Abstract
By the method of 1H NMR prescreening and tracing the diagnostic proton signals of the methyl groups, three additional new triquinane-type sesquiterpenoids—chondrosterins K–M (1–3) and the known sesquiterpenoid anhydroarthrosporone (4)—were isolated from the marine fungus Chondrostereum sp. Their structures were elucidated on the basis of MS, 1D, and 2D NMR data. Chondrosterin K is a rare hirsutane sesquiterpenoid, in which a methyl group was migrated from C-2 to C-6 and has a double bond between C-2 and C-3. Compounds 1–3 showed significant cytotoxicities against various cancer cell lines in vitro.
Keywords: Chondrostereum sp., chondrosterin, hirsutane, sesquiterpenoid, cytotoxicity
1. Introduction
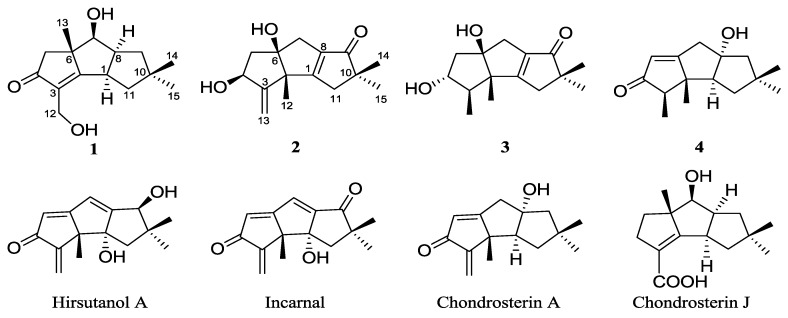
In recent decades, a large number of novel compounds were isolated from the soft corals collected from the South China Sea, which have significant biological activities, such as antitumor, antivirus, anti-hypertension, anti-inflammatory, and analgesic [1]. However, the limited supply of the soft corals and their pharmaceutical lead compounds makes the drug development a very slow process; so, searching for alternative drug resources has become a crucial task. Marine fungi associated with the soft corals can be expected to metabolize biologically interesting and chemically diverse compounds and draw much attention [2,3]. Naturally occurring sesquiterpenoids with hirsutane frameworks are the typical metabolites of some fungi. Up to now, about fifty hirsutane-type compounds have been reported, and some of them have significant biological activities, such as antibacterial [4,5,6,7], cytotoxic [6,7,8,9], and antimalarial activities [9]. The fungal strain Chondrostereum sp. was isolated from the soft coral of Sarcophyton tortuosum. Previous isolation of metabolites led to the discovery of hirsutane sesquiterpenoid compounds, chondrosterins A–F [10,11], I–J [12], hirsutanols A [13], C [10], E [13], and F [13], incarnal [11], and arthrosporone [11]. Among them, hirsutanol A, incarnal, and chondrosterin A and J (Figure 1) showed potent cytotoxicities. Hirsutanol A inhibited the growth of cancer cells by increasing the level of reactive oxygen species (ROS) [14,15,16].
Figure 1.
Chemical structures of compounds 1–4, hirsutanol A, incarnal, and chondrosterins A and J.
In our continued research project, the fungal strain Chondrostereum sp. was cultured in a large-scale glucose–peptone–yeast (GPY) medium. By tracing the characteristic proton NMR signals of the methyl groups around 1.00–1.40 ppm, chemical investigation of the extract led to the discovery of three new triquinane-type sesquiterpenoids, chondrosterins K–M (1–3), and the known sesquiterpenoid anhydroarthrosporone (4) (Figure 1) from the fungal culture extract. The structures of these metabolites were assigned on the basis of the detailed NMR and MS spectroscopic analysis. The isolation, structure identification, and cytotoxicities of these compounds are reported herein.
2. Results and Discussion
2.1. Structure Elucidation
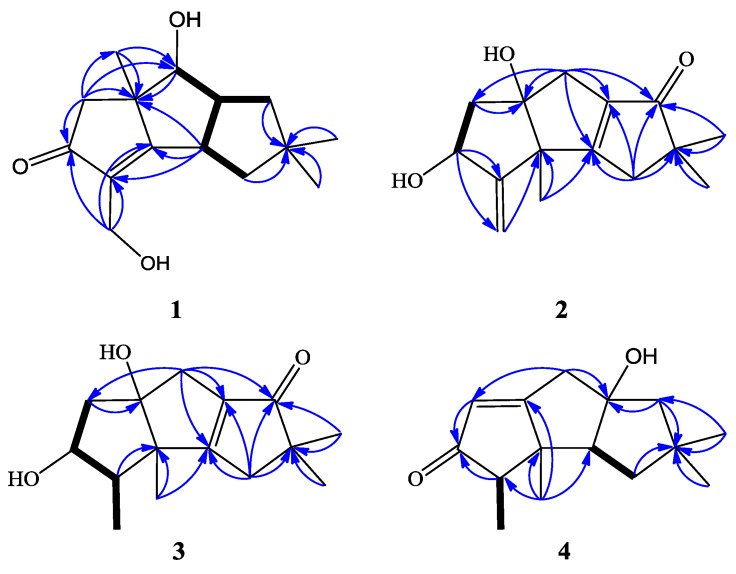
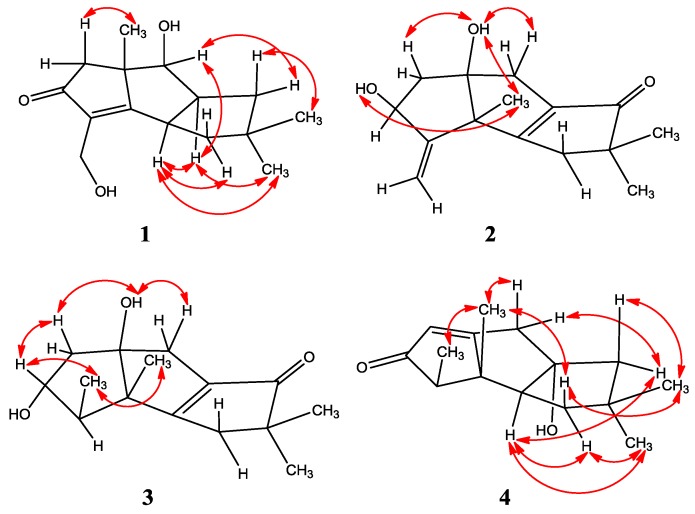
Chondrosterin K (1) was isolated as a colorless oil. The HR-EI-MS data at m/z 250.1568 [M]+ (Supplementary Figure S1), along with the NMR data (Table 1 and Table 2, Supplementary Figures S2–S7) revealed the molecular formula of compound 1 to be C15H22O3, and the degrees of unsaturation are five. The UV absorption at λmax 241 nm indicated a conjugated system formed by the carbonyl group and the double bond. So, this molecule must be tricyclic to count the five degrees of unsaturation. According to the 1H and 13C NMR and DEPT data (Table 1 and Table 2), compound 1 had three methyls, four methylenes, three methines, and five quaternary carbons. The typical functional groups included one carbonyl carbon (δC 210.1), one tetrasubstituted double bond (δC 186.4 and 135.2), three methyl group singlets (δH 1.01, 1.16, and 1.33), and two hydroxyl groups (δH 2.15, brs, 2H). The 1H–1H COSY cross-peaks of H-7 (CH, δH 4.05)/H-8 (CH, δH 3.16); H-8/H-9 (CH2, δH 1.92, 1.55), H-8/H-1 (CH, δH 3.47), and H-1/H-11 (CH2, δH 1.87, 1.63) deduced the fragment of –CHCH(CH2)CHCH2–. The HMBC correlations of H-1/C-2, H-1/C-6, H-5/C-4, H-5/C-6, H-5/C-13, H-7/C-6, H-7/C-13, H-9/C-10, H-11/C-10, H-12/C-3, H-12/C-4, H-13/C-5, H-13/C-6, H-13/C-7, H-14/C-10, and H-15/C-10 established the planar structure of compound 1 (Figure 2). The hydroxyl group at δH 2.15 (brs) connected with the methylene (CH2, δH 4.34, d, J = 13.2 Hz; 4.29, d, J = 13.2 Hz), and another hydroxyl group at δH 2.15 (brs) was connected to the methine group (C-7, δC 77.8). The NOESY correlations of H-1/H-8, H-1/H-11α, H-1/H-15, H-7/H-8, H-8/H-9α, and H-8/H-15 (Figure 3) revealed that H-1, H-7, H-8, and H-15 have an α-orientation. No NOESY correlation between H-13 and H-1, H-8 was observed, so C-13 was placed at the β position. Compound 1 is an unprecedented hirsutane-type sesquiterpenoid having a C-2/C-3 double bond in the molecule.
Table 1.
13C NMR data of compounds 1–4, δ in ppm.
| Position | 1 a | 2 | 3 | 4 | |||
|---|---|---|---|---|---|---|---|
| In CDCl3 | In CDCl3 a | In Acetone-d6 b | In CDCl3 b | In Acetone-d6 b | In CDCl3 a | In Acetone-d6 b | |
| 1 | 42.6, CH | 184.8, C | 184.6 | 183.7, C | 184.7 | 63.4, CH | 63.6 |
| 2 | 186.4, C | 59.3, C | 59.4 | 59.1, C | 59.0 | 53.4, C | 53.9 |
| 3 | 135.2, C | 156.8, C | 158.8 | 52.1, CH | 52.8 | 57.7, CH | 58.1 |
| 4 | 210.1, C | 75.9, CH | 73.4 | 77.7, CH | 76.1 | 211.6, C | 210.8 |
| 5 | 53.3, CH2 | 46.1, CH2 | 48.7 | 48.5, CH2 | 50.6 | 122.9, CH | 122.8 |
| 6 | 52.6, C | 93.2, C | 91.4 | 91.2, C | 90.1 | 190.8, C | 192.7 |
| 7 | 77.8, CH | 36.4, CH2 | 40.2 | 40.1, CH2 | 42.0 | 43.9, CH2 | 44.5 |
| 8 | 50.2, CH | 139.1, C | 140.2 | 141.2, C | 141.9 | 92.7, C | 92.7 |
| 9 | 41.6, CH2 | 209.7, C | 208.3 | 209.1, C | 208.4 | 55.9, CH2 | 56.2 |
| 10 | 43.2, C | 48.7, C | 48.9 | 48.8, CH | 49.0 | 43.3, C | 43.7 |
| 11 | 46.1, CH2 | 39.2, CH2 | 39.4 | 42.5, CH3 | 42.8 | 41.8, CH2 | 42.3 |
| 12 | 56.1, CH2 | 19.9, CH3 | 18.4 | 19.4, CH3 | 19.0 | 20.8, CH3 | 21.1 |
| 13 | 22.6, CH3 | 110.9, CH2 | 107.4 | 14.5, CH3 | 14.0 | 9.5, CH3 | 9.7 |
| 14 | 28.5, CH3 | 25.224, CH3 | 25.5 | 25.6, CH3 | 25.8 | 30.2, CH3 | 30.5 |
| 15 | 26.8, CH3 | 25.211, CH3 | 25.4 | 25.2, CH3 | 25.4 | 28.1, CH3 | 28.4 |
a Measured at 100 MHz; b Measured at 150 MHz.
Table 2.
1H NMR data of compounds 1 and 2, δ in ppm, J in Hz.
| Position | 1 a | 2 | |
|---|---|---|---|
| In CDCl3 a | In Acetone-d6 b | ||
| 1 | 3.47, ddd (10.4, 9.6, 9.2) | ||
| 4 | 4.45, dd (4.4, 2.0) | 4.32, dddd (7.8, 6.0, 1.8, 1.8) | |
| 5 | 2.35, s | β: 1.87, dd (14.0, 4.4) α: 2.08, dd (14.4, 2.0) |
β: 1.85, dd (12.6, 7.8) α: 2.17, dd (12.6, 6.0) |
| 7 | 4.05, d (9.2) | β: 2.43, dt (16.0, 3.2) α: 2.61, dt (16.0, 2.0) |
β: 2.45, ddd (16.8, 3.6, 3.0) α: 2.54, ddd (16.8, 3.6, 2.4) |
| 8 | 3.16, dddd (9.6, 9.6, 9.2, 9.2) | ||
| 9 | β: 1.55, ddd (12.8, 9.2, 2.0) α: 1.92, dd (12.8, 9.6) |
||
| 11 | β: 1.63, dd (12.8, 10.4) α: 1.87, ddd (12.8, 9.2, 2.0) |
2.31, dd (3.2, 2.0) | β: 2.27, ddd (18.0, 3.6, 3.0) α: 2.36, ddd (18.0, 3.6, 2.4) |
| 12 | β: 4.29, d (13.6) α: 4.34, d (13.6) |
1.31, s | 1.28, s |
| 13 | 1.33, s | 5.35, s 5.10, s |
5.23, d (1.8) 5.07, d (1.8) |
| 14 | 1.16, s | 1.12, s | 1.00, s |
| 15 | 1.01, s | 1.06, s | 1.07, s |
| 4-OH | 2.97, brs | 4.16, brs | |
| 6-OH | 2.97, brs | 2.98, brs | |
| 7-OH | 2.15, brs | ||
| 12-OH | 2.15, brs | ||
a Measured at 400 MHz; b Measured at 600 MHz.
Figure 2.
1H–1H COSY (bold line) and main HMBC (arrow) correlations of 1–4.
Figure 3.
Selected key NOESY correlations of 1–4.
Chondrosterin L (2) was isolated as a yellowish oil. The molecular formula of compound 2 was determined as C15H20O3 based on the HR-EI-MS data at m/z 248.1410 [M]+ (Supplementary Figure S8) and the NMR data (Table 1 and Table 2, Supplementary Figures S9–S16). The degrees of unsaturation are six. The UV spectrum peak at λmax 242 nm revealed an α,β-unsaturated carbonyl chromophore. Compound 2 contained three methyls, four methylenes, one methine, and seven quaternary carbons. The NMR data recorded in CDCl3 and Acetone-d6 are almost identical. The 1H–1H COSY spectrum recorded in CDCl3 showed the cross peak of H-4 (δH 4.45)/H-5 (δH 1.87 and 2.08) and established the structural fragment –CHCH2–. The carbonyl carbon at δC 209.7 connected the tetrasubstituted double bond (δC 184.8 and 139.1) forming a conjugated system and interpreted the UV absorption and the chemical shifts of the double bond at the lower shielding surrounding. The C-3 and C-13 formed one terminal double bond (δC 156.8, C; and δC 110.9, CH2). The HMBC correlations of H-4/C-3, H-4/C-13, H-5/C-6, H-7/C-6, H-7/C-8, H-7/C-9, H-11/C-1, H-11/C-9, H-11/C-10, H-12/C-1, H-12/C-2, H-13/C-2, H-14/C-9, H-14/C-10, H-15/C-9, and H-15/C-10 established the planar structure of 2 (Figure 2). C-4 and C-6 connected with the hydroxyl groups. Based on the NOESY correlations of 4-OH/H-12 (δH 1.31), 6-OH/H-5β (δH 1.87), 6-OH/H-7β (δH 2.43), and 6-OH/H-12 (Figure 3), 4-OH, 6-OH, and H-12 (CH3) were assigned as β positions.
Chondrosterin M (3) was isolated as a bright yellow oil. Its molecular formula was determined as C15H22O3 based on the HR-EI-MS data (m/z 250.1561 [M]+, Supplementary Figure S17), along with the NMR data (Table 1 and Table 3, Supplementary Figures S18–S25). Compound 3 has four methyls, three methylenes, two methines, and six quaternary carbons. 1H NMR data recorded in CDCl3 revealed three methyl groups with singlets (δH 1.12, 1.15, and 1.21), and one methyl group with doublet (δH 1.06) which connected with the methine carbon at C-3 (δC 52.1, δH 1.93); these are the diagnostic resonance signals of hirsutane sesquiterpenoids. By comparison, looking at the NMR data with compound 2, quick identification was made that a fragment of the CH3CH– in compound 3 was substituted the terminal C=C double bond in compound 2. The 1H–1H COSY cross-peaks of H-3 (CH, δH 1.93)/H-13(CH3, δH 1.06), H-3/H-4 (δH 3.63), and H-4/H-5 (CH, δH 1.91 and 2.28) (Figure 2) established the fragment CH3CHCHCH2–. The HMBC correlations of H-3/C-2, H-5/C-6, H-7/C-1, H-7/C-5, H-7/C-6, H-7/C-8, H-7/C-9, H-11/C-1, H-11/C-8, H-11/C-9, H-11/C-10, H-12/C-1, H-12/C-2, H-14/C-9, H-14/C-10, H-15/C-9, and H-15/C-10 (Figure 2) established the planar structure of 3. The NOESY correlations of 6-OH (δH 2.53, brs)/H-5 (δH 1.91), 6-OH/H-7 (δH 2.45), 6-OH/H-12 (δH 1.21, CH3), H-4/H-13, and H-12/H-13 (Figure 3) deduced that 6-OH, H-12, and H-13 were placed at β position, whereas 4-OH was placed at α position.
Table 3.
1H NMR data of compounds 3 and 4, δ in ppm, J in Hz.
| Position | 3 | 4 | ||
|---|---|---|---|---|
| In CDCl3 b | In Acetone-d6 b | In CDCl3 a | In Acetone-d6 b | |
| 1 | 2.38, dd (10.5, 8.4) | 2.42, dd (10.2, 9.0) | ||
| 3 | 1.93, dq (6.4, 7.2) | 1.76, dq (9.6, 7.2) | 2.32, q (7.2) | 2.27, q (7.2) |
| 4 | 3.63, dd (6.4, 5.6, 5.6) | 3.40, ddd (10.2, 9.6, 6.6) | ||
| 5 | β: 1.91, dd (13.2, 5.6) α: 2.28, dd (13.2, 5.6) |
β: 1.82, dd (12.6, 10.2) α: 2.24, dd (12.6, 6.6) |
5.82, d (1.8) | 5.69, d (1.8) |
| 7 | β: 2.45, d (10.0) α: 2.66, d (10.0) |
β: 2.45, d (10.0) α: 2.66, d (10.0) |
β: 2.71, dd (15.6, 1.8) α: 2.79, d (15.6) |
β: 2.75, d (15.6) α: 2.81, dd (15.6, 1.8) |
| 9 | β: 1.66, d (13.8) α: 1.86, dd (13.8, 1.2) |
β: 1.70, d (13.8) α: 1.85, dd (13.8, 1.2) |
||
| 11 | β: 2.38, d (10.0) α: 2.44, d (10.0) |
β: 2.39, d (10.0) α: 2.41, d (10.0) |
β: 1.46, dd (12.9, 10.5) α: 1.70, ddd (12.9, 8.4, 1.2) |
β: 1.51, dd (12.6, 10.2) α: 1.67, ddd (12.6, 9.0, 1.2) |
| 12 | 1.21, s | 1.16, s | 0.91, s | 0.92, s |
| 13 | 1.06, d (7.2) | 1.06, d (7.2) | 1.08, d (7.2) | 1.00, d (7.2) |
| 14 | 1.15, s | 1.08, s | 1.11, s | 1.08, s |
| 15 | 1.12, s | 1.03, s | 1.20, s | 1.19, s |
| 4-OH | 2.15, brs | 4.17, brs | ||
| 6-OH | 2.53, brs | 4.07, brs | ||
| 8-OH | 1.98, brs | 3.93, brs | ||
a Measured at 400 MHz; b Measured at 600 MHz.
Compound 4 has a molecular formula of C15H22O2 established by HR-EI-MS (m/z 234.1613) (Supplementary Figure S26) and NMR (Table 1 and Table 3, Supplementary Figures S27 and S28) data. It contains four methyls, three methylenes, three methines, and five quaternary carbons. The typical functional groups included one carbonyl carbon (δC 211.6), one tetrasubstituted double bond (δC 190.8 and 122.9), three methyl group singlets (δH 0.91, 1.11, and 1.20), and a methyl doublet at δH 1.08. The 1H–1H COSY spectra displayed the following cross-peaks: H-3 (δH 2.32)/H-13 (CH3, δH 1.08) and H-1(δH 2.38)/H-11(CH2, δH β: 1.46; α: 1.70), so the fragments CH3CH– and –CHCH2– were established. The HMBC correlations of H-3/C-4, H-5/C-4, H-7/C-5, H-7/C-6, H-7/C-8, H-9/C-8, H-9/C-10, H-11/C-10, H-12/C-1, H-12/C-2, H-12/C-3, H-12/C-6, H-14/C-9, H-14/C-10, H-14/C-11, H-15/C-9, H-15/C-10, and H-15/C-11 (Figure 2) established the planar structure of 6. The NOESY correlations of H-12/H-7β (δH 2.71), H-12/H-11β (δH 1.46), H-12/H-13, and H-14/H-11β (Figure 2) established C-12, C-13, and C-14 as β-oriented. In addition, NOESY correlations between H-1/H-9α (δH 1.86), H-1/H-11α (δH 1.70), and H-1/H-15 (Figure 3) allowed assignment of H-1 and H-15 in α-orientation. Compound 4 was identified as anhydroarthrosporone, which was firstly isolated by Amouzou E and co-workers from a basidiomycete fungus Ceratocystis ulmi [17]. Our NMR data are obviously different from the reference data, although both of them were recorded in the same solvent (CDCl3). For example, our 13C NMR data of C-1, C-3, C-6, C-7, and C-9 are 63.4, 57.7, 190.8, 43.9, and 55.9, respectively. As a comparison, the corresponding reference values are 57.7, 63.4, 177.0, 55.9, and 44.0, respectively [17].
2.2. Biological Evaluation
Seven cancer cell lines were used to examine the cytotoxicities of compounds 1–4 in vitro. This assay revealed that 1–3 had significant cytotoxic effects (Table 4). In contrast, 4 were apparently inactive in this assay (IC50 values > 100 μM). Hirsutanol A was used as a positive control.
Table 4.
Cytotoxicities of compounds 1–3, IC50 (μM)
| Cancer Cell Lines | 1 | 2 | 3 | Hirsutanol A |
|---|---|---|---|---|
| CNE1 | 17.66 | 33.55 | 42.00 | 10.08 |
| CNE2 | 12.03 | 22.50 | 44.08 | 12.72 |
| HONE1 | 22.06 | 34.60 | 46.11 | 17.40 |
| SUNE1 | 16.44 | 30.40 | 58.83 | 3.50 |
| A549 | 23.51 | 29.67 | 49.58 | 11.96 |
| GLC82 | 18.08 | 37.47 | 55.90 | 10.11 |
| HL7702 | 22.14 | 34.26 | 56.40 | 9.76 |
3. Materials and Methods
3.1. General Experimental Procedures
Preparative HPLC was performed using a Shimadzu LC-20AT HPLC pump (Shimadzu Corporation, Nakagyo-ku, Kyoto, Japan) equipped with an SPD-20A dual λ absorbance detector (Shimadzu Corporation, Nakagyo-ku, Kyoto, Japan) and a Shim-pack PRC-ODS HPLC column (250 mm × 20 mm, Shimadzu Corporation, Nakagyo-ku, Kyoto, Japan). Optical rotations were measured using a Schmidt and Haensch Polartronic HNQW5 optical rotation spectrometer (SCHMIDT + HAENSCH GmbH & Co., Berlin, Germany). UV spectra were recorded on a Shimadzu UV-VIS-NIR spectrophotometer (Shimadzu Corporation, Nakagyo-ku, Kyoto, Japan). IR spectra were recorded on a PerkinElmer Frontier FT-IR spectrophotometer (PerkinElmer Inc., Waltham, MA, USA). 1D and 2D NMR spectra were recorded on Bruker Avance III 400 and IIIT 600 HD spectrometers (Bruker BioSpin AG, Industriestrasse 26, Fällanden, Switzerland). The chemical shifts are relative to the residual solvent signals (CDCl3: δH 7.26 and δC 77.0; acetone-d6: δH 2.05 and δC 29.92). The low- and high-resolution EI mass spectra were obtained on Thermo DSQ and Thermo MAT95XP mass spectrometers (Thermo Fisher Scientific, Waltham, MA, USA), respectively.
3.2. Fungal Material
The marine fungus Chondrostereum sp. was isolated from the inner tissue of a soft coral of the species Sarcophyton tortuosum collected from the Hainan Sanya National Coral Reef Reserve, China. This fungal strain was deposited at School of Chemistry and Chemical Engineering, Sun Yat-sen University, Guangzhou 510275, China, and maintained in sterile aqueous solution of 15% (v/v) glycerol at −80 °C.
3.3. Fermentation, Extraction, and Isolation
The mycelia of Chondrostereum sp. were aseptically transferred to 500 mL Erlenmeyer flasks containing 200 mL of the sterilized GPY (glucose 10 g/L, peptone 5 g/L, yeast extract 2 g/L, NaCl 23 g/L) liquid medium. The flasks were then incubated at 28 °C on a rotary shaker (120 rpm) for 20 days. The cultures (200 L) were filtered through clean cheese cloth. The filtrate was extracted with ethyl acetate four times. The extract (31.6 g) was purified on a silica gel column with petroleum ether–EtOAc (100:0–0:100) and then EtOAc–MeOH (100:0–0:100) as the mobile phase to afford 12 fractions (code Fr. 1–Fr. 12). Fr. 6–7 were further purified by RP HPLC with an eluent of H2O–MeOH (40:60, v/v) to afford compounds 1 (8 mg), 2 (6 mg), and 3 (11 mg); compound 4 was obtained from Fr. 3 by Sephadex LH-20 gel column chromatography and repeated RP-HPLC eluted with H2O–MeCN (60:40, v/v).
Chondrosterin K (1): Colorless oil; [α] −31.1 (c 0.1, MeOH); UV (MeOH) λmax (log ε) 241 nm (4.02); IR (KBr) νmax 3356, 2926, 2856, 1690, 1650, 1513, 1455, 1367, 1262, 1228, 1109, 1058, 1033, 896, 829 cm−1; 1H and 13C NMR data, see Table 1; LR-EI-MS m/z 250, 232, 217, 199, 175, 123, 91, 77, 55; HR-EI-MS m/z 250.1568 [M]+ (calcd. for C15H22O3, 250.1563), 232.1456 [M − H2O]+ (calcd. for C15H20O2, 232.1458).
Chondrosterin L (2): Yellowish oil; [α] +66.5 (c 0.1, MeOH); UV (MeOH) λmax (log ε) 242 nm (3.86); IR (KBr) νmax 3375, 2926, 2855, 1683, 1629, 1514, 1456, 1383, 1264, 1229, 1107, 1061, 996, 909, 831, 829 cm−1; 1H and 13C NMR data, see Table 1; LR-EI-MS m/z 250, 232, 217, 199, 175, 123, 91, 77, 55; HR-EI-MS m/z 248.1410 [M]+ (calcd. for C15H20O3, 248.1414).
Chondrosterin M (3): Yellowish oil; [α] −1.17 (c 0.1, MeOH); UV (MeOH) λmax (log ε) 241 nm (3.46); IR (KBr) νmax 3357, 2928, 2856, 1681, 1626, 1452, 1432, 1388, 1283, 1234, 1110, 1049, 998, 922 cm−1; 1H and 13C NMR data, see Table 1; LR-EI-MS m/z 250, 232, 217, 199, 175, 123, 91, 77, 55; HR-EI-MS m/z 250.1561 [M]+ (calcd. for C15H22O3, 250.1563).
3.4. Cytotoxic Assay
The in vitro cytotoxicities of 1–4 were determined by means of the colorimetric 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay. The tested human cancer cell lines were seeded in 96-well plates at a density of 3 × 107 cells/L, and the compounds were added at various concentrations (0.125–50 mg/L). After 48 h, MTT was added to the culture medium at a final concentration of 0.5 mg/mL, and the plates were incubated for 4 h at 37 °C. The supernatant was removed. The formazan crystals were dissolved in DMSO (150 µL) with gentle shaking at room temperature. The absorbance at 570 nm was recorded with a microplate reader (Bio-Rad, Hercules, CA, USA), and the data were analyzed with the SPSS 13.0 software package. Hirsutanol A—a potent anticancer agent isolated from marine fungal metabolites—was used as a positive control, and its cytotoxicities against the tested cancer cell lines are shown in Table 4.
4. Conclusions
The marine fungus Chondrostereum sp. was cultured in PD medium and afforded three new hirsutane-type sesquiterpenoids, chondrosterins K–M (1–3), and the known compound anhydroarthrosporone (4). These results further indicated that the metabolites produced by Chondrostereum sp. in GPY [12,13] medium were different from those in PD [10,11] medium. By altering the fermentation conditions (e.g., carbon and nitrogen sources, inorganic salts), Chondrostereum sp. can produce highly functionalized hirsutane derivatives with a surprising chemodiversity. Furthermore, the metabolites isolation work based on 1H NMR screening seems to effectively obtain the novel hirsutane-type compounds.
Acknowledgments
This project is financially supported by Guangdong Provincial Science and Technology Research Program (Nos. 2013B021100010, 2013B021100012, 2014A020217004, 2015A020216007, and 2016A020222004), Guangzhou Science and Technology Research Program (No. 2014J4100059), and the Fundamental Research Funds for the Central Universities (Nos. 15ykpy05 and 14yksh01).
Supplementary Materials
The MS and NMR data of compounds 1–4 are available online at http://www.mdpi.com/1660-3397/14/9/157/s1. Figure S1: HR-EI-MS spectrum of chondrosterin K (1), Figure S2: 1H NMR spectrum of chondrosterin K (1) in CDCl3 (400 MHz), Figure S3: 13C NMR spectrum of chondrosterin K (1) in CDCl3 (100 MHz), Figure S4: HMQC spectrum of chondrosterin K (1), Figure S5: 1H–1H COSY spectrum of chondrosterin K (1), Figure S6: HMBC spectrum of chondrosterin K (1), Figure S7: NOESY spectrum of chondrosterin K (1), Figure S8: HR-EI-MS spectrum of chondrosterin L (2), Figure S9: 1H NMR spectrum of chondrosterin L (2) in CDCl3 (400 MHz), Figure S10: 13C NMR spectrum of chondrosterin L (2) in CDCl3 (100 MHz), Figure S11: HMQC spectrum of chondrosterin L (2), Figure S12: 1H–1H COSY spectrum of chondrosterin L (2), Figure S13: HMBC spectrum of chondrosterin L (2), Figure S14: NOESY spectrum of chondrosterin L (2), Figure S15: 1H NMR spectrum of chondrosterin L (2) in Acetone-d6 (600 MHz), Figure S16: 13C NMR spectrum of chondrosterin L (2) in Acetone-d6 (150 MHz), Figure S17: HR-EI-MS spectrum of chondrosterin M (3), Figure S18: 1H NMR spectrum of chondrosterin M (3) in CDCl3 (600 MHz), Figure S19: 13C NMR spectrum of chondrosterin M (3) in CDCl3 (150 MHz), Figure S20: HMQC spectrum of chondrosterin M (3), Figure S21: 1H–1H COSY spectrum of chondrosterin M (3), Figure S22: HMBC spectrum of chondrosterin M (3), Figure S23: NOESY spectrum of chondrosterin M (3), Figure S24: 1H NMR spectrum of chondrosterin M (3) in Acetone-d6 (600 MHz), Figure S25: 13C NMR spectrum of chondrosterin M (3) in Acetone-d6 (150 MHz), Figure S26: HR-EI-MS spectrum of anhydroarthrosporone (4), Figure S27: 1H NMR spectrum of anhydroarthrosporone (4) in CDCl3 (400 MHz), Figure S28: 13C NMR spectrum of anhydroarthrosporone (4) in CDCl3 (100 MHz), Figure S29: HMQC spectrum of anhydroarthrosporone (4), Figure S30: 1H–1H COSY spectrum of anhydroarthrosporone (4), Figure S31: HMBC spectrum of anhydroarthrosporone (4), Figure S32: NOESY spectrum of anhydroarthrosporone (4), Figure S33: 1H NMR spectrum of anhydroarthrosporone (4) in Acetone-d6 (600 MHz), Figure S34: 13C NMR spectrum of anhydroarthrosporone (4) in Acetone-d6 (150 MHz).
Author Contributions
Xiao-Feng Zhu and Hou-Jin Li conceived and designed the experiments; Lei Huang, Wen-Jian Lan, Rong Deng, Gong-Kan Feng, Qing-Yan Xu, Zhi-Yu Hu performed the experiments; Wen-Jian Lan and Hou-Jin Li wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Liang L.F., Guo Y.W. Terpenes from the soft corals of the genus Sarcophyton: Chemistry and biological activities. Chem. Biodiver. 2013;10:2161–2196. doi: 10.1002/cbdv.201200122. [DOI] [PubMed] [Google Scholar]
- 2.Hasan S., Ansari M.I., Mishra M., Ahmad A. Major bioactive metabolites from marine fungi: A Review. Bioinformation. 2015;11:176–181. doi: 10.6026/97320630011176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rateb M.E., Ebel R. Secondary metabolites of fungi from marine habitats. Nat. Prod. Rep. 2011;28:290–344. doi: 10.1039/c0np00061b. [DOI] [PubMed] [Google Scholar]
- 4.Stadler M., Anke T., Dasenbrock J. Phellodonic acid, a new biologically active hirsutane derivative from Phellodon melaleucus (Thelephoraceae, Basidiomycetes) Z. Naturforschung C. 1993;48:545–549. doi: 10.1515/znc-1993-7-803. [DOI] [PubMed] [Google Scholar]
- 5.Wang G.Y.S., Abrell L.M., Avelar A. New hirsutane based sesquiterpenes from salt water cultures of a marine sponge-derived fungus and the terrestrial fungus Coriolus consors. Tetrahedron. 1998;54:7335–7342. doi: 10.1016/S0040-4020(98)00398-6. [DOI] [Google Scholar]
- 6.Kupka J., Anke T., Giannetti B.M., Steglich W. Isolation and biological characterization of hypnophilin, pleurotellol, and pleurotellic acid from Pleurotellus hypnophilus (Berk.) Sacc. Arch. Microbiol. 1981;130:223–227. doi: 10.1007/BF00459523. [DOI] [Google Scholar]
- 7.Birnbacher J., Schüffler A., Deininger F. Isolation and biological activity of new norhirsutanes from Creolophus cirrhatus. Z. Naturforschung C. 2008;63:203–206. doi: 10.1515/znc-2008-3-407. [DOI] [PubMed] [Google Scholar]
- 8.Liermann J.C., Schüffler A., Wollinsky B. Hirsutane-type sesquiterpenes with uncommon modifications from three basidiomycetes. J. Org. Chem. 2010;75:2955–2961. doi: 10.1021/jo100202b. [DOI] [PubMed] [Google Scholar]
- 9.Rukachaisirikul V., Tansakul C., Saithong S., Pakawatchai C., Isaka M., Suvannakad R. Hirsutane sesquiterpenes from the fungus Lentinus connatus BCC8996. J. Nat. Prod. 2005;68:1674–1676. doi: 10.1021/np0502286. [DOI] [PubMed] [Google Scholar]
- 10.Li H.J., Xie Y.L., Xie Z.L., Chen Y., Lam C.K., Lan W.J. Chondrosterins A–E, triquinane-type sesquiterpenoids from soft coral-associated fungus Chondrostereum sp. Mar. Drugs. 2012;10:627–638. doi: 10.3390/md10030627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li H.J., Chen T., Xie Y.L., Chen W.D., Zhu X.F., Lan W.J. Isolation and structure elucidation of Chondrosterins F–H from the marine fungus Chondrostereum sp. Mar. Drugs. 2013;11:551–558. doi: 10.3390/md11020551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li H.J., Jiang W.H., Liang W.L., Huang J.X., Mo Y.F., Ding Y.Q., Lam C.K., Qian X.J., Zhu X.F., Lan W.J. Induced marine fungus Chondrostereum sp. as a means of producing new sesquiterpenoids Chondrosterins I and J by using glycerol as the carbon source. Mar. Drugs. 2014;12:167–175. doi: 10.3390/md12010167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li H.J., Lan W.J., Lam C.K., Yang F., Zhu X.F. Hirsutane sesquiterpenoids from the marine-derived fungus Chondrostereum sp. Chem. Biodiver. 2011;8:317–324. doi: 10.1002/cbdv.201000036. [DOI] [PubMed] [Google Scholar]
- 14.Yang F., Chen W.D., Deng R., Li D.D., Wu K.W., Feng G.K., Li H.J., Zhu X.F. Hirsutanol A induces apoptosis and autophagy via reactive oxygen species accumulation in breast cancer MCF-7 cells. J. Pharm. Sci. 2012;119:214–220. doi: 10.1254/jphs.11235FP. [DOI] [PubMed] [Google Scholar]
- 15.Yang F., Chen W.D., Deng R., Zhang H., Tang J., Wu K.W., Li D.D., Feng G.K., Lan W.J., Li H.J., et al. Hirsutanol A, a novel sesquiterpene compound from fungus Chondrostereum sp., induces apoptosis and inhibits tumor growth through mitochondrial-independent ROS production: Hirsutanol A inhibits tumor growth through ROS production. J. Transl. Med. 2013;11 doi: 10.1186/1479-5876-11-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang F., Gao Y.H., Wu K.W., Deng R., Li D.D., Wei Z.X., Jiang S., Wu X.Q., Feng G.K., Li H.J., et al. A novel sesquiterpene Hirsutanol A induces autophagical cell death in human hepatocellular carcinoma cells by increasing reactive oxygen species. Chin. J. Cancer. 2010;29:655–660. doi: 10.5732/cjc.009.10702. [DOI] [PubMed] [Google Scholar]
- 17.Etchri A., William A.A., Lois M.B. Antifungal sesquiterpenoids from an arthroconidial fungus. J. Nat. Prod. 1989;52:1042–1054. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.