Abstract
Background: Plasma D-dimer is closely related to prognosis in several cancers. The aim of the current study was to determine the prognostic value of plasma D-dimer in patients with resectable esophageal squamous cell carcinoma (ESCC).
Methods: A total of 337 patients with resectable ESCC were enrolled in this retrospective study. The 5-year cancer-specific survival (CSS) was calculated by Kaplan-Meier method. Cox regression analyses were performed to evaluate the prognostic factors. A nomogram model was also performed to predict the cancer prognosis.
Results: In our study, there were 242 patients (71.8%) with plasma D-dimer ≤ 0.5 μg/ml and 95 patients (28.2%) with plasma D-dimer > 0.5 μg/ml. There was a significantly better 5-year CSS in patients with plasma D-dimer ≤ 0.5 μg/ml than patients with plasma D-dimer > 0.5 μg/ml (35.5% vs. 21.1%, P < 0.001). Multivariate analyses reported that plasma D-dimer was an independent prognostic factor in patients with resectable ESCC (P < 0.001). In addition, a nomogram was also performed to predict the CSS. The Harrell's c-index was 0.68.
Conclusion: We conclude that plasma D-dimer was an independent prognostic biomarker in patients with resectable ESCC.
Keywords: plasma D-dimer, biomarker
Introduction
In China, the cancer incidence and mortality have been increasing 1. Esophageal cancer (EC) remains the 4th leading cause of cancer death worldwide 2. Esophageal squamous cell carcinoma (ESCC) is the most common pathological type (90-95%) in China 3,4. Therefore, a study that takes into account the ESCC in China is very important.
In recent years, there were some studies reporting the relationship between plasma D-dimer and prognosis for several cancers 5-7. However, few studies regarding plasma D-dimer in patients with EC are available, and the clinical significance and prognostic value of plasma D-dimer in EC remain controversial 8,9. Therefore, the aim of the current study was to determine the prognostic value of plasma D-dimer in patients with resectable ESCC. In addition, we initially used a nomogram to predict the prognostic value of D-dimer in patients with resectable ESCC.
Patients and Methods
A retrospective analysis was conducted for patients with ESCC in our hospital from January 2005 to December 2008. The eligibility criteria were included: (1) ESCC was confirmed by histopathology; (2) patients with curative esophagectomy and standard lymphadenectomy; (3) patients without distant metastasis; (4) patients without preoperative neoadjuvant therapy; and (5) preoperative plasma D-dimer was obtained before surgery within one week. Patients who had a history of venous thrombosis or anticoagulation therapy, cardiovascular or cerebrovascular disease were excluded. At last, 337 patients were enrolled in our study. This study was approved by the Ethical Committees of Zhejiang Cancer Hospital (Hangzhou, China).
The plasma D-dimer were extracted in a retrospective medical records in our hospital. The D-dimer was assayed with a latex-enhanced immunoturbidimetric assay. The reference value for D-dimer was less than 0.5 μg/ml (≤ 0.5μg/ml). Then, it was categorized into two groups: normal (D-dimer ≤ 0.5μg/ml) and high (D-dimer > 0.5 μg/ml).
In our hospital, patients were followed up in out-patient department every 3-6 months for the first 2 years after initial surgery, then annually. In the current study, a cancer-specific survival (CSS) analysis was ascertained. The last follow-up was 30 June 2013.
Statistical analysis
The 5-year CSS was analysed by the Kaplan-Meier method. Univariate and multivariate Cox analyses were performed to analyse the prognostic factors. A receiver operating characteristic (ROC) curve was also plotted to determine the accuracy of D-dimer for prognosis prediction (survival vs. death). The area under curve (AUC) was used as an estimation of diagnostic accuracy. A nomogram for possible prognostic factors (including age and sex) associated with CSS was established by R software with the Harrell's concordance index (c-index) 10,11. A P < 0.05 was considered to be statistically significant. Statistical analysis was conducted with R 3.2.3 software (Institute for Statistics and Mathematics, Vienna, Austria) and SPSS 17.0 (SPSS Inc., Chicago, IL, USA).
Results
Of the 337 patients, 294 were males and 43 were females. The mean age was 59.0 ± 7.7 years (range 36-80 years). The mean plasma D-dimer was 0.86 ± 0.98 μg/ml (range 0.12-6.22 μg/ml). There were 242 patients (71.8%) with D-dimer ≤ 0.5 μg/ml and 95 patients (28.2%) with D-dimer > 0.5 μg/ml. Clinicopathologic characters between the normal (≤ 0.5 μg/ml) and high (> 0.5 μg/ml) groups for plasma D-dimer were shown in Table 1. In our study, however, there were no significant differences between plasma D-dimer and clinical factors.
Table 1.
Comparison of baseline clinical characteristics based on D-dimer
| Cases (n) | D-dimer (μg/ml) | P-value | D-dimer (μg/ml) | P-value | |
|---|---|---|---|---|---|
| (mean ± SD) | ≤ 0.5 (n) > 0.5 (n) | ||||
| Age (years) | 0.802 | 0.591 | |||
| ≤60 | 198 | 0.87 ± 0.98 | 140 58 | ||
| >60 | 139 | 0.84 ± 1.00 | 102 37 | ||
| Gender | 0.384 | 0.441 | |||
| Female | 43 | 0.74 ± 1.06 | 33 10 | ||
| Male | 294 | 0.88 ± 0.98 | 209 85 | ||
| Tumor length (cm) | 0.241 | 0.958 | |||
| ≤ 3.0 | 88 | 0.75 ± 0.78 | 63 25 | ||
| > 3.0 | 249 | 0.90 ± 1.05 | 179 70 | ||
| Tumor location | 0.786 | 0.696 | |||
| Upper/Middle | 176 | 0.84 ± 0.95 | 128 48 | ||
| Lower | 161 | 0.87 ± 1.04 | 114 47 | ||
| Vessel invasion | 0.739 | 0.835 | |||
| Negative | 279 | 0.87 ± 1.02 | 201 78 | ||
| Positive | 58 | 0.82 ± 0.81 | 41 17 | ||
| Differentiation | 0.740 | 0.513 | |||
| Well/Moderate | 269 | 0.87 ± 1.00 | 191 78 | ||
| Poor | 68 | 0.82 ± 0.92 | 51 17 | ||
| T stage | 0.358 | 0.290 | |||
| T1-2 | 114 | 0.79 ± 0.94 | 86 28 | ||
| T3-4 | 223 | 0.89 ± 1.01 | 156 67 | ||
| N stage | 0.820 | 0.584 | |||
| N0 | 180 | 0.87 ± 0.98 | 127 53 | ||
| N1-3 | 157 | 0.85 ± 1.00 | 115 42 |
Univariate analyses revealed that tumor length (P = 0.004), vessel invasion (P = 0.003), T stage (P < 0.001), N stage (P < 0.001) and D-dimer (P < 0.001) were predictive of CSS. In multivariate analyses, we demonstrated that plasma D-dimer was an independent prognostic factor in patients with resectable ESCC (P < 0.001) (Table 2).
Table 2.
Univariate and multivariate analyses of CSS in ESCC patients
| CSS % | P-value | Univariate analysis | P-value | Multivariate analysis | P-value | |
|---|---|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | |||||
| Age (years) | 0.674 | 0.677 | - | - | ||
| ≤ 60 | 31.8 | 1.000 | ||||
| > 60 | 30.9 | 1.057 (0.814-1.374) | ||||
| Gender | 0.510 | 0.514 | - | - | ||
| Female | 37.2 | 1.000 | ||||
| Male | 30.6 | 1.143 (0.765-1.708) | ||||
| Tumor length (cm) | 0.003 | 0.004 | 0.402 | |||
| ≤ 3.0 | 42.0 | 1.000 | 1.000 | |||
| > 3.0 | 27.7 | 1.588 (1.163-2.168) | 1.159 (0.821-1.638) | |||
| Tumor location | 0.764 | 0.766 | - | - | ||
| Upper/Middle | 29.0 | 1.000 | ||||
| Lower | 34.2 | 0.962 (0.742-1.246) | ||||
| Vessel invasion | 0.003 | 0.003 | 0.414 | |||
| Negative | 34.4 | 1.000 | 1.000 | |||
| Positive | 17.2 | 1.616 (1.175-2.223) | 1.152 (0.821-1.616) | |||
| Differentiation | 0.105 | 0.109 | - | - | ||
| Well/Moderate | 32.7 | 1.000 | ||||
| Poor | 26.5 | 1.292 (0.944-1.767) | ||||
| T stage | <0.001 | <0.001 | 0.012 | |||
| T1-2 | 45.6 | 1.000 | 1.000 | |||
| T3-4 | 24.2 | 1.949 (1.455-2.611) | 1.523 (1.095-2.120) | |||
| N stage | <0.001 | <0.001 | <0.001 | |||
| N0 | 42.8 | 1.000 | 1.000 | |||
| N1-3 | 18.5 | 2.060 (1.586-2.676) | 1.763 (1.326-2.344) | |||
| D-dimer (μg/ml) | <0.001 | <0.001 | <0.001 | |||
| ≤ 0.5 | 35.5 | 1.000 | 1.000 | |||
| > 0.5 | 21.1 | 1.738 (1.318-2.291) | 1.834 (1.389-2.422) |
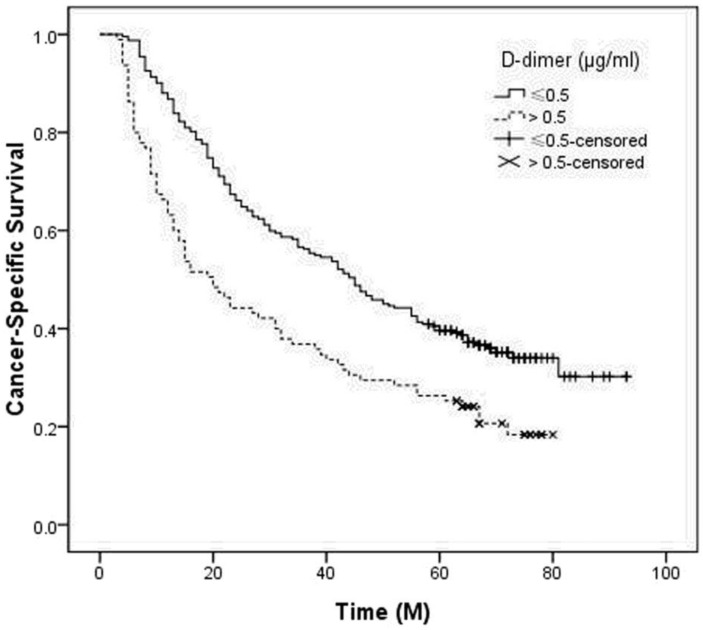
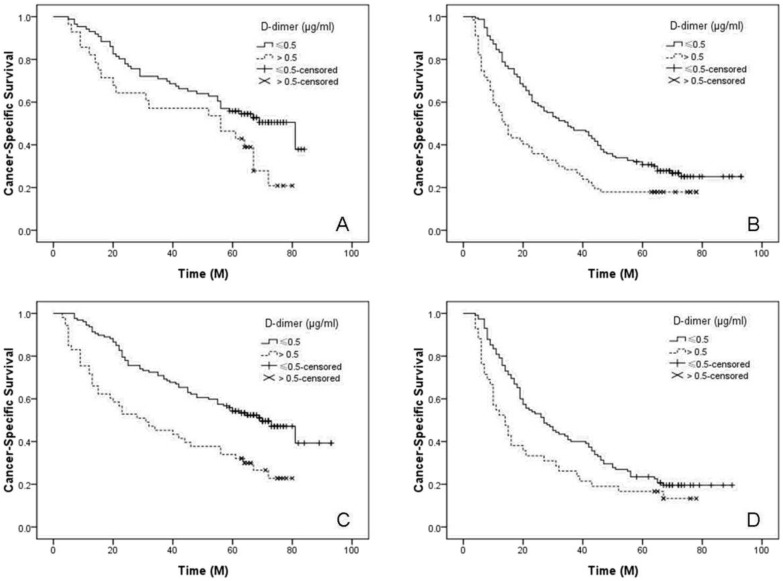
In our study, the 5-year CSS was 31.5%. Patients with normal D-dimer (≤ 0.5 μg/ml) had a significantly better 5-year CSS than patients with high D-dimer (> 0.5 μg/ml) (35.5% vs. 21.1%, P < 0.001) (Figure 1). Then, we further stratified patients into different groups based on T stage (T1-2 vs. T3-4) and N stage (N0 vs. N1-3) (Figure 2).
Figure 1.
Kaplan-Meier CSS curves stratified by D-dimer. Patients with D-dimer≤ 0.5 μg/ml had a significantly better 5-year CSS than patients with D-dimer > 0.5 μg/ml (35.5% vs. 21.1%, P < 0.001).
Figure 2.
Kaplan-Meier CSS curves stratified by T stage and N stage. For subgroup analysis, the predictive value of D-dimer was significant in patients with T1-2 (P = 0.033, A), T3-4 (P < 0.001, B), N0 (P < 0.001, C) and N1-3 (P = 0.020, D).
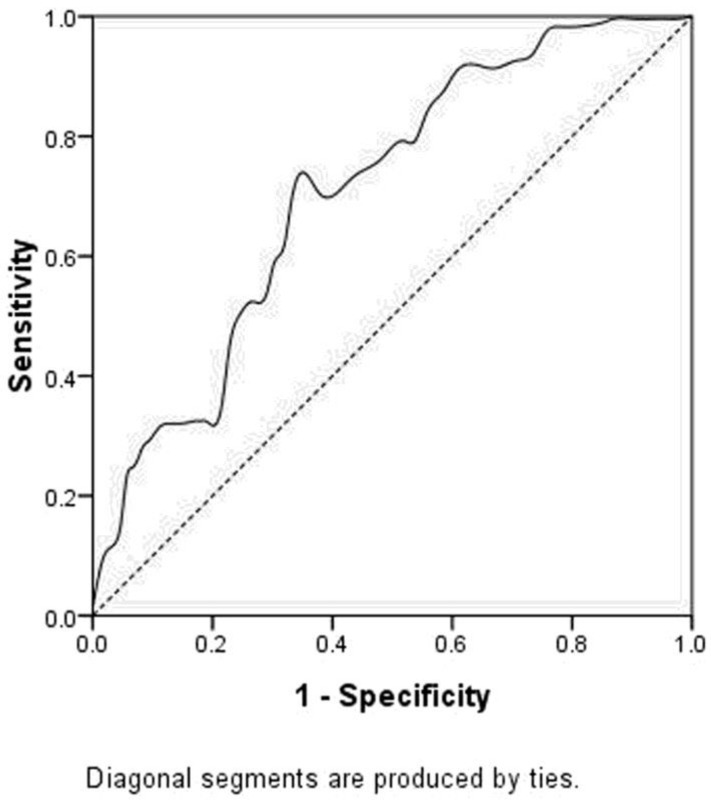
A ROC curve was also plotted to determine the accuracy of plasma D-dimer for prognosis prediction. The AUC for D-dimer was 0.708 (P<0.001). It demonstrated that D-dimer predicts cancer prognosis with a sensitivity of 35.5% and a specificity of 78.3% (Figure 3).
Figure 3.
ROC curve for CSS prediction. The area under curve (AUC) for D-dimer weas 0.708 (P<0.001). It demonstrated that D-dimer predicts cancer prognosis with a sensitivity of 35.5% and a specificity of 78.3%.
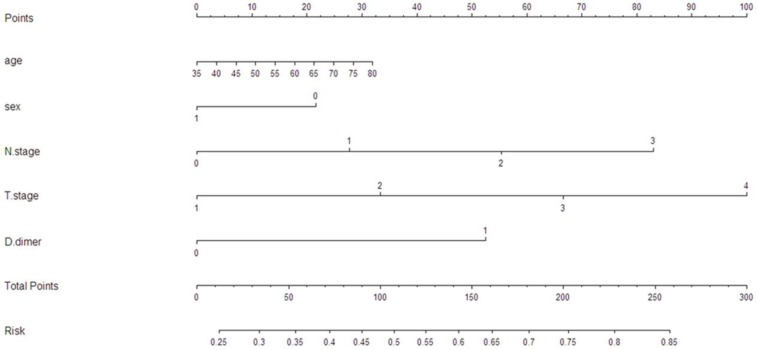
To predict the survival risk for patients with resectable ESCC, a nomogram model was performed by multivariate Cox regression model (Figure 4). It can predict the probability of death for ESCC patients after initial surgery. The Harrell's c-index for survival risk was 0.68.
Figure 4.
A nomogram predicts survival risk based on D-dimer and other prognostic factors in patients with ESCC. The nomogram is used by totaling the points identified at the top of the scale for each independent factor. This total point score is then identified on the total points scale to determine the probability of risk prediction. The Harrell's c-index for CSS prediction was 0.68.
Discussion
In our study, we determined the prognostic role of plasma D-dimer in predicting prognosis for patients with resectable ESCC. We concluded that plasma D-dimer was an independent prognostic factor in patients with resectable ESCC. Moreover, this study was the first attempt to establish a predictive nomogram to improve predictive accuracy based on D-dimer.
It is well-known that plasma D-dimer is a stable fibrin degradation product. Plasma D-dimer is usually used for the assessment of suspect venous thromboembolism 12. In addition, some studies reported that high levels of plasma D-dimer are also observed in myocardial infarction, infectious disease and trauma 13,14. In recent years, there were some studies reporting the relationship between plasma D-dimer and prognosis for several cancers 5-7. However, few studies regarding plasma D-dimer in patients with EC are available 8,9. In our study, D-dimer was an independent prognostic factor in patients with resectable ESCC. Our result is quite consistent with the previous study showing that D-dimer and albumin was a prognostic factor for patients with ESCC 9, but is contrary to the result of Zhang et al. 8, who suggested that D-dimer was not a prognostic factor.
So far, the exact mechanism between plasma D-dimer and cancer remains unclear. Several studies revealed that plasma D-dimer, as a stable fibrin degradation product, was shown to be closely associated with cancer progression 15,16. In addition, Fukumoto et al.17 reported that plasma D-dimer may reflect the micrometastases or circular tumor cells, which may be responsible for cancer recurrence.
In the current study, we attempted to perform a nomogram model to predict the survival risk based on D-dimer and other prognostic factors. Based on our model, we believe that it could be a simple and easy tool for estimating the survival risk for patients with ESCC. In our study, the Harrell's c-index was 0.68.
Limitations should be acknowledged. Firstly, our study was a retrospective study. Secondly, we excluded patients with neoadjuvant treatment. Additionally, the value of plasma D-dimer was measured only once in each patient. Therefore, further studies are needed to demonstrate the relationship between plasma D-dimer and prognosis in patients with ESCC.
In summary, based on the results of the current study, we conclude that plasma D-dimer was an independent prognostic biomarker in patients with resectable ESCC. The nomogram based on D-dimer could be a simple and easy tool for estimating the prognosis.
References
- 1.Chen W, Zheng R, Zeng H, Zhang S, He J. Annual report on status of cancer in China, 2011. Chin J Cancer Res. 2015;27:2–12. doi: 10.3978/j.issn.1000-9604.2015.01.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA: Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 3.Lin Y, Totsuka Y, He Y, Kikuchi S, Qiao Y, Ueda J. et al. Epidemiology of esophageal cancer in Japan and China. J Epidemiol. 2013;23:233–42. doi: 10.2188/jea.JE20120162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keditsu KK, Jiwnani S, Karimundackal G, Pramesh CS. Multimodality management of esophageal cancer. Indian J Surg Oncol. 2013;4:96–104. doi: 10.1007/s13193-013-0216-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Antoniou D, Pavlakou G, Stathopoulos GP, Karydis I, Chondrou E, Papageorgiou C. et al. Predictive value of D-dimer plasma levels in response and progressive disease in patients with lung cancer. Lung Cancer. 2006;53:205–10. doi: 10.1016/j.lungcan.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 6.Dirix LY, Salgado R, Weytjens R, Colpaert C, Benoy I, Huget P. et al. Plasma fibrin D-dimer levels correlate with tumour volume, progression rate and survival in patients with metastatic breast cancer. Br J Cancer. 2002;86:389–95. doi: 10.1038/sj.bjc.6600069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma X, Li Y, Zhang J, Huang J, Liu L. Prognostic role of D-dimer in patients with lung cancer: a meta-analysis. Tumour Biol. 2014;35:2103–9. doi: 10.1007/s13277-013-1279-9. [DOI] [PubMed] [Google Scholar]
- 8.Zhang F, Chen Z, Wang P, Hu X, Gao Y, He J. Combination of platelet count and mean platelet volume (COP-MPV) predicts postoperative prognosis in both resectable early and advanced stage esophageal squamous cell cancer patients. Tumour Biol. 2016 doi: 10.1007/s13277-015-4774-3. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu DQ, Li FF, Jia WH. Cumulative scores based on plasma D-dimer and serum albumin levels predict survival inesophageal squamous cell carcinoma patients treated with transthoracic esophagectomy. Chin J Cancer. 2016;35:11. doi: 10.1186/s40880-015-0062-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kattan MW. Validating a prognosting model. Cancer. 2006;107:2523–4. doi: 10.1002/cncr.22314. [DOI] [PubMed] [Google Scholar]
- 11.Harrell FE. Regression modeling strategies. New York: Springer; 2001. [Google Scholar]
- 12.Pabinger I, Ay C. Biomarkers and venous thromboembolism. Arterioscler Thromb Vasc Biol. 2009;29:332–6. doi: 10.1161/ATVBAHA.108.182188. [DOI] [PubMed] [Google Scholar]
- 13.Durczynski A, Kumor A, Hogendorf P, Szymanski D, Grzelak P, Strzelczyk J. Preoperative high level of D-dimers predicts unresectability of pancreatic head cancer. World J Gastroenterol. 2014;20:13167–71. doi: 10.3748/wjg.v20.i36.13167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lippi G, Franchini M, Targher G, Favaloro EJ. Help me, Doctor! My D-dimer is raised. Annal Med. 2008;40:594–605. doi: 10.1080/07853890802161015. [DOI] [PubMed] [Google Scholar]
- 15.Blackwell K, Haroon Z, Broadwater G, Berry D, Harris L, Iglehart JD. et al. Plasma D-dimer levels in operable breast cancer patients correlate with clinical stage and axillary lymph node status. J Clin Oncol. 2000;18:600–8. doi: 10.1200/JCO.2000.18.3.600. [DOI] [PubMed] [Google Scholar]
- 16.Kasthuri RS, Taubman MB, Mackman N. Role of tissue factor in cancer. J Clin Oncol. 2009;27:4834–8. doi: 10.1200/JCO.2009.22.6324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fukumoto K, Taniguchi T, Usami N, Kawaguchi K, Fukui T, Ishiguro F. et al. The preoperative plasma D-dimer level is an independent prognostic factor in patients with completely resected non-small cell lung cancer. Surg Today. 2015;45:63–7. doi: 10.1007/s00595-014-0894-4. [DOI] [PubMed] [Google Scholar]