‘well’ as a random factor nested within ‘pCO2 treatment’ × ‘tray’ × ‘tub’ (juvenile growth rates and egg volume only). Additionally, body size was used as a covariate for MO2 data. Random, covariate factors and interactions that had no significant effect (P > 0.05) were systematically removed one at a time (highest P value first) from the analysis, until only significant factors and the main effects were left. ‘Well’ had a significant effect on juvenile growth rates (F 34, 1097 = 2.71, P < 0.001). ‘Tub’ had a significant effect on juvenile survival, adult size, metabolic rates and egg volume (min F 10,170 = 2.09, P = 0.028). However, removing these factors from the analysis did not change the patterns of significance of the main factors, and thus effects were considered marginal. Data from the control transplants of the reciprocal transplant assay experiment in generation F7 were included in the analyses. The effect of reciprocal transplant assays (experiment two) on all traits was analysed using the same design, but with ‘exposure treatment’ and ‘assay treatment’ set as fixed factors.
Except for fecundity and metabolic rate data (max. KS36 = 0.106, P = 0.200, Kolmogorov–Smirnov's test), data did not meet the assumptions of normality despite Log10 transformations (min. KS229 = 0.144, P < 0.001). Variances were homogenous for adult size in experiment one and for all data in experiment two (max. F 3,67 = 2.36, P = 0.079, Levene's test), except egg volume (F 3,706 = 3.42, P = 0.017). For all other datasets assumptions were not met following Log10 transformations (min. F 11,2077 = 2.45, P = 0.005). However, given the size of our experiment and replication, we assumed the GLM design employed should be tolerant to deviation from the assumptions of normality and heteroscedasticity (Sokal and Rohlf 1995; Underwood 1997). Nonetheless, we also tested the residuals from each analysis against the factors tested with GLM's and no significant relationships were detected (P > 0.05). All pair‐wise comparisons were conducted using 95% confidence interval levels (95% CI). All analyses were conducted using SPSS 21 (IBM, Armonk, NY, USA).
Results
Experiment one: multi‐generational exposure
All data produced from the multi‐generational exposure experiment are presented in Table S1. The only trait significantly affected by multi‐generational exposure to low pCO2 was fecundity (Fig. 3A; F 5,197 = 3.53, P = 0.004). In generation F1, mean fecundity in the elevated pCO2 treatment (6.70 eggs chaetiger−1) was significantly greater than in the low pCO2 treatment (3.55 eggs chaetiger−1; P < 0.05). Similarly, in generation F2 fecundity was significantly greater in the elevated pCO2 treatment (8.12 eggs chaetiger−1) compared to the low pCO2 treatment (6.82 eggs chaetiger−1; P < 0.05). However, this difference did not re‐appear in subsequent generations (Fig. 3A; P > 0.05).
Figure 3.
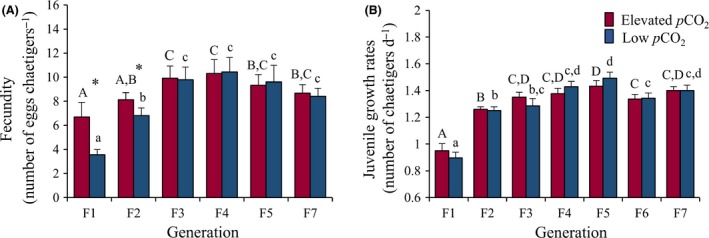
Experiment 1: effect of the multi‐generational exposure to elevated and low p CO 2 on mean fecundity (A) and juvenile growth rates (B) of O. labronica. Asterisk (*) indicates significant differences (P < 0.05) between p CO 2 treatments within a generation. Capital and lower case letters represent significant differences (P < 0.05) between generations for the elevated and low p CO 2 treatments, respectively. Bar charts represent mean values ±95% CI. Numbers of replicates are reported in Table S1.
For all traits, except egg volume (F 5,2078 = 0.25, P = 0.942), a significant generation effect was observed (Table 2; min. F 5,180 = 2.67, P = 0.023). Mean juvenile growth rates, fecundity and adult size all significantly increased across generations. For all traits, mean values in generation F1 were significantly lower compared to generation F7 (P < 0.05). However, despite a significant generation effect in juvenile survival and metabolic rate data, no clear trends were present; for both these traits, generation F1 values being statistically similar to generation F7 (P > 0.05). For an explanation on observed significant generation effects please refer to Supporting information.
Table 2.
Mean generation values ±95% CI for all traits measured for the marine polychaete Ophryotrocha labronica
| Trait | Generation | ||||||
|---|---|---|---|---|---|---|---|
| F1 | F2 | F3 | F4 | F5 | F6 | F7 | |
| Juvenile growth rates (number of chaetigers day−1) | 0.92 ± 0.04A (80) | 1.25 ± 0.02B (180) | 1.31 ± 0.03C (175) | 1.41 ± 0.03E (180) | 1.46 ± 0.03E (170) | 1.34 ± 0.03C,D (180) | 1.40 ± 0.03D,E (180) |
| Juvenile survival (%) | 79.69 ± 10.10A,B (16) | 83.33 ± 3.78A,B (36) | 80.29 ± 4.96A (35) | 87.22 ± 4.29A,B (36) | 89.71 ± 3.51B (34) | 88.61 ± 2.80B (36) | 83.47 ± 2.46A,B (36) |
| Adult size (number of chaetigers) | 14.21 ± 0.34A (34) | 15.37 ± 0.30B (35) | 15.44 ± 0.28B (34) | 15.89 ± 0.21B (35) | 15.75 ± 0.33B (36) | 15.40 ± 0.22B (35) | |
| Fecundity (number of eggs chaetiger−1) | 5.13 ± 0.83A (34) | 7.45 ± 0.48B (35) | 9.85 ± 0.72C,D (34) | 10.38 ± 0.81D (35) | 9.46 ± 0.82C,D (36) | 8.55 ± 0.48C (35) | |
| Egg volume (×10−3 mm3) | 0.61 ± 0.01 (340) | 0.62 ± 0.01 (350) | 0.62 ± 0.01 (340) | 0.61 ± 0.01 (350) | 0.61 ± 0.01 (360) | 0.61 ± 0.01 (350) | |
| Metabolic rates (μmol O2 h−1 STPD) | 0.23 ± 0.02A (34) | 0.27 ± 0.03A,B (35) | 0.27 ± 0.02A,B (34) | 0.27 ± 0.02A,B (35) | 0.29 ± 0.02B (36) | 0.29 ± 0.04A,B (18) | |
Capital letters represent significant differences (P < 0.05) between generations. Numbers of replicates are provided in parentheses.
Finally, for mean fecundity and juvenile growth rates there was a significant difference in the rate of increase between pCO2 treatments across generations (Fig. 3A,B). Although maximum mean fecundity and juvenile growth rates values were statistically similar for both pCO2 treatments (P > 0.05), they were reached a generation faster in the elevated pCO2 treatment. In the elevated pCO2 treatment, maximum mean fecundity values and juvenile growth rates were reached in generation F2 and F3, respectively, whereas in the low pCO2 treatment, they were reached in generation F3 and F4 respectively. These differences in rate of increase are highlighted by significant pCO2 × generation interactions (min. F 6,1131 = 2.93, P = 0.008).
Experiment two: reciprocal transplant assays
All data are presented by pCO2 treatment in Table 3. As in experiment one, fecundity was the only trait that was significantly affected by reciprocal transplants between pCO2 treatments indicated by a significant exposure × assay interaction (Fig. 4; F 1,66 = 14.83, P < 0.001). Mean fecundity was 22.25 eggs chaetiger−1 in the elevated‐elevated pCO2 transplant and was significantly greater than the elevated‐low and low‐elevated pCO2 cross (P < 0.05), where mean fecundity was 19.54 and 18.67 eggs chaetiger−1, respectively. However, there was no significant difference in mean fecundity between the low‐low pCO2 transplant (21.44 eggs chaetiger−1) and low‐elevated pCO2 transplant (18.66 eggs chaetiger−1) (P > 0.05). There were also no significant differences in fecundity between respective control (i.e. elevated‐elevated versus low‐low) and reciprocal (elevated‐low versus low‐elevated) transplant assays (P > 0.05 in each case).
Table 3.
Mean values ± 95% CI for all traits measured in the marine polychaete O. labronica in the reciprocal transplant assay experiment
| Trait | Transplant assay | |||
|---|---|---|---|---|
| Elevated‐Elevated | Elevated‐Low | Low‐Low | Low‐Elevated | |
| Juvenile growth rates (number of chaetigers day−1) | 1.40 ± 0.03 (90) | 1.40 ± 0.04 (85) | 1.40 ± 0.04 (90) | 1.39 ± 0.04 (90) |
| Juvenile survival (%) | 82.22 ± 4.09 (18) | 78.23 ± 4.59 (17) | 84.72 ± 2.91 (18) | 79.44 ± 5.43 (18) |
| Adult size (number of chaetigers) | 15.41 ± 0.29 (17) | 15.12 ± 0.33 (17) | 15.39 ± 0.32 (18) | 14.94 ± 0.24 (18) |
| Fecundity (number of eggs chaetiger−1) | 22.25 ± 1.33A (17) | 19.54 ± 1.21B (17) | 21.44 ± 1.38A,B (18) | 18.67 ± 1.61B (18) |
| Egg volume (×10−3 mm3) | 0.62 ± 0.02 (170) | 0.59 ± 0.02 (170) | 0.60 ± 0.02 (180) | 0.60 ± 0.02 (180) |
| Metabolic rates (μmol O2 h−1 STPD) | 0.28 ± 0.05 (9) | 0.31 ± 0.03 (9) | 0.29 ± 0.06 (9) | 0.27 ± 0.03 (9) |
Capital letters represent significant differences (P < 0.05) between treatments. Numbers of replicates are provided in parentheses.
Figure 4.
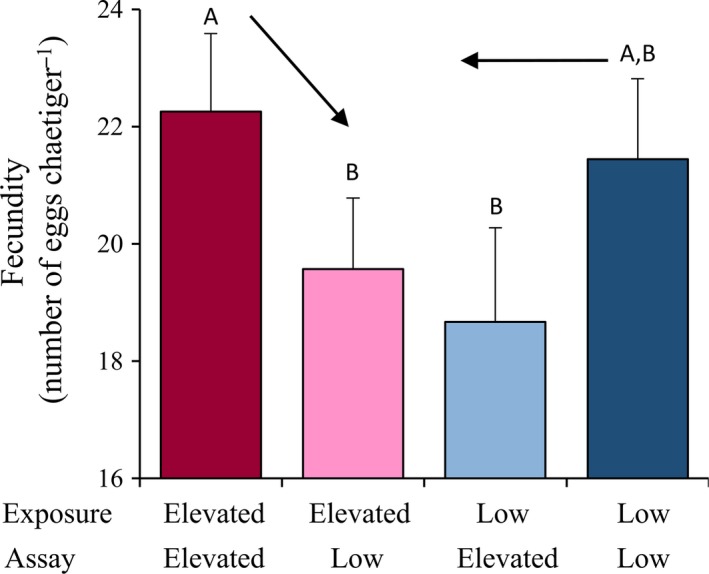
Experiment 2: reciprocal transplant assays. Mean fecundity of O. labronica individuals of the F7 generation exposed to either an elevated or low p CO 2 and then transplanted to same (control assay) or other p CO 2 treatments (reciprocal assay). Capital letters represent significant differences (P < 0.05) between transplant treatments. Bar charts represent mean values ±95% CI. Arrows represent reaction norms between control and reciprocal transplants. Numbers of replicates are reported in Table 3.
Discussion
The O. labronica strain used in our study had acquired tolerance to elevated pCO2 during the pre‐experimental phase, through multiple generations of culturing under low/variable pH conditions in the laboratory. Individuals exhibited greater fitness (i.e. fecundity) under elevated pCO2 (generations F1 and F2). However, we show that between generations F1 and F3 worms were able to increase their fitness under low pCO2 conditions to levels similar to those observed in the elevated pCO2 treatment, and that this was likely driven by TGP. From generation F3, worms were able to maintain their restored fitness levels for multiple generations (F3–F7). Hereafter, we discuss the consequences of long‐term culture under a low and variable pH regime, the initial TGP response and the consequences of multi‐generational exposure to low pCO2 conditions.
Consequences of laboratory culture under a low and variable pH regime
The worms used in this study exhibited significantly reduced fitness (i.e. fecundity) when initially exposed to low pCO2 conditions (generations F1 and F2). However, we cannot fully discount that our observed tolerance to elevated pCO2 conditions was driven by pre‐experimental conditions in the laboratory, other than pH, as we did not have access to worms that had been maintained in culture under stable high pH conditions. Nonetheless, long‐term laboratory culture resulted in worms that performed better under elevated pCO2 conditions. Additionally, we found that adult size and metabolic rates were unaffected by exposure to low pCO2. Whilst this shows that individuals were able to acclimate morphologically and physiologically to low pCO2 conditions, it suggests a change in energy allocation (Stumpp et al. 2011; Fitzer et al. 2012; De Wit et al. 2015; Thor and Dupont 2015) away from reproduction to maintain growth and cellular homeostasis. This shift in energy allocation is consistent with life‐history theory for invertebrates where growth continues after maturation, such as O. labronica, as investment into growth increases future reproductive performance; fecundity being related to female size (Reznick 1983; Berglund 1991). Ophryotrocha labronica females can allocate up to 84% of available energy into reproduction under favourable conditions (Cassai and Prevedelli 1999). The reduced reproductive output under low pCO2 conditions is therefore likely due to a portion of this energy being used to support an increased energetic cost of living in a stressful environment, possibly related to disruptions in acid‐base regulation and ion transport (Pörtner et al. 2004; Pörtner 2008). Indeed, physiological alterations caused by acclimation (i.e. phenotypic plasticity) are often associated with fitness costs/life‐history trade‐offs (Hoffmann 1995; Angilletta et al. 2003).
The initial TGP response
Although fecundity was reduced in individuals exposed to low pCO2 conditions in both generations F1 and F2, the magnitude of this difference was reduced across generations (F1 = 47.0% vs. F2 = 16.0%). Furthermore, from generation F3 onwards there was no longer a significant difference in fecundity between treatments, suggesting that routine energy allocation had been restored. In contrast, the negative effects of elevated pCO2 on larval development and naupliar production in the copepod Tisbe battagliai remained after multi‐generational exposure (Fitzer et al. 2012). It is possible that evolutionary adaptation could have occurred after only two generations (e.g. Christie et al. 2012), however, we argue that TGP was likely the underlying mechanism for our observed restoration in fecundity. In support of this suggestion, we observed no significant differences in juvenile survival between the two pCO2 treatments, from which we inferred that no evolutionary adaptation, via natural selection, had occurred.
Grandparental, in addition to parental, environment may have influenced the performance of individuals from generation F3. This was evidenced by the full restoration of fecundity requiring two generations of exposure to low pCO2. The influence TGP may have on the next generation can depend on whether the parental population has experienced within‐generation acclimation (Donelson et al. 2012; Dupont et al. 2013; Suckling et al. 2014), as previously discussed. Within‐generation acclimation can refer to both nonpermanent responses (i.e. reversible acclimation), that occur when an organism is exposed to short‐term environmental fluctuations, and permanent, irreversible responses that are mainly established during early ontogeny (i.e. developmental acclimation) (West‐Eberhard 2003; Angilletta 2009). Ophryotrocha labronica undergoes direct development. In generation F1, offspring were initially moved to low pCO2 conditions 3 days posthatching. As a consequence, juvenile worms may have experienced limited developmental acclimation. Therefore, it is possible that the acclimation to low pCO2 conditions we observed in generation F1 was reversible, and consequently not sufficient for TGP to be fully expressed across one generation. Phenotypic plasticity may take at least two generations to be fully expressed, as seen in this study, due to the cumulative effects of different forms of acclimation (e.g. developmental and trans‐generational) (Munday et al. 2013).
Potential mechanism for the observed TGP
Trans‐generational plasticity can occur through a variety of mechanisms, including the transfer of nutritional (e.g. maternal provisioning) and molecular (e.g. epigenetic) material (Bonduriansky et al. 2012). In this study, there was no difference in egg volume (a proxy for egg quality) between pCO2 treatments in any generation, indicating no apparent differences in maternal provisioning (see also Miller et al. 2012; Shama et al. 2014). Trans‐generational epigenetic effects (Jablonka and Raz 2009; Ho and Burggren 2010) may therefore have been the causative mechanism for restoring and maintaining routine energy allocation, and subsequently fecundity, under low pCO2 conditions. Trans‐generational epigenetic effects were also thought to be responsible for mediating the negative impacts that elevated pCO2 had on juvenile anemonefish, A. melanopus (Miller et al. 2012). The extent to which epigenetic effects are induced, is suggested to be dependent upon which life‐stage experiences the stressor, being greater in earlier life stages (Burton and Metcalfe 2014). Consequently, the fact that offspring were initially exposed to low pCO2 in generation F1, 3 days posthatching, may have meant that epigenetic effects were not fully induced. This again could potentially explain why it took two generations for our observed TGP to be fully expressed. Irrespective of the mechanism involved, our study adds to the growing body of evidence showing that TGP can be an effective mechanism in buffering populations of marine metazoans against the negative effects of changes in pCO2 and other climate change stressors (e.g. Jensen et al. 2014; Munday 2014; Pedersen et al. 2014; Parker et al. 2015).
Consequences of multi‐generational exposure to low pCO2 conditions
Reciprocal transplant assay experiment: TGP or evolutionary adaptation?
A reciprocal transplant assay experiment was performed in order to determine whether TGP had persisted across several generations, or if evolutionary adaptation had occurred (Calosi et al. 2013; Sunday et al. 2014; Thor and Dupont 2015). Fecundity levels were significantly lower in individuals transplanted from low to elevated pCO2 (low‐elevated) compared to those individuals from the elevated pCO2 control line (elevated‐elevated). This change in reaction norm of the experimental lines lends support to the idea that evolutionary adaptation to low pCO2 may have occurred after six generations. Indeed, it is now widely recognized that evolutionary adaptation can occur over very short ecological timescales (Reznick and Ghalambor 2001; Stockwell et al. 2003). However, the low CO2 lines showed no significant changes in fecundity when exposed to elevated CO2 conditions (Fig. 4), suggesting that what we have observed could be irreversible effects of TGP persisting across generations. In fact, there is growing evidence to show that trans‐generational epigenetic effects can span across multiple (10+) generations (Jablonka and Raz 2009; Ho and Burggren 2010). In conclusion, the ambiguous nature of the results from our reciprocal transplant assay experiment prevents us from us pinpointing the underlying mechanism (i.e. phenotypic plasticity or evolutionary adaptation) which enabled worms to maintain fitness under low pCO2 conditions through to generation F7. Irrespective of the mechanism responsible, worms were able to maintain fitness levels across several generations.
TGP as a mechanism for facilitating evolutionary adaptation
If evolutionary adaptation to low pCO2 conditions had occurred, it was likely driven by TGP instead of natural selection. We base this conclusion on the lack of significant differences in juvenile survival between pCO2 treatments in any generation. Furthermore, all pairs produced viable offspring with no evidence of unfertilized eggs or delayed development. Together, these observations demonstrate that the strength of the selection environment was low. We predict that for species which are more resistant to rapid changes in pCO2, evolutionary adaptation may be driven primarily by TGP as opposed to natural selection.
Overall, O. labronica had a high capacity to exhibit phenotypic plasticity, both within and across generations. This is not surprising, as O. labronica typically occurs in highly heterogeneous environments, where phenotypic plasticity is generally evolutionary favoured (Ghalambor et al. 2007). Thus, it is reasonable to assume that the high levels of plasticity we observed in response to changes in pCO2 had evolved as a result of culturing worms under a highly variable pH regime for ~33 generations. In addition, many coastal environments experience substantial fluctuations in pH on a daily or seasonal basis that can be as large, or even greater, than the decrease in pH projected to occur over the next 50–100 years (Hofmann et al. 2011; Shaw et al. 2012; Melzner et al. 2013). Consequently, many coastal organisms may possess a high capacity to exhibit TGP. Indeed, a recent study showed that natural populations of the Atlantic silverside, Menidia menidia, were able to condition their offspring to seasonally acidifying environments (Murray et al. 2014). Such findings are important as TGP could buffer populations against the negative impacts of rapid changes in pCO2 allowing time for evolutionary adaptation to catch up (Chevin et al. 2010), or even facilitate evolutionary adaptation through processes such as the Baldwin effect and/or genetic assimilation (i.e. genetic accommodation) (Pigliucci et al. 2006; Crispo 2007).
No apparent costs associated with the multi‐generational responses
Plastic and evolutionary responses are often associated with costs and trade‐offs to life‐history traits and fitness (Hoffmann 1995; Angilletta et al. 2003). However, whilst we observed costs of within‐generation plasticity (i.e. reduced fecundity in generation F1 and F2), we detected no apparent costs for our observed multi‐generational responses. Although it is still possible that costs exist, potentially affecting traits such as maximum size, longevity and total life‐span reproductive output, all of which we were unable to determine with our experimental design. There is urgent need to identify the potential costs of TGP and evolutionary adaptation in response to changes in pCO2 and other climate change stressors (i.e. warming, hypoxia, combined stressors) if we are to more accurately predict whether current populations levels of marine metazoans will be able to persist under rapidly changing conditions. For example, if marine metazoans are able to persist at the cost of reduced body size (Gardner et al. 2011; Sheridan and Bickford 2011; Calosi et al. 2013; Garilli et al. 2015); body size‐dependent traits and processes (e.g. fecundity, competitive and predator‐prey interactions ‐ Peters 1983; Arendt 2007) and subsequently ecosystem functions may still be negatively impacted (Solan et al. 2004; Sheridan and Bickford 2011). Where feasible, future trans‐generational and multi‐generational studies should therefore characterize performance over entire life spans to better identify potential costs.
Summary
Plastic and evolutionary responses are increasingly recognized as primary rescue mechanisms that could prevent species’ extinctions in the face of rapid climate change (Hoffmann and Sgrò 2011; Godbold and Calosi 2013; Gonzalez et al. 2013; Munday et al. 2013; Salinas et al. 2013; Sunday et al. 2014). Here, we show that a laboratory strain of O. labronica was able to rapidly (within two generations) restore its fitness levels, via TGP, and maintain these levels for a further four generations, after experiencing a change in pCO2. Regardless of whether TGP or evolutionary adaptation was the mechanism responsible for the results from our reciprocal transplant assay experiment, we provide evidence to suggest that marine metazoans may be capable of coping with drastic changes in pH conditions over multiple generations. Our study supports the idea that multi‐generational experiments are required to accurately predict how marine organisms will respond to climate change associated stressors (including pCO2) predicted to occur over the coming centuries (Munday et al. 2013; Sunday et al. 2014). Investigations that span across multiple generations will be valuable for the planning of socio‐economic and environmental buffering for the duration of the ‘detrimental’ phase (i.e. whilst fitness is reduced), while planning intervention actions that may preserve and/or speed up the ‘recovery’ phase. Finally, laboratory multi‐generational experiments could be used as a valuable conservation tool to select for strains tolerant to specific pCO2 conditions, with the scope to prevent the extinctions of keystone species in the face of rapid changes in pCO2 via the process of assisted evolution (Van Oppen et al. 2015).
Data archiving statement
Life‐history, metabolic and water chemistry data have been deposited in the British Oceanographic Data Centre (http://www.bodc.ac.uk, doi:10.5285/22b54764‐2448‐1318‐e053‐6c86abc01ae1).
Supporting information
Table S1. Mean values ±95 % CI for all traits measured for the marine polychaete Ophryotrocha labronica at elevated (grey rows) and low (white rows) p CO 2 conditions across generations.
Appendix S1. Acclimation/selection to experimental conditions.
Acknowledgements
This work was supported by a NERC‐Defra‐DEC UKOA Research Programme grant NE/H017127/1 to J.I.S. and P.C. A.R.R. was supported by a Junta de Andalucía (Spanish Regional Government) grant RNM‐3924. P.C. is supported by a NSERC Discovery Program grant and G.M.N. is supported by a EU Marie Curie‐Skłodowska Research Fellowship (EVOLMARIN‐GA 659359). We thank Marie Hawkins from MBERC for her help during setting up the system and experiment, and Daniela Prevedelli and Roberto Simonini from Università di Modena e Reggio Emilia for supplying individuals of O. labronica. We thank Oliver Tills for allowing us use of his bio‐imaging system. Finally, we thank Leela Chakravarti and the anonymous reviewers for their valuable comments which ultimately improved the quality of our paper.
Contributor Information
Araceli Rodríguez‐Romero, Email: araceli.rodriguezromero@icman.csic.es.
Michael D. Jarrold, Email: mdjarrold@hotmail.com.
Piero Calosi, Email: Piero_Calosi@uqar.ca.
Literature cited
- Åkesson, B. 1976. Temperature and life cycle in Ophryotrocha labronica (Polychaeta, Dorvilleidae). Ophelia 15:37–47. [Google Scholar]
- Allan, B. J. M. , Miller G. M., McCormick M. I., Domenici P., and Munday P. L.. 2014. Parental effects improve escape performance of juvenile reef fish in a high‐CO2 world. Proceedings of the Royal Society of London Series B: Biological Sciences 281:20132179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen, R. M. , and Marshall D.. 2014. Egg size effects across multiple life‐history stages in the marine annelid Hydroides diramphus . PLoS One 9:e102253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angilletta, M. J. Jr . 2009. Thermal Adaptation: A Theoretical and Empirical Synthesis. Oxford University Press, New York. [Google Scholar]
- Angilletta Jr, M. J. , Wilson R. S., Navas C. A., and James R. S.. 2003. Tradeoffs and the evolution of thermal reaction norms. Trends in Ecology and Evolution 18:234–240. [Google Scholar]
- Arendt, J. . 2007. Ecological correlates of body size in relation to cell size and cell number: patterns in flies, fish, fruits and foliage. Biological Reviews 82:241–256. [DOI] [PubMed] [Google Scholar]
- Badyaev, A. V. , and Uller T.. 2009. Parental effects in ecology and evolution: mechanisms, processes and implications. Philosophical Transactions of the Royal Society B: Biological Sciences 364:1169–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berglund, A. 1991. To change or not to change sex: a comparison between two Ophryotrocha species (Polychaeta). Evolutionary Ecology 5:128–135. [Google Scholar]
- Bickle, M. J. 2009. Geological carbon storage. Nature Geoscience 2:815–818. [Google Scholar]
- Bonduriansky, R. , Crean A. J., and Troy D.. 2012. The implications of nongenetic inheritance for evolution in changing environments. Evolutionary Applications 5:192–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton, T. , and Metcalfe N. B.. 2014. Can environmental conditions experienced in early life influence future generations? Proceedings of the Royal Society of London Series B: Biological Sciences 281:20140311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calosi, P. , Rastrick S. P. S., Lombardi C., de Guzman H. J., Davidson L., Jahnke M., Giangrande A. et al. 2013. Metabolic adaptation and acclimatisation to ocean acidification in marine ectotherms: an in situ transplant experiment at a shallow CO2 vent system. Philosophical Transactions of the Royal Society B: Biological Sciences 368:20120444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassai, C. , and Prevedelli D.. 1999. Fecundity and reproductive effort in Ophryotrocha labronica (Polychaeta: Dorvilleidae). Marine Biology 133:489–494. [Google Scholar]
- Chevin, L.‐M. , Lande R., and Mace G. M.. 2010. Adaptation, plasticity, and extinction in a changing environment: towards a predictive theory. PLoS Biology 8:e1000357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie, M. R. , Marine M. L., French R. A., and Blouin M. S.. 2012. Genetic adaptation to captivity can occur in a single generation. Proceedings of the National Academy of Sciences of the United States of America 109:238–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cripps, G. , Lindeque P., and Flynn K. J.. 2014. Have we been underestimating the effects of ocean acidification in zooplankton? Global Change Biology 20:3377–3385. [DOI] [PubMed] [Google Scholar]
- Crispo, E. . 2007. The Baldwin effect and genetic assimilation: revisiting two mechanisms of evolutionary change mediated by phenotypic plasticity. Evolution 61:2469–2479. [DOI] [PubMed] [Google Scholar]
- De Wit, P. , Dupont S., and Thor P.. 2015. Selection on oxidative phosphorylation and ribosomal structure as a multigenerational response to ocean acidification in the common copepod Pseudocalanus acuspes . Evolutionary Applications 9:1112–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson, A. G. , and Millero F. J. A.. 1987. Comparison of the equilibrium constants for the dissociation of carbonic‐acid in seawater media. Deep Sea Research Part A. Oceanographic Research Papers 34:1733–1743. [Google Scholar]
- Donelson, J. M. , Munday P. L., McCormick M. I., and Pitcher C. R.. 2012. Rapid transgenerational acclimation of a tropical reef fish to climate change. Nature Climate Change 2:30–32. [Google Scholar]
- Doney, S. C. , Fabry V. J., Feely R. A., and Kleypas J. A.. 2009. Ocean acidification: the other CO2 problem. Annual Review of Marine Science 1:169–192. [DOI] [PubMed] [Google Scholar]
- Dupont, S. , Dorey N., Stumpp M., Melzner F., and Thorndyke M.. 2013. Long‐term and trans‐life‐cycle effects of exposure to ocean acidification in the green sea urchin Strongylocentrotus droebachiensis . Marine Biology 160:1835–1843. [Google Scholar]
- Fitzer, S. C. , Caldwell G. S., Close A. J., Clare A. S., Upstill‐Goddard R. C., and Bentley M. G.. 2012. Ocean acidification induces multi‐generational decline in copepod naupliar production with possible conflict for reproductive resource allocation. Journal of Experimental Marine Biology Ecology 418–419:30–36. [Google Scholar]
- Foo, S. A. , Dworjanyn S. A., Poore A. G., and Byrne M.. 2012. Adaptive capacity of the habitat modifying sea urchin Centrostephanus rodgersii to ocean warming and ocean acidification: performance of early embryos. PLoS One 7:e42497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frölicher, T. L. , and Joos F.. 2010. Reversible and irreversible impacts of greenhouse gas emissions in multi‐century projections with the NCAR global coupled carbon cycle‐climate model. Climate Dynamics 35:1439–1459. [Google Scholar]
- Gardner, J. L. , Peters A., Kearney M. R., Joseph L., and Heinsohn R.. 2011. Declining body size: a third universal response to warming? Trends in Ecology and Evolution 26:285–291. [DOI] [PubMed] [Google Scholar]
- Garilli, V. , Rodolfo‐Metalpa R., Scuderi D., Brusca L., Parrinello D., Rastrick S. P. S., Foggo A. et al. 2015. Physiological advantages of dwarfing in surviving extinctions in high‐CO2 oceans. Nature Climate Change 5:678–682. [Google Scholar]
- Ghalambor, C. K. , McKay J. K., Carroll S. P., and Reznick D. N.. 2007. Adaptive versus non‐adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments. Functional Ecology 21:394–407. [Google Scholar]
- Godbold, J. A. , and Calosi P.. 2013. Ocean acidification and climate change: advances in ecology and evolution. Philosophical Transactions of the Royal Society B: Biological Sciences 368:20120448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez‐Mestre, I. , and Jovani R.. 2013. A heuristic model on the role of plasticity in adaptive evolution: plasticity increases adaptation, population viability and genetic variation. Proceedings of the Royal Society of London Series B: Biological Sciences 280:20131869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez, A. , Ronce O., Ferriere R., and Hochberg M. E.. 2013. Evolutionary rescue: an emerging focus at the intersection between ecology and evolution. Philosophical Transactions of the Royal Society B: Biological Sciences 368:20120404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green, E. J. , and Carrit D. E.. 1967. New table for oxygen saturation of seawater. Journal of Marine Research 25:140–147. [Google Scholar]
- Ho, D. H. , and Burggren W. W.. 2010. Epigenetics and transgenerational transfer: a physiological perspective. Journal of Experimental Biology 213:3–16. [DOI] [PubMed] [Google Scholar]
- Hoffmann, A. A. . 1995. Acclimation: increasing survival at a cost. Trends in Ecology and Evolution 10:1–2. [Google Scholar]
- Hoffmann, A. A. , and Sgrò C.. 2011. Climate change and evolutionary adaptation. Nature 470:479–485. [DOI] [PubMed] [Google Scholar]
- Hofmann, G. E. , Smith J. E., Johnson K. S., Send U., Levin L. A., Micheli F., Paytan A. et al. 2011. High‐frequency dynamics of ocean pH: a multi‐ecosystem comparison. PLoS One 6:e28983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IPCC , 2013. Climate Change 2013: The Physical Science Basis: Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, New York. [Google Scholar]
- Jablonka, E. , and Raz G.. 2009. Trans‐generational epigenetic inheritance: prevalence, mechanisms, and implications for the study of heretic and evolution. The Quarterly Review of Biology 84:131–176. [DOI] [PubMed] [Google Scholar]
- Jensen, N. , Allen R. M., and Marshall D. J.. 2014. Adaptive maternal and paternal effects: gamete plasticity in response to parental stress. Functional Ecology 28:724–733. [Google Scholar]
- Kelly, M. W. , Padilla‐Gamino J. L., and Hofmann G. E.. 2013. Natural variation, and the capacity to adapt to ocean acidification in the keystone sea urchin Strongylocentrotus purpuratus . Global Change Biology 19:2536–2546. [DOI] [PubMed] [Google Scholar]
- Klironomos, F. D. , Berg J., and Collins S.. 2012. How epigenetic mutations can affect genetic evolution: model and mechanisms. BioEssays 35:571–578. [DOI] [PubMed] [Google Scholar]
- Kroeker, K. J. , Kordas R. L., Crim R., Hendriks I. E., Ramajo L., Singh G. S., Duarte C. M. et al. 2013. Impacts of ocean acidification on marine organisms: quantifying sensitivities and interactions with warming. Global Change Biology 19:1884–1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Greca, M. , and Bacci G.. 1962. Una nuova specie di Ophryotrocha delle coste tirreniche. Bolletino di zoologia 29:13–23. [Google Scholar]
- Lande, R. , and Shannon S.. 1996. The role of genetic variation in adaptation and population persistence in a changing environment. Evolution 50:434–437. [DOI] [PubMed] [Google Scholar]
- Lewis, C. N. , Brown K. A., Edwards L. A., Cooper G., and Findlay H. S.. 2013. Sensitivity to ocean acidification parallels natural pCO2 gradients experienced by Artic copepods under winter sea ice. Proceedings of the National Academy of Sciences of the United States of America 110:E4960–E4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas, A. E. , Wishner K. F., and Seibel B. A.. 2012. The metabolic response of pteropods to acidification reflects natural CO2‐exposure in oxygen minimum zones. Biogeosciences 9:747–757. [Google Scholar]
- Malvezzi, A. J. , S Murray C., Feldheim K. A., DiBattista J. D., Garant D., Gobler C. J., Chapman D. D. et al. 2015. A quantitative genetic approach to assess the evolutionary potential of a coastal marine fish to ocean acidification. Evolutionary Applications 8:352–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall, D. J. , and Uller T.. 2007. When is a maternal effect adaptive? Oikos 116:1957–1963. [Google Scholar]
- Massamba‐N'Siala, G. , Simonini R., Cossu P., Maltagliati F., Castelli A., and Prevedelli D.. 2011. Life‐history and demographic spatial variation in Mediterranean populations of the opportunistic polychaete Ophryotrocha labronica (Polychaeta, Dorvilleidae). Marine Biology 158:1523–1535. [Google Scholar]
- Massamba‐N'Siala, G. , Calosi P., Bilton D. T., Prevedelli D., and Simonini R.. 2012. Life‐history and thermal tolerance traits display different thermal plasticities and relationships with temperature in the marine polychaete Ophryotrocha labronica La Greca and Bacci (Dorvilleidae). Journal of Experimental Marine Biology and Ecology 438:109–117. [Google Scholar]
- Mehrbach, C. , Culberson C. H., Hawley J. E., and Pytkowicz R. M.. 1973. Measurement of the apparent dissociation constants of carbonic acid in seawater at atmospheric pressure. Limnology and Oceanography 18:897–907. [Google Scholar]
- Melzner, F. , Thomsen J., Koeve W., Oschlies A., Gutowska M. A., Bange H. W., Hansen H. P. et al. 2013. Future ocean acidification will be amplified by hypoxia in coastal habitats. Marine Biology 160:1875–1888. [Google Scholar]
- Miller, G. M. , Watson S.‐A., Donelson J. M., McCormick M. I., and Munday P. L.. 2012. Parental environment mediates impacts of elevated CO2 on a coral reef fish. Nature Climate Change 2:858–861. [Google Scholar]
- Miller, N. A. , Paganini A. W., and Stillman J. H.. 2013. Differential thermal tolerance and energetic trajectories during ontogeny in porcelain crabs, genus Petrolisthes . Journal of Thermal Biology 38:79–85. [Google Scholar]
- Mousseau, T. A. , and Fox C. W.. 1998. The adaptive significance of maternal effects. Trends in Ecology and Evolution 13:403–407. [DOI] [PubMed] [Google Scholar]
- Munday, P. L. . 2014. Transgenerational acclimation of fishes to climate change and ocean acidification. F1000 Prime Reports 6:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munday, P. L. , Warner R. R., Monro K., Pandolfi J. M., and Marshall D. J.. 2013. Predicting evolutionary responses to climate change in the sea. Ecology Letters 16:1488–1500. [DOI] [PubMed] [Google Scholar]
- Murray, C. S. , Malvezzi A., Gobler C. J., and Baumann H.. 2014. Offspring sensitivity to ocean acidification changes seasonally in a coastal marine fish. Marine Ecology Progress Series 504:1–11. [Google Scholar]
- Parker, L. M. , O'Connor W. A., Raftos D. A., Pörtner H.‐O., and Ross P. M.. 2015. Persistence of positive carryover effects in the Oyster, Saccostrea glomerata, following transgenerational exposure to ocean acidification. PLoS One 10:e0132276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen, S. A. , Håkedal O. J., Salaberria I., Tagliati A., Gustavson L. M., Jenssen B. M., Olsen A. J. et al. 2014. Multigenerational exposure to ocean acidification during food limitation reveals consequences for copepod scope for growth and vital rates. Environmental Science and Technology 48:12275–12284. [DOI] [PubMed] [Google Scholar]
- Pespeni, M. H. , Sanford E., Gaylord B., Hill T. M., Hosfelt J. D., Jaris H. K., LaVigne M. et al. 2013. Evolutionary change during experimental ocean acidification. Proceedings of the National Academy of Sciences of the United States of America 110:6937–6942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters, R. H. 1983. The Ecological Implications of Body Size. Cambridge University Press, Cambridge. [Google Scholar]
- Pierrot, D. , Lewis E., and Wallace D. W. R. 2006. CO2SYS DOS program developed for CO2 system calculations. ORNL/CDIAC‐105. Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory. US Department of Energy, Oak Ridge, Tennessee. [Google Scholar]
- Pigliucci, M. , Murren C. J., and Schlichting C. D.. 2006. Phenotypic plasticity and evolution by genetic assimilation. Journal of Experimental Biology 209:2362–2367. [DOI] [PubMed] [Google Scholar]
- Pistevos, J. C. A. , Calosi P., Widdicombe S., and Bishop J. D. D.. 2011. Will variation among genetic individuals influence species response to global climate change? Oikos 120:675–689. [Google Scholar]
- Pörtner, H.‐O. . 2008. Ecosystem effects of ocean acidification in times of ocean warming: a physiologist's view. Marine Ecology Progress Series 373:203–217. [Google Scholar]
- Pörtner, H.‐O. , Langenbuch M., and Reipschläger A.. 2004. Biological impact of elevated ocean CO2 concentrations: lessons from animal physiology and earth history. Journal of Oceanography 60:705–718. [Google Scholar]
- Prevedelli, D. , Massamba‐N'Siala G., and Simonini R.. 2005. The seasonal dynamics of six species of Dorvilleidae (Polychaeta) in the harbour of La Spezia (Italy). Marine Ecology 26:286–293. [Google Scholar]
- Reusch, T. B. H. . 2014. Climate change in the oceans: evolutionary versus phenotypically plastic responses of marine animals and plants. Evolutionary Applications 7:104–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reznick, D. . 1983. The structure of guppy life histories: the trade‐off between growth and reproduction. Ecology 64:862–873. [Google Scholar]
- Reznick, D. N. , and Ghalambor C. K.. 2001. The population ecology of contemporary adaptations: what empirical studies reveal about the conditions that promote adaptive evolution. Genetica 112–113:183–198. [PubMed] [Google Scholar]
- Salinas, S. , Brown S. C., Mangel M., and Munch S. B.. 2013. Non‐genetic inheritance and changing environments. Non‐Genetic Inheritance 1:38–50. [Google Scholar]
- Shama, L. N. S. , Strobel A., Mark F. C., and Wegner K. M.. 2014. Transgenerational plasticity in marine stickleback: maternal effects mediate impacts of a warming ocean. Functional Ecology 28:1482–1493. [Google Scholar]
- Shaw, E. C. , McNeil B. I., and Tilbrook B.. 2012. Impacts of ocean acidification in naturally variable coral reef flat ecosystems. Journal of Geophysical Research 117:C03038. [Google Scholar]
- Sheridan, J. A. , and Bickford D.. 2011. Shrinking body size as an ecological response to climate change. Nature Climate Change 1:401–406. [Google Scholar]
- Simonini, R. , and Prevedelli D.. 2003. Life history and demography of three populations of Ophryotrocha japonica (Polychaeta: Dorvilleidae). Marine Ecology Progress Series 258:171–180. [Google Scholar]
- Simonini, R. , Grandi V., Massamba‐N'Siala G., and Prevedelli D.. 2009. Distribution of the genus Ophryotrocha (Polychaeta) in Italy: new reports and comments on the biogeography of Mediterranean species. Vie et Milieu, 59:79–88. [Google Scholar]
- Sokal, R. R. , and Rohlf F. J. 1995. Biometry: The Principles and Practice of Statistics in Biological Research, 3rd edn. Freeman W. H, New York. [Google Scholar]
- Solan, M. , Cardinale B. J., Downing A. L., Engelhardt K. A. M., Ruesink J. L., and Srivastava D. S.. 2004. Extinction and ecosystem function in the marine benthos. Science 306:1177–1180. [DOI] [PubMed] [Google Scholar]
- Stearns, S. C. 1992. The Evolution of Life Histories. Oxford University Press, New York. [Google Scholar]
- Stockwell, C. A. , Hendry A. P., and Kinnison M. T.. 2003. Contemporary evolution meets conservation biology. Trends in Ecology and Evolution 18:94–101. [Google Scholar]
- Strong, A. L. , Kroeker K. J., Teneva L. T., Mease L. A., and Kelly R. P.. 2014. Ocean acidification 2.0: managing our changing coastal ocean chemistry. BioScience 64:581–592. [Google Scholar]
- Stumpp, M. , Wren J., Melzner F., Thorndyke M. C., and Dupont S. T.. 2011. CO2 induced seawater acidification impacts sea urchin larval development I: elevated metabolic rates decrease scope for growth and induce developmental delay. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology 160:331–340. [DOI] [PubMed] [Google Scholar]
- Suckling, C. C. , Clark M. S., Beveridge C., Brunner L., Hughes A. D., Harper E. M., Cook E. J. et al. 2014. Experimental influence of pH on the early life‐stages of sea urchins II: increasing parental exposure times gives rise to different responses. Invertebrate Reproduction and Development 58:161–175. [Google Scholar]
- Sunday, J. M. , Crim R. N., Harley C. D. G., and Hart M. W.. 2011. Quantifying rates of evolutionary adaptation in response to ocean acidification. PLoS One 6:e22881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunday, J. M. , Calosi P., Dupont S., Munday P. L., Stillman J. H., and Reusch T. B. H.. 2014. Evolution in an acidifying ocean. Trends in Ecology and Evolution 29:117–125. [DOI] [PubMed] [Google Scholar]
- Szulczewski, M. L. , W MacMinn C., Herzog H. J., and Juanes R.. 2012. Lifetime of carbon capture and storage as a climate‐change mitigation technology. Proceedings of the National Academy of Sciences of the United States of America 109:5185–5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thor, P. , and Dupont S.. 2015. Transgenerational effects alleviate severe fecundity loss during ocean acidification in a ubiquitous planktonic copepod. Global Change Biology 21:2261–2271. [DOI] [PubMed] [Google Scholar]
- Tills, O. , Bitterli T., Culverhouse P., Spicer J. I., and Rundle S. D.. 2013. A novel application of motion analysis for detecting stress responses in embryos at different stages of development. BMC Bioinformatics 14:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underwood, A. J. 1997. Experiments in Ecology. Cambridge University Press, Cambridge. [Google Scholar]
- Van Oppen, M. J. H. , Oliver J. K., Putnam H. M., and Gates R. D.. 2015. Building coral reef resilience through assisted evolution. Proceedings of the National Academy of Sciences of the United States of America 112:2307–2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West‐Eberhard, M. J. 2003. Developmental Plasticity and Evolution. Oxford University Press, Oxford. [Google Scholar]
- Wittmann, A. C. , and Pörtner H.‐O.. 2013. Sensitivities of extant animal taxa to ocean acidification. Nature Climate Change 3:995–1001. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Mean values ±95 % CI for all traits measured for the marine polychaete Ophryotrocha labronica at elevated (grey rows) and low (white rows) p CO 2 conditions across generations.
Appendix S1. Acclimation/selection to experimental conditions.


