Abstract
Ataxia-telangiectasia mutated (ATM) kinase is a central component involved in the signal transduction of the DNA damage response (DDR) and thus plays a critical role in the maintenance of genomic integrity. Although the primary functions of ATM are associated with the DDR, emerging data suggest that ATM has many additional roles that are not directly related to the DDR, including the regulation of oxidative stress signaling, insulin sensitivity, mitochondrial homeostasis, and lymphocyte development. Patients and mice lacking ATM exhibit growth retardation and lower bone mass; however, the mechanisms underlying the skeletal defects are not fully understood. In the present study, we generated mutant mice in which ATM is specifically inactivated in osteoclasts. The mutant mice did not exhibit apparent developmental defects but showed reduced bone mass due to increased osteoclastic bone resorption. Osteoclasts lacking ATM were more resistant to apoptosis and showed a prolonged lifespan compared to the controls. Notably, the inactivation of ATM in osteoclasts resulted in enhanced NF-κB signaling and an increase in the expression of NF-κB-targeted genes. The present study reveals a novel function for ATM in regulating bone metabolism by suppressing the lifespan of osteoclasts and osteoclast-mediated bone resorption.
Ataxia-telangiectasia mutated (ATM) is a 350 kDa Ser/Thr protein kinase that exists basally as an inactive dimer. Upon DNA damage, ATM is recruited to the damaged site and activated to initiate a cellular signal that regulates cell cycle checkpoints and promotes DNA repair1,2,3. The activation of ATM also leads to cellular senescence and apoptosis through the phosphorylation of p53 under certain conditions. Consistent with this, patients with mutations in the ATM gene, which cause a rare inherited disorder called ataxia-telangiectasia (A-T), are highly susceptible to DNA damage caused by ionizing radiation and exhibit various defects, including cerebellar ataxia, premature aging, and increased predisposition to malignancy4,5,6. While ATM is best characterized as a master regulator of the DNA damage response (DDR), emerging data have demonstrated that ATM has extensive functions in maintaining cellular homeostasis that are not directly related to the DDR. These additional roles include the regulation of oxidative stress7, metabolic signaling pathways (including insulin sensitivity and glucose metabolism)8,9,10,11, mitochondrial homeostasis12,13, immunity (most importantly, V(D)J recombination and class switch recombination)14,15,16,17,18,19, gonadal and germline development14,20,21,22, and telomere elongation23, among others. These observations clearly underscore the crucial functions of ATM not only in managing the DDR but also in regulating development and homeostasis in various types of cells and tissues.
Although A-T patients develop growth retardation, there are only a few studies describing the potential function of ATM in skeletal development. In the growth plates, ATM indirectly regulates chondrocyte proliferation and differentiation by regulating the amount of reactive oxygen species (ROS)24. The systemic abrogation of Atm in mice also results in decreased bone mass, most likely due to a steroid hormone deficiency caused by gonad developmental defects20,25. Whereas these studies show that mice lacking ATM exhibit reduced bone formation and increased bone resorption, the potential cell-autonomous functions of ATM in osteoblasts and osteoclasts are not yet fully understood.
Osteoclasts are multinucleated cells that are essential for bone resorption and remodeling in vivo26,27,28. The progenitors of osteoclasts are derived from the monocyte/macrophage cell lineage and express the receptor activator of NF-κB (RANK). Binding of RANK with RANK ligand (RANKL), a membrane-bound ligand expressed on osteoblasts and osteocytes, triggers intracellular signaling, which ultimately leads to osteoclast differentiation. Past studies have revealed that the differentiation and activity of osteoclasts are tightly regulated by various hormones and cytokines produced by immune cells and osteoblasts. However, despite the intricate mechanisms involved in maintaining skeletal homeostasis in vivo, the activity of osteoclasts is often dysregulated, resulting in overt bone resorption and reduced bone mass, as observed in skeletal disorders such as osteoporosis, bone metastasis, and arthritic bone destruction29,30,31. Therefore, it is clinically relevant and crucial to learn more about the regulatory mechanisms behind the differentiation and activity of osteoclasts.
In the present study, we generated mutant mice in which Atm is specifically abrogated under the control of the cathepsin K promoter (AtmCtsk mice) to elucidate the potential function of ATM in regulating osteoclast activity. These mice did not exhibit overt abnormalities but showed reduced bone mass due to increased bone resorption. Osteoclasts lacking ATM were more resistant to apoptosis and had an extended lifespan compared to control cells. Of note, the NF-κB pathway, a critical regulator of osteoclast activity and longevity32, was significantly enhanced in osteoclasts derived from AtmCtsk mice compared to those from control mice. These results show an unexpected role of ATM as a negative regulator of osteoclast longevity through suppressing NF-κB signaling in vivo and may shed novel light on the biology of ATM and skeletal homeostasis.
Results
Atm Ctsk mice exhibit reduced bone volume
In a preliminary experiment, we found that ATM is activated at a higher level in mature osteoclasts than in bone marrow-derived macrophages (BMMs), indicating that ATM is involved in a later stage of osteoclastogenesis or in the maintenance of osteoclast functions (Fig. 1A). The activation of ATM in mature osteoclasts was, in part, dependent on ROS production as a treatment with a potent ROS scavenger N-acetyl cysteine suppressed the phosphorylation of ATM (Supplementary Fig. 1A). On the other hand, DDR was not markedly induced in osteoclasts under the present experimental settings (Supplementary Fig. 1B). These results suggest that the activation of ATM is, at least in part, dependent on ROS production but not derived from DSB in mature osteoclasts.
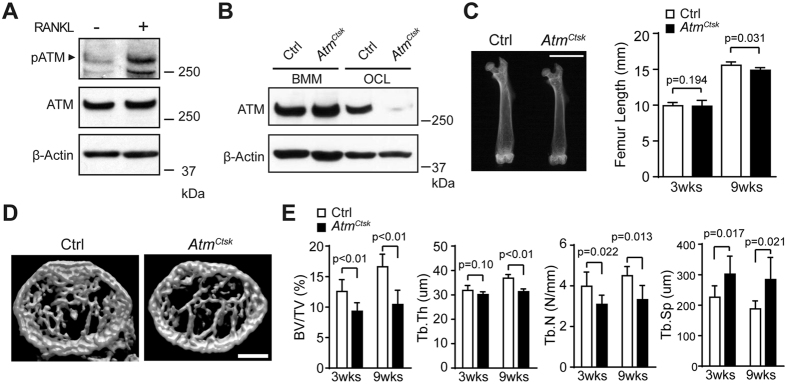
Figure 1. Conditional inactivation of ATM in osteoclasts results in decreased bone mass.
(A) Western blots showing Phosphorylated-ATM (p-ATM) and total ATM in BMMs incubated with (+) or without (−) sRANKL for 5 d. Representative images of 3 independent experiments are shown. (B) Immunoblots of the cell lysates collected from BMMs and osteoclasts (OCL) probed for ATM. (C) X-ray images of the femurs of 9-week-old Ctrl and AtmCtsk mice (left panel). Scale bar, 5 mm. The lengths of the femurs collected from 3- and 9-week-old Ctrl and AtmCtsk mice are shown (right panel). (D) Reconstructed three-dimensional μCT images of the distal femurs of Ctrl and AtmCtsk mice. Scale bar, 500 μm. (E) Bone morphometric analysis of the femurs collected from 3- and 9-week-old Ctrl and AtmCtsk mice. BV/TV, bone volume/tissue volume; Tb.Th, trabecular thickness; Tb.N, trabecular number; Tb.Sp, trabecular space. n = 7 and 6 for 3-week-old Ctrl and AtmCtskmice, respectively, and n = 5 mice for each genotype at 9 weeks old.
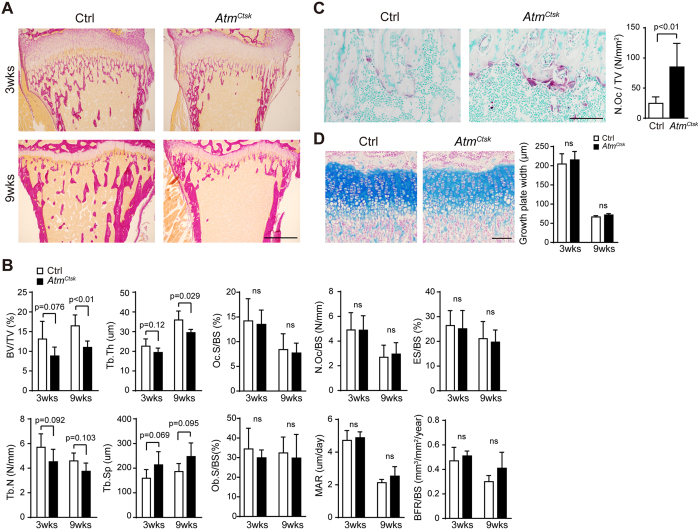
To elucidate the potential role of ATM in osteoclasts, we generated mutant mice in which Atm is abrogated under the control of a Ctsk (encodes the osteoclast-specific proteolytic enzyme cathepsin K) promoter. AtmCtsk mice did not show any gross defects under unchallenged conditions (data not shown). Atmflox/+, Atmflox/flox, and Atmflox/+/Ctsk-Cre mice exhibited no apparent pathological phenotype and were used as control (Ctrl) animals in the present study. Western blot analysis showed that ATM was expressed in both BMMs and mature osteoclasts from Ctrl mice. The expression of ATM was also observed in AtmCtsk mouse-derived BMMs; however, this expression was markedly suppressed when the cells were induced into osteoclasts, demonstrating that the Atm alleles were successfully excised specifically in osteoclasts (Fig. 1B). Although AtmCtsk mice showed no significant difference in body size or weight (data not shown), the length of their long bones was approximately 5% shorter compared to Ctrl mice at 9 weeks of age (Fig. 1C). We next performed micro-computed tomography (μCT) analysis and found that AtmCtsk mice exhibited reduced bone mass compared to Ctrl mice, as highlighted by decreased BV/TV, Tb.Th, and Tb.N, and increased Tb.Sp values (Fig. 1D,E). Structural histomorphometric parameters (BV/TV, Tb.Th, Tb.N, and Tb.Sp) of the tibial sections at the secondary spongiosa showed a similar trend (Fig. 2A,B). Notably, we observed a significant increase in the osteoclast number in AtmCtsk mice at the edge of primary spongiosa (Fig. 2D). On the other hand, there was no significant differences in the bone formative or resorptive parameters in histomorphometric, inducing Oc.S/BS, Oc.N/BS, ES/BS, Ob.S/BS, MAR, and BFR/BS, in the secondary spongiosa (Fig. 2B), or in the thickness of the growth plates (Fig. 2D). These observations indicate that AtmCtsk mice exhibit reduced bone mass primarily due to an increase in osteoclastic bone resorption at the edge of primary spongiosa, whereas bone resorption at the secondary spongiosa is not overtly enhanced.
Figure 2. AtmCtsk mice have increased osteoclast numbers.
(A) Sections of the proximal tibia of 3-week- (upper panel) and 9-week-old (lower panel) Ctrl and AtmCtsk mice stained with van Gieson stain. Scale bar: 500 μm. (B) Bone histomorphometry of the proximal tibia. BV/TV, bone volume/tissue volume; Tb.Th, trabecular thickness; Tb.N, trabecular number; Tb.Sp, trabecular space; Oc.S/BS, osteoclast surface/bone surface; N.Oc/BS, number of osteoclasts/bone surface; ES/BS, erosion surface/bone surface; Ob.S/BS, osteoblast surface/bone surface; MAR, mineral apposition rate; BFR/BS, bone formation rate/bone surface. (C) Sections of the tibia (3-week-old mice) stained for TRAcP and counterstained with methyl green. Images of the primary spongiosa are shown. Scale bar, 100 μm. The number of osteoclasts/tissue volume (N.Oc/TV) at the edge of tibial primary spongiosa of 3-week-old mice. (D) Sections of the tibia (3-week-old mice) stained with Alcian blue. Images of the growth plate are shown. Scale bar, 100 μm. Growth plate width of 3-week- (upper panel) and 9-week-old Ctrl and AtmCtsk mice was measured. n = 7 and 6 for 3-week-old Ctrl and AtmCtsk mice, respectively, and n = 5 mice for each genotype at 9 weeks old. ns, not significant.
BMMs derived from Atm Ctsk mice exhibit no apparent defects in differentiation
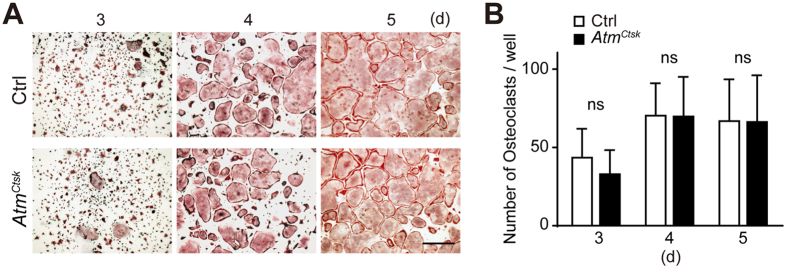
The lower bone mass and increased osteoclast number observed in AtmCtsk mice at the primary spongiosa suggested that osteoclastogenesis was enhanced in these mice. BMMs collected from Ctrl and AtmCtsk mice were incubated in the presence of soluble RANKL (sRANKL) and macrophage colony-stimulating factor (M-CSF) for 3–5 d and stained for tartrate-resistant acid phosphatase (TRAcP). Contrary to our expectations, there was no difference in the number of multinucleated osteoclasts between Ctrl and AtmCtsk mouse-derived BMMs at any time period (Fig. 3). We also found no changes in the expression levels of genes involved in osteoclastogenesis (including Nfatc1, Fos, Acp5, and Dcstamp) between Ctrl and AtmCtsk osteoclasts (data not shown). These observations show that conditional abrogation of Atm under the control of the Ctsk gene promoter does not have any marked impact on osteoclast differentiation in vitro, as has been previously described20,25.
Figure 3. BMMs prepared from AtmCtsk bone marrow cells show no defect in osteoclast differentiation.
(A) BMMs prepared from Ctrl and AtmCtsk bone marrow cells were incubated for 3, 4, or 5 d and stained for TRAcP. Scale bar: 1 mm. (B) The number of TRAcP-positive multinucleated cells in each well. n = 5 and 6 replicates for Ctrl and AtmCtsk, respectively. ns, not significant.
Osteoclasts lacking ATM exhibit prolonged survival
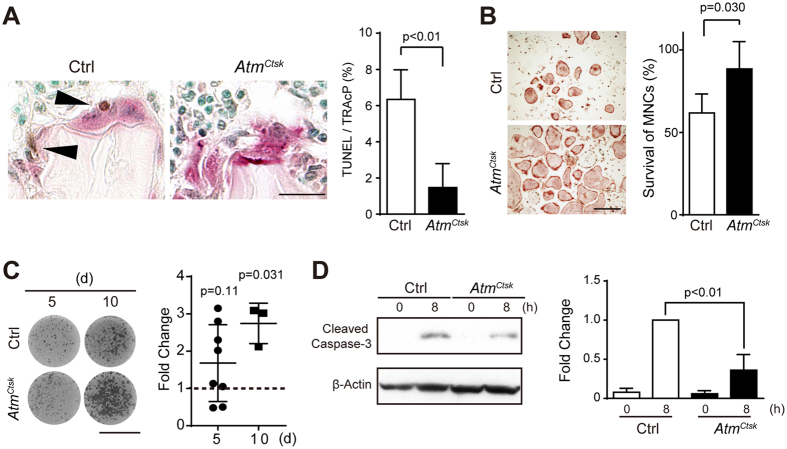
Because BMMs derived from AtmCtsk mice were not defective in osteoclast differentiation, we next asked if the lack of ATM had any impact on the lifespan of osteoclasts. Tibial sections prepared from Ctrl and AtmCtsk mice were stained for apoptotic cells using the terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) method and also stained for TRAcP. We enumerated the TRAcP-positive cells and assessed whether these cells contained nuclei that were positive for TUNEL. As shown in Fig. 4A, we found that the ratio of TRAcP-positive multinucleated cells containing at least one TUNEL-positive nucleus was significantly higher in AtmCtsk mice than in Ctrl mice, indicating that osteoclasts in AtmCtsk mice are less prone to apoptosis than those in Ctrl mice. To confirm this finding, we next induced osteoclasts in vitro using BMMs prepared from Ctrl and AtmCtsk mice and further cultured these cells under sRANKL- and M-CSF-depleted conditions for 8 h to induce apoptosis. At the end of the incubation, the cells were stained for TRAcP, and the number of surviving cells was enumerated. As shown in Fig. 4B, we found that more TRAcP-positive multinucleated cells remained on the plates with AtmCtsk mouse-derived cells than with control cells, indicating that osteoclasts derived from AtmCtsk BMMs were more resistant to growth factor-deprived conditions. In parallel with these findings, the amount of resorption, as evaluated with a pit formation assay, was increased in AtmCtsk osteoclasts compared to Ctrl osteoclasts, which was statistically significant at day 10 of incubation (Fig. 4C). This observation indicates that a lack of ATM in osteoclasts does not enhance the bone resorption activity per se, but the prolonged lifespan results in an overall increase in the amount of resorption. Furthermore, the protein level of cleaved caspase-3, a marker for apoptotic cells, was decreased in AtmCtsk cells compared to control cells under growth factor-deprived conditions, suggesting that osteoclasts lacking ATM are more resistant to apoptosis under the current experimental conditions (Fig. 4D).
Figure 4. Osteoclasts lacking ATM are less prone to apoptosis.
(A) Tibial sections from 3-week-old Ctrl and AtmCtsk mice dually stained for TUNEL and TRAcP (left panel). The ratio of TUNEL-positive osteoclasts in the tibial sections of 3-week-old control and AtmCtsk mice is shown (right panel). Scale bar: 20 μm. n = 7 for control and 6 for AtmCtsk mice, respectively. At least 50 osteoclasts were evaluated in each genotype. (B) Representative images of TRAcP-stained osteoclasts (left panel) and the ratio of remaining osteoclasts (right panel) 8 h after the withdrawal of sRANKL and M-CSF. The number of osteoclasts before the withdrawal of growth factors was set to 100%. n = 4 and 5 replicates for Ctrl and AtmCtsk, respectively. Scale bar: 0.5 mm. (C) Representative images of osteoclast-formed pits (left) and the ratio of the pit area between Ctrl and AtmCtsk cells (right) on days 5 and 10 after sRANKL stimulation are shown. n = 8 and 3 for day 5 and 10, respectively. Each value was calculated from of 3-4 replicates. (D) Immunoblots for cleaved caspase-3 in Ctrl and AtmCtsk osteoclasts 0 and 8 h after the withdrawal of growth factors (left panel). Quantification of the relative changes in the signal intensity of cleaved caspase-3 (right panel). The signal intensity of cleaved caspase-3 in Ctrl cells 8 h after the starvation was normalized to 1. The data were obtained from 3 independent experiments.
NF-κB activity is enhanced in osteoclasts lacking ATM
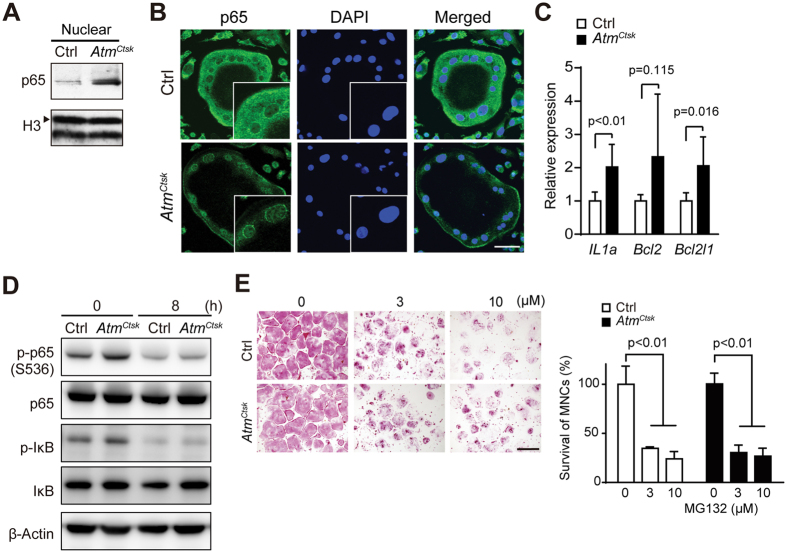
To further elucidate the potential mechanism behind the pro-apoptotic effect of ATM in osteoclasts, we next asked whether the lack of ATM in osteoclasts affects the activity of the NF-κB pathway, a signaling pathway critically involved in osteoclast survival28,32. Western blots using nuclear extracts from osteoclasts showed an increase in the level of p65 (encoded by Rela), one of the major components of the NF-κB complex, in AtmCtsk osteoclasts compared to Ctrl osteoclasts (Fig. 5A). Consistent with this, immunostaining for p65 revealed nuclear accumulation of p65 in AtmCtsk osteoclasts, which reflects increased NF-κB activity in the absence of ATM (Fig. 5B). Furthermore, there was an increase in the transcripts for anti-apoptotic genes, including Il1a, Bcl2, and Bcl2l1 (encodes Bcl-XL), which are all regulated by the NF-κB pathway (Fig. 5C). In parallel with these findings, we also confirmed the increased levels of phosphorylated-IκB and phosphorylated-p65 (Ser536) (Fig. 5D, both of which reflect the activity of the NF-κB pathway. Of note, we found that the levels of both phosphorylated-IκB and phosphorylated-p65 (Ser536) decreased after sRANKL- and M-CSF-depletion, yet the expression of these molecules remained higher in AtmCtsk osteoclasts than in Ctrl cells (Fig. 5D). In addition, as have been previously described33,34, inhibition of p65 activity by a proteasome inhibitor MG-132 significantly suppressed the survival of both AtmCtsk and Ctrl osteoclasts (Fig. 5E), indicating that the survival of osteoclasts are highly dependent on the activity of the NF-κB pathway. Taken together, these observations suggest that the abrogation of Atm in osteoclasts extends the lifespan of osteoclasts most likely by enhancing NF-κB activity.
Figure 5. Enhanced NF-κB signaling in osteoclasts lacking ATM.
(A) Immunoblots of the nuclear extracts of Ctrl and AtmCtsk osteoclasts probed for p65. Representative images of 3 independent experiments are shown. The blots for histone H3 (H3, arrowhead) serve as an internal control. (B) Ctrl and AtmCtsk osteoclasts stained for p65 and DAPI. The insets show a magnified image of the nuclei. Scale bar, 50 μm. (C) Relative expression of the transcripts of NF-κB target genes determined by quantitative PCR. n = 6 replicates. The average expression levels of each transcript in the Ctrl sample are set to 1. (D) Immunoblots for p65, IκB and their phosphorylated forms in Ctrl and AtmCtsk osteoclasts 0 and 8 h after the withdrawal of growth factors. Representative images of 3 independent experiments are shown. (E) TRAcP staining of Ctrl and AtmCtsk osteoclasts treated with MG-132 (0, 3, and 10 μM). The number of TRAcP-positive multinucleated cells in each well. n = 4 replicates for Ctrl and AtmCtsk, respectively. Scale bar, 500 μm.
Discussion
Our data show that the conditional abrogation of Atm in osteoclasts leads to decreased bone mass, potentially due to prolonged osteoclast survival. We found that fewer osteoclasts in AtmCtsk mice stained positive for TUNEL compared to those in Ctrl mice, indicating that a lack of ATM renders osteoclasts less prone to apoptosis. Consistently, osteoclasts from AtmCtsk mice were more resistant to apoptosis under sRANKL- and M-CSF-deprived conditions than those from Ctrl mice. Furthermore, we found that nuclear accumulation of p65 was enhanced in AtmCtsk osteoclasts, suggesting that the prolonged longevity of AtmCtsk osteoclasts was due to increased activation of the NF-κB pathway. Taken together, the present study reveals an unexpected role for ATM in regulating osteoclast longevity and bone volume.
The observation that the loss of ATM promotes the survival of mature osteoclasts was somewhat unexpected. Because ATM plays a protective role against genomic damage and the accumulation of intracellular ROS, we initially assumed that osteoclasts lacking ATM would be more prone to apoptosis and hence would have a shorter lifespan. In the developing central nervous system, radiation-induced cell death is largely dependent on ATM activity, and it has been shown that the loss of ATM renders neurons more resistant to apoptosis35. In a similar vein, our data suggest that the induction of apoptosis under physiological conditions is in part dependent on ATM activity in osteoclasts. However, a lack of ATM in osteoclasts does not have marked effects on the efficiency of bone resorption under physiological conditions in adult mice.
As a potential mechanism behind the enhanced survival of osteoclasts lacking ATM, we observed an increase in the activity of NF-κB in AtmCtsk osteoclasts. Because the activation of NF-κB can be triggered by ATM36,37, our data may appear counterintuitive. Nonetheless, a recent study has shown that the loss of ATM can induce NF-κB signaling, at least in certain cell types. Hathcock et al. demonstrated that systemic abrogation of ATM promotes the development of B-cell lymphoma in mice lacking T cells and that the NF-κB-pathway was significantly enhanced in these lymphoma cells38. The study also showed that the survival of the ATM-deficient lymphoma cells was dependent on NF-κB activity using pharmacological inhibitors of IKK. Although it is not fully understood how the loss of ATM enhances NF-κB activity in lymphocytes, the study supports the idea that ATM can both positively and negatively regulate NF-κB activity in a context- or cell-dependent manner. Given that the primary function of ATM is to induce DDR upon DNA double-strand break, it is also possible that the extended life-span was derived from the failure to induce DDR-mediated apoptosis. However, our data indicate that DDR is not noticeably induced in mature osteoclasts (Supplementary Fig. 1B), arguing against this hypothesis.
How ATM is activated in mature osteoclasts and how ATM negatively regulates the activity of NF-κB remain to be addressed. Our data suggest that ROS production is partially responsible for the activation of ATM39. On the other hand, we did not find any marked DDR in mature osteoclasts, indicating that DSB is not the major contributor for ATM activation. The potential mechanisms behind the suppression of NF-κB activity by ATM may include the inhibition of p65 activity via the phosphorylation of Ser547 by ATM40; the reciprocal inhibition of NF-κB and p53, where the abrogation of the ATM-p53 axis reversely enhances NF-κB activity41; the competitive binding of ATM and NF-κB to protein phosphatase 2A, which exhibits inhibitory activity towards both ATM and NF-κB42,43. In the present study we found the levels of the phosphorylation of p65 (Ser536) and IκB were increased in AtmCtsk osteoclasts compared to that in Ctrl (Fig. 5D). This observation indicates that the NF-κB pathway is activated through the conventional pathway which involves phosphorylation and degradation of IκB in AtmCtsk osteoclasts. On the other hand, it remains to be clarified what the direct substrates of ATM in osteoclasts and whether other factors than phosphorylation of IκB and p65 are involved in the activation of the NF-κB pathway in osteoclasts. Screening of the potential ATM substrates in osteoclasts using mass spectrometry may help to address these issues.
There are limitations in the present study. First, most importantly, because all of the animal experiments were performed under unchallenged conditions, it is not clear whether the results of the present study reflect the pathological conditions of skeletal diseases with enhanced bone resorption. Second, because we used mice of mixed genetic background (129Sv and C57BL/6) for morphometric analyses the data potentially have a large dispersion. Nevertheless, the data from 3 week-old mice showed a similar trend with those of 9 week-old mice, indicating that our results were consistent among different sets of animals. Of note, a recent study has shown that the Atm protein and transcript levels decline with aging in splenocytes44. Accordingly, our preliminary observations indicate that this is also the case for bone marrow cells and osteoclasts (data not shown). Because a loss of ATM in osteoclasts results in enhanced bone resorption, it is tempting to speculate that the decrease in ATM activity is causally related to the pathogenesis of senile osteoporosis.
In summary, the present study identifies ATM as a novel negative regulator of osteoclastic bone resorption. The loss of ATM in osteoclasts leads to enhanced NF-κB activity and promotes their survival, leading to an increase in overall bone resorption. Our data therefore reveal an unexpected role for ATM, a critical molecule involved in the maintenance of genome integrity, in osteoclast longevity and bone resorption.
Methods
Mice
The Atmflox/flox mice and Ctsk-Cre transgenic mice were generated as previously described16,45. The mice used in the present study are of a mixed genetic background (129Sv and C57BL/6). All animal experiments were approved by the Institutional Animal Care and Use Committee of the Keio University School of Medicine (approval number, 09101) and performed in accordance with the approved guidelines. Atmflox/flox mice and Ctsk-Cre transgenic mice were kindly provided by Dr. Fred Alt (Harvard Univ.) and Dr. Shigeaki Kato (Tokyo Univ.), respectively. Histology and morphometric analyses were performed using male mice. Bone marrow cells were collected from both male and female mice.
Reagents and antibodies
Anti-ATM (2C1), anti-p65 (C-20), anti-p53 (FL-393), and anti-β actin (C4) antibodies were obtained from Santa Cruz Biotechnology (Dallas, TX). Anti-cleaved caspase-3 (Asp175, 9661), anti-histone H3, anti-phosphorylated p65 (Ser536, 3031), anti- phosphorylated IκB (Ser32/36, 9246), anti-phosphorylated p53 (Ser15, 9284), anti-phosphorylated H2AX (Ser139, 9718), and anti-IκB antibodies were purchased from Cell Signaling Technology (Danvers, MA). Anti-phospho-ATM (Ser1981) antibody (10H11.E12) was obtained from Rockland Immunochemicals (Limerick, PA). To detect phospho-ATM, blocking was performed using phosphoBLOCKER Blocking Reagent (Cell Biolabs, San Diego, CA). M-CSF and sRANKL were purchased from Kyowa Kirin (Tokyo, Japan) and PeproTech (Rocky Hill, NJ), respectively.
μCT and histomorphometric analysis
Three-dimensional images of the distal femurs were reconstructed using a μCT scanner (R_mCT2, Rigaku, Yokohama, Japan) and the 3D software TRI/3D-BON (Ratoc System Engineering, Tokyo, Japan). The ethanol-fixed tibiae were embedded in glycol methacrylate resin and sectioned into 5-μm slices. For histomorphometric analyses, an area 1.2 mm above the growth plate (1.62–2.34 mm2 in size) at the proximal metaphysis was evaluated. For the assessment of osteoclasts, tibial sections were stained for TRAcP and with van Gieson’s solution and Alcian blue. All of the measurements were performed in a blinded manner.
Osteoclast culture
BMMs were prepared as previously described46 and used as osteoclast precursors. The BMMs were cultured in the presence of sRANKL (50 ng/ml) and M-CSF (3.3 × 103 U/ml) for 5 d to induce osteoclastogenesis. TRAcP staining was performed as previously described46. For the osteoclast survival assay, osteoclasts induced in vitro were cultured for 8 h in the absence of sRANKL and M-CSF as previously described47,48. At the end of the incubation, the cells remaining on the dishes were stained for TRAcP, and the TRAcP-positive multinucleated cells (>3 nuclei) were enumerated. To examine the inhibitory effect of MG-132 on osteoclast survival, mature osteoclasts formed in vitro were treated with MG-132 (0, 3, and 10 μM) for 18 h. The surviving cells were fixed and stained for TRAcP.
RNA isolation and quantitative RT-PCR
Total RNAs were extracted from cells using Sepasol reagent (Nacalai Tesque, Kyoto, Japan) and used for RT-PCR analyses. The nucleotide sequences of the oligos used in the present study are presented in Supplementary Table 1. The relative gene expression was determined by SYBR Green–based real-time PCR using a 7300/7500 Fast real-time PCR system (Applied Biosystems, Foster City, CA) and a LightCycler II (Roche, Risch-Rotkreuz, Switzerland). The expression levels were normalized to the Gapdh transcript expression levels.
Resorption pit assay
Osteoclast activity was evaluated using an Osteo Assay Surface (Corning, Corning, NY) according to the manufacturer’s instructions. Stripwells were scanned using a flatbed scanner, and osteoclast-formed pits were analyzed using ImageJ software (http://imagej.nih.gov/ij/index.html).
Immunofluorescence microscopy
The cells were fixed with 4% paraformaldehyde for 15 min and permeabilized in 0.3% Triton X-100 for 10 min. The fixed cells were incubated with a primary antibody overnight at 4 °C and subsequently with a secondary antibody (AlexaFluor 488–conjugated anti-rabbit IgG or AlexaFluor 546/555–conjugated anti-mouse IgG, Invitrogen, Carlsbad, CA) for 1 h at 37 °C. The nuclei were stained with DAPI (Vectashield HardSet Mounting Medium with DAPI, Vector Laboratories, Burlingame, CA). Images were captured using a FluoView FV10i confocal microscope (Olympus, Tokyo, Japan) and the Olympus FluoView Viewer software (Ver. 3.1). The acquired images were processed with Adobe Photoshop CS6 (Adobe Systems, San Jose, CA) for contrast and black balance correction.
Evaluation of apoptotic multinucleated cells
The sections of the tibiae collected from 3-week-old AtmCtsk and Ctrl mice were stained for TRAcP and TUNEL. TUNEL staining was performed using an In situ Apoptosis Detection Kit (Takara Bio, Shiga, Japan) according to the manufacturer’s instructions. The nuclei were counterstained with methyl green. At least 50 TRACP-positive osteoclasts in each section were evaluated for the presence of TUNEL-positive nuclei, and the ratio of TUNEL-positive multinucleated cells was calculated.
Statistics
All statistical analyses were performed using GraphPad Prism 7 (GraphPad Software, La Jolla, CA). Two-tailed Student’s t-tests for 2 samples assuming equal variances were used to calculate the p values except for those in Fig. 5E. One-way ANOVA was used to calculate the p values in Fig. 5E. Values of p < 0.05 were considered significant. The values are presented as the mean ± SD.
Additional Information
How to cite this article: Hirozane, T. et al. Conditional abrogation of Atm in osteoclasts extends osteoclast lifespan and results in reduced bone mass. Sci. Rep. 6, 34426; doi: 10.1038/srep34426 (2016).
Supplementary Material
Acknowledgments
We would like to thank Akemi Ito (Bone Science Institute) for her advice on histomorphometric analysis and Shizue Yamanashi, Kaori Sue, Mayumi Ito, and Naoko Goto for technical support. This study was supported in part by MEXT KAKENHI (24390358 to K.H.) and JSPS KAKENHI (16J02900 to T.H.).
Footnotes
Author Contributions T.H. performed almost all of the experiments, prepared the Figures, and wrote the manuscript. T.T. and Y.M. supported in vitro experiments. M.S. and Y.K. supported histological analyses. M.M., H.M. and M.N. supervised the project. K.H. designed the project, prepared the Figures, and wrote the manuscript. All authors reviewed the manuscript.
References
- Stracker T. H., Roig I., Knobel P. A. & Marjanovic M. The ATM signaling network in development and disease. Front Genet 4, 37 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guleria A. & Chandna S. ATM kinase: Much more than a DNA damage responsive protein. DNA Repair (Amst) 39, 1–20 (2016). [DOI] [PubMed] [Google Scholar]
- Shiloh Y. & Ziv Y. The ATM protein kinase: regulating the cellular response to genotoxic stress, and more. Nat Rev Mol Cell Biol 14, 197–210 (2013). [PubMed] [Google Scholar]
- Kastan M. B., Lim D. S., Kim S. T. & Yang D. ATM–a key determinant of multiple cellular responses to irradiation. Acta Oncol 40, 686–688 (2001). [DOI] [PubMed] [Google Scholar]
- McKinnon P. J. ATM and the molecular pathogenesis of ataxia telangiectasia. Annu Rev Pathol 7, 303–321 (2012). [DOI] [PubMed] [Google Scholar]
- Lavin M. F. Ataxia-telangiectasia: from a rare disorder to a paradigm for cell signalling and cancer. Nat Rev Mol Cell Biol 9, 759–769 (2008). [DOI] [PubMed] [Google Scholar]
- Ditch S. & Paull T. T. The ATM protein kinase and cellular redox signaling: beyond the DNA damage response. Trends Biochem Sci 37, 15–22 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schalch D. S., McFarlin D. E. & Barlow M. H. An unusual form of diabetes mellitus in ataxia telangiectasia. N Engl J Med 282, 1396–1402 (1970). [DOI] [PubMed] [Google Scholar]
- Yang D. Q. & Kastan M. B. Participation of ATM in insulin signalling through phosphorylation of eIF-4E-binding protein 1. Nat Cell Biol 2, 893–898 (2000). [DOI] [PubMed] [Google Scholar]
- Armata H. L. et al. Requirement of the ATM/p53 tumor suppressor pathway for glucose homeostasis. Mol Cell Biol 30, 5787–5794 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peretz S., Jensen R., Baserga R. & Glazer P. M. ATM-dependent expression of the insulin-like growth factor-I receptor in a pathway regulating radiation response. Proc Natl Acad Sci USA 98, 1676–1681 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton J. S., Lin Z. P., Sartorelli A. C., Bonawitz N. D. & Shadel G. S. Ataxia-telangiectasia mutated kinase regulates ribonucleotide reductase and mitochondrial homeostasis. J Clin Invest 117, 2723–2734 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentin-Vega Y. A. et al. Mitochondrial dysfunction in ataxia-telangiectasia. Blood 119, 1490–1500 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow C. et al. Atm-deficient mice: a paradigm of ataxia telangiectasia. Cell 86, 159–171 (1996). [DOI] [PubMed] [Google Scholar]
- Xu Y. et al. Targeted disruption of ATM leads to growth retardation, chromosomal fragmentation during meiosis, immune defects, and thymic lymphoma. Genes Dev 10, 2411–2422 (1996). [DOI] [PubMed] [Google Scholar]
- Zha S. et al. Ataxia telangiectasia-mutated protein and DNA-dependent protein kinase have complementary V(D)J recombination functions. Proc Natl Acad Sci USA 108, 2028–2033 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankovic M. et al. 53BP1 alters the landscape of DNA rearrangements and suppresses AID-induced B cell lymphoma. Mol Cell 49, 623–631 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann M., Lottersberger F., Buonomo S. B., Sfeir A. & de Lange T. 53BP1 regulates DSB repair using Rif1 to control 5′ end resection. Science 339, 700–704 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredemeyer A. L. et al. ATM stabilizes DNA double-strand-break complexes during V(D)J recombination. Nature 442, 466–470 (2006). [DOI] [PubMed] [Google Scholar]
- Rasheed N., Wang X., Niu Q. T., Yeh J. & Li B. Atm-deficient mice: an osteoporosis model with defective osteoblast differentiation and increased osteoclastogenesis. Hum Mol Genet 15, 1938–1948 (2006). [DOI] [PubMed] [Google Scholar]
- Barlow C. et al. Atm deficiency results in severe meiotic disruption as early as leptonema of prophase I. Development 125, 4007–4017 (1998). [DOI] [PubMed] [Google Scholar]
- Pandita T. K. et al. Atm inactivation results in aberrant telomere clustering during meiotic prophase. Mol Cell Biol 19, 5096–5105 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. S., Bohrson C., Pike A. M., Wheelan S. J. & Greider C. W. ATM Kinase Is Required for Telomere Elongation in Mouse and Human Cells. Cell Rep 13, 1623–1632 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita K. et al. Reactive oxygen species induce chondrocyte hypertrophy in endochondral ossification. J Exp Med 204, 1613–1623 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hishiya A. et al. Ataxia telangiectasia mutated (Atm) knockout mice as a model of osteopenia due to impaired bone formation. Bone 37, 497–503 (2005). [DOI] [PubMed] [Google Scholar]
- Teitelbaum S. L. Bone resorption by osteoclasts. Science 289, 1504–1508 (2000). [DOI] [PubMed] [Google Scholar]
- Edwards J. R. & Mundy G. R. Advances in osteoclast biology: old findings and new insights from mouse models. Nat Rev Rheumatol 7, 235–243 (2011). [DOI] [PubMed] [Google Scholar]
- Nakashima T., Hayashi M. & Takayanagi H. New insights into osteoclastogenic signaling mechanisms. Trends Endocrinol Metab 23, 582–590 (2012). [DOI] [PubMed] [Google Scholar]
- Baschuk N., Rautela J. & Parker B. S. Bone specific immunity and its impact on metastasis. Bonekey Rep 4, 665 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raisz L. G. Pathogenesis of osteoporosis: concepts, conflicts, and prospects. J Clin Invest 115, 3318–3325 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamopoulos I. E. & Mellins E. D. Alternative pathways of osteoclastogenesis in inflammatory arthritis. Nat Rev Rheumatol 11, 189–194 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimi E. et al. Activation of NF-kappaB is involved in the survival of osteoclasts promoted by interleukin-1. J Biol Chem 273, 8799–8805 (1998). [DOI] [PubMed] [Google Scholar]
- Ang E. et al. Proteasome inhibitors impair RANKL-induced NF-kappaB activity in osteoclast-like cells via disruption of p62, TRAF6, CYLD, and IkappaBalpha signaling cascades. J Cell Physiol 220, 450–459 (2009). [DOI] [PubMed] [Google Scholar]
- Jono H. et al. NF-kappaB is essential for induction of CYLD, the negative regulator of NF-kappaB: evidence for a novel inducible autoregulatory feedback pathway. J Biol Chem 279, 36171–36174 (2004). [DOI] [PubMed] [Google Scholar]
- Herzog K. H., Chong M. J., Kapsetaki M., Morgan J. I. & McKinnon P. J. Requirement for Atm in ionizing radiation-induced cell death in the developing central nervous system. Science 280, 1089–1091 (1998). [DOI] [PubMed] [Google Scholar]
- Hadian K. & Krappmann D. Signals from the nucleus: activation of NF-kappaB by cytosolic ATM in the DNA damage response. Sci Signal 4, pe2 (2011). [DOI] [PubMed] [Google Scholar]
- Wu Z. H., Shi Y., Tibbetts R. S. & Miyamoto S. Molecular linkage between the kinase ATM and NF-kappaB signaling in response to genotoxic stimuli. Science 311, 1141–1146 (2006). [DOI] [PubMed] [Google Scholar]
- Hathcock K. S. et al. ATM deficiency promotes development of murine B-cell lymphomas that resemble diffuse large B-cell lymphoma in humans. Blood 126, 2291–2301 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee N. K. et al. A crucial role for reactive oxygen species in RANKL-induced osteoclast differentiation. Blood 106, 852–859 (2005). [DOI] [PubMed] [Google Scholar]
- Sabatel H. et al. Phosphorylation of p65(RelA) on Ser(547) by ATM represses NF-kappaB-dependent transcription of specific genes after genotoxic stress. PLoS One 7, e38246 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culmsee C. et al. Reciprocal inhibition of p53 and nuclear factor-kappaB transcriptional activities determines cell survival or death in neurons. J Neurosci 23, 8586–8595 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T. et al. PHF20 regulates NF-kappaB signalling by disrupting recruitment of PP2A to p65. Nat Commun 4, 2062 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodarzi A. A. et al. Autophosphorylation of ataxia-telangiectasia mutated is regulated by protein phosphatase 2A. EMBO J 23, 4451–4461 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Z. et al. Declining p53 function in the aging process: a possible mechanism for the increased tumor incidence in older populations. Proc Natl Acad Sci USA 104, 16633–16638 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T. et al. Estrogen prevents bone loss via estrogen receptor alpha and induction of Fas ligand in osteoclasts. Cell 130, 811–823 (2007). [DOI] [PubMed] [Google Scholar]
- Tohmonda T. et al. IRE1alpha/XBP1-mediated branch of the unfolded protein response regulates osteoclastogenesis. J Clin Invest 125, 3269–3279 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama K. et al. 5-Azacytidine-induced protein 2 (AZI2) regulates bone mass by fine-tuning osteoclast survival. J Biol Chem 290, 9377–9386 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasawa M. et al. The antiapoptotic protein Bcl-xL negatively regulates the bone-resorbing activity of osteoclasts in mice. J Clin Invest 119, 3149–3159 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.