Abstract
Aminopeptidases have emerged as new promising drug targets for the development of novel anti-parasitic drugs. An aspartyl aminopeptidase-like gene has been identified in the Toxoplasma gondii genome (TgAAP), although its function remains unknown. In this study, we characterized TgAAP and performed functional analysis of the gene product. Firstly, we expressed a functional recombinant TgAAP (rTgAAP) protein in Escherichia coli, and found that it required metal ions for activity and showed a substrate preference for N-terminal acidic amino acids Glu and Asp. Then, we evaluated the function and drug target potential of TgAAP using the CRISPR/Cas9 knockout system. Western blotting demonstrated the deletion of TgAAP in the knockout strain. Indirect immunofluorescence analysis showed that TgAAP was localized in the cytoplasm of the wild-type parasite, but was not expressed in the knockout strain. Phenotype analysis revealed that TgAAP knockout inhibited the attachment/invasion, replication, and substrate-specific activity in T. gondii. Finally, the activity of drug CID 23724194, previously described as targeting Plasmodium and malarial parasite AAP, was tested against rTgAAP and the parasite. Overall, TgAAP knockout affected the growth of T. gondii but did not completely abolish parasite replication and growth. Therefore, TgAAP may comprise a useful adjunct drug target of T. gondii.
Protozoan parasites from the phylum Apicomplexa are a global health threat because of their ability to infect humans and animals. Toxoplasma gondii, which causes toxoplasmosis, infects a wide range of warm-blooded animals, including humans. There are no clear symptoms of acute toxoplasmosis in healthy adults1,2. However, immunocompromised people, such as those infected with HIV, immunocompromised individuals receiving certain types of chemotherapy, or those who have recently received an organ transplant, may develop severe toxoplasmosis. Severe toxoplasmosis can cause encephalitis in the brain or retinochoroiditis in the eyes3. Latent toxoplasmosis produces no readily observable symptoms in most immunocompetent people4, during which tissue cysts can form in the retinas, lungs, heart, skeletal muscle, and brain. Cysts form in the central nervous system upon infection with T. gondii and persist for the lifetime of the host5. Recent studies have suggested that infection of toxoplasma causes behavioral changes6. It was estimated that 30–50% of the global population has been exposed to and may be chronically infected with latent toxoplasmosis according to serological studies7,8. The currently available therapeutic drugs for toxoplasmosis treatment elicit hypersensitivity syndrome and/or are toxic9,10. Also, no vaccine is currently available that would block the transmission of this parasite. Thus, identifying drugs with new modes and/or targets of action is essential for creating safer and more effective drugs that are urgently needed to treat toxoplasmosis.
Peptidase-catalyzed protein degradation is one of the most important processes providing amino acids for an organism11. Aminopeptidases catalyze the cleavage of N-terminal amino acids in proteins and peptides. The T. gondii genome appears to encode at least ten aminopeptidases: one leucyl aminopeptidase (LAP), one aspartyl aminopeptidase, one prolyl aminopeptidase, three aminopeptidases N, and four methionyl aminopeptidases. AAP belongs to the M18 family of peptidases that has been proposed as a novel antimalarial drug target12. The M18 family is widely distributed in bacteria and eukaryotes, and consists of metalloaminopeptidases that use metal ions to increase their substrate specificity for N-terminal acidic amino acids, beyond aspartic and glutamic acids. Until now, only a few M18 family peptidases have been characterized in detail, including Saccharomyces cerevisiae aminopeptidase I (MEROPS: M18.001)13, mammalian AAP (MEROPS: M18.002)14,15,16, and Plasmodium falciparum AAP (PfM18AAP) (MEROPS: M18.003)12. Kinetic measurements and structure determination of AAP have been performed in Pseudomonas aeruginosa17. An aminopeptidase from the M18 peptidase family is predicted to be an AAP (ToxoDB: TGGT1_297970) in T. gondii, although its structure is unknown and its characterization has yet to be performed.
Important functional characterization of AAPs has been performed in parasites. AAP gene disruption/truncation experiments indicated that the enzyme was dispensable for malarial blood-stage replication but its production had an associated fitness cost for the parasite18. The protease knockdown resulted in parasite growth abnormalities, vascularization, and cell damage12. In this study, we report for the first time the cellular distribution, enzyme kinetics, and inhibition of TgAAP. We constructed a new T. gondii TgAAP deletion strain to characterize the enzyme. Our results showed that TgAAP is synthesized in the parasite cytosol. TgAAP deletion resulted in inhibition, but not abolition, of invasion and growth of T. gondii in cell culture, thus indicating that the enzyme may be a promising adjunct target for anti-toxoplasma drug development.
Results
Analysis of TgAAP amino acid sequence
To investigate the similarity between TgAAP and AAPs from other species, we cloned and sequenced the gene encoding TgAAP from the T. gondii RH strain. The TgAAP coding sequence was 1521 bp long, consistent with the predicted sequence (ToxoDB accession number TGGT1_297970). Amino acid sequence alignment was performed with predicted AAPs from apicomplexan parasites, including T. gondii, Eimeria tenella, Cryptosporidium muris, P. falciparum, and Thelleria annulata (Fig. 1Sa). The alignment revealed that the predicted AAP functional domains (gray), especially Zn-binding sites (blue; residues 109, 300, 332, 377, and 471) and substrate-binding/catalytic sites (red; residues 111 and 333), may be conserved in apicomplexan parasites. A constructed phylogenetic tree revealed that TgAAP was closely related to the predicted E. tenella AAP (51% identity; Fig. 1Sb). M18 aminopeptidases are reported to be dodecamers that form a tetrahedron19.
Purification and multimeric nature of rTgAAP
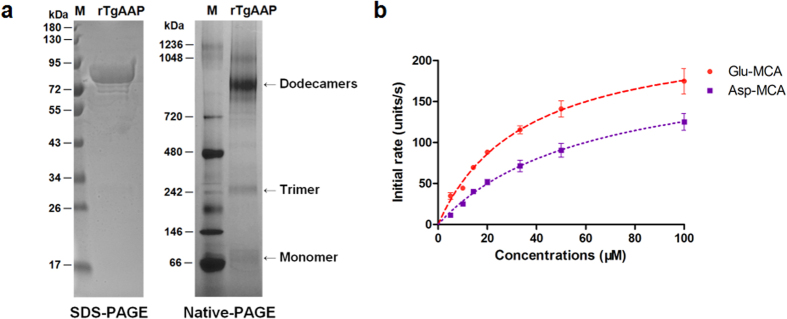
To explore the function and activity, we first expressed rTgAAP protein in E. coli. The protein tagged with glutathione S-transferase (GST) was purified by affinity chromatography. The molecular weight of the protein was estimated by polyacrylamide gel electrophoresis (PAGE). This revealed a ~80 kDa band on SDS-PAGE, consistent with the predicted size (~56 kDa TgAAP fused with the ~27 kDa GST tag; Fig. 1a). To investigate the multimeric state of the protein, rTgAAP was analyzed using native PAGE. Native PAGE revealed a minor band (about 80 kDa) as well as two major oligomeric bands (about 960 kDa and 240 kDa). These sizes might correspond to rTgAAP dodecamers and trimers.
Figure 1. Characterization of the TgAAP protein product.
(a) SDS-PAGE and native PAGE analysis of rTgAAP. Left: SDS-PAGE (10% gel). Lane 1 (M), pre-stained protein standard (10–180 kDa). Lane 2, rTgAAP [the protein was heated in 4× protein loading buffer (Solarbio, Beijing) at 100 °C for 5 min before loading]. Right: native PAGE (3–12% gel). Lane 1 (M), unstained protein standard (20–1200 kDa). Lane 2, rTgAAP. (b) Enzyme activity of purified rTgAAP. An initial assay with different concentrations of fluorogenic peptide substrates H-Glu-MCA and H-Asp-MCA is shown. One activity unit is defined as MCA (pM) released per mg of recombinant protein.
Enzyme activity of rTgAAP
Next, rTgAAP enzyme activity was analyzed against various synthesized peptidase substrates. The protein concentration (0.3 μM) at which the reaction rate was linear (25.72 μg/ml) was chosen for the assay. Data collected for the specific substrates H-Glu/Asp-MCA (7-methoxycoumarin-4-acetic acid) conformed to the Michaelis-Menten kinetics (Fig. 1b). The initial reaction rate of rTgAAP with H-Glu-MCA was higher compared with the specific substrate H-Asp-MCA (Student’s t-test, P < 0.01, n = 3). Metal ion sensitivity was then investigated by assaying rTgAAP activity after 30 min pre-incubation at 37 °C in Tris-HCl (50 mM, pH 7.5) containing bivalent metal chlorides (Sigma-Aldrich, St. Louis, USA). The enzyme was markedly activated by the addition of the bivalent metal cations Co2+, Mn2+, Fe2+, Ni2+, Mg2+, Ca2+, Cu2+, and Zn2+ (Table 1). Particularly with Co2+ (377.76 ± 56.36 U/s, initial rate ± STD), the initial rate increased more than 6-fold compared with the absence of metal ions (59.39 ± 2.72 U/s; Student’s t-test, P < 0.01, n = 3), followed by Mn2+ (151.3 ± 26.44 U/s), which enhanced enzymatic activity more than 2-fold (Student’s t-test, P < 0.01, n = 3). These results indicated that Co2+ was the most effective metal ion cofactor of rTgAAP.
Table 1. Effect of divalent metal ions on rTgAAP activity.
| Metal ion | Concentration (mM) | Activity (units/s)a |
|---|---|---|
| Co2+ | 1 | 377.76 ± 56.36 |
| Mn2+ | 1 | 151.3 ± 26.44 |
| Fe2+ | 1 | 133.45 ± 68.28 |
| Ni2+ | 1 | 116.89 ± 39.34 |
| Mg2+ | 1 | 62.47 ± 7.08 |
| Ca2+ | 1 | 44.52 ± 9.12 |
| Cu2+ | 1 | 3.78 ± 0.478 |
| Zn2+ | 1 | 2.81 ± 0.516 |
| Control (No metalion) | — | 59.39 ± 2.72 |
aOne unit was defined as picomoles of MCA released per milligram of recombinant protein. Data represent means ± SD from three independent experiments.
Substrate specificity of rTgAAP
rTgAAP enzyme kinetics with fluorogenic synthetic substrates confirmed its classification as a member of the M18 AAP family. Acidic amino acids Glu and Asp were efficiently cleaved by rTgAAP from the N-termini of synthetic substrates. Compared with H-Asp-MCA (kcat/Km = 85.66 mM−1 s−1), the catalytic efficiency of rTgAAP was higher with H-Glu-MCA (kcat/Km = 190.16 mM−1 s−1; Student’s t-test, P < 0.01, n = 3) (Table 2). With Asn-MCA, rTgAAP catalytic efficiency was even lower (kcat/Km = 19.86 mM−1 s−1; Student’s t-test, P < 0.01, n = 3). rTgAAP had no preference or activity toward other synthesized substrates, such as His-, Ser-, and Pro-MCA, with enzyme efficiency values (kcat/Km) 2.35, 1.33, and 0.28 mM−1 s−1, respectively.
Table 2. Kinetic parameters for the hydrolysis of peptide substrates by rTgAAP.
| Substrate | kcat (×10−2 s−1)a | Km (μM)a | kcat/Km (mM−1 s−1) |
|---|---|---|---|
| H-Glu-MCA | 139.67 ± 30.65 | 7.35 ± 0.73 | 190.16 |
| H-Asp-MCA | 109.99 ± 6.48 | 12.84 ± 0.21 | 85.66 |
| H-Asn-MCA | 48.35 ± 1.26 | 24.32 ± 0.26 | 19.86 |
| H-His-MCA | 20.84 ± 0.43 | 88.70 ± 2.53 | 2.35 |
| H-Ser-MCA | 12.35 ± 0.86 | 92.87 ± 3.38 | 1.33 |
| H-Pro-MCA | 27.59 ± 2.37 | 98.55 ± 1.96 | 0.28 |
aData represent means ± SD from three independent experiments.
Generation of TgAAP-knockout and complemented parasites
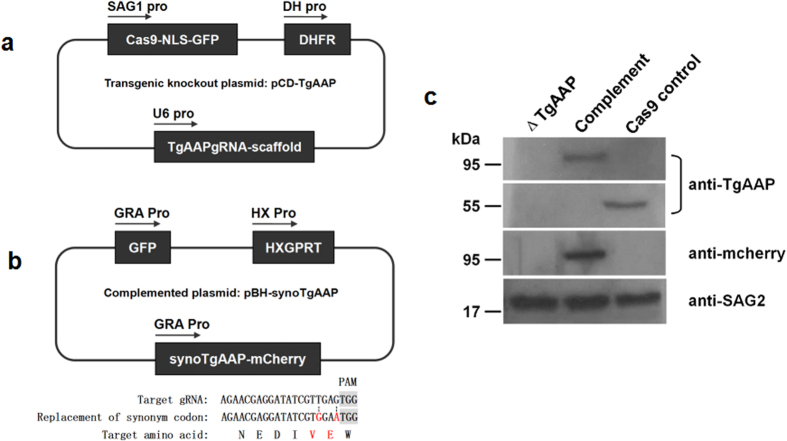
To investigate the function of AAPs in T. gondii, we proceeded to generate a TgAAP-knockout mutant (ΔTgAAP). A TgAAP gene-targeting plasmid, designated pCD-TgAAP (Fig. 2a), was constructed using specific gRNA with a dihydrofolate reductase (DHFR) selectable marker cassette. Plasmid pBluescript II containing codons synonymous to TgAAP fused with mCherry and a hypoxanthine xanthine guanine phosphoribosyl transferase (HXGPRT) expression cassette derived from pHXNTPHA plasmid, designated pBH, were used to construct a TgAAP-complementing plasmid pBH-synoTgAAP (Fig. 2b). After electroporation and selection, ΔTgAAP, complemented, and Cas9 control strains were verified by western blot analysis (Fig. 2c). Protein band ~56 kDa was observed in the lysate of the Cas9 control cell line, but not in the ΔTgAAP strain. The size corresponded to the predicted size (55897 Da) in ToxoDB. An ~95 kDa (TgAAP fused with the mcherry tag) band was observed after probing the complemented strain lysate with anti-TgAAP serum. This was confirmed using anti-mCherry monoclonal antibody. We also performed a PCR assay (Fig. 2S) to verify TgAAP knockout. All these experiments confirmed the absence of TgAAP in the ΔTgAAP strain.
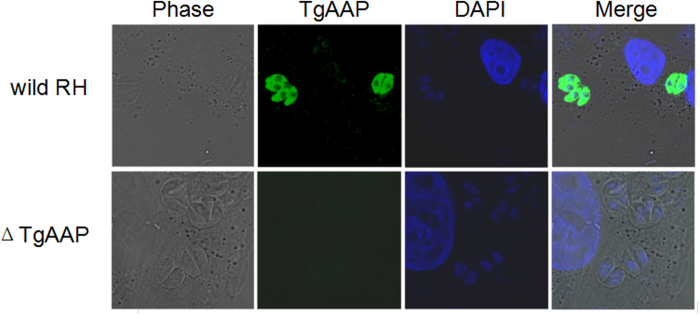
Figure 2. Cellular localization of TgAAP.
(a) Indirect immunofluorescence detection of TgAAP in T. gondii RH strain. TgAAP was detected with anti-rTgAAP polyclonal antibodies (green). Host cell nuclei were visualized with DAPI (blue).
Cellular localization of TgAAP
Confocal laser scanning microscopy detection of TgAAP with fluorescently labeled anti-TgAAP mouse polyclonal antibodies indicated that TgAAP localized in the cytoplasm of the T. gondii wild-type RH strain, but was not expressed in the ΔTgAAP strain (Fig. 3).
Figure 3. Knocking out TgAAP.
(a) Schematic of the knockout plasmid. Knockout plasmid pCD-TgAAP was constructed by inserting DHFR coding sequence and TgAAP-specific single guide RNA (gRNA) into plasmid pSAG1::CAS9-U6::sgUPRT (Addgene, #54467). The plasmid contains Cas9, nuclear localization signal (NLS), and expressed GFP fusion element. (b) Schematic of the complementing plasmid. To avoid recognition by Cas9, synonymous codon substitutions (not affecting TgAAP amino acid sequence) were introduced in the TgAAP target located near a protospacer adjacent motif (PAM) in the complementing plasmid pGH-synoTgAAP. mCherry tag was used to identify the complementing TgAAP. HXGPRT expression element was used to screen for complemented parasite lines. (c) Western blotting analysis of TgAAP-knockout (ΔTgAAP) and complemented parasites. TgAAP was detected with a mouse anti-TgAAP serum in Cas9 control and complemented strains but not in ΔTgAAP. The enzyme was also detected with a mouse monoclonal anti-mCherry antibody in the complemented strain. TgSAG2, detected using mouse anti-TgSAG2 serum, was used as a loading control. Primary antibodies were targeted by horseradish peroxidase-labeled goat anti-mouse secondary antibodies (Sigma, St Louis, USA). Pre-stained protein marker (10–180 kDa) is shown.
Loss of TgAAP affects attachment/invasion and growth in vitro
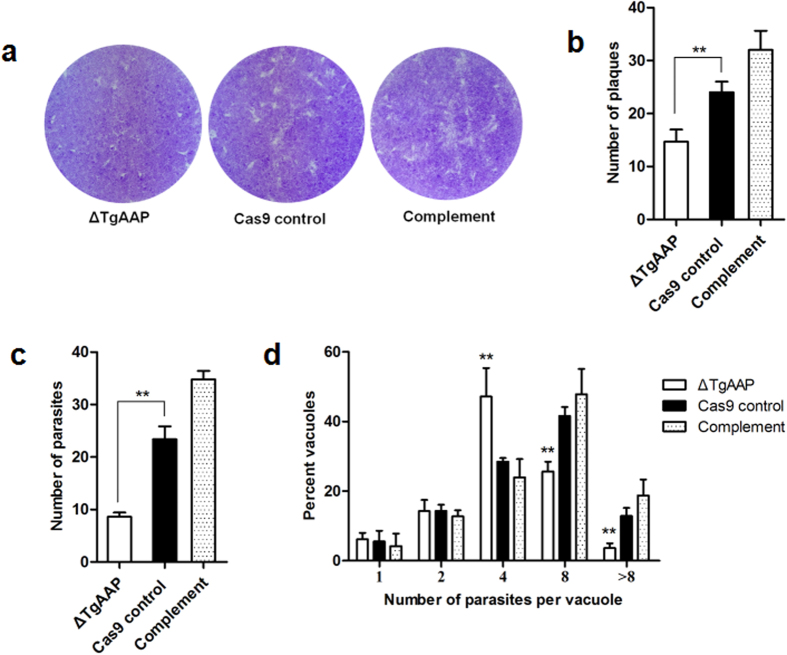
Aminopeptidases are exopeptidases that catalyze sequential removal of amino acids from the N-termini of peptides. These enzymes play major roles in regulating the balance between catabolism and anabolism in all living cells20. Since aminopeptidases can affect cellular metabolism, we anticipated that TgAAP plays a role in parasite growth. To assess this, we tested the ability of TgAAP-knockout strains to form plaques on human foreskin fibroblast (HFF) monolayers. The ΔTgAAP strain was able to produce plaques (Fig. 4a), suggesting that the TgAAP gene is not essential for growth. During repeat experiments, plaques produced by the ΔTgAAP strain were always smaller and less numerous (Fig. 4a,b) than those produced by the Cas9 control strain (Student’s t-test, P < 0.05, n = 3). These results suggested that the parasite attachment/invasion and replication were reduced after TgAAP deletion. To further investigate whether the complemented strain would recover the ability to invade, intracellular parasite numbers were scored by microscopic observation (Fig. 4c). The mean number of parasites per field of view was significantly lower in the ΔTgAAP strain when compared with the Cas9 control strain 2 h post infection (Student’s t-test, P < 0.01, n = 3). These phenomena were reversed in the complemented strain. We also performed growth assays and scored the number of parasites per vacuole (Fig. 4d). Growth assays showed that a significant percentage of vacuoles in the ΔTgAAP-infected group contained tachyzoites that exhibited a reduced ability to divide 24 h post infection. Unlike the control and complemented strains, significantly more vacuoles containing four tachyzoites were seen in ΔTgAAP compared with the Cas9 control strain (Student’s t-test, P < 0.01, n = 3), with a significantly reduced number of eight-tachyzoite vacuoles (Student’s t-test, P < 0.01, n = 3). More parasitophorous vacuoles (PVs) containing more than eight tachyzoites were detected in the Cas9 control and complemented parasites. By contrast, fewer PVs containing more than eight tachyzoites were detected in the cells inoculated with ΔTgAAP parasites (Student’s t-test, P < 0.01, n = 3). Together, these results indicate that TgAAP plays an important role in parasite attachment/invasion and growth.
Figure 4. TgAAP affects parasite invasion and growth in vitro.
(a) Plaque assay examining the growth of parasites in HFFs. Plaques are visible as clear zones on a crystal violet-stained HFF monolayer background. (b) Quantification of ΔTgAAP, Cas9 control, and complement plaques using the digital images. (c) Invasion assay. HFF monolayers were inoculated (2 h) with Cas9 control, ΔTgAAP, or complemented parasites, and the mean number of parasites per field (y-axis) was counted after washing off the unattached parasites. At least 60 fields were scored for each strain. (d) Replication assay. Cas9 control, ΔTgAAP, or complemented parasite strains were grown for 24 h, and then the number of parasites per vacuole (x-axis) was counted. At least 100 vacuoles were scored for each strain. The differences between samples were evaluated by Student’s t-test (n = 3). *P < 0.05, **P < 0.01.
Specific enzyme activity in ΔTgAAP
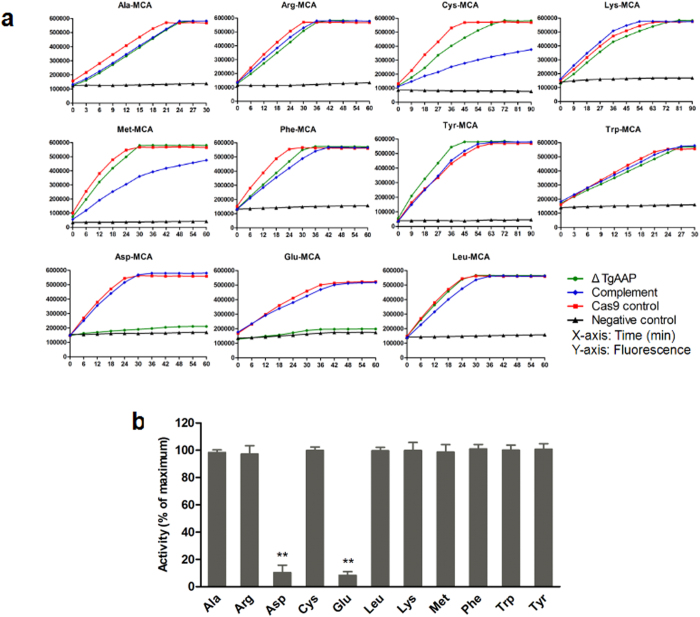
Previous studies demonstrated that AAPs have a strict preference for the N-terminal acidic amino acids Glu and Asp12. We performed substrate-cleaving assays to investigate this specific enzymatic activity in the TgAAP deletion strain. Specific fluorescent substrates H-Asp-MCA and H-Glu-MCA were used. H-Leu-MCA and eight other substrates were used to detect the activities of other enzymes in ΔTgAAP, Cas9 control, and complemented strains. The results indicated that TgAAP deletion led to a significant decrease in AAP-specific enzymatic activity compared with the Cas9 control (Vmax; Student’s t-test, P < 0.01, n = 3) but the cleavage of H-Glu-MCA and H-Asp-MCA in the complemented strain was restored to Cas9 control levels (Fig. 5a). Exopeptidase activities and reaction rates with respect to Ala-, Arg-, Cys-, Lys-, Met-, Phe-, Tyr-, Trp-, and Leu-MCA were higher in ΔTgAAP, Cas9 control, and complemented strains. To further examine whether TgAAP knockout impacted other enzyme activities in T. gondii RH, enzyme activity in ΔTgAAP was assessed using 11 substrates (Fig. 5a) and compared with the Cas9 control (Fig. 5b). Except for a decreased activity toward H-Glu- and H-Asp-MCA (8.33% and 10.37% of the Cas9 control, respectively), no significant difference was observed between the two strains with respect to enzymatic activities toward other substrates, thus indicating the substrate specificity of TgAAP.
Figure 5. Enzyme activity in knockout and complemented parasite strains.
(a) Cellular aminopeptidase activity with different fluoropeptide substrates, including AAP-specific substrates H-Asp-MCA and H-Glu-MCA. Enzyme activity curves of whole protein lysates from various parasite strains are shown and were generated using GraphPad Prism v. 5.0c (GraphPad Software, San Diego, USA). Enzyme activities were recorded at 3 min intervals until maximum activity levels were reached. Samples devoid of protein lysates were used as negative controls. All experiments were independently repeated three times (each in triplicate), and representative curves are shown. (b) Activities in TgAAP-knockout parasites toward different substrates (x-axis) calculated as percentages of the activity in Cas9 control lysates (y-axis). Student’s t-test was used for calculation of significance between groups. *P < 0.05, **P < 0.01 (n = 3).
Inhibitor assay
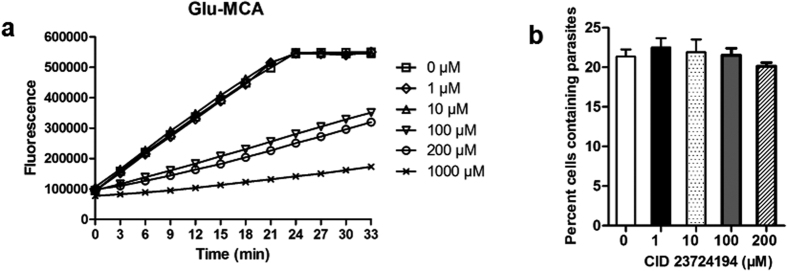
In vitro inhibition assay of rTgAAP using CID 23724194, identified as a PfM18AAP inhibitor21, is shown in Fig. 6a. rTgAAP activity was partly inhibited by 100 μM CID 23724194. The activity of rTgAAP was inhibited by more than 50% in the presence of 1 mM drug. The effect of drug concentration on parasite growth is shown in Fig. 6b. As the concentration of the drug was increased, no change in the percentage of Toxoplasma-infected host cells was observed (Student’s t-test, P > 0.05, n = 3). This indicated that CID 23724194 inhibited rTgAAP, but this did not affect the growth of the parasite.
Figure 6. Susceptibility of rTgAAP and T. gondii to CID 23724194.
(a) rTgAAP inhibition by CID 23724194 (Vitas-M Laboratory). The inhibition was studied at final drug concentrations of 0, 1, 10, 100, and 1000 μM, in reaction mixtures containing AAP substrate H-Glu-MCA. Data points indicate mean activity ± SD (n = 3). (b) Parasite growth inhibition as assayed by FACS. Toxoplasma-infected host cells were quantified after 3 days of incubation with final CID 23724194 concentrations of 0, 1, 10, 100, and 200 μM. The experiments were repeated independently three times, each repeated in triplicate. Representative curves are shown in (a).
Discussion
Similarly to PfM18AAP, TgAAP is the only AAP encoded in the genome of T. gondii. A recent study proved that T. gondii ingests and digests host cytosolic proteins. Disruption of this process attenuates the virulence of the parasites22. In addition, deletion of TgLAP severely affects the growth of the parasites. Taken together, these phenomena suggest the importance of protein digestion by these enzymes in parasite development. This study reports the characterization of TgAAP. The amino acid sequence of TgAAP was predicted to contain conserved Zn-binding sites and substrate-binding/catalytic sites. The structure of PfM18AAP revealed a dodecameric assembly that forms a tetrahedron shape23. The dodecameric enzyme is formed by interactions of four PfM18AAP trimers with an internal M18 family active site cavity comprising four trimer cones, each with three active sites19. To assess the multimeric nature of TgAAP, rTgAAP was analyzed by native PAGE, which revealed that our recombinant enzyme exists in solution mainly as a dodecamer, partially in a trimeric and monomeric form. The multimeric nature of TgAAP is similar to that of PfM18AAP. We also predicted a 3D model of TgAAP that exhibited the highest homology with the crystal structure of human AAP (DNPEP, template: 4dyo.1.A, sequence identity: 44.9%)24 using the auto-model method (Fig. 3S). It is risky to develop new drugs targeting this enzyme. However, it is possible to identify drugs that target the non-conserved part of this enzyme. TgAAP trimers and monomer structures were predicted separately from the tetrahedron by comparison with the X-ray crystal structure of a PfM18AAP monomer. Structural prediction of the TgAAP model suggested that the protein is a canonical member of the M18 aminopeptidase family. PfM18AAP is expressed in the cytosol and is also trafficked out of the parasite into the surrounding PVs. It has therefore been speculated that PfM18AAP plays a role in processing proteins or peptides in transit from the PV to the parasite cytosol12. We found that TgAAP is also expressed in the cytosol. We previously characterized another toxoplasma aminopeptidase, M17 leucine aminopeptidase TgLAP25, an exopeptidase with specificity toward N-terminal hydrophobic residues Leu and Phe that also localizes to the PVs. These two enzymes function in concert in protein catabolism in mammalian cells15. The free amino acids released during the process are likely used in anabolism and facilitate protein synthesis in the rapidly growing intracellular parasite.
Previous biochemical assays of the malarial AAP, PfM18AAP, demonstrated that the enzyme requires metal ions for its activity and has a strict preference for N-terminal acidic amino acids Glu and Asp12. To investigate substrate specificity of TgAAP, we performed H-Glu-/Asp-MCA substrate activity assays using recombinant TgAAP expressed in E. coli. The results of an assay testing the effect of metal ions on enzyme activity indicated that Co2+ was the preferred metal cofactor for rTgAAP, followed by Mn2+. This is different from the reported activity of PfM18AAP12, enhanced only by Co2+. The substrate specificity assay indicated that rTgAAP had a strict preference for H-Asp-MCA and H-Glu-MCA, although the catalytic efficiency of the enzyme was higher with H-Glu-MCA than with H-Asp-MCA. This was consistent with the finding that the initial reaction rate with H-Glu-MCA was higher than with H-Asp-MCA.
In P. falciparum, antisense knockdown experiments had previously identified a lethal phenotype associated with PfM18AAP, suggesting that the enzyme is a valid target for new antimalarial therapies26. Another study reported that this enzyme is not dispensable for parasite growth through the disruption of this gene in the genome of Plasmodium18. To assess whether TgAAP may be a candidate drug target for parasite growth control, we constructed a TgAAP-knockout strain and analyzed its phenotype. The absence of TgAAP hindered parasite invasion and growth but was not lethal to T. gondii. Therefore, we conclude that either the low activity of other aminopeptidases is able to sustain slower growth of the parasites or the AAP activity is not essential for parasite growth. High throughput screening was used to identify potent and selective inhibitors of AAPs. Compound CID 23724194 showed anti-parasite potency in malaria growth assays11. CID 23724194 efficiently inhibited PfM18AAP activity in parasite lysates (96% at 10 μM) and efficiently inhibited parasite growth (IC50 = 1.3 μM). However, this inhibitor is almost completely ineffective against TgAAP (more than 50% Glu-MCA cleavage inhibition at 1 mM). This may be due to differences in the protein sequence or spatial structure between PfM18AAP and TgAAP. The parasite growth inhibition assay revealed no significant difference between the percentages of Toxoplasma-infected host cells with increasing drug concentrations. In general, CID 23724194 is not a lead compound. Therefore, our results suggest that TgAAP may only be used as an adjuvant drug target. Previously, we reported the function of LAP by deleting TgLAP using the CRISPR/Cas9 genome editing technique. Our results indicated that TgLAP was not essential, but played important roles in growth, invasion, and virulence. Accordingly, from the perspective of new anti-parasite drug development, M18AAP, along with M1 and M17, remains a protein of high interest27,28. Until now, we have only tested two metal aminopeptidases, TgLAP and TgAAP, and neither was essential to the parasite. Considering the importance of aminopeptidases for parasite growth and invasion, we believe that TgLAP and TgAAP, together or with other aminopeptidases, may serve as ideal targets. Recently, an “open collaboration” has been pursued in search of new drugs to manage neglected tropical diseases. Such initiatives advance the screening of additional molecular targets in Plasmodium, including PfM18AAP, PfM17LAP, and PfM128. These studies confirmed the feasibility of simultaneous targeting of two or more drug targets. It is yet to be determined whether aminopeptidases are suitable candidates for such drug development strategies for toxoplasmosis treatment.
It has been reported that AAPs of the M18 family are required for the removal of N-terminal acidic amino acid residues, Glu and Asp, that cannot be removed by M17 LAP or other aminopeptidases29,30. Similar conclusions may be drawn from our study: the substrate activity (H-Glu- and H-Asp-MCA) was almost completely lost in the ΔTgAAP strain. This also indicated that no other enzyme complemented the TgAAP activity. These studies revealed the presence of additional aminopeptidases that could remove amino acids such as Ala, Arg, and Lys, not cleaved by TgAAP or TgLAP. Other aminopeptidases encoded in the malaria genome, for example, M1 membrane aminopeptidase N30, can cleave off these amino acids (Ala, Arg, and Lys), and thus complete the line-up of exopeptidases required for the total degradation of proteins to free amino acids in the parasite cytosol.
To investigate the essentiality of TgAAP in T. gondii, we created a knockout/transgenic parasite line using the new DNA editing technology, the CRISPR/Cas9 system31,32,33. TgAAP deletion was successful, as evidenced by the absence of a corresponding specific protein band in the western blotting experiment and significantly decreased AAP-specific enzyme activity when compared with Cas9 control parasites. The method was also highly specific because the relative activity of TgLAP was unaffected in the TgAAP deletion strain. Phenotype assay revealed that TgAAP deletion inhibited cell growth, leading to smaller parasite plaques, and resulting in a reduced substrate cleavage rate. However, the phenotype of the TgAAP deletion mutant was not as pronounced as the phenotype of the corresponding PfM18AAP knockdown mutant12. Whether knocking out TgAAP or TgLAP would impact the anabolism of critical invasive proteins involved in parasite invasion requires further investigation.
Aminopeptidases have been treated as tractable Plasmodium targets for antimalarial drug development34,30,28. The function of other T. gondii aminopeptidases should also be investigated in the future, to establish a foundation for designing multiple-target anti-toxoplasma drugs to explore malaria treatments.
Materials
Parasite strains and growth conditions
The T. gondii RH strain (with HXGPRT deleted by homologous recombination; the strain is a generous gift from Dr. X. Xuan) and parasite mutants used in this study were maintained by growth in Vero cells or HFFs cultured in Dulbecco’s Modified Eagle Medium (DMEM, Gibco, Invitrogen) supplemented with fetal bovine serum (FBS; 2%), penicillin/streptomycin (1%), gentamicin (10 μg/mL), and glutamine (10 mM; Thermo, Fisher Scientific, Waltham, MA), at 37 °C in an air/CO2 (5%) environment. To purify T. gondii tachyzoites, parasites and host cells were washed in cold phosphate-buffered saline (PBS), and the final pellets were suspended in cold PBS and passed three times through a 27-gauge needle. The parasites were then passed through 5.0 μm-pore filters (Millipore, USA), washed twice with PBS, and stored at −80 °C until use.
Sequencing T. gondii RH TgAAP
The TgAAP full-length sequence was amplified from a T. gondii RH strain cDNA library. Primers TgAAPFwd (BamHI site underlined) (5′-CGCGGATCCATGCAGACTGGCACAGAACTC-3′) and TgAAPRev (HindIII site underlined) (5′-CCCAAGCTTTTACATGCCCTTGTAGCTATTGTC-3′) were designed based on the predicted TgAAP sequence (ToxoDB accession number: TGGT1_297970), upstream from the predicted start site and downstream from the predicted stop codon, respectively. The TgAAP fragment was amplified using KOD High-Fidelity DNA polymerase (TOYOBO Shanghai Co., Ltd.), cloned into pGEM-T Easy vector (Promega, France), and sequenced.
Recombinant TgAAP and anti-TgAAP polyclonal antibody production
TgAAP cDNA was amplified using the following primers: ColdTgAAPFwd, 5′-CTCGAGGGATCCGAATTCATGCAAATGCAGACTGGCAC-3′ (EcoRI site underlined); and pColdTgAAPRev, 5′-GTCGACAAGCTTGAATTCTTACATGCCCTTGTAGCTAT-3′ (EcoRI site underlined). The PCR fragment fused with GST at the N-terminus was cloned into the pCold III vector (Takara Bio Inc., Dalian, China). The verified plasmids were transformed into E. coli BL21. The culture were induced by treatment with 1 mM IPTG at an OD600 of ~0.5 and cultivated at 25 °C for 20 h. After centrifugation, cells were resuspended in PBS and lysed by ultrasonic treatment. Purification of rTgAAP was performed using glutathione resin (GenScript, Piscataway, USA), according to the manufacturer’s instructions. rTgAAP fused with GST was eluted with a buffer containing reduced glutathione (20 mM; GE Healthcare, Piscataway, USA) and dialyzed against Tris-HCl (50 mM, pH 8.0). The purified rTgAAP was resolved on SDS-PAGE and native PAGE (Novex® System, Invitrogen), as previously described35. The concentration of purified rTgAAP was measured with the BCA Protein Assay Kit (Thermo Scientific Pierce, USA). Mice were immunized three times at 2 week intervals with GST-tagged rTgAAP peptide (100 μg per injection) formulated in Freund’s Complete and Incomplete Adjuvant6. Sera were collected 14 days after the last immunization.
Enzyme activity and kinetics
Enzymatic activity of rTgAAP was determined by measuring the rate of Glu or Asp release from fluorogenic substrates H-Glu-MCA or H-Asp-MCA (Bachem, Bubendorf, Switzerland), respectively. MCA release was measured using the EnSpire Multimode Plate Reader (PerkinElmer, Turku, Finland), at 355 and 460 nm for both emission and excitation. The experimental data were plotted using GraphPad Prism v. 5.0c (GraphPad Software, San Diego, USA). To determine enzyme sensitivity to metal ions, TgAAP activity was investigated after pre-incubating the enzyme (0.3 μM) at 37 °C for 30 min in Tris-HCl (50 mM, pH 7.5) containing 1 mM specified Co2+ (Sigma-Aldrich, St Louis, USA).
Km (Michaelis-Menten constant) and Vmax (maximum velocity) values of rTgAAP were determined by incubating the enzyme (0.3 μM) in the presence of increasing concentrations of various fluorogenic substrates (Bachem, Bubendorf, Switzerland) at 37 °C. The initial velocity was calculated from the slope of the linear range of fluorescence vs. time curve. The average Km and Vmax values were calculated with standard errors from three independent experiments.
Construction of T. gondii transgenic knockout and complementing plasmids
The structure of the pSAG1::CAS9-U6::sgUPRT plasmid (Addgene, #54467) has been described previously31. In this vector, Cas9 protein fused with GFP is expressed under the control of a SAG1 promotor. We constructed a knockout vector targeting TgAAP by modifying this plasmid. The backbone sequence, except the single guide RNA (gRNA) cassette, was amplified from pSAG1::CAS9-U6::sgUPRT and self-ligated after digestion with PmeI. A DHFR cassette was then inserted into the KpnI site, and the resultant plasmid was designated pCD. Finally, a gRNA cassette targeting TgAAP (gRNA sequence: 5′-AGAACGAGGATATCGTTGAG-3′) under the control of the TgU6 promoter was cloned into the PmeI site. The final plasmid was designated pCD-TgAAP.
Plasmid pBluescript II containing the HXGPRT expression cassette (derived from pHXNTPHA plasmid, a generous gift from Dr. X. Xuan) and a GFP cassette under the control of the GRA1 promotor (derived from PDMG33) was used to construct the complementing plasmid. To validate and identify the monoclonal complemented strain, the GFP expression cassette was inserted into a the plasmid. The plasmid was designated pBH. To construct the complementing plasmid, the synonymous codon TgAAP gene (synoTgAAP, containing synonymous codons GTG and GAA in the target gRNA sequence adjacent to the PAM site) was amplified using primers synoTgAAPFwd (5′-GAACGAGGATATCGTGGAATGGGACTTGTG-3′) and synoTgAAPRev (5′-CACAAGTCCCATTCCACGATATCCTCGTTC-3′), and inserted into the PmeI site of the pBH vector using the ClonExpress™ II One Step Cloning Kit. In the constructed plasmid, synoTgAAP expression was regulated by the GRA1 promoter. The C-terminus of synoTgAAP was fused to mCherry tag (synthesized by GeneScript Co., Ltd.) by hierarchical fusion PCR. The final plasmid was designated pBH-synoTgAAP.
Transient transformation and Cas9-mediated gene disruption and complementation
Transient transformation with the plasmid (pCD-TgAAP) was achieved by transfecting freshly lysed tachyzoites with circular DNA (10 μg)36, and immediate infection of Vero cells. Selection of the transfected parasites that acquired resistance to pyrimethamine (1 μM, in ethanol) was performed as described earlier37. For cloning purposes, the transformed parasites were serially diluted into a culture of Vero cells grown on 96-well plates without selection. Single-plaque wells were replica-passaged for propagation or for western blotting, and indirect immunofluorescence detection with anti-rTgAAP antibodies was used to determine whether the TgAAP gene was disrupted. To generate ΔTgAAP/synoTgAAP complemented parasites, 107 ΔTgAAP parasites were transfected with pBH-synoTgAAP plasmid (10 μg) and selected with mycophenolic acid (25 μg/mL) and xanthine (50 μg/mL). After three generations, parasites expressing synoTgAAP were used in in vitro invasion and replication assays. We also constructed and screened a T. gondii RH strain that was transformed with plasmid pCD-HXGPRT (HXGPRT gRNA sequence: 5′-GTGGAGACCATTGGGTCACT-3′), named Cas9 control. The resultant strain was used as a control.
Western blotting
Samples for western blotting were obtained by gentle centrifugation of extracellular parasites and incubating them with RIPA buffer (50 mM Tris-HCl, pH 8.0, 150 mM NaCl, 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, and 1 mM EDTA) containing a protease inhibitor cocktail (Calbiochem, USA) for 20 min on ice, to lyse them. Next, the samples were centrifuged for 10 min at 15,000 × g at 4 °C, and Laemmli buffer was added to the supernatant. Unless indicated otherwise, an equivalent of 107 parasites was loaded per SDS-polyacrylamide gel lane and immunoblotting was performed. Briefly, parasite extracts were resolved on a 10% SDS-polyacrylamide gel, transferred to a PVDF membrane, and probed with mouse anti-rTgAAP serum (1:500) as a primary antibody. The mCherry protein was detected using an anti-mCherry mouse monoclonal antibody (1:5000) (Abbkine, California, USA) as the primary antibody. Then, a horseradish peroxidase-conjugated goat anti-mouse antibody (1:5000) (Jackson Immunoresearch, West Grove, Pennsylvania, USA) was used as the secondary antibody. Chemiluminescent signals were developed with the SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific, Waltham, USA).
Immunofluorescence
Immunofluorescence assays were carried out as described previously25. Briefly, after 16–24 h of incubation, HFF monolayers infected with T. gondii wild-type RH or ΔTgAAP parasites were fixed with paraformaldehyde (4%) prepared in PBS for 15 min and then permeabilized with Triton X-100 (0.3%) prepared in PBS for 10 min. Cells were then incubated with mouse anti-rTgAAP serum (1:200) as a primary antibody. An Alexa 488-conjugated goat anti-mouse fluorescent antibody (1:500) (Jackson ImmunoResearch) was used as the secondary antibody. The parasite nuclei were visualized with DAPI. Samples were examined with a confocal microscope.
Attachment/invasion assay
To measure parasite attachment/invasion rates, freshly lysed Cas9 control, knockout, and complemented parasites were filtered and used to inoculate HFF cell monolayers in 6-well culture plates (Costar, USA; 106 parasites/well, three wells per each strain). Parasites were allowed to invade HFF cells for 2 h (37 °C, 5% CO2), and extracellular parasites were washed off with PBS. Then, mean numbers of parasites per field (60 fields/well) were counted under a microscope. Three independent experiments were performed, and each strain was assayed in triplicate within each experiment.
Replication assay
To directly compare the growth of Cas9 control and knockout parasites, plaque assays were performed. Purified parasites were used to infect fresh HFF monolayers seeded in 6-well plates and grown for 7 days without shaking (100 parasites/well). Cells were then fixed with ethanol (70%) and stained with crystal violet (0.1%). Plaques were scanned using an Epson scanner and analyzed as previously reported31. Three independent experiments were performed, and each strain was assayed in triplicate within each experiment. To investigate replication rates of ΔTgAAP, Cas9 control, and complemented parasite lines, freshly lysed tachyzoites were collected, filtered, and used to inoculate Vero cell monolayers in 6-well culture plates (106 parasites/well). Parasites were allowed to invade for 2 h under normal growth conditions (37 °C, 5% CO2), following which, extracellular parasites were washed away with PBS, and the parasites were incubated for another 24 h. The number of vacuoles containing the indicated number of parasites (i.e., 1, 2, 4, 8, or >8 cells) was counted in ≥100 vacuoles from three separate wells per experiment. Three independent experiments were conducted, and the results were combined and graphed.
Native enzyme activity assay
To determine the native TgAAP enzyme activity in the knockout and complemented lines, freshly lysed tachyzoites were purified and collected by centrifugation. Proteins were extracted using lysis buffer (20 mM Tris-HCl, pH 8.0, 137 mM NaCl, 1% Nonidet P-40, and 2 mM EDTA) and quantified using a BCA Protein Assay Kit (Pierce, Bonn, Germany). Parasite proteins (10 μg) were added to 200 μL of Tris-HCl buffer (50 mM, pH 7.5) before a specific substrate (0.1 mM) was added. Relative fluorescence levels were assessed every 3 min for a total of 60 min. To determine the effect of TgAAP deletion on the activity of other cellular aminopeptidases, 11 substrates were used. For each substrate, maximum enzyme activities of ΔTgAAP and Cas9 control strains were compared.
Inhibition assay
To determine the inhibitory effect of CID 23724194 (Vitas-M Laboratory, Catalog # STK091533), rTgAAP was pre-incubated with CID 23724194 for 1 h at 37 °C before the addition of the fluorogenic substrate H-Glu-MCA. Relative enzyme inhibition levels were assessed with various final CID 23724194 concentrations (1, 10, 100, 200, and 1000 μM). To determine the inhibitory effect of CID 23724194 on parasite growth, Cas9 control strain cultured in Vero cells was incubated with the compound at various final concentrations (1, 10, 100, and 200 μM) for 3 days. The percentages of Toxoplasma-infected host cells were then evaluated using FACS.
Additional Information
How to cite this article: Zheng, J. et al. Characterization of aspartyl aminopeptidase from Toxoplasma gondii. Sci. Rep. 6, 34448; doi: 10.1038/srep34448 (2016).
Supplementary Material
Acknowledgments
This work was supported by a grant awarded to Honglin Jia by the National Natural Science Foundation of China (no. 31101811). We thank Dr. Joiner, K. A. (Yale University) for providing pHXNTPHA and pDMG plasmids generated by Dr. Xuan, X. (Obihiro University of Agriculture and Veterinary Medicine). We also thank Dr. Jiang, L. for help with molecular protein analyses.
Footnotes
Author Contributions J.Z. and Z.C. conducted the experiments. J.Z. drafted the manuscript. H.J. conceived the study. Y.Z. read and approved the final manuscript.
References
- Dupont C. D., Christian D. A. & Hunter C. A. Immune response and immunopathology during toxoplasmosis. Seminars in Immunopathology. 34, 793–813 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey J. P. & Jones J. L. Toxoplasma gondii infection in humans and animals in the United States. International Journal for Parasitology. 38, 1257–1278 (2008). [DOI] [PubMed] [Google Scholar]
- Jones J. L. et al. Toxoplasma gondii infection in the United States: seroprevalence and risk factors. American Journal of Epidemiology. 154, 357–365 (2001). [DOI] [PubMed] [Google Scholar]
- Flegr J., Prandota J., Sovičková M. & Israili Z. H. Toxoplasmosis–a global threat. Correlation of latent toxoplasmosis with specific disease burden in a set of 88 countries. Plos one 9, e90203 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard N., Dunay I. R. & Schlüter D. Persistence of Toxoplasma gondii in the central nervous system: a fine-tuned balance between the parasite, the brain and the immune system. Parasite Immunol. 37, 150–158 (2015). [DOI] [PubMed] [Google Scholar]
- Gardiner D. L. et al. Overexpression of leucyl aminopeptidase in Plasmodium falciparum parasites. Target for the antimalarial activity of bestatin. J Biol Chem. 281, 1741–1745 (2005). [DOI] [PubMed] [Google Scholar]
- Parlog A., Schlüter D. & Dunay I. R. Toxoplasma gondii-induced neuronal alterations. Parasite Immunol. 37, 159–170 (2015). [DOI] [PubMed] [Google Scholar]
- Pappas G., Roussos N. & Falagas M. E. Toxoplasmosis snapshots: global status of Toxoplasma gondii seroprevalence and implications for pregnancy and congenital toxoplasmosis. International Journal for Parasitology. 39, 1385–1394 (2009). [DOI] [PubMed] [Google Scholar]
- Dannemann B. et al. Treatment of toxoplasmic encephalitis in patients with AIDS-a randomized trial comparing pyrimethamine plus clindamycin to pyrimethamine plus sulfadiazine. Ann. Intern. Med. 116, 33–34 (1992). [DOI] [PubMed] [Google Scholar]
- Jacobson J. M. et al. Pyrimethamine pharmacokinetics in human immunodeficiency virus-positive patients seropositive for Yoxoplasma gondii. Antimicrob. Agents Chemother. 40, 1360–1365 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper N. M. & Lendeckel U. Aminopeptidases in Biology and Disease (Proteases in Biology and Disease). Springer, New York (2004). [Google Scholar]
- Teuscher F. et al. The M18 aspartyl aminopeptidase of the human malaria parasite Plasmodium falciparum. J. Biol. Chem. 282, 30817–30826 (2007). [DOI] [PubMed] [Google Scholar]
- Chang Y. H. & Smith J. A. Molecular cloning and sequencing of genomic DNA encoding aminopeptidase I from Saccharomyces cerevisiae. J. Biol. Chem. 264, 6979–6983 (1989). [PubMed] [Google Scholar]
- Wilk S., Wilk E. & Magnusson R. P. Purification, characterization, and cloning of a cytosolic aspartyl aminopeptidase. J. Biol. Chem. 273, 15961–15970 (1998). [DOI] [PubMed] [Google Scholar]
- Wilk S., Wilk E. & Magnusson R. P. Identification of histidine residues important in the catalysis and structure of aspartyl aminopeptidase. Arch. Biochem. Biophys. 407, 176–183 (2002). [DOI] [PubMed] [Google Scholar]
- Chen Y. et al. Insights into substrate specificity and metal activation of mammalian tetrahedral aspartyl aminopeptidase. J. Biol. Chem. 287, 13356–13370 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen D. D. et al. Structural and kinetic bases for the metal preference of the M18 aminopeptidase from Pseudomonas aeruginosa. Biochem Biophys Res Commun. 447, 101–107 (2014). [DOI] [PubMed] [Google Scholar]
- Dalal S. & Klemba M. Roles for two aminopeptidases in vacuolar hemoglobin catabolism in Plasmodium falciparum. J. Biol. Chem. 282, 35978–35987 (2007). [DOI] [PubMed] [Google Scholar]
- Dura M. A. et al. The structural and biochemical characterizations of a novel TET peptidase complex from Pyrococcus horikoshii reveal an integrated peptide degradation system in hyperthermophilic Archaea. Mol. Microbiol. 72, 26–40 (2009). [DOI] [PubMed] [Google Scholar]
- Kang J. M., Ju H. L., Sohn W. M. & Na B. K. Molecular cloning and characterization of a M17 leucine aminopeptidase of Cryptosporidium parvum. Parasitology. 138, 682–690 (2011). [DOI] [PubMed] [Google Scholar]
- Spicer T. et al. Identification of Potent and Selective Inhibitors of the Plasmodium falciparum M18 Aspartyl Aminopeptidase (PfM18AAP) of Human Malaria via High-Throughput Screening. J. Biomol. Screen. 19, 1107–1115 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou Z., McGovern O. L., Di, Cristina M. & Carruthers V. B. Toxoplasma gondii ingests and digests host cytosolic proteins. MBio. 15, e01188–14 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivaraman K. K. et al. X-ray crystal structure and specificity of the Plasmodium falciparum malaria aminopeptidase PfM18AAP. J. Mol. Biol. 422, 495–507 (2012). [DOI] [PubMed] [Google Scholar]
- Chaikuad A. et al. Structure of human aspartyl aminopeptidase complexed with substrate analogue: insight into catalytic mechanism, substrate specificity and M18 peptidase family. BMC. Struct. Biol. 12, 14 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia H. et al. Characterization of a leucine aminopeptidase from Toxoplasma gondii. Mol. Biochem. Parasitol. 170, 1–6 (2010). [DOI] [PubMed] [Google Scholar]
- Rosenthal P. J., Sijwali P. S., Singh A. & Shenai B. R. Cysteine proteases of malaria parasites: targets for chemotherapy. Curr Pharm Des. 8, 1659–1672 (2002). [DOI] [PubMed] [Google Scholar]
- Drag M. & Salvesen G. S. Emerging principles in protease-based drug discovery. Nat. Rev. Drug Discov. 9, 690–701 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paiardini A. et al. & McGowan S. Screening the Medicines for Malaria Venture “Malaria Box” against the Plasmodium falciparum Aminopeptidases, M1, M17 and M18. PLoS One. 10, e0115859 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stack C. M. et al. Characterization of the Plasmodium falciparum M17 leucyl aminopeptidase. A protease involved in amino acid regulation with potential for antimalarial drug development. J. Biol. Chem. 282, 2069–2080 (2007). [DOI] [PubMed] [Google Scholar]
- McGowan S. Working in concert: the metalloaminopeptidases from Plasmodium falciparum. Current Opinion in Structural Biology. 23, 828–835 (2013). [DOI] [PubMed] [Google Scholar]
- Shen B., Brown K. M., Lee T. D. & Sibley L. D. Efficient gene disruption in diverse strains of Toxoplasma gondii using CRISPR/CAS9. MBio. 5, e01114–e01114 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidik S. M. et al. Efficient genome engineering of Toxoplasma gondii using CRISPR/Cas9. PLoS One. 9, e100450 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J., Jia H. L. & Zheng Y. H. Knockout of leucine aminopeptidase in Toxoplasma gondii using CRISPR/Cas9. Int. J. Parasitol. 45, 141–148 (2015). [DOI] [PubMed] [Google Scholar]
- Spangenberg T. et al. The open access malaria box: a drug discovery catalyst for neglected diseases. PLoS One 8, e62906 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schägger H. Blue Native Gels to Isolate Protein Complexes from Mitochondria. Meth. Cell Biol. 65, 231–244 (2001). [DOI] [PubMed] [Google Scholar]
- Soldati D. & Boothroyd J. C. Transient transfection and expression in the obligate intracellular parasite Toxoplasma gondii. Science. 260, 349–352 (1993). [DOI] [PubMed] [Google Scholar]
- Reynolds M. G., Oh J. & Roos D. S. In vitro generation of novel pyrimethamine resistance mutations in the Toxoplasma gondii dihydrofolate reductase. Antimicrob. Agents Chemother. 45, 1271–1277 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.