Abstract
Objectives:
To evaluate the epidemiology, risk factors, and antibiotic resistance of Gram negative bacteria (GNB) in patients with hematologic or solid organ malignancies.
Methods:
This is a retrospective study of 61 episodes of GNB bacteremia occurring in 56 patients with malignancy admitted to the Oncology Units in King Khalid University Hospital, Riyadh. Kingdom of Saudi Arabia during the period from January 2013 to October 2015. Data were retrieved from the computerized database of the microbiology laboratory and the patient’s medical records.
Results:
Hematological malignancies accounted for 30 (54%) and solid tumors accounted for 26 (46%). The most common hematological malignancies were leukemia 23 (77%), followed by lymphoma 6 (20%). Among solid tumors, colorectal cancer 9 (34.6) and breast cancer 6 (23%) were the most common. The most predominant pathogen was Escherichia coli (E. coli) (29.5%) followed by Acinetobacter baumannii (A. baumannii) (18%). The extended-spectrum beta-lactamases producers rate of E. coli and Klebsiella pneumonia was (34.6%). Imipenem resistance among Pseudomonas aeruginosa/A. baumannii was high (52.4%). The multi-resistant organisms rate was (43.5%). Risk factors associated with the bacteremia were ICU admission (32.1%), post-surgery (23.2%), and placement of central line (21.4%). The overall 30-day mortality rate of the studied population was high (32.1%).
Conclusion:
In light of the high resistant rate among the GNB isolated from malignancy patients from our institution, careful selection of antimicrobial treatment based on antimicrobial susceptibility testing is recommended.
Blood stream infection caused by Gram-negative bacilli (GNB) is a significant threats to hospitalized patients, cancer patients are particularly prone to hospital-acquired bacteremia. This can be due to the effect of chemotherapy on their immune system.1 Data over the past decade have found a higher prevalence of Gram-positive organisms as the predominant etiologic agent causing nosocomial bacteremia among patients with malignancy. However recent reports have shown a considerable change in the spectrum and antibiotic susceptibility pattern of organisms causing bacteremia with reemergence of Gram-negative bacteria in cancer patients.2,3 In recent years, a notable increase in antibiotic resistance among Gram-negative bacteria has been reported, especially in critically ill patients, including patients with malignancy.4 Limited information is available regarding the spectrum and microbiology of these infections in cancer patients in our country.5-8 We aim to evaluate the epidemiology and risk factors of acquiring GNB bacteremia in febrile cancer patients at a university hospital in Saudi Arabia, emphasizing the emergence of multi-resistant organisms and their antibiotic resistance patterns.
Methods
Setting, patients, and study design
A retrospective study was conducted in King Khalid University Hospital, a 200 bed hospital admitting children and adult cancer patients, Riyadh, Saudi Arabia. The study included all hospitalized cancer patients diagnosed with solid and hematologic malignancy with at least one episode of bacteremia from January 2013 to October 2015. Variables of interest included age, gender, presence of solid tumor or hematologic disease, underlying disease, type of infection, and causative microorganisms. The presence of the following comorbid conditions were also documented: recent operation, corticosteroid use, immunosuppressant use, indwelling urinary catheter, cancer status, central venous catheter use, length of intensive care unit (ICU) stay, and the 7- and 30-day mortality.
Exclusion criteria
gram-negative isolates from patients having bacteremia in the same admission during the study period were excluded.
Definitions
Bacteremia is defined as isolation of the same bacterial or pathogen from at least one set of blood cultures (2 bottles taken at the same time). Bacteremia is considered polymicrobial if at least 2 organisms from the same blood culture on 2 occasions are isolated, or more than one organism each in at least 2 separate blood cultures within 48 hours.10 Bacteremia occurring more than 14 days after a previous episode and separated by repeatedly negative blood cultures was considered a separate episode. Fever was defined as oral temperature of 38°C or above for at least one hour.9 Each new hospital admission for cancer patients with fever was defined as a separate episode.
Microbiological identification and susceptibility testing
Blood cultures received from all febrile cancer patients in the study period were included. Blood cultures were performed using BACTEC 9240 automated system (Becton-Dickinson Microbiology Systems Sparks, MD, USA). The GNB were identified and their antibiotic susceptibility was tested using commercial panels from the MicroScan system (Siemens Healthcare Diagnostics Inc., West Sacramento, CA, USA). Negative blood culture bottles were incubated for 7 days before being reporting negative. Interpretive criteria (breakpoints) for susceptible, intermediate, and resistant bacterial isolates were those included in the Clinical and Laboratory Standards Institute guidelines (CLSI).11 Strains showing “intermediate” antimicrobial susceptibility profiles were considered to be resistant. Antimicrobial susceptibility testing and extended-spectrum beta-lacamases (ESBL) confirmatory testing were performed using an automated system for the modified broth microdilution method or the disk diffusion method according to the recommendations of the CLSI.11 Sensitivity testing for non-Enterobacteriaceae was carried out by using agar dilution method as recommended by CLSI.11 In this study, gram-negative bacteria were considered multidrug-resistant, when resistant to third and fourth generation cephalosporins, fluoroquinolones, aminoglycosides, and carbapenems, including Stenotrophomonas maltophilia (S. maltophilia), carbapenem-resistant Pseudomonas aeruginosa (P. aeruginosa), and Acinetobacter baumannii (A. baumannii).12
Statistical analysis
Data were reported as the mean standard deviation (SD) or number of patients (percentage) using the Statistical Package for the Social Sciences version 23 (IBM Corp., Armonk, NY, USA).
Results
In the present study, both patients with hematologic malignancies (leukemic patients) and patients with solid tumors were included in the study. In addition, we detected the risk factors, mortality rates attributed to nosocomial infections caused by gram-negative isolates. Almost half (29/51%) of our studied population were elderly above 50 years of age. Among the 61 microbiologically documented febrile episodes in 56 patients with malignancy, hematological malignancies accounted for 30 (54%), while solid tumors accounted for 26 (46%). The most common hematological malignancies were leukemia 23 (77%), followed by Hodgkin’s and non-Hodgkin’s lymphoma 6 (20%). The distribution of solid tumor in malignancy patients was as followed: Colorectal cancer 9 (34.6%) followed by breast cancer 6 (23%), brain cancer 4 (15.3%), bladder cancer 3 (11.5%), gall bladder cancer 3.27% (2/61%), Cholengiocarcinoma 2 (7.6%), pancreatic cancer and sarcoma 1 (4%) for each. Significant risk factors associated with the bacteremia were ICU admission 32.1% (18/56), post-surgical 23.2% (13/56), and central line 21.4% (12/56) (Table 1). The multi-resistant organisms (MRO) represent 43.5 of all isolates. The ESBL rate of Escherichia coli (E. coli) and Klebsiella pneumonia (K. pneumonia), was 34.6% (9/26). The overall 30-day mortality rate of the study population was high 32.1% (18/56). The microbial spectrum of gram-negative bacteria isolated from various infection sites in hospitalized cancer patients is shown in Figure 1. The spectrum studied was not limited to the most common gram-negative bacteria, but included less-frequent gram-negative bacteria as well. The most predominant pathogen was E. coli 29.5% (18/61) followed by A. baumannii. 18.0% (11/61), Pseudomonas spp. 16.3% (10/61), and K. pneumonia 13.1% (8/61). Other organisms included, Salmonella spp. (6.5%), Serratia marcescens (4.9%), Enterobacter spp. (3.24%) proteus mirabilis, Aeromonas hydrophila, Salmonella typhi, Citrobacter koseri and S. maltophilia contributed to (1.6%) with one isolate each. The resistance profile of the isolated fermentative gram-negative bacteria was examined (Table 2). The highest sensitivity was shown to imipenem and meropenem. Nearly 50% of E. coli isolates and 25% of Salmonella spp. were resistant to ciprofloxacin. The susceptibility profile of A. baumannii and P. aeruginosa is shown in Table 3. Imipenem resistance among P. aeruginosa/A. baumannii was high 52.4% (11/21). Multi-resistant organisms rate among the isolates of P. aeruginosa/A. baumannii was 31.1% (19/61).
Table 1.
Epidemiological and clinical characteristic of 56 malignancy patients with GNB bacteremia.

Figure 1.
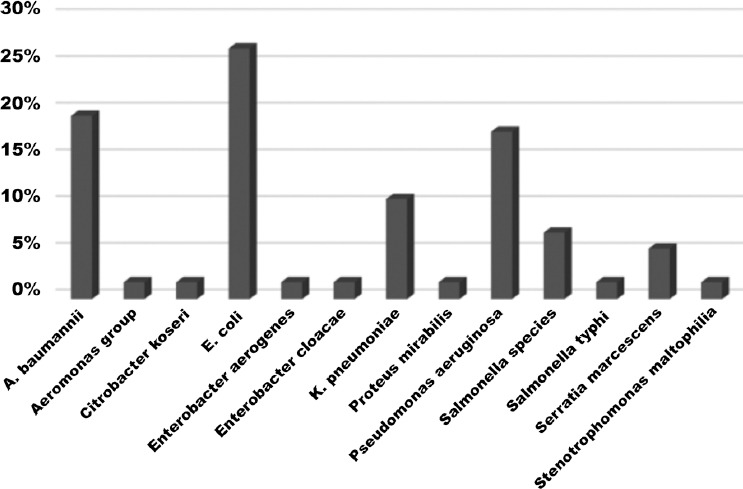
The microbial spectrum of Gram-negative bacteria causing bacteremia in patients with malignancy, A. baumannii - Acinetobacter baumannii, E. coli - Escherichia coli, K. pneumoniae - Klebsiella pneumoniae
Table 2.
Susceptibility of fermentative gram-negative isolates from blood stream infection of patient with malignancy.

Table 3.
Susceptibility of A. baumnii and P. aeruginosa causing blood stream infection in malignancy patients.

Discussion
Bacteremia is a major cause of life threatening and poor outcome in patients with cancer, particularly patients with hematologic malignancies, such as leukemia and lymphoma. The spectrum of microorganisms isolated from blood culture have been significantly changed over the past decades, with reemergence of GNB as the leading causative agents.13,14 The current study was carried out with the intention of testing the etiology of bacteremia in febrile cancer patients, and to describe in more detail the clinical characteristics and outcome of patients. Our study revealed that E. coli followed by A. baumannii and P. aeruginosa as the predominantly isolated pathogens. This result is similar to previous studies,15,16 where E. coli was the most prevalent organism.
In a recent study from Lebanon,15 E. coli represent (39.5%) of all gram negative organisms. In another study from Pakistan16 to evaluate drug resistance amongst bacteremic isolates of febrile neutropenic patients, E. coli was found to be the most predominant organism of the Enterobacteriaceae group while P. aeruginosa and Acinetobacter species were the most common isolates among the non-Enterobacteriaceae group.16 In contrast to this finding, a study from Greece17 revealed that, Pseudomonas spp. was the most common cause (19%), followed closely by E. coli (18%) and K. pneumoniae (17%). Recently, there is an emergence of drug-resistant GNB, such as ESBL producing GNB, MRO P. aeruginosa, A. baumannii, S. maltophilia, and carbapenemase-producing GNB.18,19 The emergence of carbapenemase-producing K. pneumoniae (KPC-Kp) blood stream infection among patients with hematologic malignancies is a major concern. The KPC-Kp has contributed to 26 (18%) of all 147 blood stream infections caused by gram negative bacteria in patients with hematologic malignancies in Italy.20 In this study, the extended-spectrum beta-lactamases producers (ESBLs) rate was 34.6%. This rate is considerably higher than that reported by Kang et al21 (23.7%). This finding might be related to the extensive use of ß-lactam agents in the management of oncology patients in our institution. The increasing incidence of ESBL-producing bacteremia in cancer patients could contribute to increasing rates of treatment failure and poor outcome in such patients with severe infectious complications.22 In a Korean study,23 approximately 40% of patients factors associated with ESBL-producing bacteremia were nosocomial acquisition, ICU care, and prior use of antibiotics. Gudiol and Carratala,24 reported that the risk factors of bacteremia due to GNB in febrile neutropenic cancer patients vary depending on the type of organism, duration of hospitalization, and antibiotic therapy. Several factors have been implicated for the emergence of multi-resistant GNB, namely the increased placement of indwelling catheters, the administration of antimicrobial prophylaxis, the nature of chemotherapeutic regimens currently in use, as well as several environmental conditions that are still poorly identified. Intensive care unit admission, placement of central line, and post-surgery were significant factors associated with bacteremia in this study. The impact of antimicrobial resistance, ESBL-producers, and MRO, on outcome in patients with malignancy is still a controversial issue.25,26 Furthermore, the association between multi-resistant organisms and ESBL producing GNB, and serious infection in patients with malignancy has not been fully established. A major concern is the progressive emerging resistance to carbapenem group of antimicrobial agents, which are considered to be the mainstay treatment of blood stream infection caused by resistant GNB. In a study from Pakistan,27 rising trend of resistance against this group of antibiotics was observed among Enterobacteriaceae including P. aeruginosa. Acinetobacter species were highly resistant against imipenem/meropenem.27 We observe in our study that 52.4% of P. aeruginosa/A. baumannii were imipenem resistant and the overall MRO resistant rate was 43.5%. In addition, the hospital mortality rate was relatively high 32.1% in the present analysis compared with 20% in the 2 previous studies from lebanon.28,29 These results are higher than a recent published data documenting mortality rates ranging between 4-7%.30-32
In conclusion, the pattern of infecting organisms in febrile malignancy patients has been not been well studied in Saudi Arabia. Our data suggest that there is a rising trend of highly resistant organisms stresses the increasing importance of continuous surveillance system and stewardship of antibiotics as strategies in the overall management of patients with malignancy and determining the optimal empiric antimicrobial therapy.
Footnotes
www.smj.org.sa.
Saudi Medical Journal Online features
-
*
Instructions to Authors
-
*
Uniform Requirements
-
*
STARD
-
*
Free access to the Journal's Current issue
-
*
Future Contents
-
*
Advertising and Subscription Information
All Subscribers have access to full text articles in HTML and PDF format. Abstracts and Editorials are available to all Online Guests free of charge.
References
- 1.Arnan M, Gudiol C, Calatayud L, Liñares J, Dominguez MÁ, Batlle M, et al. Risk factors for, and clinical relevance of, faecal extended-spectrum ß-lactamase producing Escherichia coli (ESBL-EC) carriage in neutropenic patients with haematological malignancies. Eur J Clin Microbiol Infect Dis. 2011;30:355–360. doi: 10.1007/s10096-010-1093-x. [DOI] [PubMed] [Google Scholar]
- 2.Wojak I, Gospodarek E. Analysis of microorganisms isolated from febrile neutropenic children with neoplastic disease. Med Dosw Mikrobiol. 2004;56:411–419. [PubMed] [Google Scholar]
- 3.Haupt R, Romanengo M, Fears T, Viscoli C, Castagnola E. Incidence of septicaemias and invasive mycoses in children undergoing treatment for solid tumours: a 12-year experience at a single Italian institution. Eur J Cancer. 2001;37:2413–2419. doi: 10.1016/s0959-8049(01)00274-x. [DOI] [PubMed] [Google Scholar]
- 4.Fridkin SK, Gaynes RP. Antimicrobial resistance in intensive care units. Clin Chest Med. 1999;20:303–316. doi: 10.1016/s0272-5231(05)70143-x. [DOI] [PubMed] [Google Scholar]
- 5.Eltahawy AT. Febrile neutropenia Etiology of infection, empirical treatment and prophylaxis. Saudi Med J. 2003;24:331–336. [PubMed] [Google Scholar]
- 6.Babay HA. Bacterial isolates from fatal cases of bloodstream infections at a university hospital in Central, Saudi Arabia. Saudi Med J. 2007;28:231–235. [PubMed] [Google Scholar]
- 7.Mansoor Sirkhazi, Azmi Sarriff, Noorizan Abd Aziz, Fatma Almana, Osama Arafat, Mahmoud Shorman. Bacterial Spectrum, Isolation Sites and Susceptibility Patterns of Pathogens in Adult Febrile Neutropenic Cancer Patients at a Specialist Hospital in Saudi Arabia. World J Oncol. 2014;5:196–203. doi: 10.14740/wjon850w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al-Ahwal MS. Pattern of febril neutropenia in solid tumors - A hospital based study. Pak J Med Sic. 2005;21:249–252. [Google Scholar]
- 9.Glasmacher A, Von Lilienfeld-Toal M, Schulte S, Hahn C, Schmidt-Wolf IG, Prentice A. An evidence-based evaluation of important aspects of empirical antibiotic therapy in febrile neutropenic patients. Clin Microbiol Infect. 2005;11:17–23. doi: 10.1111/j.1469-0691.2005.01239.x. [DOI] [PubMed] [Google Scholar]
- 10.Reuben AG, Musher DM, Hamill RJ, Broucke I. Polymicrobial bacteremia: clinical and microbiologic patterns. Rev Infect Dis. 1989;11:161–183. doi: 10.1093/clinids/11.2.161. [DOI] [PubMed] [Google Scholar]
- 11.National Committee for Clinical Laboratory Standards. Tenth informational supplement M100-S10. Wayne, PA: National Committee for Clinical Laboratory Standards; 2000. Performance Standards for Antimicrobial Susceptibility Testing. [Google Scholar]
- 12.Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 13.Gudiol C, Calatayud L, Garcia-Vidal C, Lora-Tamayo J, Cisnal M, Duarte R, et al. Bacteremia due to extended-spectrum beta-lactamase-producing Escherichia coli (ESBL-EC) in cancer patients: clinical features, risk factors, molecular epidemiology and outcome. J Antimicrob Chemother. 2010;65:333–341. doi: 10.1093/jac/dkp411. [DOI] [PubMed] [Google Scholar]
- 14.Gudiol C, Tubau F, Calatayud L, Garcia-Vidal C, Cisnal M, Sanchez-Ortega I, Duarte R, et al. Bacteraemia due to multidrug-resistant Gram-negative bacilli in cancer patients: risk factors, antibiotic therapy and outcomes. J Antimicrob Chemother. 2011;66:657–663. doi: 10.1093/jac/dkq494. [DOI] [PubMed] [Google Scholar]
- 15.Moghnieh R, Estaitieh N, Mugharbil A, Jisr T, Abdallah DI, Ziade F, et al. Third generation cephalosporin resistant Enterobacteriaceae and multidrug resistant gram-negative bacteria causing bacteremia in febrile neutropenia adult cancer patients in Lebanon, broad spectrum antibiotics use as a major risk factor, and correlation with poor prognosis. Front Cell Infect Microbiol. 2015;5:11. doi: 10.3389/fcimb.2015.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Irfan S, Idrees F, Mehraj V, Habib F, Adil S, Hasan R. Emergence of Carbapenem resistant Gram negative and vancomycin resistant Gram positive organisms in bacteremic isolates of febrile neutropenic patients: a descriptive study. BMC Infect Dis. 2008;8:80. doi: 10.1186/1471-2334-8-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Samonis G, Vardakas KZ, Maraki S, Tansarli GS, Dimopoulou D, Kofteridis DP, et al. A prospective study of characteristics and outcomes of bacteremia in patients with solid organ or hematologic malignancies. Support Care Cancer. 2013;21:2521–2526. doi: 10.1007/s00520-013-1816-5. [DOI] [PubMed] [Google Scholar]
- 18.Musollino G, Mastrolonardo G, Prezioso R, Pagano L, Primignani P, Carestia C, et al. Molecular mechanisms of a novel ß-thalassaemia mutation due to the duplication of tetranucleotide ‘AGCT’at the junction IVS-II/exon 3. Ann Hematol. 2012;91:1695–1701. doi: 10.1007/s00277-012-1526-y. [DOI] [PubMed] [Google Scholar]
- 19.Montassier E, Batard E, Gastinne T, Potel G, deLaCochetière MF. Recent changes in bacteremia in patients with cancer: a systematic review of epidemiology and antibiotic resistance. Eur J Clin Microbiol Infect Dis. 2013;32:841–850. doi: 10.1007/s10096-013-1819-7. [DOI] [PubMed] [Google Scholar]
- 20.Pagano L, Caira M, Trecarichi EM, Spanu T, Di Blasi R, Sica S, et al. Carbapenemase-producing Klebsiella pneumoniae and hematologic malignancies. Emerg Infect Dis. 2014;20:1235–1236. doi: 10.3201/eid2007.130094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kang CI, Chung DR, Ko KS, Peck KR, Song JH Korean Network for Study of Infectious Diseases. Risk factors for infection and treatment outcome of extended-spectrum ß-lactamase-producing Escherichia coli and Klebsiella pneumoniae bacteremia in patients with hematologic malignancy. Ann Hematol. 2012;91:115–121. doi: 10.1007/s00277-011-1247-7. [DOI] [PubMed] [Google Scholar]
- 22.Trecarichi EM, Tumbarello M, Spanu T, Caira M, Fianchi L, Chiusolo P, Fadda G, Leone G, Cauda R, Pagano L. Incidence and clinical impact of extended-spectrum-beta-lactamase (ESBL) production and fluoroquinolone resistance in bloodstream infections caused by Escherichia coli in patients with hematological malignancies. J Infect. 2009;58:299–307. doi: 10.1016/j.jinf.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 23.Kang CI, Chung DR, Ko KS, Peck KR, Song JH. The Korean Network for the Study of Infectious Diseases (KONSID). Risk factors for mortality and impact of broad-spectrum cephalosporin resistance on outcome in bacteraemic intra-abdominal infections caused by Gram-negative bacilli. Scand J Infect Dis. 2011;43:202–208. doi: 10.3109/00365548.2010.539257. [DOI] [PubMed] [Google Scholar]
- 24.Gudiol C, Carratala J Antibiotic resistance in cancer patients. Expert Rev Anti Infect Ther. Expert Rev Anti Infect Ther. 2014;12:1003–1016. doi: 10.1586/14787210.2014.920253. [DOI] [PubMed] [Google Scholar]
- 25.Chong Y, Yakushiji H, Ito Y, Kamimura T. Cefepime-resistant Gram-negative bacteremia in febrile neutropenic patients with hematological malignancies. Int J Infect Dis. 2010;14(Suppl 3):e171–e175. doi: 10.1016/j.ijid.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 26.Trecarichi EM, Tumbarello M, Spanu T, Caira M, Fianchi L, Chiusolo P, Fadda G, et al. Incidence a nd clinical impact of extended-spectrum-beta-lactamase (ESBL) production and fluoroquinolone resistance in bloodstream infections caused by Escherichia coli in patients with hematological malignancies. J Infect. 2009;58:299–307. doi: 10.1016/j.jinf.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 27.Khan MA, Siddiqui BK, Shamim A, Yosuf MA, Ahmed A, Zakiullah N, et al. Emerging bacterial resistance pattern in febrile neutropenic patients: experience at a tertiary care hospital in Pakistan. J Pak Med Assoc. 2004;54:357–360. [PubMed] [Google Scholar]
- 28.Hamzeh F, Kanj SS, Uwaydah M. Febrile neutropenia in cancer patients in a tertiary care medical center in Lebanon: microbial spectrum and outcome. J Med Liban. 2000;48:136–142. [PubMed] [Google Scholar]
- 29.Kanafani ZA, Dakdouki GK, El-Chammas KI, Eid S, Araj GF, Kanj SS. Bloodstream infections in febrile neutropenic patients at a tertiary care center in Lebanon: a view of the past decade. Int J Infect Dis. 2007;11:450–453. doi: 10.1016/j.ijid.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 30.Khan MA, Siddiqui BK, Shamim A, Yosuf MA, Ahmed U, Zakiullah N, et al. Emerging bacterial resistance patterns in febrile neutropenic patients: experience at a tertiary care hospital in Pakistan. J Pak Med Assoc. 2004;54:357–360. [PubMed] [Google Scholar]
- 31.Cherif H, Bjorkholm M, Engervall P, Johansson P, Ljungman P, Hast R, et al. A prospective, randomized study comparing cefepime and imipenem--cilastatin in the empirical treatment of febrile neutropenia in patients treated for haematological malignancies. Scand J Infect Dis. 2004;36:593–600. doi: 10.1080/00365540410017590. [DOI] [PubMed] [Google Scholar]
- 32.Rossini F, Terruzzi E, Verga L, Larocca A, Marinoni S, Miccolis I, et al. A randomized clinical trial of ceftriaxone and amikacin versus piperacillin tazobactam and amikacin in febrile patients with hematological neoplasia and severe neutropenia. Support Care Cancer. 2005;13:387–392. doi: 10.1007/s00520-004-0753-8. [DOI] [PubMed] [Google Scholar]


