Abstract
Background
Intermittent smoking is prevalent among Hispanics, but little is known about whether this smoking pattern associates with increased chronic kidney disease (CKD) risk in this population. The objective of the present study is to identify patterns of exposure associated with CKD in US Hispanics.
Methods
We used cross-sectional data on 15 410 participants of the Hispanics Community Health Study/the Study of Latinos, a population-based study of individuals aged 18–74 years, recruited in 2008 to 2011 from four US field centers (Bronx, NY; Chicago, IL; Miami, FL; San Diego, CA). Smoking exposure was obtained through a questionnaire. CKD was defined by an estimated glomerular filtration rate of <60 mL/min/1.73 m2 or a urine albumin-to-creatinine ratio of ≥30 mg/g.
Results
Approximately 14% of individuals were daily and 7% were intermittent smokers, and 16% were past smokers. There was a significant interaction between smoking status and pack-years of exposure (P = 0.0003). In adjusted models, there was an increased odds of CKD among daily, intermittent and past smokers by pack-years compared with never smokers. The association of intermittent smokers was significant at 10 pack-years [odds ratio (OR) = 1.38, 95% confidence intervals (CI) 1.06, 1.81], whereas for daily smokers this association was observed at 40 pack-years (OR = 1.43, 95% CI 1.09, 1.89).
Conclusions
Our findings of increased risk of CKD among Hispanics who are intermittent smokers support screening and smoking cessation interventions targeted to this population for the prevention of CKD. It also suggests novel mechanistic pathways for kidney toxicity that should be further explored in future studies.
Keywords: chronic kidney disease, Hispanics/Latinos, intermittent smoking
INTRODUCTION
Epidemiologic studies have shown that smoking is associated with reduced kidney function [1–3], development of microalbuminuria and clinical proteinuria [4–6], and progression to end-stage kidney disease (ESKD) [7]. Adding to this evidence, a recent study showed an excess of death due to renal disease attributable to current smoking [8]. Among smokers, mortality due to renal failure was increased by 2-fold, and death due to essential hypertension and hypertensive renal disease was increased by 2.4- to 2.6-fold [8]. Smoking is associated with atherosclerotic vascular disease [9–12] and metabolic syndrome [13]; both conditions are also known risk factors for chronic kidney disease (CKD). Experimental evidence suggests that smoking promotes endothelial dysfunction, oxidative stress and inflammation [14–18]. Nicotine has been shown to worsen the severity of kidney injury in animal models of diabetes [19] and acute nephritis [16]. Nicotine promotes mesangial cell proliferation and hypertrophy via nonneuronal nicotinic acetylcholine receptors [20] and podocyte injury in cell culture (L. Raij, personal communication). Overall, the evidence supports biologic mechanisms linking smoking to CKD causally.
Although smoking prevalence has declined in the US population [21], the rates of smoking among Hispanics/Latinos are higher compared with national averages, particularly among individuals of Puerto Rican and Cuban backgrounds [22, 23]. Hispanics also have a high prevalence of intermittent smoking compared with other racial/ethnic groups [24, 25]. Prior studies have shown the association of smoking duration and total smoking exposure on CKD [26–28]. However, these studies have not accounted for the effects of regular versus intermittent exposures on CKD risk or fully characterized the risk among past smokers, which have shown either a reduction or no CKD risk after smoking cessation [26, 28, 29].
We hypothesized that, among Hispanics, patterns of exposure among current smokers, in addition to total smoking exposure, explain the association of smoking with CKD. The characterization of harmful effects of smoking at lower levels of exposure in this population is important as this information could inform public health preventive programs and clinicians in their effort to reduce the burden of CKD. Here, we describe important interactions between smoking status and cumulative smoking exposure in Hispanics/Latino individuals, the largest minority population in the USA, who were part of the Hispanics Community Health Study/the Study of Latinos (HCHS/SOL).
MATERIALS AND METHODS
Study population
This is a cross-sectional study of the HCHS/SOL, a population-based cohort study of 16 415 self-identified Hispanics/Latino persons aged 18–74 years at the time of screening from four US field centers (Bronx, NY; Chicago, IL; Miami, FL; San Diego, CA) [30]. The cohort includes participants who self-identified as having Hispanics/Latino background, the largest groups being Central American (n = 1730), Cuban (n = 2348), Dominican (n = 1460), Mexican (n = 6471), Puerto Rican (n = 2728) and South American (n = 1068). The baseline examination during the period of 2008–11 included a clinical visit with comprehensive biological, behavioral and socio-demographic assessments [30]. The sample design and cohort selection have been described previously [31]. Briefly, a stratified two-stage area probability sample of households was selected in each of the four field centers. The first sampling stage randomly selected census block groups with stratification based on Hispanics/Latino concentration and proportion of high/low socioeconomic status. The second sampling stage randomly selected households, with stratification, from US Postal Service registries that covered the randomly selected census block groups. Both stages oversampled certain strata to increase the likelihood that a selected address yielded a Hispanics/Latino household. After households were sampled, in-person or telephone contacts were made to screen eligible households and to roster its members. Lastly, the study oversampled the 45–74 age group (n = 9714, 59.2%) to facilitate examination of target outcomes. This study is based on cross-sectional analysis of baseline visit exposures and outcomes. All data were obtained during a clinical visit, which included a physical exam, collection of biospecimens (urine, blood) and administration of a questionnaire for lifestyle and clinical measures [30]. The study was approved by the Institutional Review Boards at the coordinating center and each field center where all subjects gave written consent. Informed consent was obtained for all participants. The study adheres to the principles of the Declaration of Helsinki.
Anthropometrics and blood pressure were obtained using standardized protocols during a clinical visit, in addition to blood and urine samples for bioassays. Creatinine was measured in serum and urine on a Roche Modular P Chemistry Analyzer (Roche Diagnostics Corporation, Indianapolis, IN, USA) using a creatinase enzymatic method (Roche Diagnostics Corporation). Serum and urine creatinine measurements are traceable to isotope dilution mass spectrometry reference measurement procedures. Albumin was measured in urine using an immunoturbidimetric method on the ProSpec nephelometric analyzer (Siemens Healthcare Diagnostics, Tarrytown, NY, USA, formerly Dade Behring). Serum cystatin C was measured using a turbidimetric method on the Roche Modular P Chemistry Analyzer (Gentian AS, Moss, Norway).
Exposures
Self-reported tobacco exposure was defined as current, past or never smoking from information collected through questionnaires based on the following questions: ‘Have you ever smoked at least 100 cigarettes in your entire life?’ Participants responding ‘yes’ were then asked if they now smoke daily (daily smokers), some days (intermittent smokers) or not at all (former smokers). Tobacco use measures included age first started to smoke cigarettes regularly, number of years smoked cigarettes, and, on average, number of cigarettes per day. Lifetime pack-years was used as an estimate of long-term exposure of cigarette smoking and calculated based on age at smoking initiation, periods of quitting and average lifetime cigarettes per day, as the average number of packs of cigarettes smoked per day multiplied by the number of years smoked. The reproducibility of smoking variables was assessed in a sample of 56 individuals through repeated study visits (kappa = 0.93 for smoking status, reliability coefficients of 0.89, 0.92 and 0.83 for age started smoking, current cigarettes/day and lifetime average cigarettes a day, respectively) [22].
Outcomes
CKD was defined based on a composite outcome that included an estimated glomerular filtration rate (eGFR) of <60 mL/min/1.73 m2 (Stage 3 CKD) or a urine albumin-to-creatinine ratio (UACR) of ≥30 mg/g. eGFR was estimated using the equation developed by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) working group, which includes serum creatinine, age, sex and race components [32–34]. We also calculated eGFR using the CKD-EPI equation based on serum creatinine and cystatin C, but results were unchanged. Therefore, we reported CKD based on serum creatinine eGFR. The CKD-EPI equations have recently been shown to more accurately estimate ESKD and mortality risk than did the Modification of Diet in Renal Disease equation [32]; however, these eGFR equations have not been validated in Hispanics.
Statistical analysis
For the descriptive analysis, the mean and the 95% confidence interval (CI) were generated for quantitative variables with normal distributions. The median and the interquartile range were generated for the continuous variables, which were not normally distributed. For categorical variables, the column percentages and 95% CI were presented. We applied survey logistic regression to study the association between the smoking exposure and a CKD composite outcome. This procedure was used to account for the complex study design as previously reported [30]. We tested the interaction between smoking status and cumulative pack-years, age and sex at the significance level of 0.05. We also tested models using an indicator variable for blood pressure lowering medications including angiotensin-converting enzyme inhibitor and angiotensin II receptor blockers. The final models were adjusted for age, sex, body mass index, history of diabetes, history of prevalent coronary heart disease, systolic blood pressure, history of alcohol intake, high- and low-density lipoprotein cholesterols, hypertension, and cross-classification of center and Hispanics/Latino background group, and included the interaction between smoking status and cumulative pack-years. Education was not significantly associated with CKD, and it was not included in the final models. The adjusted prevalence of CKD was estimated based on survey logistic regression estimates using predicted marginal results for the subgroups of current daily smokers, intermittent smokers, past smokers and never smokers, adjusting to the distribution of target population of other covariates. We then estimated the odds ratios (OR) of CKD for smokers (current daily, intermittent and past) relative to never smokers across levels of cumulative smoking in pack-years. All reported measures were weighted to account for the complex sampling design. OR and 95% CI were reported. All analyses were performed using SAS 9.3 software (SAS Institute, Cary, NC, USA) and SUDAAN software release 11 (RTI International, Research Triangle Park, NC, USA).
RESULTS
The characteristics of the HCHS/SOL target population by smoking exposure are shown in Table 1. Approximately 21% of individuals reported current smoking, of which 14% reported daily smoking, and 7% reported intermittent smoking, and 16% were past smokers. Intermittent smokers had lower mean pack-years smoking exposure and smoking years than those reporting daily or past smoking. In addition, they were younger, more often men (except when compared with past smokers) and had a lower prevalence of comorbidities compared with never smokers and the other smoker groups, except for history of cardiovascular disease, which was least prevalent among never smokers. The mean eGFR was comparable among intermittent smokers and never smokers, while the median UACR was similar between current daily smokers and never smokers.
Table 1.
Descriptive characteristics by smoking exposure: the HCHS/SOL: 2008–11
| Characteristic | Smoking exposure |
|||||||
|---|---|---|---|---|---|---|---|---|
| Daily smoker |
Intermittent smoker |
Past smoker |
Never smoker |
|||||
| Mean/percent | (95% CI) | Mean/percent | (95% CI) | Mean/percent | (95% CI) | Mean/percent | (95% CI) | |
| Unweighted sample size | 2023 | 897 | 2887 | 9603 | ||||
| Mean cigarette pack-years | 18.3 | (16.7, 19.8) | 5.2 | (4.3, 6.1) | 13.6 | (12.6, 14.7) | 0.0 | (0.0, 0.0) |
| Mean age, years | 42.6 | (41.6, 43.6) | 35.7 | (34.4, 36.9) | 48.3 | (47.4, 49.2) | 39.5 | (38.9, 40.0) |
| Female sex, % | 40.8 | (37.9, 43. 8) | 39.8 | (33.7, 46.2) | 39.1 | (36.6, 41.7) | 60.5 | (59.1, 61.9) |
| Mean BMI, kg/m2 | 28.8 | (28.4, 29.2) | 29.5 | (28.5, 30.5) | 30.3 | (29.9, 30.7) | 29.2 | (29.0, 29.4) |
| ACE inhibitors, % | 8.2 | (7.0, 10.0) | 4.0 | (2.8, 5.8) | 14.7 | (12.9, 16.8) | 7.5 | (6.8, 8.3) |
| Angiotensin II receptor antagonists, % | 2.4 | (1.7, 3.8) | 2.1 | (1.1, 4.0) | 5.2 | (4.3, 6.4) | 3.3 | (2.9, 3.8) |
| Diabetes, % | 12.6 | (11.0, 14.4) | 11.5 | (8.8, 14.9) | 21.5 | (19.6, 23.6) | 13.3 | (12.4, 14.3) |
| History of CVD, % | 5.6 | (4.5, 6.9) | 5.8 | (3.3, 9.9) | 6.1 | (4.9, 7.5) | 3.7 | (3.15, 4.24) |
| Hypertension, % | 21. 7 | (19.2, 24.3) | 13.0 | (10.3, 16.4) | 32.2 | (29.6, 34.9) | 19.7 | (18.5, 21.0) |
| Mean systolic blood pressure | 120.7 | (119. 7, 121.8) | 117.4 | (115.7, 119.1) | 123.6 | (122.7, 124.5) | 118.8 | (118.2, 119.4) |
| Mean diastolic blood pressure | 72.8 | (72.1, 73.6) | 71.1 | (70.0, 72.2) | 73.8 | (73.2, 74.3) | 71.6 | (71.2, 72.0) |
| Alcohol, % | ||||||||
| Current | 11.6 | (9.9, 13.5) | 5.3 | (3.7, 7.6) | 11.3 | (9.7, 13.2) | 23.8 | (22.0, 25.7) |
| Past | 27.9 | (25.3, 30.6) | 16.6 | (13.3, 20.4) | 36.1 | (33.3, 38.9) | 30.5 | (28.8, 32.2) |
| Never | 60.5 | (57.7, 63.3) | 78.1 | (73.5, 82.1) | 52.6 | (49.6, 55.6) | 45.7 | (43.8, 47.6) |
| Mean HDL cholesterol, mg/g | 46.8 | (46.0, 47.7) | 48.0 | (46.8, 49.3) | 48.0 | (47.3, 48.7) | 49.6 | (49.2, 50.0) |
| Mean LDL cholesterol, mg/g | 120.0 | (117.7, 122.2) | 118.0 | (114.6, 121.4) | 126.6 | (124.6, 128.5) | 118.2 | (117.0, 119.3) |
| Mean eGFRcr, mL/min/1.73 m2 | 97.5 | (96.4, 98.6) | 103.2 | (101.4, 105.0) | 93.7 | (92.5, 94.9) | 102.3 | (101.6, 103.0) |
| Smoking years | 24.6 | (23.6, 25.6) | 15.3 | (14.1, 16.6) | 17.2 | (16.5, 17.9) | 0 | |
| Median UACR (IQR), mg/g | 6.0 | (4.2, 10.0) | 5.5 | (4.0, 8.9) | 6.2 | (4.2, 11.5) | 6.1 | (4.3, 10.8) |
BMI, body mass index; ACE, angiotensin-converting enzyme; CVD, cardiovascular disease; HDL, high-density lipoprotein; LDL, low-density lipoprotein; eGFRcr, estimated glomerular filtration rate based on serum creatinine; UACR, urine albumin-to-creatinine ratio; IQR, interquartile range.
Among Hispanics/Latinos meeting our criteria of CKD, 17% had an eGFR of <60 mL/min/1.73 m2, 73% had UACR ≥30 mg/g and 10% had both reduced eGFR and increased UACR. CKD prevalences among daily smokers, intermittent smokers, past smokers and never smokers were 10, 9, 13 and 10%, respectively. The survey-adjusted prevalence estimate of CKD by smoking categories was 8% for daily smokers (95% CI 7, 10), 12% for intermittent smokers (9, 15), 11% for past smokers (9, 12) and 10% for never smokers (9, 11).
There was a significant interaction between smoking status (daily and intermittent, past compared to never) with pack-years of exposure (P = 0.0003) on CKD risk. Based on the model that accounted for this interaction and adjusted for other CKD risk factors, we calculated the OR associated with smoking at 10, 20 and 40 pack-years of smoking for daily and past smokers, and at 5, 10 and 20 pack-years for intermittent smokers compared with never smokers (Table 2). Lower numbers of pack-years were chosen for intermittent smokers because that group had lower cumulative smoking exposure.
Table 2.
Adjusted ORa and 95% CI of CKD by smoking categories and pack-years
| OR | 95% CI | |
|---|---|---|
| Estimation for daily smokers | ||
| Daily smokers with 10 pack-years | 0.87 | (0.71, 1.07) |
| Daily smokers with 20 pack-years | 1.03 | (0.85, 1.25) |
| Daily smokers with 40 pack-years | 1.43 | (1.09, 1.89) |
| Estimation for intermittent smokers | ||
| Intermittent smokers with 5 pack-years | 1.21 | (0.92, 1.58) |
| Intermittent smokers with 10 pack-years | 1.38 | (1.06, 1.81) |
| Intermittent smokers with 20 pack-years | 1.81 | (1.24, 2.65) |
| Estimation for past smokers | ||
| Past smokers with 5 pack-years | 1.17 | (0.98, 1.39) |
| Past smokers with 10 pack-years | 1.19 | (1.00, 1.42) |
| Past smokers with 20 pack-years | 1.23 | (0.96, 1.57) |
aOR of CKD associated with smoking (daily, intermittent and past) compared with never smokers by pack-years of smoking based on models adjusted for age, sex, cross-classification of center and Hispanics/Latino background, body mass index, history of diabetes, history of prevalent coronary heart disease, systolic blood pressure, history of alcohol intake, high- and low-density lipoprotein cholesterols, hypertension and blood pressure lowering medications including angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers. P-value for interaction of smoking categories with pack-years = 0.0003.
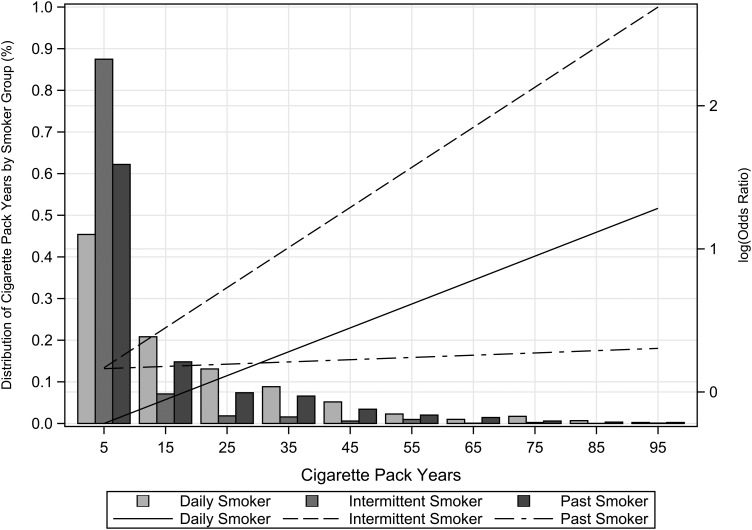
Daily and intermittent smokers showed an increase in the odds of CKD compared with never smokers with increasing pack-years of exposure, but intermittent smokers had a steeper increase (Figure 1). For example, with 20 pack-years, intermittent smoking was associated with an 80% higher odds of CKD compared with never smokers, whereas daily smokers had a non-significant 3% higher odds of CKD (Table 2). In addition, the association for intermittent smoking was statistically significant at 10 pack-years of smoking, whereas for daily smokers this association was observed only at 40 pack-years.
FIGURE 1:
Distribution of cumulative pack-years by smoker group among Hispanics/Latinos. The histogram shows the percent of cigarette pack-year groups (0–10, 10–20 and so on) by smoker groups (current daily, intermittent and past smokers). Lines are the adjusted OR of CKD by smoker groups (current daily, intermittent and past versus never smokers) (see Table 2 for covariates). The x-axis uses the midpoint of the cigarette pack-year group interval.
The OR comparing past smokers with never smokers increased linearly across pack-years of exposure at a very low rate, and it was not significant (Figure 1). We also tested the association of time since quitting smoking and CKD among past smokers in models replacing pack-years with this time variable, but the association was not significant. In addition, there was no interaction of smoking categories with age (P = 0.06) or sex (P = 0.69) on the risk of CKD.
DISCUSSION
Our main findings are the significant interactions among smoking status and cumulative pack-year exposure in Hispanics/Latino individuals, which have not been previously reported. Current smokers showed increased odds of CKD associated with higher level of cumulative pack-years of exposure compared with never smokers, in agreement with prior studies [26–28]. Our new findings are related to a subset of current smokers who had an intermittent pattern of smoking, which is more common among Hispanics populations [24]. Although these individuals had low mean cumulative pack-years, were younger and had fewer overall risk factors for CKD, their odds of CKD compared with never smokers were higher than daily smokers across various pack-year levels (Table 2, Figure 1). These findings suggest that intermittent smoking exposure has deleterious effects in the kidneys, the mechanisms of which may differ from those related to daily smoking patterns. Given users of nicotine delivery devices such as e-cigarettes, estimated as 3–7% of the US population, often continue smoking intermittently [35–38], these findings could be relevant to this population. However, our study does not have information on e-cigarette use, and this issue will need to be addressed in future studies.
Mechanisms related to CKD in intermittent smokers include transitory increases in blood pressure due to acute vascular effects of smoking [39, 40]. Blood pressure variability has been previously associated with kidney organ damage including CKD among hypertensive individuals [41]. In addition to hemodynamic changes, intermittent exposure of the kidney to toxic compounds present in cigarettes may also play a role in CKD. Experimental research suggests that nicotine stimulates mesangial cell proliferation and hypertrophy [20], inflammation and atherosclerosis [18], and induces oxidative stress pathways, which could promote kidney damage. Cadmium, a pollutant present in tobacco smoking, accumulates in kidneys and has been associated with hypertension [42]. Chronic smoking exposure induces enzymes related to the metabolism and excretion of nicotine and cadmium, potentially reducing their circulating levels and acute tissue toxicity. Therefore, intermittent smokers could be exposed to a higher level of these toxins acutely, although the exposure may be for a short period. In the HCHS/SOL study, intermittent smokers more often were men of Mexican background [22], although we did not find significant differences in the observed association between smoking and CKD by sex, and we accounted for Hispanics/Latino background. Future work is needed to identify causal pathways relating intermittent smoking patterns to CKD.
The pattern of risk for past smokers was constant across cumulative pack-years, with an ∼20% higher odds of CKD compared with never smokers, regardless of the time since quitting. Although not significant, these findings suggest a remaining residual risk of CKD among individuals who quit smoking. Our findings are in agreement with prior studies that showed a lower risk of CKD among past smokers, but we did not observe a lack of risk among past smokers, which suggests that smoking exposure has a long-lasting harmful effect on kidneys.
This is the largest study of Hispanics/Latinos to examine the relationship of smoking exposure patterns—including daily and intermittent smoking—with CKD risk. Our study is limited to cross-sectional analyses, and therefore no causal inferences should be drawn. We also lack data on secondhand smoke exposure and the question of whether intermittent smokers maintain the smoking habit over their entire life. Ongoing HCHS/SOL follow-up visits will allow the study of these associations prospectively. Most of the CKD cases in our composite outcome were due to increased albuminuria, which is the most common manifestation of CKD, particularly in a young population such as the HCHS/SOL target population. Microalbuminuria is a strong and independent predictor of ESKD and cardiovascular health outcomes [43, 44].
Our findings suggest that intermittent smoking exposure is associated with an increased odds of CKD. These findings have important implications for public health, especially for Hispanics who have a high prevalence of intermittent smoking, estimated at 30% in the National Health and Nutrition Examination Survey 1999–2000 and 2001–2 [24]. This intermittent pattern may be unrecognized by healthcare providers, thereby missing opportunities for prevention. Our findings support public health efforts to avoid smoking exposure [45], particularly among young populations such as Hispanics/Latinos. Future studies should examine the relationship of secondhand smoke exposure to CKD risk in addition to the effect of intermittent smoking among e-cigarette users on CKD outcomes.
CONCLUSIONS
Our study shows significant associations of current smoking with CKD across increasing cumulative smoking exposure (pack-years) among Hispanics/Latinos, which was significant among intermittent smokers at lower pack-years of smoking. There was a remaining non-significant risk of CKD among past smokers even years after quitting smoking, which could reflect prior damage or continuous exposure through passive smoking. Further work is needed to identify the mechanisms associated with these findings and the risk of secondhand and active intermittent smoking exposure on CKD, and in addition to determine the temporal relationship of these associations using longitudinal data. Our research supports efforts to target intermittent smoking exposure among Hispanics/Latinos through public health interventions and extend these current recommendations to CKD outcomes.
CONFLICT OF INTEREST STATEMENT
None declared. An abstract of this study was presented at the 2015 American Society of Nephrology Renal Week meeting. (See related article by Plantinga and Gander. Intermittent smoking and chronic kidney disease. Nephrol Dial Transplant 2016; 31: 1558–1560)
ACKNOWLEDGEMENTS
The HCHS/SOL was carried out as a collaborative study supported by contracts from the National Heart, Lung, and Blood Institute (NHLBI) to the University of North Carolina (N01-HC65233), University of Miami (N01-HC65234), Albert Einstein College of Medicine (N01-HC65235), Northwestern University (N01-HC65236) and San Diego State University (N01-HC65237). The following institutes, centers or offices contribute to the HCHS/SOL through a transfer of funds to the NHLBI: National Center on Minority Health and Health Disparities, the National Institute on Deafness and Other Communications Disorders, the National Institute of Dental and Craniofacial Research, the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Neurological Disorders and Stroke, and the Office of Dietary Supplements. A.C.R. and J.P.L. are funded by the National Institute of Diabetes and Digestive and Kidney Diseases (K23 DK094829, A.C.R.; K24 DK092290, J.P.L.). N.F. and J.C. had full access to the data and take responsibility for the integrity of the data and the accuracy of the data analysis. N.F. is supported by the National Institutes of Health grant R21HL123677-01.
REFERENCES
- 1.Shankar A, Klein R, Klein BE. The association among smoking, heavy drinking, and chronic kidney disease. Am J Epidemiol 2006; 164: 263–271 [DOI] [PubMed] [Google Scholar]
- 2.Stengel B, Tarver-Carr ME, Powe NR et al. Lifestyle factors, obesity and the risk of chronic kidney disease. Epidemiology 2003; 14: 479–487 [DOI] [PubMed] [Google Scholar]
- 3.Fox CS, Larson MG, Leip EP et al. Predictors of new-onset kidney disease in a community-based population. JAMA 2004; 291: 844–850 [DOI] [PubMed] [Google Scholar]
- 4.Orth SR, Hallan SI. Smoking: a risk factor for progression of chronic kidney disease and for cardiovascular morbidity and mortality in renal patients—absence of evidence or evidence of absence? Clin J Am Soc Nephrol 2008; 3: 226–236 [DOI] [PubMed] [Google Scholar]
- 5.Yoon HJ, Park M, Yoon H et al. The differential effect of cigarette smoking on glomerular filtration rate and proteinuria in an apparently healthy population. Hypertens Res 2009; 32: 214–219 [DOI] [PubMed] [Google Scholar]
- 6.Bash LD, Astor BC, Coresh J. Risk of incident ESRD: a comprehensive look at cardiovascular risk factors and 17 years of follow-up in the Atherosclerosis Risk in Communities (ARIC) Study. Am J Kidney Dis 2010; 55: 31–41 [DOI] [PubMed] [Google Scholar]
- 7.Orth SR, Stockmann A, Conradt C et al. Smoking as a risk factor for end-stage renal failure in men with primary renal disease. Kidney Int 1998; 54: 926–931 [DOI] [PubMed] [Google Scholar]
- 8.Carter BD, Abnet CC, Feskanich D et al. Smoking and mortality—beyond established causes. N Engl J Med 2015; 372: 631–640 [DOI] [PubMed] [Google Scholar]
- 9.Bartecchi CE, MacKenzie TD, Schrier RW. The human costs of tobacco use (1). N Engl J Med 1994; 330: 907–912 [DOI] [PubMed] [Google Scholar]
- 10.Koh HK, Sebelius KG. Ending the tobacco epidemic. JAMA 2012; 308: 767–768 [DOI] [PubMed] [Google Scholar]
- 11.Tonelli M, Wiebe N, Culleton B et al. Chronic kidney disease and mortality risk: a systematic review. J Am Soc Nephrol 2006; 17: 2034–2047 [DOI] [PubMed] [Google Scholar]
- 12.Teo KK, Ounpuu S, Hawken S et al. Tobacco use and risk of myocardial infarction in 52 countries in the INTERHEART study: a case-control study. Lancet 2006; 368: 647–658 [DOI] [PubMed] [Google Scholar]
- 13.Ishizaka N, Ishizaka Y, Toda E et al. Association between cigarette smoking, metabolic syndrome, and carotid arteriosclerosis in Japanese individuals. Atherosclerosis 2005; 181: 381–388 [DOI] [PubMed] [Google Scholar]
- 14.Raij L, DeMaster EG, Jaimes EA. Cigarette smoke-induced endothelium dysfunction: role of superoxide anion. J Hypertens 2001; 19: 891–897 [DOI] [PubMed] [Google Scholar]
- 15.Jaimes EA, DeMaster EG, Tian RX et al. Stable compounds of cigarette smoke induce endothelial superoxide anion production via NADPH oxidase activation. Arterioscler Thromb Vasc Biol 2004; 24: 1031–1036 [DOI] [PubMed] [Google Scholar]
- 16.Jaimes EA, Tian RX, Joshi MS et al. Nicotine augments glomerular injury in a rat model of acute nephritis. Am J Nephrol 2009; 29: 319–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jaimes EA, Tian RX, Raij L. Nicotine: the link between cigarette smoking and the progression of renal injury? Am J Physiol Heart Circ Physiol 2007; 292: H76–H82 [DOI] [PubMed] [Google Scholar]
- 18.Zhou MS, Chadipiralla K, Mendez AJ et al. Nicotine potentiates proatherogenic effects of oxLDL by stimulating and upregulating macrophage CD36 signaling. Am J Physiol Heart Circ Physiol 2013; 305: H563–H574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hua P, Feng W, Ji S et al. Nicotine worsens the severity of nephropathy in diabetic mice: implications for the progression of kidney disease in smokers. Am J Physiol Renal Physiol 2010; 299: F732–F739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rezonzew G, Chumley P, Feng W et al. Nicotine exposure and the progression of chronic kidney disease: role of the alpha7-nicotinic acetylcholine receptor. Am J Physiol Renal Physiol 2012; 303: F304–F312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jamal A, Agaku IT, O'Connor E et al. Current cigarette smoking among adults—United States, 2005–2013. MMWR Morb Mortal Wkly Rep 2014; 63: 1108–1112 [PMC free article] [PubMed] [Google Scholar]
- 22.Kaplan RC, Bangdiwala SI, Barnhart JM et al. Smoking among U.S. Hispanic/Latino adults: the Hispanic Community Health Study/Study of Latinos. Am J Prev Med 2014; 46: 496–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Daviglus ML, Pirzada A, Talavera GA. Cardiovascular disease risk factors in the Hispanic/Latino population: lessons from the Hispanic Community Health Study/Study of Latinos (HCHS/SOL). Prog Cardiovasc Dis 2014; 57: 230–236 [DOI] [PubMed] [Google Scholar]
- 24.Everhart J, Ferketich AK, Browning K et al. Acculturation and misclassification of tobacco use status among Hispanic men and women in the United States. Nicotine Tob Res 2009; 11: 240–247 [DOI] [PubMed] [Google Scholar]
- 25.Zhu SH, Pulvers K, Zhuang Y et al. Most Latino smokers in California are low-frequency smokers. Addiction 2007; 102 (Suppl 2): 104–111 [DOI] [PubMed] [Google Scholar]
- 26.Hallan SI, Orth SR. Smoking is a risk factor in the progression to kidney failure. Kidney Int 2011; 80: 516–523 [DOI] [PubMed] [Google Scholar]
- 27.Yamamoto R, Nagasawa Y, Shoji T et al. Cigarette smoking and progression of IgA nephropathy. Am J Kidney Dis 2010; 56: 313–324 [DOI] [PubMed] [Google Scholar]
- 28.Jin A, Koh WP, Chow KY et al. Smoking and risk of kidney failure in the Singapore Chinese Health Study. PloS One 2013; 8: e62962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maeda I, Hayashi T, Sato KK et al. Cigarette smoking and the association with glomerular hyperfiltration and proteinuria in healthy middle-aged men. Clin J Am Soc Nephrol 2011; 6: 2462–2469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lavange LM, Kalsbeek WD, Sorlie PD et al. Sample design and cohort selection in the Hispanic Community Health Study/Study of Latinos. Ann Epidemiol 2010; 20: 642–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sorlie PD, Aviles-Santa LM, Wassertheil-Smoller S et al. Design and implementation of the Hispanic Community Health Study/Study of Latinos. Ann Epidemiol 2010; 20: 629–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levey AS, Stevens LA, Schmid CH et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Inker LA, Schmid CH, Tighiouart H et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 2012; 367: 20–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levey AS, Stevens LA. Estimating GFR using the CKD Epidemiology Collaboration (CKD-EPI) creatinine equation: more accurate GFR estimates, lower CKD prevalence estimates, and better risk predictions. Am J Kidney Dis 2010; 55: 622–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kralikova E, Kubatova S, Truneckova K et al. The electronic cigarette: what proportion of smokers have tried it and how many use it regularly? Addiction 2012; 107: 1528–1529 [DOI] [PubMed] [Google Scholar]
- 36.Kralikova E, Novak J, West O et al. Do e-cigarettes have the potential to compete with conventional cigarettes?: a survey of conventional cigarette smokers’ experiences with e-cigarettes. Chest 2013; 144: 1609–1614 [DOI] [PubMed] [Google Scholar]
- 37.Zhu SH, Gamst A, Lee M et al. The use and perception of electronic cigarettes and snus among the U.S. population. PloS One 2013; 8: e79332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bhatnagar A, Whitsel LP, Ribisl KM et al. Electronic cigarettes: a policy statement from the American Heart Association. Circulation 2014; 130: 1418–1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zamir Z, Mahmud A, Feely J. Acute haemodynamic effects of cigarette smoking in healthy young subjects. Irish J Med Sc 2006; 175: 20–23 [DOI] [PubMed] [Google Scholar]
- 40.Omvik P. How smoking affects blood pressure. Blood Pressure 1996; 5: 71–77 [DOI] [PubMed] [Google Scholar]
- 41.Parati G, Faini A, Valentini M. Blood pressure variability: its measurement and significance in hypertension. Curr Hypertens Rep 2006; 8: 199–204 [DOI] [PubMed] [Google Scholar]
- 42.Staessen JA, Kuznetsova T, Roels HA et al. Exposure to cadmium and conventional and ambulatory blood pressures in a prospective population study. Public Health and Environmental Exposure to Cadmium Study Group. Am J Hypertens 2000; 13: 146–156 [DOI] [PubMed] [Google Scholar]
- 43.Astor BC, Matsushita K, Gansevoort RT et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with mortality and end-stage renal disease. A collaborative meta-analysis of kidney disease population cohorts. Kidney Int 2011; 79: 1331–1340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van der Velde M, Matsushita K, Coresh J et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. A collaborative meta-analysis of high-risk population cohorts. Kidney Int 2011; 79: 1341–1352 [DOI] [PubMed] [Google Scholar]
- 45.Jha P, Peto R. Global effects of smoking, of quitting, and of taxing tobacco. N Engl J Med 2014; 370: 60–68 [DOI] [PubMed] [Google Scholar]