Abstract
Ticks carry several human pathogenic microbes including Borreliae and Flavivirus causing tick-born encephalitis. Ticks can also carry DNA of Chlamydia-like organisms (CLOs). The purpose of this study was to investigate the occurrence of CLOs in ticks and skin biopsies taken from individuals with suspected tick bite. DNA from CLOs was detected by pan-Chlamydiales-PCR in 40% of adult ticks from southwestern Finland. The estimated minimal infection rate for nymphs and larvae (studied in pools) was 6% and 2%, respectively. For the first time, we show CLO DNA also in human skin as 68% of all skin biopsies studied contained CLO DNA as determined through pan-Chlamydiales-PCR. Sequence analyses based on the 16S rRNA gene fragment indicated that the sequences detected in ticks were heterogeneous, representing various CLO families; whereas the majority of the sequences from human skin remained “unclassified Chlamydiales” and might represent a new family-level lineage. CLO sequences detected in four skin biopsies were most closely related to “uncultured Chlamydial bacterium clones from Ixodes ricinus ticks” and two of them were very similar to CLO sequences from Finnish ticks. These results suggest that CLO DNA is present in human skin; ticks carry CLOs and could potentially transmit CLOs to humans.
Keywords: Chlamydiales, Chlamydia-like organisms (CLOs), ticks, phylogeny, 16S rRNA, PCR, skin
1. Introduction
A total of nine families have so far been recognised as members of the order Chlamydiales [1,2]. The most widely studied is the Chlamydiaceae-family that includes the well-known human pathogens Chlamydia trachomatis and C. pneumoniae, as well as several animal pathogens (some with zoonotic potential). Members of the remaining eight families (Clavichlamydiaceae, Criblamydiaceae, Piscichlamydiaceae, Parachlamydiaceae, Rhabdochlamydiaceae, Simkaniaceae, Waddliaceae, and Parilichlamydiaceae) are called Chlamydia-like organisms (CLOs). They share intracellular lifestyle, biphasic developmental cycle and a large core gene set (the “Pan-Genome of the Chlamydiae”) with the genus Chlamydia [3]. A variety of CLOs have been detected in various environmental (water and soil) samples, in amoebae and in animals, such as bats, deer, seabirds, snakes, arthropods, isopods and fish [2,4,5,6,7,8,9,10]. The role of CLOs as human pathogens is currently being explored: Recent publications have reported association between Waddlia chondrophila and tubal factor infertility [11], adverse pregnancy outcome [12] and lower respiratory tract infections [13]; Simkania negevensis and Rhabdochlamydia spp. may be associated with respiratory infections [14,15,16], and Parachlamydia acanthamoebae with pneumonia [17,18,19].
Ixodes ricinus, the most common species of tick in Europe, is known to carry and transmit several microbes pathogenic to animals and humans, including Borrelia burgdorferi sensu lato, the causative agent of Lyme disease, B. miyamotoi, causing relapsing fever, Anaplasma phagocytophilum, the etiologic agent of human anaplasmosis, and Babesia sp. causing babesiosis [20,21,22]. In addition, I. ricinus and Ixodes persulcatus can transmit tick-borne encephalitis (TBE) virus. The life cycle of the Ixodes tick involves four stages: egg, larva, nymph and adult. The Ixodes tick needs a blood meal during every post-hatching life stage, and thus needs to find a new host at each stage. Therefore, I. ricinus might transmit pathogens forward during the subsequent meal. While some Ixodes species are host specific, I. ricinus feeds on various species including humans. In Europe, I. ricinus is the most common vector known to transmit a variety of pathogenic microbes to humans, and ticks have also been shown to be carriers of CLO DNA. In Switzerland, a substantial number of ticks were collected and studied for Chlamydial DNA by a PCR method amplifying a fragment of the 16S rRNA gene [7,23]. In both studies, ticks were found to carry DNA of members from several families of the Chlamydiales order. Two-thirds of the sequenced samples belonged to the Rhabdochlamydiaceae and the Parachlamydiaceae families [23].
The aim of this study was to investigate the prevalence of CLOs in ticks and in skin. Consequently, we analysed more than 1800 Ixodes ticks (in pools and individually) collected from southwestern Finland for the presence of CLOs by PCR. To seek evidence of possible transmission of CLOs to human via tick bite, skin biopsies screened for Borrelia burgdorferi-specific DNA obtained from individuals with suspected tick-related skin manifestation and skin biopsies from healthy individuals were analysed.
2. Materials and Methods
2.1. Ticks and DNA Extraction
Questing Ixodes ricinus (Acari: Ixodidae) of all life stages were collected in an earlier study by blanket dragging from May to September in 2012 and 2013 [20,24]. Ticks (n = 1823) were collected from two rural islands, Seili and Boskär, located in the inner archipelago of the Archipelago Sea in southwestern Finland. Southwestern Finland is a region endemic to Lyme borreliosis spirochetes, where increasing tick abundance has recently been observed [20,24,25]. Furthermore, three adult Ixodes persulcatus samples were provided by Ritva Penttinen (Zoological Museum, University of Turku, Turku, Finland) for analysis. Total DNA was extracted from collected tick samples (n = 1826) using NucleoSpin® TriPrep-kits (Macherey-Nagel, Düren, Germany), following the protocol in the kit manual. More comprehensive description of tick sampling, DNA extraction, and other pathogens found in these ticks can be found in Sormunen, et al. [20,24].
2.2. Skin Samples and DNA Extraction
To gain support on whether the CLOs could be transmitted from ticks to humans through a tick bite, we studied a total of 80 archived DNA samples extracted from diagnostic skin biopsies obtained from patients with a suspected tick-borne skin reaction [26]. All above skin biopsies were obtained for routine histopathological analysis and Borrelia burgdorferi sensu lato DNA detection by PCR methods (16S rRNA and ospA as targets) as described earlier [27,28]. Of the specimens from individuals with suspected tick bite, 39 with PCR-confirmed B. burgdorferi infection and 41 PCR-negative were included in this study. As a control group, we studied 39 archived DNA samples extracted from healthy skin [29,30]. These samples were obtained from healthy adults during arthroscopy due to joint trauma (a small sample of skin was simultaneously collected from the arthroscopy wound edge at Dextra Medical Center, Helsinki, Finland), and from healthy hospital or laboratory staff (a punch biopsy). All subjects gave their informed consent for inclusion before they participated in the study. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of Helsinki and Uusimaa Hospital District (Dnro HUS 553/E6/01).
2.3. DNA Amplification and Sequencing
A total of 326 DNA samples extracted from ticks and pooled ticks, and 119 samples extracted from skin were screened for the presence of CLOs, by using a Chlamydiales-specific Pan-Chlamydiales real-time TaqMan PCR targeting the 16S rRNA-encoding gene [31]. This PCR method amplifies an approximately 200 bp fragment of the 16S rRNA-encoding gene, and has been shown to detect a wide range (at least all the 15 Chlamydial reference strains tested) of different members of the Chlamydiales order, and sensitive for at least five DNA copies per reaction of the positive control (with an efficiency of 75%). A PCR reaction of 25 μL contained 12.5 μL Maxima Probe/ROX qPCR Master Mix (2X) (Thermo Scientific, Waltham, MA, USA), 100 nM of primers (panCh-Fwd: 5′-ccgcaacactgggact-3′, panCh-R: 5′-ggagttagccggtgcttctttac-3′) and 100 nM probe (panCh-Probe: 5′-FAM-ctacgggaggctgcagtcgagaatc-BHQ1). Primers and probe were purchased from Integrated DNA technologies. Amplification was performed with 7500 Real-Time PCR system (Applied Biosystems). Cycling conditions were 50 °C/2 min, 95 °C/10 min, and 45 cycles of 95 °C/15 s, 60 °C/1 min.
Sequence analysis of 16S rRNA-encoding gene is a widely used preliminary method for bacterial species classification and identification. Thus, we also amplified and sequenced regions of Chlamydiales 16S rRNA-encoding gene from ticks and skin biopsies, and compared those to the reference sequences deposited in GenBank. Amplicons of the Pan-Chlamydiales qPCR were purified by Illustra ExoProStar 1-Step (GE Healthcare, Buckinghamshire, UK) as instructed by the manufacturer and sequenced using primer panFseq [31]. Sequencing was performed in the sequencing unit of Institute for Molecular Medicine Finland (https://www.fimm.fi/en/services/technology-centre/sequencing). BLAST analysis was performed in order to compare the gained CLO sequences to the known sequences in NCBI database. Sequence data has been deposited into the NCBI database (GenBank) and the accession numbers are provided in Table S1. A maximum likelihood tree was constructed of the 16S rRNA sequences and is shown in Figure S1 [32,33].
3. Results
3.1. Prevalence and Sequence Analysis of Chlamydia-Like Organisms (CLOs) in Ticks Collected from Finland
Altogether 326 DNA samples extracted from individual ticks and pooled ticks were analysed by Pan-Chlamydiales PCR targeting the 16S rRNA gene. CLO prevalence was 40% for adult ticks (19/47) (Table 1).
Table 1.
Prevalence of Chlamydiales DNA in Ixodes tick life stages.
| Tick Life Stage | No. of Ticks | No. of Positive Specimens 1 | No. of Samples Examined 2 | No. of Positive Samples/Total No. of Individuals (Prevalence of CLO DNA in Individual Ticks %) | No. of Positive Samples/ Total No. of Individuals (Minimum Infection Rate 3) |
|---|---|---|---|---|---|
| Adult | 47 | 19 | 47 | 19/47 (40.4%) | |
| Nymph | 497 | 30 | 215 | 30/497 (6.0%) | |
| Larva | 1282 | 22 | 63 | 22/1282 (1.7%) | |
| Total | 1826 | 71 | 325 | 52/1779 (2.9%) |
1 Five adult ticks and ten nymph pools also carried Borrelia DNA [24]; 2 Adult samples contained a single individual; nymph and larval samples were pools that contained 1–139 individuals; 3 Number of positive pools/total number of ticks.
The minimum infection rate (number of positive pools/total number of ticks) was 6.0% (30/497) for pooled nymph samples (215 pools, 1–14 individuals per pool, altogether 497 nymphs) and 1.7% (22/1282) for pooled larval samples (163 pools, 1–139 individuals per pool, altogether 1282 larval ticks).
All PCR products were sent for sequencing, and readable sequence (approximately 170 bp) data was obtained from 35 samples. Classification criteria (cut-offs of 97%, 95% and 90% of sequence identity within the 16S rRNA gene can be used for designation of species, genus and family levels of the Chlamydiales order, respectively) published by Everett [1] were used to classify CLOs [7,23]. When compared to Chlamydiales 16S rRNA gene sequences, 32 tick-derived CLO sequences showed above 90% identity with the earlier described strains allowing identification at the family level (Figure 1, Table 2).
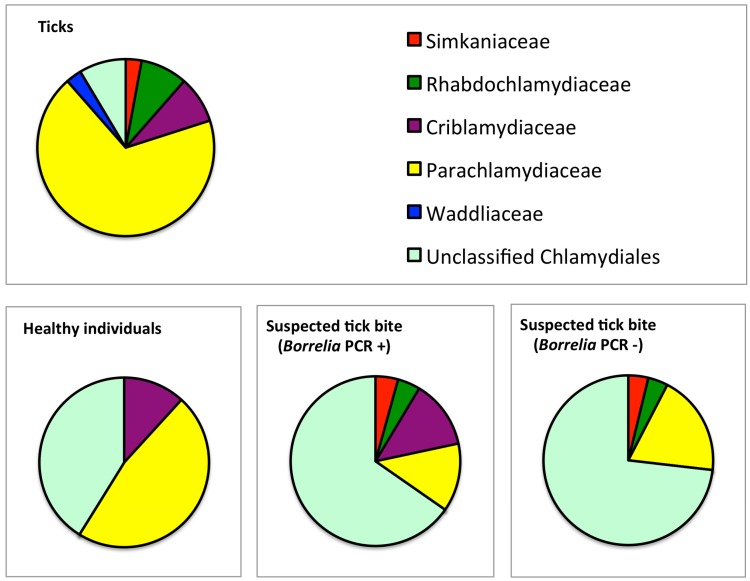
Figure 1.
Chlamydiales families in the sequenced pan-Chlamydiales PCR-positive tick and skin samples. CLOs belonging to the Parachlamydiaceae family were the most common family-level lineage in ticks. Unclassified Chlamydiales were prevalent in skin biopsies.
Table 2.
Sequencing results and classification of the 16S rRNA gene fragment of pan-Chlamydiales PCR-positive tick samples (n = 35).
| Family-Level (≥90%) 1 | Genus-Level (≥95%) 1 | Species-Level (≥97%) 1 |
|---|---|---|
| Parachlamydiaceae (n = 24) | Parachlamydia (n = 8) | Parachlamydia acanthamoebae (n = 2) |
| Neochlamydia (n = 4) | Neochlamydia sp. | |
| Protochlamydia (n = 1) | Trut23-12-2015_Venoge-Embouchure | |
| Candidatus Metachlamydia (n = 1) | (n = 1) | |
| ND (n = 10) | ND (n = 11) | |
| Rhabdochlamydiaceae (n = 3) | Rhabdochlamydia (n = 3) | Candidatus Rhabdochlamydia porcellionis strain 15C (n = 2) ND (n = 1) |
| Criblamydiaceae (n = 3) | ND (n = 3) | |
| Waddliaceae (n = 1) | ND (n = 1) | |
| Simkaniaceae (n = 1) | ND (n = 1) | |
| Chlamydiaceae | 0 | |
| Unclassified Chlamydiales (n = 3) |
The majority of the sequences belonged to the Parachlamydiaceae family (24 sequences, 69%) (Figure 1). Three (9%) sequences belonged to Rhabdochlamydiaceae and 3 (9%) to Criblamydiaceae family (Table 2). Interestingly, the best BLAST match for only two sequences (6%) was JQ860079, “uncultured Chlamydiales bacterium isolated from tick Ixodes ricinus.” The best BLAST match for a majority of the sequences was “uncultured Chlamydiales bacterium clone” from water sources, such as domestic shower heads, raw surface water, and amoebae (14/35, 40%), and “nasopharyngeal samples from hospitalized children” (12/35, 34%). Identity percentages and the GenBank accession numbers of the best BLAST hits are shown in Table S1.
3.2. Prevalence and Sequence Analysis of CLOs in Human Skin
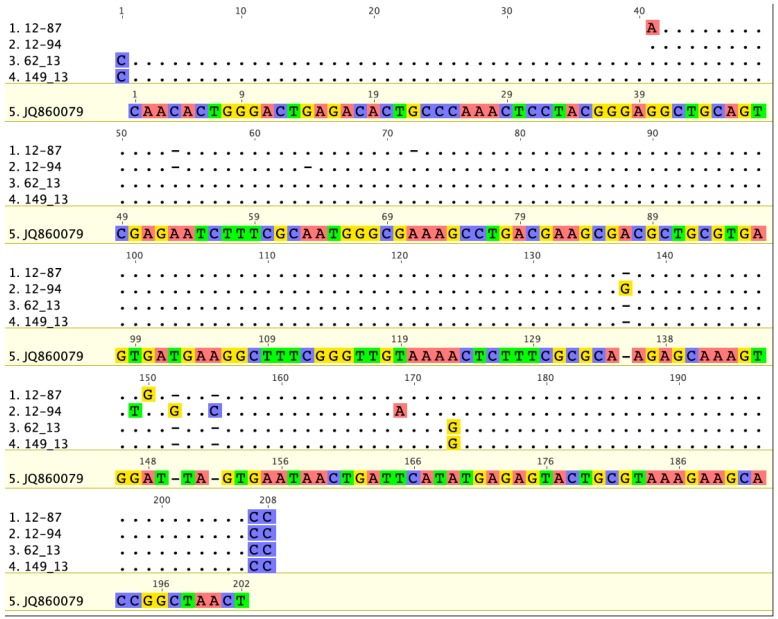
CLO DNA was detected in human skin, and the prevalence was higher in specimens from individuals with suspected tick bite (62/80, 78%) than in healthy skin (19/39, 49%). The pan-Chlamydiales PCR-positive samples (n = 81) were sent for sequencing, and sequences were obtained from 66 samples (Table S1). BLAST analysis revealed that four skin samples contained sequences that matched best with the GenBank sequences from “uncultured Chlamydiales bacterium clone” from Ixodes ricinus. Two of these sequences belonged to family Parachlamydiaceae and two to Rhabdoclamydiaceae. In addition, two of the tick-derived CLO sequences (62_13 and 149_13, Table S1) belonging to the family Rhabdochlamydiaceae were 95%–98% similar to the Rhabdochlamydiaceae CLO sequences from skin (12–87 and 12–94, Figure 2, Table S1).
Figure 2.
Comparative analysis of the CLO 16S rRNA sequences from ticks (63_13 and 149_13) and two skin biopsies (12–87 and 12–94). The sequences are 95%–98% similar. The best BLAST match for tick CLO sequences (JQ86007, from Ixodes ricinus) is shown as a reference. Geneious version 6.1 created by Biomatters. Available from http://www.geneious.com.
Sequences belonging to Rhabdochlamydiaceae or Simkaniaceae families were not detected in biopsies from healthy individuals (Figure 1, Table 3). Sequencing results showed that a majority (62%) of CLO sequences in skin showed < 90% identity not allowing classification at the family level. Again, “uncultured Chlamydiales bacterium clones” mainly from various water sources, were the best BLAST matches for the CLOs from skin. The best BLAST matches with accession numbers and identity percentages are presented in detail in Table S1.
Table 3.
Chlamydiales family-level lineages based on sequencing of the 16S rRNA gene fragment of pan-Chlamydiales PCR-positive skin biopsies (n = 66). Genus- and species-level information is shown in footnotes (if the level of classification could be determined).
| Family-Level Lineage 1 | Skin Condition (n) | ||
|---|---|---|---|
| Suspected Tick Bite (Borrelia PCR +) (n = 23) | Suspected Tick Bite (Borrelia PCR −) (n = 26) | Healthy Skin (n = 17) | |
| Parachlamydiaceae | 3 | 4 | 8 5 |
| Criblamydiaceae | 3 | 2 6 | |
| Rhabdochlamydiaceae | 1 3 | 2 4 | |
| Simkaniaceae | 1 | 1 | |
| Chlamydiaceae | 0 | 0 | 0 |
| Unclassified Chlamydiales 2 | 15 | 19 | 7 |
1 Taxonomy cutoffs defined by Everett et al. [1] and applied to classification of CLOs by Pilloux et al. [23]; 2 CLO sequences showed <90% identity not allowing classification at the family level [1]; 3 Genus: Rhabdochlamydia (n = 1); 4 Genus: Rhabdochlamydia (n = 1), Candidatus Rhabdochlamydia porcellionis strain 15C (n = 1); 5 Genus: Parachlamydia (n = 2); 6 Genus: Estrella (n = 1).
4. Discussion
The first recovery of Chlamydial organisms (called psittacosis-lymphogranuloma venereum agents at that time) in ticks was reported in 1969 [34]. Our study investigated the presence and diversity of Chlamydia-like organisms (CLO) DNA in ticks and in human skin with pan-Chlamydiales-PCR and sequence analysis of the PCR product. Altogether, 40% of the investigated adult ticks, at minimum 3% of the tick pools, and 68% of all the human skin biopsies studied were positive for CLO DNA. To our knowledge, this is the first time CLOs have been examined and found in human skin biopsies. The estimated prevalence of Chlamydiales DNA in Ixodes ricinus in an earlier study was 4%–28% [7], but a later study consisting of a larger collection of ticks estimated only < 1% of individual ticks to carry Chlamydial genetic material [23]. Of ticks harvested in Algeria, as much as 45% were shown to contain Chlamydial DNA [7]. In addition to rather high prevalence, a diversity of CLOs was observed in ticks. Among the ticks collected from southwestern Finland, most CLO sequences belonged to Parachlamydiaceae (74%), whereas a smaller proportion contained Rhabdochlamydaceae DNA (9%). Similarly, in a large number of ticks collected in Switzerland and in Algeria, the most prevalent CLO sequences detected belonged to the family Parachlamydiaceae (33%) and Rhabdoclamydiaceae (29%). The observed differences in prevalence and diversity may be due to differences in investigation strategy and methods: In the Swiss studies, ticks were analysed in large pools, and the prevalence is an estimate, whereas a portion of the Finnish and the Algerian ticks were examined individually, albeit in smaller number. Moreover, differences in the environment, including host animals of the ticks, could partly explain this.
Transmission of Chlamydia to human via tick bite was indirectly suggested earlier by development of antibodies against Chlamydia after tick bite in children [35]. To assess whether CLOs could be transmitted to humans via ticks, we studied DNA extracted from skin biopsies taken from individuals with suspected tick bite in history. CLOs could be detected in 85% of the skin biopsies from B. burgdorferi PCR-positive and 71% from B. burgdorferi PCR-negative lesions. Moreover, the two Rhabdochlamydiaceae sequences from skin specimens showed 95%–98% similarity with the sequences from Finnish ticks. This suggests that ticks, indeed, could serve as a vector of transmission. Very little is known about the clinical relevance of CLOs in general and thus, practically nothing can be said about the significance of these findings. Also, specimens from healthy individuals contained CLO DNA. This is not surprising as recent microbiome studies have shown that skin carries DNA from various microbes [36,37], although Chlamydiales DNA as such was not detected in these studies. As a matter of fact, tick bites often go unnoticed, so we cannot exclude tick exposure in the healthy individuals. Indeed, Borrelia miyamotoi infection does not necessarily cause erythema migrans-like skin symptoms [38]. However, most of the sequences observed in healthy skin were related to water-associated CLO sequences. Indeed, CLOs are found in the environment and various water sources [39,40,41,42]. The sequence analysis of CLO DNA was performed of a highly variable ≤ 200 bp fragment of the 16S rRNA gene [7,31]. Criteria proposed by Everett [1] were used to putatively classify the sequences at the family-level and genus-level lineages [7]. A majority (62%) of CLO sequences in skin showed < 90% identity with established Chlamydial strains and were unclassified Chlamydiales. Although the discriminatory power of this approach can be limited, we suggest that CLO sequences in skin represent largely unknown, potentially novel family-level lineage(s) in Chlamydiales. As more whole genome sequences of CLOs are determined, more precise analyses can be performed. We cannot yet answer whether CLO DNA is associated with pathogenesis of skin disorders or whether CLO DNA stays in the skin after acquisition, like DNA from ssDNA viruses remains as a bioportfolio [29].
It is not known how and where CLOs are acquired and how they end up in human skin. The occurrence of CLOs in wild mammals in Finland, potential hosts for ticks, has thus far been poorly investigated. Earlier studies have shown that bats can be a reservoir for a variety of pathogens, including Bartonella species [43,44] and viruses [45,46]. Our earlier study shows that CLO DNA is found in bats (Myotis daubentonii) and their prey insects [10]. Phylogenetic analysis suggested that 56% of the CLO sequences obtained from bats and 39% of those from insects belong to the family Rhabdochlamydiaceae [10]. However, most tick-derived sequences did not assemble together with the bat-associated CLO sequences, suggesting that the majority of CLOs in ticks observed in this study did not originate from bats (data not shown). Major hosts of ticks in the wild include small rodents and deer [47,48], which should be the next avenue of research to pursue. We also showed that insects carry CLO DNA and sequences belonging to families Rhabdochlamydiaceae and Parachlamydiaceae were most common [10]. Thus, other arthropods besides ticks could also serve as a vector for CLOs and spread the bacterium to human skin through bites.
Sequences identical to or resembling most closely the Chlamydia genus were not detected in ticks. This confirms the earlier notion that C. trachomatis is a human pathogen and likely transmitted only between humans with the exception of flies that can carry C. trachomatis DNA in trachoma-endemic area [49]. Other species belonging to the genus Chlamydia were not identified in ticks either, although some of them are animal pathogens. Neither C. trachomatis nor C. pneumoniae DNA was detected in the skin biopsies studied here. Contradictory evidence of Chlamydia-specific DNA sequences in some conditions, such as mycosis fungoides and keratoderma blenorrhagicum, has been presented [50,51,52].
In conclusion, CLO DNA was frequently detected in human skin and ticks in Finland. Our findings are in agreement with the earlier studies showing that ticks carry CLO DNA. Moreover, our results show that CLOs can be detected in human skin, and a remarkable sequence similarity was observed between sequences from ticks and skin. The transmission routes of CLOs remain unknown, but ticks can represent a transmitting vector. The significance of CLOs in skin remains to be investigated.
Acknowledgments
This study was supported by the Academy of Finland, project #1285975 (MP), and by research grants from Helsinki-Uusimaa Hospital District (TYH2013405/MP and TYH2015318/MP), and Ella and Georg Ehrnrooth Foundation (EJV). We thank Ritva Penttinen for providing Ixodes persulcatus samples, and Anu Kaitonen for technical assistance.
Supplementary Materials
The following are available online at www.mdpi.com/2076-2607/4/3/28/s1, Figure S1: Phylogenetic tree of the 16S rRNA sequences analyzed in this study, Table S1: Sequencing results of positive pan-Chlamydiales (16S rRNA) qPCR tick and skin specimens collected in Finland.
Author Contributions
Kati Hokynar and Mirja Puolakkainen conceived and designed the experiments; Kati Hokynar performed the experiments; Kati Hokynar, Veera Timonen and Mirja Puolakkainen analyzed the data; Eero J. Vesterinen, Annamari Ranki, Jaana Panelius and Esa K. Partio contributed materials; Kati Hokynar, Jani J. Sormunen, Eero J. Vesterinen, Thomas Lilley and Mirja Puolakkainen wrote the paper; All authors contributed to, and approved the final version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Everett K.D., Bush R.M., Andersen A.A. Emended description of the order Chlamydiales, proposal of Parachlamydiaceae fam. nov. and simkaniaceae fam. nov., each containing one monotypic genus, revised taxonomy of the family Chlamydiaceae, including a new genus and five new species, and standards for the identification of organisms. Int. J. Syst. Bacteriol. 1999;49:415–440. doi: 10.1099/00207713-49-2-415. [DOI] [PubMed] [Google Scholar]
- 2.Taylor-Brown A., Vaughan L., Greub G., Timms P., Polkinghorne A. Twenty years of research into Chlamydia-like organisms: A revolution in our understanding of the biology and pathogenicity of members of the phylum Chlamydiae. Pathog. Dis. 2015;73:1–15. doi: 10.1093/femspd/ftu009. [DOI] [PubMed] [Google Scholar]
- 3.Collingro A., Tischler P., Weinmaier T., Penz T., Heinz E., Brunham R.C., Read T.D., Bavoil P.M., Sachse K., Kahane S., et al. Unity in variety—The pan-genome of the Chlamydiae. Mol. Biol. Evol. 2011;28:3253–3270. doi: 10.1093/molbev/msr161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aaziz R., Vorimore F., Verheyden H., Picot D., Bertin C., Ruettger A., Sachse K., Laroucau K. Detection of atypical Chlamydiaceae in roe deer (Capreolus capreolus) Vet. Microbiol. 2015;181:318–322. doi: 10.1016/j.vetmic.2015.10.018. [DOI] [PubMed] [Google Scholar]
- 5.Aaziz R., Gourlay P., Vorimore F., Sachse K., Siarkou V.I., Laroucau K. Chlamydiaceae in North Atlantic seabirds admitted to a wildlife rescue center in Western France. Appl. Environ. Microbiol. 2015;81:4581–4590. doi: 10.1128/AEM.00778-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corsaro D., Venditti D. Detection of Chlamydiae from freshwater environments by PCR, amoeba coculture and mixed coculture. Res. Microbiol. 2009;160:547–552. doi: 10.1016/j.resmic.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 7.Croxatto A., Rieille N., Kernif T., Bitam I., Aeby S., Peter O., Greub G. Presence of Chlamydiales DNA in ticks and fleas suggests that ticks are carriers of Chlamydiae. Ticks Tick Borne Dis. 2014;5:359–365. doi: 10.1016/j.ttbdis.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 8.Taylor-Brown A., Ruegg S., Polkinghorne A., Borel N. Characterisation of Chlamydia pneumoniae and other novel Chlamydial infections in captive snakes. Vet. Microbiol. 2015;178:88–93. doi: 10.1016/j.vetmic.2015.04.021. [DOI] [PubMed] [Google Scholar]
- 9.Chua P.K., Corkill J.E., Hooi P.S., Cheng S.C., Winstanley C., Hart C.A. Isolation of Waddlia malaysiensis, a novel intracellular bacterium, from fruit bat (Eonycteris spelaea) Emerg. Infect. Dis. 2005;11:271–277. doi: 10.3201/eid1102.040746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hokynar V.E.J., Lilley T.M., Pulliainen A.T., Korhonen S.J., Paavonen J., Puolakkainen M. Molecular evidence of Chlamydia-like organisms in the faeces of the bat myotis daubentonii. in preparation. [DOI] [PMC free article] [PubMed]
- 11.Verweij S.P., Kebbi-Beghdadi C., Land J.A., Ouburg S., Morre S.A., Greub G. Waddlia chondrophila and Chlamydia trachomatis antibodies in screening infertile women for tubal pathology. Microbes. Infect. 2015;17:745–748. doi: 10.1016/j.micinf.2015.09.019. [DOI] [PubMed] [Google Scholar]
- 12.Baud D., Regan L., Greub G. Emerging role of Chlamydia and Chlamydia-like organisms in adverse pregnancy outcomes. Curr. Opin. Infect. Dis. 2008;21:70–76. doi: 10.1097/QCO.0b013e3282f3e6a5. [DOI] [PubMed] [Google Scholar]
- 13.Pilloux L., LeRoy D., Brunel C., Roger T., Greub G. Mouse model of respiratory tract infection induced by Waddlia chondrophila. PLoS ONE. 2016;11:28. doi: 10.1371/journal.pone.0150909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heiskanen-Kosma T., Paldanius M., Korppi M. Simkania negevensis may be a true cause of community acquired pneumonia in children. Scand. J. Infect. Dis. 2008;40:127–130. doi: 10.1080/00365540701558680. [DOI] [PubMed] [Google Scholar]
- 15.Lamoth F., Jaton K., Vaudaux B., Greub G. Parachlamydia and Rhabdochlamydia: Emerging agents of community-acquired respiratory infections in children. Clin. Infect. Dis. 2011;53:500–501. doi: 10.1093/cid/cir420. [DOI] [PubMed] [Google Scholar]
- 16.Niemi S., Greub G., Puolakkainen M. Chlamydia-related bacteria in respiratory samples in Finland. Microbes Infect. 2011;13:824–827. doi: 10.1016/j.micinf.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 17.Greub G., Boyadjiev I., La Scola B., Raoult D., Martin C. Serological hint suggesting that Parachlamydiaceae are agents of pneumonia in polytraumatized intensive care patients. Ann. N Y Acad. Sci. 2003;990:311–319. doi: 10.1111/j.1749-6632.2003.tb07381.x. [DOI] [PubMed] [Google Scholar]
- 18.Greub G., Berger P., Papazian L., Raoult D. Parachlamydiaceae as rare agents of pneumonia. Emerg. Infect. Dis. 2003;9:755–756. doi: 10.3201/eid0906.020613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greub G. Parachlamydia acanthamoebae, an emerging agent of pneumonia. Clin. Microbiol. Infect. 2009;15:18–28. doi: 10.1111/j.1469-0691.2008.02633.x. [DOI] [PubMed] [Google Scholar]
- 20.Sormunen J.J., Penttinen R., Klemola T., Hanninen J., Vuorinen I., Laaksonen M., Saaksjarvi I.E., Ruohomaki K., Vesterinen E.J. Tick-borne bacterial pathogens in Southwestern Finland. Parasit. Vectors. 2016;9:168. doi: 10.1186/s13071-016-1449-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karbowiak G., Biernat B. The role of particular tick developmental stages in the circulation of tick-borne pathogens affecting humans in central europe. 2. Tick-borne encephalitis virus. Ann. Parasitol. 2016;62:3–9. doi: 10.17420/ap6201.25. [DOI] [PubMed] [Google Scholar]
- 22.Swanson S.J., Neitzel D., Reed K.D., Belongia E.A. Coinfections acquired from Ixodes ticks. Clin. Microbiol. Rev. 2006;19:708–727. doi: 10.1128/CMR.00011-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pilloux L., Aeby S., Gaumann R., Burri C., Beuret C., Greub G. The high prevalence and diversity of Chlamydiales DNA within Ixodes ricinus ticks suggest a role for ticks as reservoirs and vectors of Chlamydia-related bacteria. Appl. Environ. Microbiol. 2015;81:8177–8182. doi: 10.1128/AEM.02183-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sormunen J.J., Klemola T., Vesterinen E.J., Vuorinen I., Hytonen J., Hanninen J., Ruohomaki K., Saaksjarvi I.E., Tonteri E., Penttinen R. Assessing the abundance, seasonal questing activity, and Borrelia and tick-borne encephalitis virus (TBEV) prevalence of Ixodes ricinus ticks in a Lyme borreliosis endemic area in Southwest Finland. Ticks Tick Borne Dis. 2016;7:208–215. doi: 10.1016/j.ttbdis.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 25.Makinen J., Vuorinen I., Oksi J., Peltomaa M., He Q., Marjamaki M., Viljanen M.K. Prevalence of granulocytic Ehrlichia and Borrelia burgdorferi sensu lato in Ixodes ricinus ticks collected from Southwestern Finland and from Vormsi Island in Estonia. APMIS. 2003;111:355–362. doi: 10.1034/j.1600-0463.2003.1110209.x. [DOI] [PubMed] [Google Scholar]
- 26.Stanek G., Wormser G.P., Gray J., Strle F. Lyme borreliosis. Lancet. 2012;379:461–473. doi: 10.1016/S0140-6736(11)60103-7. [DOI] [PubMed] [Google Scholar]
- 27.Ranki A., Aavik E., Peterson P., Schauman K., Nurmilaakso P. Successful amplification of DNA specific for finnish Borrelia burgdorferi isolates in erythema chronicum migrans but not in circumscribed scleroderma lesions. J. Investig. Dermatol. 1994;102:339–345. doi: 10.1111/1523-1747.ep12371793. [DOI] [PubMed] [Google Scholar]
- 28.Eriksson P., Schroder M.T., NiIranen K., Nevanlinna A., Panelius J., Ranki A. The many faces of solitary and multiple erythema migrans. Acta. Derm. Venereol. 2013;93:693–700. doi: 10.2340/00015555-1549. [DOI] [PubMed] [Google Scholar]
- 29.Norja P., Hokynar K., Aaltonen L.M., Chen R., Ranki A., Partio E.K., Kiviluoto O., Davidkin I., Leivo T., Eis-Hubinger A.M., et al. Bioportfolio: Lifelong persistence of variant and prototypic erythrovirus DNA genomes in human tissue. Proc. Natl. Acad. Sci. USA. 2006;103:7450–7453. doi: 10.1073/pnas.0602259103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hokynar K., Soderlund-Venermo M., Pesonen M., Ranki A., Kiviluoto O., Partio E.K., Hedman K. A new parvovirus genotype persistent in human skin. Virology. 2002;302:224–228. doi: 10.1006/viro.2002.1673. [DOI] [PubMed] [Google Scholar]
- 31.Lienard J., Croxatto A., Aeby S., Jaton K., Posfay-Barbe K., Gervaix A., Greub G. Development of a new Chlamydiales-specific real-time PCR and its application to respiratory clinical samples. J. Clin. Microbiol. 2011;49:2637–2642. doi: 10.1128/JCM.00114-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guindon S., Gascuel O. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst. Biol. 2003;52:696–704. doi: 10.1080/10635150390235520. [DOI] [PubMed] [Google Scholar]
- 33.Kearse M., Moir R., Wilson A., Stones-Havas S., Cheung M., Sturrock S., Buxton S., Cooper A., Markowitz S., Duran C., et al. Geneious basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012;28:1647–1649. doi: 10.1093/bioinformatics/bts199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eddie B., Radovsky F.J., Stiller D., Kumada N. Psittacosis-lymphogranuloma venereum (PL) agents (Bedsonia, Chlamydia) in ticks, fleas, and native mammals in california. Am. J. Epidemiol. 1969;90:449–460. doi: 10.1093/oxfordjournals.aje.a121091. [DOI] [PubMed] [Google Scholar]
- 35.Facco F., Grazi G., Bonassi S., Magnani M., di Pietro P. Chlamydial and rickettsial transmission through tick bite in children. Lancet. 1992;339:992–993. doi: 10.1016/0140-6736(92)91572-P. [DOI] [PubMed] [Google Scholar]
- 36.Grice E.A., Kong H.H., Renaud G., Young A.C., Program N.C.S., Bouffard G.G., Blakesley R.W., Wolfsberg T.G., Turner M.L., Segre J.A. A diversity profile of the human skin microbiota. Genome. Res. 2008;18:1043–1050. doi: 10.1101/gr.075549.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oh J., Byrd A.L., Park M., Program N.C.S., Kong H.H., Segre J.A. Temporal stability of the human skin microbiome. Cell. 2016;165:854–866. doi: 10.1016/j.cell.2016.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krause P.J., Narasimhan S., Wormser G.P., Rollend L., Fikrig E., Lepore T., Barbour A., Fish D. Human borrelia miyamotoi infection in the united states. N. Engl. J. Med. 2013;368:291–293. doi: 10.1056/NEJMc1215469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thomas V., Casson N., Greub G. Criblamydia sequanensis, a new intracellular Chlamydiales isolated from Seine river water using amoebal co-culture. Environ. Microbiol. 2006;8:2125–2135. doi: 10.1111/j.1462-2920.2006.01094.x. [DOI] [PubMed] [Google Scholar]
- 40.Thomas V., Loret J.F., Jousset M., Greub G. Biodiversity of amoebae and amoebae-resisting bacteria in a drinking water treatment plant. Environ. Microbiol. 2008;10:2728–2745. doi: 10.1111/j.1462-2920.2008.01693.x. [DOI] [PubMed] [Google Scholar]
- 41.Wheelhouse N., Sait M., Gidlow J., Deuchande R., Borel N., Baily J., Caldow G., Longbottom D. Molecular detection of Chlamydia-like organisms in cattle drinking water. Vet. Microbiol. 2011;152:196–199. doi: 10.1016/j.vetmic.2011.03.040. [DOI] [PubMed] [Google Scholar]
- 42.Kahane S., Greenberg D., Newman N., Dvoskin B., Friedman M.G. Domestic water supplies as a possible source of infection with simkania. J. Infect. 2007;54:75–81. doi: 10.1016/j.jinf.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 43.Veikkolainen V., Vesterinen E.J., Lilley T.M., Pulliainen A.T. Bats as reservoir hosts of human bacterial pathogen, bartonella mayotimonensis. Emerg. Infect. Dis. 2014;20:960–967. doi: 10.3201/eid2006.130956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lilley T.M., Veikkolainen V., Pulliainen A.T. Molecular detection of candidatus bartonella hemsundetiensis in bats. Vector. Borne Zoonotic. Dis. 2015;15:706–708. doi: 10.1089/vbz.2015.1783. [DOI] [PubMed] [Google Scholar]
- 45.Drexler J.F., Corman V.M., Muller M.A., Maganga G.D., Vallo P., Binger T., Gloza-Rausch F., Cottontail V.M., Rasche A., Yordanov S., et al. Bats host major mammalian paramyxoviruses. Nat. Commun. 2012;3:796. doi: 10.1038/ncomms1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rupprecht C.E., Turmelle A., Kuzmin I.V. A perspective on lyssavirus emergence and perpetuation. Curr. Opin. Virol. 2011;1:662–670. doi: 10.1016/j.coviro.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 47.Matuschka F.R., Fischer P., Musgrave K., Richter D., Spielman A. Hosts on which nymphal Ixodes ricinus most abundantly feed. Am. J. Trop. Med. Hyg. 1991;44:100–107. doi: 10.4269/ajtmh.1991.44.100. [DOI] [PubMed] [Google Scholar]
- 48.Jaenson T.G., Jaenson D.G., Eisen L., Petersson E., Lindgren E. Changes in the geographical distribution and abundance of the tick Ixodes ricinus during the past 30 years in Sweden. Parasit. Vectors. 2012;5:8. doi: 10.1186/1756-3305-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miller K., Pakpour N., Yi E., Melese M., Alemayehu W., Bird M., Schmidt G., Cevallos V., Olinger L., Chidambaram J., et al. Pesky trachoma suspect finally caught. Br. J. Ophthalmol. 2004;88:750–751. doi: 10.1136/bjo.2003.038661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abrams J.T., Vonderheid E.C., Kolbe S., Appelt D.M., Arking E.J., Balin B.J. Sezary T-cell activating factor is a Chlamydia pneumoniae-associated protein. Clin. Diagn. Lab. Immunol. 1999;6:895–905. doi: 10.1128/cdli.6.6.895-905.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rossler M.J., Rappl G., Muche M., Hasselmann D.O., Sterry W., Tilgen W., Reinhold U. No evidence of skin infection with Chlamydia pneumoniae in patients with cutaneous T cell lymphoma. Clin. Microbiol. Infect. 2003;9:721–723. doi: 10.1046/j.1469-0691.2003.00594.x. [DOI] [PubMed] [Google Scholar]
- 52.Carter J.D., Gerard H.C., Hudson A.P. Psoriasiform lesions induced by tumour necrosis factor antagonists: A skin-deep medical conundrum. Ann. Rheum. Dis. 2008;67:1181–1183. doi: 10.1136/ard.2007.082842. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.