Abstract
Objectives:
To determine seasonal variations in the vitamin D status of Saudi adolescent girls in Riyadh, Kingdom of Saudi Arabia (KSA) and its effect in biochemical and clinical characteristics.
Methods:
In this prospective study, a total of 2000 Saudi females aged 12-18 years from different schools in Riyadh, KSA participated and submitted a generalized questionnaire with clinical information. Fasting blood samples were obtained in 1618 subjects for the winter season (December to February) and only 499 subjects returned to submit fasting blood samples for the summer season (June-August). Circulating serum 25(OH)D, parathyroid hormone (PTH), and other biomarkers of bone remodeling were measured during both seasons.
Results:
Vitamin D deficiency (serum 25(OH)D <25 nmol/L) was significantly higher during summer than winter (63.5% versus 40.8%; p<0.001). Mean serum PTH was also significantly higher during summer than winter (p<0.01). In all subjects, serum PTH showed a significant inverse association with 25(OH)D at levels below 40 nmol/L (r=-0.21; p<0.001). The prevalence of subjects having clinical and metabolic manifestations suggestive of osteomalacia was 2.1% (N=33 out of 1548).
Conclusion:
Seasonal variations in the vitamin D status of Saudi adolescent females significantly modifies biochemical parameters as response to vitamin D status change. In the meantime, heightened public health awareness should be given to populations at higher risk for vitamin D deficiency.
Vitamin D deficiency has reemerged as a major health concern in the twenty-first century in both developed and developing countries.1-3 It remains the most common cause of rickets and osteomalacia, making it an important health concern for all ages.4,5 Furthermore, its occurrence in children and adolescence during skeletal growth is even more important. Vitamin D deficiency is a common clinical problem encountered in the Saudi population, including Saudi adolescent females.6-8 Chronic, severe deficiency can lead to osteomalacia during adolescence and adulthood.3-5 The condition is associated with non-specific generalized muscle and bone pain, as well as gait abnormalities which can be misdiagnosed as fibromyalgia or arthritis. The presentation of vitamin D deficiency may range from an asymptomatic state to clinical osteomalacia. To the best of our knowledge, the nutritional, biochemical and clinical impact of vitamin D deficiency among Saudi adolescent girls has not been evaluated previously, much more so with respect to seasonal changes. This study was undertaken to determine the prevalence of vitamin D deficiency among this age group and its biochemical and clinical characterization as influenced by season.
Methods
Subjects
In this prospective study, a total of 2000 school girls (aged 12-18 years) from intermediate and secondary schools in Riyadh, KSA (latitude, 24.6º N) were recruited during the period September 2011 to June 2014. Riyadh was stratified into 5 administrative regions. Proportional allocation method was used to determine the number of students recruited from each stratum. Within the stratum, one or more schools were selected randomly for recruitment. School girls from southern, northern, middle, eastern, and western regions were selected. Girls on vitamin D and calcium supplements or drugs interfering with vitamin D metabolism (for example, anticonvulsants) were excluded. Ethical approval was granted by King Abdulaziz City for Science and Technology (KACST), Riyadh, Kingdom of Saudi Arabia (KSA), in accordance to the principles of Helsinki declaration. Permission to conduct the study was also obtained from the Ministry of Education, Directorate of Schools Health. Informed consent was obtained from each student’s parent or guardian through an official letter from the principal investigator and his team. A written assent was obtained from all participants. A detailed flow diagram of subject participation is outlined in Figure 1.
Figure 1.
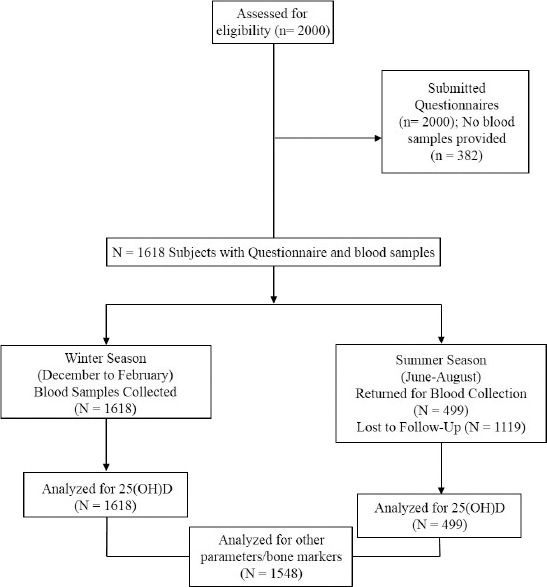
Flow diagram of subject recruitment of 2000 Saudi adolescent girls from Riyadh, Kingdom of Saudi Arabia.
All consenting subjects completed a questionnaire of 34 items, which included basic demographic data, medical history related to bone health and lifestyle data. Direct sun exposure to face and arms in minutes per day during weekdays and weekend in the last week was recorded. Dietary intake with emphasis on dietary vitamin D and calcium using the 7-day semi quantitative food frequency questionnaires were adapted for use from previous local studies.9,10 The questionnaire consisted of Saudi food list, definition of portion sizes, and frequency of consumption with emphasis on dietary calcium - in dairy or nondairy products, supplements or foods fortified with vitamin D. Subjects were also asked if they had bone pains. Pain was recorded as “present” or “absent” as perceived by the adolescent girl. Abducting shoulders against resistance tested proximal muscle weakness. Weakness was reported if muscle strength was less than grade 4 using the Medical Research Council (MRC) system.11 Waddling gait was reported if there was waddling and exaggerated lumbar lordosis, while walking on a straight line. Bowing of the legs was identified if there was (outward) bowing of the lower leg in relation to the thigh.
Anthropometrics
A general practitioner obtained anthropometric data. Height was measured in centimeters and weight (without shoes) - in kilograms. Body mass index (BMI) was calculated as weight (kg)/height in squared meters and classified according to Expert Committee Recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity.12
Blood sampling and analysis
Fasting serum samples were drawn during winter (December to February), and summer months (June to end of August) by 2 experienced nurses. Blood samples were frozen at -80ºC. Batches were analyzed within 3 days of receiving samples. The serum levels of calcium, phosphorous, and alkaline phosphatase were determined using Dimension Xpand Plus autoanalyzer (Siemens Healthcare Diagnostics, Erlangen, Germany). Serum 25(OH)D was measured using the electrochemiluminescence binding assay using Roche (Cobas e 411 analyzer, Indiana, USA). All measurements were performed in A RIQAS - Randox International Quality Assessment Scheme-participating laboratory of King Khalid University Hospital, Riyadh, KSA. The intra assay coefficient variation was 2.2-6.8, while the inter assay variablity was 3.4-13%. Serum parathyroid hormone (PTH) was measured using elecrochemiluminescence assay (Roche-modular E-170). Bone profile and creatinine were measured using the colorimetric method (Dade Behring Dimension RxL Max). Carboxy-terminal cllagen crosslinks (CTX) and osteocalcin were determined using electrchemiluminescence immunoassay by the Roche automated system.
Definitions used
Vitamin D inadequacy was defined based on the cut-off recommendations by the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) and the Institute of Medicine (IOM) of serum 25(OH)D below 50 nmol/l.13,14 The biochemical and clinical features suggestive of possible osteomalacia were vitamin D deficiency (25(OH)D <25 nmol/l) in the presence of bone pain and muscle weakness, as well as the following biochemical features:15 1) elevations in serum alkaline phosphatase; 2) normal or low serum calcium corrected for albumin; and 3) normal or low serum phosphorous level.
Statistical analysis
This sample size was calculated on the assumption that the prevalence of girls with clinical findings due to vitamin D deficiency may reach 10% (8-12%). From the calculation, a sample of N=875 girls were needed. However, due to the use of stratified random sampling method, a design effect of 2 was used and 15% for non-participation. The final sample size obtained was N=2000. The actual sample size obtained was N=1618, which was taken from subjects who submitted both questionnaires and fasting blood samples during winter season (Figure 1). The Statistical Package for Social Sciences version 21 (SPSS Inc., Chicago, IL, USA) was used for data analysis. Continuous data were presented as mean, median, and standard deviation, while frequencies were presented as percentages. Independent T-test was used to compare differences of normally distributed variables and Mann-Whitney for non-Gaussian variables. Chi square test was used to test association between categorical variables. Person coefficient correlation (R) was used to test correlation between 2 continuous variables. A scatter graph using the linear model was used to display the correlation. Significance was set at p<0.05.
Results
Table 1 highlights the demographic characteristics of the all subjects. Most of the subjects (55.8%) had minimal sun exposure (<10 minutes). Almost half of the subjects (43.5%) reported bone pain, and more than half of the subjects reported physical inactivity 56.2%). Dairy intake was also minimal, with more than half reporting <1 cup/day. The rest of the variables are reported in Table 1.
Table 1.
General characteristics of subjects.
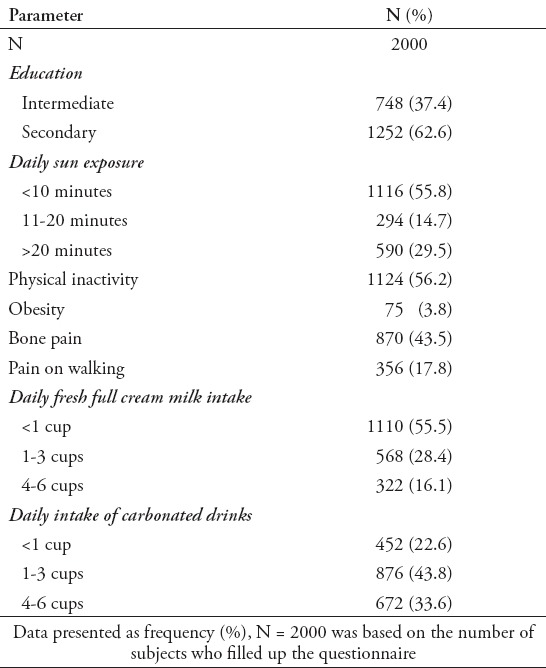
The differences in mean biochemical parameters of subjects based on season are presented in Table 2. Circulating 25(OH)D levels were significantly higher during the winter season than summer (p<0.001). Significantly higher mean serum calcium level was also observed in the winter than in the summer months (p<0.001). Furthermore, mean serum ALP and PTH levels were also significantly lower in the winter as compared with the summer season (Table 2). A significant negative correlation between serum calcium and alkaline phosphatase (r=-0.139, p<0.01) was observed (not shown in table). There were no significant changes in the levels of osteocalcin and CTX between summer and winter.
Table 2.
Demographics and differences in biochemical profile of subjects according to season.
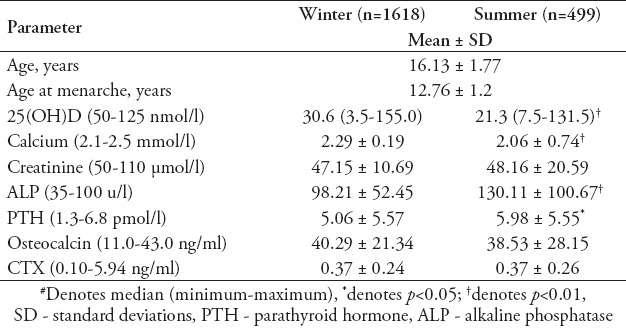
Table 3 shows the vitamin D status of subjects stratified and compared according to different risk factors. Vitamin D deficiency was most prevalent among subjects with no sun exposure (40.3%) and those with minimal intake of fresh milk (56.7%). Furthermore, there is a significant difference in the prevalence of vitamin D deficiency in relation to carbonated drinks consumption, with those consuming more having a higher percentage of vitamin D deficiency than those who consume less than once per day (p<0.001). The prevalence of 25(OH)D deficiency is significantly higher in the summer than winter season (p<0.001).
Table 3.
Vitamin D status according to risk factors.
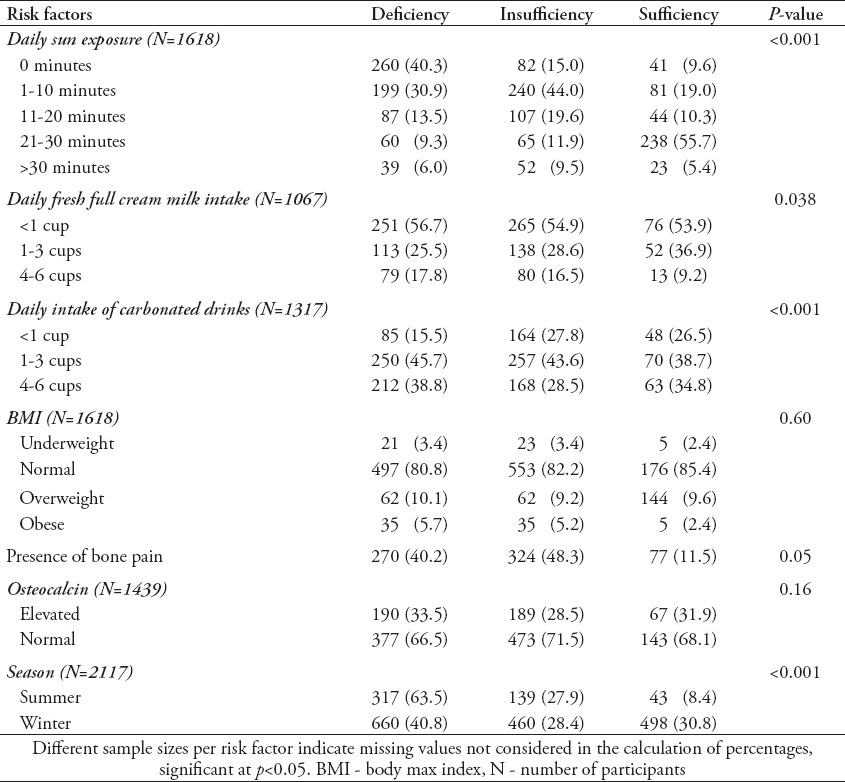
Table 4 summarizes the prevalence of vitamin D deficiency with other clinical abnormalities among subjects with complete clinical profile (N=1548). Elevated alkaline phosphatase was noted in 27.8% of subjects. The combined biochemical and clinical features suggestive of possible osteomalacia based on the criteria outlined were identified in 33 girls (2.13 %).
Table 4.
Prevalence of 25(OH)D deficiency percentage with other conditions suggestive of osteomalacia (n=1548 girls).
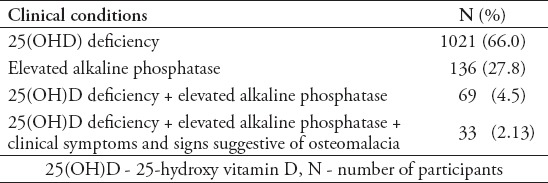
Figure 2 shows that the negative but insignificant correlation between low 25(OH)D level and PTH levels (r=0.024; p=0.351). However, at levels ≤40 nmol/l, the negative correlation significantly increased to r=0.15 (p<0.001).
Figure 2.
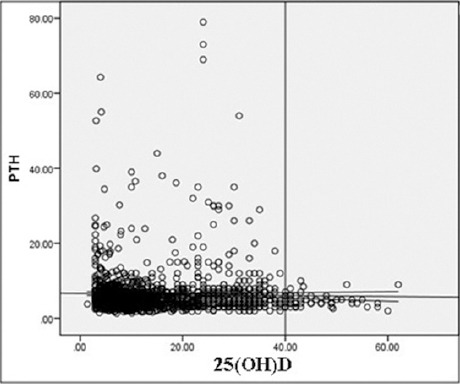
Correlation between vitamin D and parathyroid hormone (PTH) among Saudi adolescent girls in Riyadh, Kingdom of Saudi Arabia. 25(OH)D - 25-hydroxy vitamin D
Discussion
The present study documented a high prevalence of vitamin D deficiency (63.5% in summer and 40.8% in winter) among Saudi adolescent girls irrespective of season. This confirms many previous reports indicating a strikingly high prevalence of vitamin D deficiency in different age groups and in both genders of the Saudi population.16,17
The difference in the vitamin D levels between summer and winter was striking, and is opposite of what has been reported from countries at higher latitudes, which found higher vitamin D levels during summer months when people are more likely to be outdoors and obtaining increased sunlight exposure.18,19 Our findings are similar to the local observations made by Al-Daghri et al,20 which suggested that although summer sun exposure in Middle Eastern and Gulf States are minimal, an increase may occur during winter when the weather heat is less harsh, and this could raise vitamin D to modest levels.20 Worthy to note is that the optimum time for vitamin D formation in people living in the Riyadh area during summer is from 9:00Am-10: 30Am and from 2:00-3:00pm. In winter, it was suggested that the optimal time for sun exposure is from 10:00am-2:00pm.21
With regards to the seasonal variation, the Canadian Health Measures Survey (CHMS) examined 25(OH)D levels in a representative sample of the Canadian population, and in 456 girls aged 12-19 years, the mean 25(OH)D ranged from 60.8 nmol/l (winter, no supplement use) to 76.8 nmol/l (summer, no supplement use).22 The IOM report suggested that osteomalacia and rickets are most likely to be associated with 25(OH)D levels below 30 nmol/l;14 and in CHMS, this was found in 3.8% of Caucasian boys and girls, and 17.5% of “non-Caucasian” adolescents aged 12-19 years (non-Caucasian includes people of Chinese, South Asian, African, Filipino, Latin American, Southeast Asian, Arab, West Asian, Japanese, Korean, Aboriginal, and other racial backgrounds). An earlier study23 of 1753 Québec schoolchildren (871 girls) aged 9, 13, and 16 years, found a larger proportion had low vitamin D status, and in particular, 2%, 8%, and 10% of 9-, 13, and 16-year-old girls had frank vitamin D deficiency (defined as a serum 25(OH)D ≤27.5 nmol/l).23
The nutritional status of the girls, in relation to vitamin D deficiency and osteomalacia, revealed that a small number of the girls consume sufficient milk or other dairy products. This appears to be cultural or related to the currently prevailing dietary habits of girls in this age group. This low dairy intake was coupled with a relatively high intake of soft drinks. Worthy to note is that milk in KSA is fortified with vitamin D at 400 iu/L of milk. This could further exacerbate a pre-existing vitamin D insufficient state.
Our study revealed that 2.1% had vitamin D deficiency in the presence of elevations in alkaline phosphatase consistent with a clinical diagnosis of osteomalacia. It is evident that our cohort had variable degrees of vitamin D insufficiency, with evidence of secondary hyperparathyroidism observed in approximately 10% of subjects. A significant percentage of these individuals may have had elevations in serum PTH within the normal reference range.24 Ardawi et al17 found no threshold of serum 25(OH)D at which PTH levels plateaued in Saudi men. However, secondary hyperparathyroidism was evident in 18.5% and 24.6% in pre- and post-menopausal women with 25(OH)D below 50 nmol/l.16 In our study, the negative correlation between serum vitamin D levels and PTH appeared at around 40 nmol/l. This is lower than in some other studies. However, similar observations have been documented before. In Chinese premenopausal women, a serum vitamin D level of 40 nmol/l was found to be the optimum level to “suppress” PTH.25 In an older study by Lips et al,26 a level of 30 nmol/l was enough to suppress PTH level in elderly subjects. Interestingly, in Finnish adolescent girls, it was observed also that at levels of 40 nmol/l or above, PTH would start to be suppressed.27 This observation in our girls - in contrast with some other studies may be a reflection of elevations in serum PTH within the normal reference range. It is also evident that many girls were having variable degrees of hypocalcemia in summer, which could contribute to the clinical observation of osteomalacia. It is also possible that magnesium deficiency may be present in our study population, resulting in a blunted rise in PTH due to low intracellular magnesium.28,29 Intracellular reductions in magnesium can result in a paradoxical block of PTH secretion.30,31 We did not measure serum magnesium in our study population and this requires further evaluation.
This study has several limitations. Boys were not included, as the study concentrated on females who are at a higher risk for vitamin D deficiency. The presumptive diagnosis of osteomalacia was made only on biochemical and clinical grounds, and was not confirmed by bone biopsy. There is a possibility for potential biases regarding the study design, the reporting of pain, muscle weakness, and the choices in the dietary questionnaire. Nevertheless, this study documents the prevalence of vitamin D inadequacy and its potential clinical impact amongst Arab adolescent girls in the region, and raises the clinical importance of vitamin D deficiency in this under- studied population.
In conclusion, a high prevalence of vitamin D deficiency amongst Saudi adolescent girls was noted, with a greater frequency of this condition in the summer months than in the winter months. This significant difference in vitamin D status is accompanied by parallel changes in biochemical parameters of bone metabolism, suggesting seasonal influence. Serious attempts should be undertaken to address this serious health care issue and its consequences in this population. Girls should be encouraged to obtain sun exposure in schools and homes, even for a short time. More food fortification with vitamin D for different dairy products, cereals, and flour is needed in KSA. Further studies are needed to further characterize the clinical impact of severe vitamin D deficiency in adolescent Saudis.
Acknowledgment
The authors express their appreciation and gratitude to King Abdulaziz City for Science and Technology, Riyadh, Kingdom of Saudi Arabia for funding this study.
Footnotes
References
- 1.Cashman KD, Dowling KG, Škrabáková Z, Gonzalez-Gross M, Valtueña J, De Henauw S, et al. Vitamin D deficiency in Europe: pandemic? Am J Clin Nutr. 2016;103:1033–1044. doi: 10.3945/ajcn.115.120873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vaishya R, Vijay V, Agarwal AK, Jahangir J. Resurgence of vitamin D: old wine in new bottle. J Clin Orthop Trauma. 2015;6:173–183. doi: 10.1016/j.jcot.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 4.Gifre L, Peris P, Monegal A, Martinez de Osaba MJ, Alvarez L, Guañabens N. Osteomalacia revisited: a report on 28 cases. Clin Rheumatol. 2011;30:639–645. doi: 10.1007/s10067-010-1587-z. [DOI] [PubMed] [Google Scholar]
- 5.Wintermeyer E, Ihle C, Ehnert S, Stöckle U, Ochs G, de Zwart P, et al. Crucial role of vitamin D in the musculoskeletal system. Nutrients. 2016;8 doi: 10.3390/nu8060319. pii: E319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saggese G, Vierucci F, Boot AM, Czech-Kowalska J, Weber G, Camargo CA, Jr, et al. Vitamin D deficiency in childhood and adolescence: an expert position statement. Eur J Pediatr. 2015;174:565–576. doi: 10.1007/s00431-015-2524-6. [DOI] [PubMed] [Google Scholar]
- 7.Alsuwadia AO, Farag YM, Al Sayyari AA, Mousa DH, Alhejaili FF, Al-Harbi AS, et al. Prevalence of vitamin D deficiency in Saudi adults. Saudi Med J. 2013;34:814–818. [PubMed] [Google Scholar]
- 8.Al-Mogbel ES. Vitamin D status among Adult Saudi Females visiting Primary Health Care Clinics. Int J Health Sci (Qassim) 2012;6:116–126. doi: 10.12816/0005987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135:1114–1126. doi: 10.1093/oxfordjournals.aje.a116211. [DOI] [PubMed] [Google Scholar]
- 10.Al-Disi D, Al-Daghri N, Khanam L, Al-Othman A, Al-Saif M, Sabico S, et al. Subjective sleep duration and quality influence diet composition and circulating adipocytokines and ghrelin levels in teen-age girls. Endocr J. 2010;57:915–923. doi: 10.1507/endocrj.k10e-145. [DOI] [PubMed] [Google Scholar]
- 11.Miller ML. Muscle examinations in the evaluation of weakness. [Updated 2015 December 15]. Available from: http://www.uptodate.com/contents/muscle-examination-in-the-evaluation-of-weakness .
- 12.Barlow SE Expert Committee. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007;120(Suppl 4):S164–S192. doi: 10.1542/peds.2007-2329C. [DOI] [PubMed] [Google Scholar]
- 13.Rizzoli R, Boonen S, Brandi ML, Bruyère O, Cooper C, Kanis JA, et al. Vitamin D supplementation in elderly or postmenopausal women: a 2013 update of the 2008 recommendations from the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) Curr Med Res Opin. 2013;29:305–313. doi: 10.1185/03007995.2013.766162. [DOI] [PubMed] [Google Scholar]
- 14.Institute of Medicine. Dietary Reference Intakes for Calcium and Vitamin D. Washington (DC): National Academic Press (US); 2011. Available from: http://www.ncbi.nlm.nih.gov/books/NBK56070/ [Google Scholar]
- 15.Thacher TD, Clarke BL. Vitamin D insufficiency. Mayo Clin Proc. 2011;86:50–60. doi: 10.4065/mcp.2010.0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ardawi MS, Qari MH, Rouzi AA, Maimani AA, Raddadi RM. Vitamin D status in relation to obesity, bone mineral density, bone turnover markers and vitamin D receptor genotypes in healthy Saudi pre- and postmenopausal women. Osteoporosis Int. 2011;22:463–475. doi: 10.1007/s00198-010-1249-7. [DOI] [PubMed] [Google Scholar]
- 17.Ardawi MS, Sibiany AM, Bakhsh TM, Qari MH, Maimani AA. High prevalence of vitamin D deficiency among healthy Saudi Arabian men: relationship to bone mineral density, parathyroid hormone, bone turnover markers, and lifestyle factors. Osteoporosis Int. 2012;23:675–686. doi: 10.1007/s00198-011-1606-1. [DOI] [PubMed] [Google Scholar]
- 18.Manios Y, Moschonis G, Lyritis GP. Seasonal variations of vitamin D status in Greek postmenopausal women receiving enriched dairy products for 30 months: the Postmenopausal Health Study. Eur J Clin Nutr. 2011;65:412–414. doi: 10.1038/ejcn.2010.270. [DOI] [PubMed] [Google Scholar]
- 19.Luque-Fernandez MA, Gelaye B, VanderWeele T, Ferre C, Siega-Riz AM, Holzman C, et al. Seasonal variation of 25-hydroxyvitamin D among non-Hispanic black and white pregnant women from three US pregnancy cohorts. Paediatr Perinat Epidemiol. 2014;28:166–176. doi: 10.1111/ppe.12103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Al-Daghri NM, Al-Attas OS, Alokail MS, Alkharfy KM, El-Kholie E, Yousef M, et al. Increased vitamin D supplementation recommended during summer season in the gulf region: a counterintuitive seasonal effect in vitamin D levels in adult, overweight and obese Middle Eastern residents. Clin Endocrinol (Oxf) 2012;76:346–350. doi: 10.1111/j.1365-2265.2011.04219.x. [DOI] [PubMed] [Google Scholar]
- 21.Alshahrani FM, Almalki MH, Aljohani N, Alzahrani A, Alsaleh Y, Holick MF. Vitamin D: Light side and best time of sunshine in Riyadh, Saudi Arabia. Dermatoendocrinol. 2013;5:177–180. doi: 10.4161/derm.23351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Whiting SJ, Langlois KA, Vatanparast H, Greene-Finestone LS. The vitamin D status of Canadians relative to the 2011. Dietary Reference Intakes: an examination in children and adults with and without supplement use. Am J Clin Nutr. 2011;94:128–135. doi: 10.3945/ajcn.111.013268. [DOI] [PubMed] [Google Scholar]
- 23.Mark S, Gray-Donald K, Delvin EE, O'Loughlin J, Paradis G, Levy E, et al. Low vitamin D status in a representative sample of youth from Québec, Canada. Clin Chem. 2008;54:1283–1289. doi: 10.1373/clinchem.2008.104158. [DOI] [PubMed] [Google Scholar]
- 24.Heaney RP, Davies KM, Chen TC, Holick MF, Barger-Lux MJ. Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am J Clin Nutr. 2003;77:204–210. doi: 10.1093/ajcn/77.1.204. [DOI] [PubMed] [Google Scholar]
- 25.Bacon CJ, Woo J, Lau EM, Lam CW, Gamble GD, Reid IR. Effects of 25-hydroxyvitamin D level and its change on parathyroid hormone in premenopausal Chinese women. Osteoporos Int. 2010;21:1935–1941. doi: 10.1007/s00198-009-1163-z. [DOI] [PubMed] [Google Scholar]
- 26.Lips P, Wiersinga A, van Ginkel FC, Jongen MJ, Netelenbos JC, Hackeng WH, et al. The effect of vitamin D supplementation on vitamin D status and parathyroid function in elderly subjects. J Clin Endocrinol Metab. 1988;67:644–650. doi: 10.1210/jcem-67-4-644. [DOI] [PubMed] [Google Scholar]
- 27.Outila TA, Kärkkäinen MU, Lamberg-Allardt CJ. Vitamin D status affects serum parathyroid hormone concentrations during winter in female adolescents: associations with forearm bone mineral density. Am J Clin Nutr. 2001;74:206–210. doi: 10.1093/ajcn/74.2.206. [DOI] [PubMed] [Google Scholar]
- 28.Sahota O, Mundey MK, San P, Godber IM, Hosking DJ. Vitamin D insufficiency and the blunted PTH response in established osteoporosis: the role of magnesium deficiency. Osteoporos Int. 2006;17:1013–1021. doi: 10.1007/s00198-006-0084-3. [DOI] [PubMed] [Google Scholar]
- 29.Steen O, Khan A. Role of magnesium in parathyroid physiology. In: Bradi ML, Brown EM, editors. Hypoparathyroidism. Springer; 2015. pp. 61–68. [Google Scholar]
- 30.Quitterer U, Hoffmann M, Freichel M, Lohse MJ. Paradoxical block of parathormone secretion is mediated by increased activity of G alpha subunits. J Biol Chem. 2001;276:6763–6739. doi: 10.1074/jbc.M007727200. [DOI] [PubMed] [Google Scholar]
- 31.Mori S, Harada S, Okazaki R, Inoue D, Matsumoto T, Ogata E. Hypomagnesemia with increased metabolism of parathyroid hormone and reduced responsiveness to calcitropic hormones. Intern Med. 1992;31:820–824. doi: 10.2169/internalmedicine.31.820. [DOI] [PubMed] [Google Scholar]


