Abstract
AIM
To assess the effect of neutral (NC) and positive (PC) oral contrast use on patient dose in low-dose abdominal computed tomography (CT).
METHODS
Low-dose clinically indicated CTs were performed on 79 Crohn’s patients (35 = PC, 1 L 2% gastrografin; 44 = NC, 1.5 L polyethylene glycol). Scanner settings for both acquisitions were identical apart from 25 s difference in intravenous contrast timing. Body mass index (BMI), scan-ranges, dose-length product and size-specific dose estimated were recorded. Data was reconstructed with pure model-based iterative reconstruction. Image quality was objectively and subjectively analysed. Data analysis was performed with Statistical Package for Social Scientists.
RESULTS
Higher doses were seen in neutral contrast CTs (107.60 ± 78.7 mGy.cm, 2.47 ± 1.21 mGy vs 85.65 ± 58.2 mGy.cm, 2.18 ± 0.96 mGy). The difference was significant in 2 of 4 BMI groups and in those that had both NC and PC investigations. Image-quality assessment yielded 6952 datapoints. NC image quality was significantly superior (P < 0.001) (objective noise, objective signal to noise ratio, subjective spatial resolution, subjective contrast resolution, diagnostic acceptability) at all levels. NC bowel distension was significantly (P < 0.001) superior.
CONCLUSION
The use of polyethylene glycol as a neutral OC agent leads to higher radiation doses than standard positive contrast studies, in low dose abdominal CT imaging. This is possibly related to the osmotic effect of the agent resulting in larger intraluminal fluid volumes and resultant increased overall beam attenuation.
Keywords: Radiation dose, Low dose computed tomography, Abdominal imaging, Oral contrast, Computed tomography
Core tip: The use of neutral oral contrast agent results in higher radiation doses than standard positive contrast studies when performed low dose abdominopelvic computed tomography imaging. This likely relates to the osmotic effect of the agent resulting in larger intraluminal fluid volumes and resultant increased overall beam attenuation.
INTRODUCTION
The industry and profession-wide drive for computed tomography (CT) dose reduction has resulted in considerable progress towards substantial dose reduction for abdominopelvic CT. These developments have been achieved as a result of improved detectors, tailored protocols, automated exposure control (AEC) and more recently newer reconstruction techniques such as iterative reconstruction. All elements of the acquisition process are now under scrutiny as part of the overall dose reduction strategies. The use of intraluminal contrast agents in the setting of abdominal imaging is one such factor. Traditionally, positive oral contrast agents were favoured but there is a significant body of evidence that suggests that negative or no oral contrast have a similar efficacy both in the trauma[1-3] and non-trauma setting[4,5]. In addition, neutral contrast agents are superior for bowel wall assessment, particularly in the setting of CT enterography (CTE)[6-11] such that CTE or magnetic resonance enterography are recommended as first-line investigations in diagnosing Crohn’s disease (CD) or in detecting Crohn’s complications[12,13].
Positive oral contrast universally contains either a dilute iodine-containing compound, e.g., 2% gastrografin or dilute barium. Negative oral contrast agents include water, polyethylene glycol (PEG), very dilute (0.1%) barium, methylcellulose, mannitol and milk. Water alone is not favoured as an oral contrast agent in the setting of CTE as bowel distension is suboptimal due to absorption. Most of the commonly utilised negative contrast agents contain a substance that retains or increases the intraluminal fluid volume thus improving bowel distension. A bulking agent such as PEG is amongst the most commonly employed. PEG ingestion entails consuming 1 to 1.5 L of a water-based solution over approximately 45 min preceding the scan. The intraluminal volume is further increased by osmosis due to the high effective osmolality of the consumed solution.
It is generally assumed that positive oral contrast agents lead to higher radiation doses than negative oral contrast agents, due to the increased radiation attenuation as a result of the increased density. In a prior study, Wang et al[14] demonstrated that the use of water resulted in decreased radiation doses when compared with utilization of positive oral contrast, in a phantom model. No published study has examined the effect that PEG oral contrast has on radiation dose when compared to positive contrast in vivo or in vitro.
To this end, we designed a study to examine the influence that positive oral contrast has on patient radiation dose on low dose abdominal imaging when compared with PEG neutral oral contrast.
MATERIALS AND METHODS
The institutional ethics research committee approved the study. Seventy-nine low-dose clinically indicated CTs were performed on patients with histologically diagnosed CD over a 3-year period. All patients were suspected of having an exacerbation of CD and CT was performed in order to assess the extent and severity of CD and to assess for fibrostenotic or extraluminal complications. Exclusion criteria included patients with CD who were less than sixteen years of age, pregnancy, patients with a contra-indication to intravenous contrast medium, patients presenting acutely via the emergency department and those without histological confirmation of CD. A positive oral contrast CT examination was performed on patients referred for CT in the first 18-mo of this study period and a negative contrast study was undertaken on patients referred for CT in the latter 18-mo of the study period. Written informed consent was obtained from all patients. Patients had their weight and height measured using a digital device (Seca electronic measuring station, Model 763, Seca Medical, Hamburg, Germany) and individual body mass indices (BMI) were recorded.
CT acquisition
All CT images were acquired using a 64-slice multidetector General Electric Lightspeed VCT-XTe (GE Healthcare, GE Medical Systems, Milwaukee, WI). Written consent was obtained from all patients and each one consented to having two contrast-enhanced CT scans of the abdomen and pelvis contemporaneously. The initial CT scan was a low dose scan which imparted approximately 10%-20% of a standard CT dosage regimen and the second scan was a conventional dosage scan which imparted approximately 80%-90% of a standard dosage regimen. The low dose studies only, performed with positive and neutral oral contrast, represented the imaging studies which underwent analysis as part of the current study.
All low dose scans were acquired with the following parameters: Tube voltage 100 kV, noise index 70, Z-axis tube current modulation range 20-350 mA and rotation time 0.5 s. For the positive contrast studies, the scan was commenced on arrested inspiration 45 s after peak aortic enhancement of 100 hounsfield units (HU). In the case of the negative contrast studies, to ensure a more “enteric phase” of enhancement, the scan commenced 20 s after peak aortic enhancement of 100 HU was surpassed on arrested inspiration.
Type of contrast used
Each patient was given either positive or neutral oral contrast as follows: With regard to positive contrast use, 2% Gastrografin solution was given as 1 L over 1 h as per departmental protocol. In contradistinction, neutral contrast, PEG “Klean Prep”, was ingested as a 1.5 L solution over 45 min. Along with this, each patient was given a single 100 mL bolus of intravenous contrast (Iohexol, Omnipaque 300, GE Healthcare, Mississauga, ON) at a flow rate of 2.5 mL/s.
CT image reconstruction and CT dosage calculation
Images acquired were acquired at 0.625 mm thickness and reconstructed with a slice thickness of 2 mm. Low dose images were reconstructed using pure model based iterative reconstruction (MBIR) (GE Healthcare) in addition to hybrid iterative reconstruction (Adaptive Statistical Iterative Reconstruction, (ASiR, GE Heathcare).
The imaging performance and assessment in CT patient dosimetry calculator (ImPACT version 0.99x, London, England) was used to calculate effective dosage (ED) in all studies. Size specific dose estimates (SSDE) were also calculated, by a single observer, by multiplying the CTDIvol by multiplication factors as per American Association of Physicists in Medicine[15].
Subjective and objective evaluation of CT image quality
The low dose MBIR images were objectively and subjectively analysed. Spherical regions of interest (ROI’s) (10 mm diameter; 519 mm3 volume) were used to calculate objective noise and signal to noise ratio at multiple levels. Mean attenuation in HU and standard deviation (SD) of the mean attenuation were recorded for all datasets. The standard deviation served as an objective measure of noise with mean attenuation divided by SD serving as a measure of signal to noise ratio (SNR)[16,17]. The regions used to assess subjective and objective image quality were the liver at the right hemi-diaphragm level (level 1), liver at the porta hepatis (level 2), right renal cortex at the renal hilum (level 3), psoas muscle at the iliac crest (level 4), and gluteus maximus at the level of the acetabular roof (level 5). Subjective parameters assessed were spatial resolution, contrast resolution, streak artefact, subjective noise and diagnostic acceptability utilizing a previously employed grading system[17-19] adopted from the European Guidelines on Quality Criteria for CT document[20]. Subjective parameter assessments were performed by 2 readers (KPM, MMM) in consensus. Image quality of the solid organs, large bowel, small bowel, peri-colonic fat as well as the peri-enteric fat was subjectively assessed to ascertain diagnostic acceptability. Other subjective evaluations were performed at the 5 levels used in the objective analyses. All subjective parameters apart from streak artefact were scored using a 1 to 10 ranking system where 1 indicated poor image quality, 5 was deemed as acceptable, and 10 indicated excellent imaging quality for the relevant factor. The presence of streak artefact was assessed using a 3-point key where 0 represented no streak artefact, 1 corresponds to the presence of streak artefact that is not affecting the image quality and 3 represents interference with image quality.
Finally, the level of bowel distension was scored on a 3-point scale whereby 0 indicates unacceptable distension, 1 implies distension that is acceptable and 2 specifies excellent and complete distension. The jejunum, ileum, terminal ileum, caecum, ascending colon, transverse colon, descending colon, sigmoid colon and rectum were individually scored.
Statistical analysis
All statistical tests were performed with the Statistical Package for Social Scientists (SPSS) version 20.0 (IBM, Armonk, NY). Wilcoxon signed rank test was used for statistical analysis to compare the qualitative parameters (diagnostic acceptability, image noise, streak artefact, spatial resolution and contrast resolution). Normally distributed quantitative indices were compared using a paired t test. A difference with a P value of < 0.05 was considered statistically significant. All data are presented as mean ± SD unless otherwise stated.
RESULTS
Patient BMI, radiation dose and scan range
Seventy nine scans were performed over a 3-year period. Thirty-five positive contrast (PC) studies were acquired on patients with a mean age of 37.8 ± 13.7 years (range = 16-74 years) and mean BMI of 24.7 ± 4.97 kg/m2 (range = 17.4-38.8 kg/m2). Forty-four patients underwent negative contrast (NC) studies. A mean age of 38.5 ± 12.98 years and a mean BMI of 22.17 ± 5.31 kg/m2 (range 14.4-38.5 kg/m2) were recorded in this cohort. Six patients underwent both positive and negative contrast studies over the 3-year period. The NC cohort had, on average, lower BMIs than the PC group, though this difference was not statistically significant (24.44 ± 5.05 kg/m2 vs 22.17 ± 5.32 kg/m2, P = 0.064).
The PC studies had the following mean radiation exposure parameters: Dose-length product (DLP) 85.65 ± 58.2 mGy.cm; ED 1.28 ± 0.87 mSv; SSDE 2.18 ± 0.96 mGy. Mean NC exposure parameters were: DLP 107.60 ± 78.7 mGy.cm; ED 1.61 ± 1.18 mSv; 2.47 ± 1.21 mGy. Averages for all exposure measures were greater for NC examinations but these differences were not statistically significant (DLP, P = 0.173; ED, P = 0.173; SSDE, P = 0.268) (Figures 1 and 2). No significant difference (P = 0.939) was observed between both groups in terms of scan range - positive: 429.78 ± 33.4 mm; negative: 430.41 ± 39.5 mm.
Figure 1.
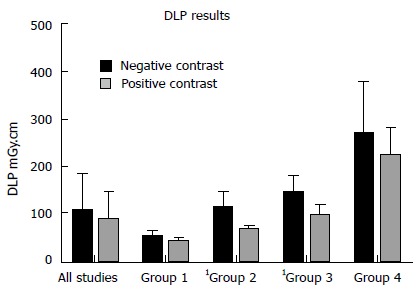
Comparison of dose-length product measurements for all studies in the neutral and positive cohorts and by body mass index range. Group 1: BMI < 20 kg/m2; group 2: BMI 20-25 kg/m2; group 3: BMI 25-30 kg/m2; group 4: BMI > 30 kg/m2. Significant differences are denoted by1. BMI: Body mass index.
Figure 2.
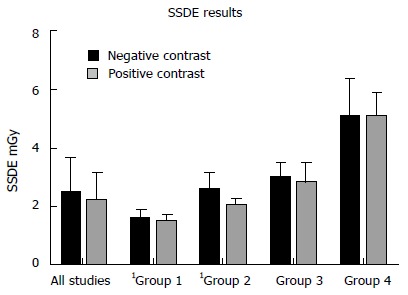
Comparison of size-specific dose estimated measurements for all studies in the neutral and positive cohorts and by body mass index range. Group 1: BMI < 20 kg/m2; group 2: BMI 20-25 kg/m2; group 3: BMI 25-30 kg/m2; group 4: BMI > 30 kg/m2. Significant differences are denoted by1. BMI: Body mass index.
Radiation exposures (DLP and SSDE) by BMI range were compared between the cohorts - group 1: BMI < 20 kg/m2; group 2: BMI 20-25 kg/m2; group 3: BMI 25-30 kg/m2; group 4: BMI > 30 kg/m2. The results are depicted in Table 1 and Figures 1 and 2. DLPs were significantly higher for the NC studies in groups 2 and 3 and SSDEs were significantly higher for NC patients in groups 1 and 2. Other comparisons were not significant.
Table 1.
Comparison of dose-length product and size-specific dose estimated measurements by body mass index range
| Group | n | Parameter | PC | NC | P value |
| 1 | PC n = 7 | DLP (mGy.cm) | 40.08 ± 6.42 | 52.07 ± 12.09 | 0.355 |
| NC n = 18 | SSDE (mGy) | 1.46 ± 0.29 | 1.58 ± 0.30 | 0.0211 | |
| 2 | PC n = 15 | DLP (mGy.cm) | 64.57 ± 9.98 | 2.03 ± 0.26 | 0.0021 |
| NC n = 18 | SSDE (mGy) | 111.68 ± 34.38 | 2.56 ± 0.58 | < 0.0011 | |
| 3 | PC n = 9 | DLP (mGy.cm) | 94.75 ± 33.54 | 145.75 ± 33.54 | 0.0171 |
| NC n = 3 | SSDE (mGy) | 2.79 ± 0.70 | 2.95 ± 0.57 | 0.719 | |
| 4 | PC n = 4 | DLP (mGy.cm) | 224.09 ± 57.69 | 269.94 ± 111.24 | 0.951 |
| NC n = 5 | SSDE (mGy) | 5.09 ± 0.82 | 5.04 ± 1.32 | 0.483 |
Significant differences are denoted by1. Group 1: BMI < 20 kg/m2; group 2: BMI 20-25 kg/m2; group 3: BMI 25-30 kg/m2; group 4: BMI > 30 kg/m2. DLP: Dose-length product; SSDE: Size-specific dose estimated; BMI: Body mass index; PC: Positive contrast; NC: Negative contrast.
Of the 6 patients that had both PC and NC studies, all dose measurements were higher for all NC studies. Mean increases were as follows: DLP 21.11 mGy.cm (range 10.31-38.38 mGy.cm), ED 0.317 mSv (range 0.155-0.576 mSv) and SSDE 0.246 mGy (range 0.013-0.414 mGy). When mean dose indices were compared for these 6 patients, significant differences were seen for DLP (P = 0.005) and ED (P = 0.005) but SSDE was not significant (P = 0.175). The BMIs of these patients showed minimal change between study acquisitions - the BMI was lower for the NC studies in 4 cases (range 0.1-3.12 kg/m2) and higher in 2 cases (range 1.2-5.7 kg/m2). The effective abdominal diameter changed by < 2 cm in all cases.
Objective and subjective image quality analysis
Objective noise was measured at each of the 5 previously mentioned levels. The NC group had a significantly reduced objective noise when compared to the PC group at each of the 5 measured locations. Results as follows - liver at the hemi-diaphragm: 21.75 ± 3.27 HU vs 83.61 ± 13.47 HU; liver at the porta hepatis: 23.19 ± 3.07 HU vs 82.35 ± 8.15 HU; renal hilum: 44.57 ± 16.09 HU vs 73.72 ± 11.11 HU; psoas muscle at the iliac crest: 25.36 ± 4.21 HU vs 72.68 ± 12.27 HU; gluteus maximus at acetabular roof: 20.05 ± 2.33 HU vs 76.30 ± 11.55 HU; (P < 0.05 for all values) (Figure 3).
Figure 3.
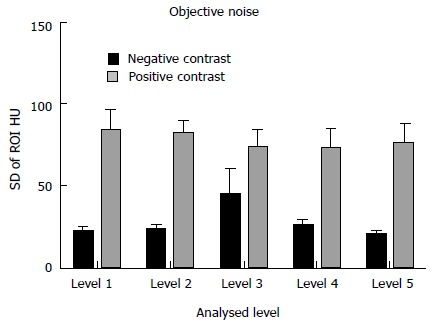
Comparison of objective noise measurements between the neutral and positive studies. Level 1: Liver at the right hemi-diaphragm level; level 2: Liver at the porta hepatis; level 3: Right renal cortex at the renal hilum; level 4: Psoas muscle at the iliac crest; level 5: Gluteus maximus at the level of the acetabular roof. All neutral measurements were significantly superior. ROI: Regions of interest.
The objective signal to noise ratio (SNR) at each of the 5 locations was significantly superior for the NC studies - liver at the hemi-diaphragm: 4.17 ± 1.22 HU vs 1.54 ± 0.35 HU; liver at the porta hepatis: 4.24 ± 0.84 HU vs 1.64 ± 0.32 HU; renal hilum: 4.71 ± 1.58 HU vs 0.93 ± 0.27 HU; psoas muscle at the iliac crest: 2.98 ± 0.85 HU vs 0.930 ± 0.22 HU; gluteus maximus at acetabular roof: 2.98 ± 0.85 HU vs 0.66 ± 0.27 HU; (P < 0.05 for all comparisons) (Figure 4).
Figure 4.
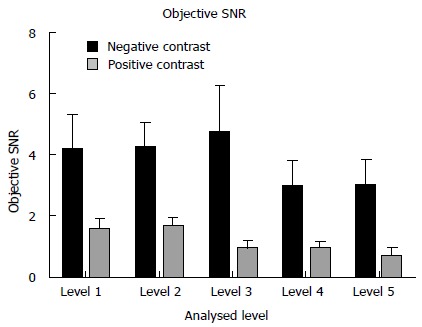
Comparison of objective signal to noise ratio measurements between the neutral and positive studies. Level 1: Liver at the right hemi-diaphragm level; level 2: Liver at the porta hepatis; level 3: Right renal cortex at the renal hilum; level 4: Psoas muscle at the iliac crest; level 5: Gluteus maximus at the level of the acetabular roof. All neutral measurements were significantly superior.
Subjective image noise, contrast resolution, spatial resolution and diagnostic acceptability were superior for the NC studies when compared with the PC examinations (P < 0.001 for all comparisons). NC streak artefact was insignificantly superior (P = 0.051) to PC streak artefact. Results are depicted in Figure 5.
Figure 5.
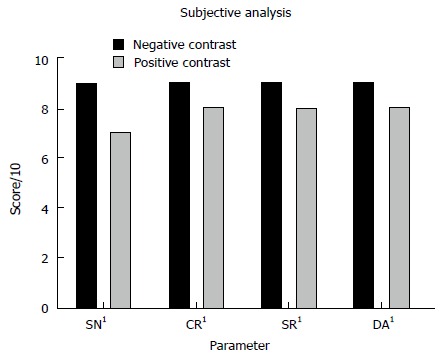
Comparison of median subjective image quality parameters for all neutral and positive studies. 1All neutral measurements were significantly superior. SN: Subjective noise; CR: Contrast resolution; SR: Spatial resolution; DA: Diagnostic acceptability.
Bowel distension was significantly superior in the NC studies (median score 2, interquartile range 0) (P < 0.001) than the PC examinations (median score 1, IQR 1).
DISCUSSION
We found that negative PEG oral contrast examinations had significantly higher radiation doses than positive contrast studies, for most BMI subgroups and in those that had both NC and PC examinations, despite matched scan ranges. On the other hand, the resultant NC images reconstructed with MBIR were significantly superior to the PC MBIR images in terms of objective noise, objective signal to noise ratio, subjective noise, subjective contrast resolution, subjective spatial resolution and subjective diagnostic acceptability. NC subjective streak artefact was also insignificantly superior.
In terms of protocol parameter differences, settings for both the NC and PC studies were identical apart from the type of oral contrast and the intravenous (IV) contrast phase. The NC studies utilised an enteric phase IV contrast and the PC CT examinations utilised a more portovenous phase. It is worth noting firstly that the overall volume of IV contrast within the scan range is the same for both protocols hence this should not alter radiation dose. Secondly, when automated tube current modulation (ATCM) is employed, as was the case in these examinations, the selected mA gets chosen from the scanned projection radiograph before IV contrast is administered, hence the phase of IV contrast does not influence this aspect.
Hence, the type of oral contrast is the most important and perhaps only significant reason as to why the NC examinations had higher radiation doses than the PC scans.
Their results also indicated that bowel distension and hence intraluminal volume was greater with PEG oral contrast. It is likely that the overall attenuation influence of PEG oral contrast, when the total volume of fluid and per unit attenuation value are taken into account, particularly when used in combination with AEC/ATCM is in fact greater than positive oral contrast.
Future studies may involve the comparison of other negative contrast media and calculation of the overall intraluminal volume increase that results when PEG is consumed.
The greatest difference between each cohort with regard to radiation dose was observed in patients with a BMI of less than 25 kg/m2. Therefore, choice of contrast agent is especially important in this cohort as it may have a greater impact on radiation dose than in overweight patients. The reason for this remains unclear but it is possible that patients with a high BMI may behave differently with regard to other parameters such as ATCM performance. A further consideration in patients with a high BMI is that as intra-peritoneal inflammatory conditions often manifest as abnormalities of the adipose tissue adjacent to the inflamed organ, a larger quantity of adipose may a result in inflammatory changes being more readily apparent. This had led some authors to suggest the omission of oral contrast when scanning patients with a high BMI[21].
The results of the current study are important. As stated previously, major progress has been made in recent years in the area of radiation exposure reduction in abdomino-pelvic CT and the era of sub-millisievert abdomino-pelvic CT is fast approaching. This substantial progress has been achieved as a result of improved detectors, tailored protocols, AEC and more recently newer reconstruction techniques such as iterative reconstruction. Future reductions in radiation exposure will likely be smaller and will rely on finer protocol modifications. All elements of the acquisition process are now under scrutiny as part of the overall dose reduction strategy. The results of this study suggest that choice of oral contrast agent influences radiation exposure and should be added to a list of factors, which are worthy of further study.
In conclusion, our results suggest that PEG solution, when used as a negative oral contrast agent, results in significantly higher radiation doses in low dose abdominal CT imaging when compared with positive oral contrast, despite it being of lower density. This is likely attributable to the greater intraluminal volume and thus bowel distension achieved with PEG. This however, may have led to the significantly greater image quality recorded with the PEG studies. The choice of oral contrast agent should be tailored to the individual clinical scenario. In cases where bowel assessment is the key consideration, we suggest the use of a NC agent where in cases where bowel assessment may be a secondary consideration, a PC agent may be more appropriate so as to optimise overall radiation dose. Our study is novel and further examination of the relationship between oral contrast, radiation dose and diagnostic performance is warranted.
COMMENTS
Background
There has been considerable industry and profession-wide drive to reduce the radiation dose incurred by patients form diagnostic imaging with computed tomography. All elements of the acquisition process including technical parameters and reconstruction algorithm are now under scrutiny as part of an overall dose reduction strategy. The use of intraluminal contrast agents in the setting of abdominal imaging is another factor that warrants further assessment. Traditionally, positive oral contrast agents were favoured but there is a significant body of evidence that suggests that negative or no oral contrast have a similar efficacy. It is generally assumed that positive oral contrast agents lead to higher radiation doses than negative oral contrast agents, due to the increased radiation attenuation as a result of the increased density. Positive oral contrast universally contains either a dilute iodine-containing compound, e.g., 2% gastrografin or dilute barium. Negative oral contrast agents include water, polyethylene glycol (PEG), very dilute (approximately 0.1%) barium, methylcellulose, mannitol and milk. The authors designed a study to examine the influence that positive oral contrast has on patient radiation dose on low dose abdominal imaging when compared with PEG neutral oral contrast.
Research frontiers
A single previous study demonstrated that the use of water resulted in decreased radiation doses when compared with utilization of positive oral contrast, in a phantom model. No published study has examined the effect that PEG oral contrast has on radiation dose when compared to positive contrast in vivo or in vitro.
Innovations and breakthroughs
The results suggest that PEG solution, when used as a negative oral contrast agent, results in significantly higher radiation doses in low dose abdominal computed tomography (CT) imaging when compared with positive oral contrast, despite it being of lower density.
Applications
All elements of the acquisition process are now under scrutiny as part of an overall dose reduction strategy in CT. The results of this study suggest that choice of oral contrast agent influences radiation exposure and should be added to a list of factors when considering how best to optimise patient radiation dose from CT.
Terminology
PEG is a negative oral contrast agent.
Peer-review
The paper is interesting and clinically relevant.
Footnotes
Institutional review board statement: The study received institutional board approval from the Cork Clinical Research Ethics Committee, Lancaster Hall, 6 Little Hanover Street, Cork, Ireland.
Informed consent statement: Informed consent was not deemed necessary for this study. Clinical and radiological data was collected prospectively in an anonymised fashion and no patient underwent additional procedures or investigations as a result of inclusion in the study. No potential risks to patients were identified. The clinical research ethics committee granted approval for the study without a requirement of consent from each patient.
Conflict-of-interest statement: All authors declare no conflicts of interest.
Data sharing statement: Technical appendix, statistical code, and dataset available from the corresponding author at fiachramoloney@hotmail.com.
Manuscript source: Invited manuscript
Specialty type: Radiology, nuclear medicine and medical imaging
Country of origin: Ireland
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
Peer-review started: April 15, 2016
First decision: May 19, 2016
Article in press: August 15, 2016
P- Reviewer: Cerwenka HR, Li YZ, Shen J, Yu TH S- Editor: Qiu S L- Editor: A E- Editor: Wu HL
References
- 1.Allen TL, Mueller MT, Bonk RT, Harker CP, Duffy OH, Stevens MH. Computed tomographic scanning without oral contrast solution for blunt bowel and mesenteric injuries in abdominal trauma. J Trauma. 2004;56:314–322. doi: 10.1097/01.TA.0000058118.86614.51. [DOI] [PubMed] [Google Scholar]
- 2.Stafford RE, McGonigal MD, Weigelt JA, Johnson TJ. Oral contrast solution and computed tomography for blunt abdominal trauma: a randomized study. Arch Surg. 1999;134:622–626; discussion 626-627. doi: 10.1001/archsurg.134.6.622. [DOI] [PubMed] [Google Scholar]
- 3.Stuhlfaut JW, Soto JA, Lucey BC, Ulrich A, Rathlev NK, Burke PA, Hirsch EF. Blunt abdominal trauma: performance of CT without oral contrast material. Radiology. 2004;233:689–694. doi: 10.1148/radiol.2333031972. [DOI] [PubMed] [Google Scholar]
- 4.Hill BC, Johnson SC, Owens EK, Gerber JL, Senagore AJ. CT scan for suspected acute abdominal process: impact of combinations of IV, oral, and rectal contrast. World J Surg. 2010;34:699–703. doi: 10.1007/s00268-009-0379-6. [DOI] [PubMed] [Google Scholar]
- 5.Lee SY, Coughlin B, Wolfe JM, Polino J, Blank FS, Smithline HA. Prospective comparison of helical CT of the abdomen and pelvis without and with oral contrast in assessing acute abdominal pain in adult Emergency Department patients. Emerg Radiol. 2006;12:150–157. doi: 10.1007/s10140-006-0474-z. [DOI] [PubMed] [Google Scholar]
- 6.Ilangovan R, Burling D, George A, Gupta A, Marshall M, Taylor SA. CT enterography: review of technique and practical tips. Br J Radiol. 2012;85:876–886. doi: 10.1259/bjr/27973476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paulsen SR, Huprich JE, Fletcher JG, Booya F, Young BM, Fidler JL, Johnson CD, Barlow JM, Earnest F. CT enterography as a diagnostic tool in evaluating small bowel disorders: review of clinical experience with over 700 cases. Radiographics. 2006;26:641–657; discussion 657-662. doi: 10.1148/rg.263055162. [DOI] [PubMed] [Google Scholar]
- 8.Mazzeo S, Caramella D, Battolla L, Melai L, Masolino P, Bertoni M, Giusti P, Cappelli C, Bartolozzi C. Crohn disease of the small bowel: spiral CT evaluation after oral hyperhydration with isotonic solution. J Comput Assist Tomogr. 2001;25:612–616. doi: 10.1097/00004728-200107000-00017. [DOI] [PubMed] [Google Scholar]
- 9.Aiyappan SK, Kalra N, Sandhu MS, Kochhar R, Wig JD, Khandelwal N. Comparison of neutral and positive enteral contrast media for MDCT enteroclysis. Eur J Radiol. 2012;81:406–410. doi: 10.1016/j.ejrad.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 10.Hara AK, Swartz PG. CT enterography of Crohn’s disease. Abdom Imaging. 2009;34:289–295. doi: 10.1007/s00261-008-9443-1. [DOI] [PubMed] [Google Scholar]
- 11.Minordi LM, Vecchioli A, Mirk P, Bonomo L. CT enterography with polyethylene glycol solution vs CT enteroclysis in small bowel disease. Br J Radiol. 2011;84:112–119. doi: 10.1259/bjr/71649888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huprich JE, Rosen MP, Fidler JL, Gay SB, Grant TH, Greene FL, Lalani T, Miller FH, Rockey DC, Sudakoff GS, et al. ACR Appropriateness Criteria on Crohn’s disease. J Am Coll Radiol. 2010;7:94–102. doi: 10.1016/j.jacr.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 13.Van Assche G, Dignass A, Panes J, Beaugerie L, Karagiannis J, Allez M, Ochsenkühn T, Orchard T, Rogler G, Louis E, et al. The second European evidence-based Consensus on the diagnosis and management of Crohn’s disease: Definitions and diagnosis. J Crohns Colitis. 2010;4:7–27. doi: 10.1016/j.crohns.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 14.Wang ZJ, Chen KS, Gould R, Coakley FV, Fu Y, Yeh BM. Positive enteric contrast material for abdominal and pelvic CT with automatic exposure control: what is the effect on patient radiation exposure? Eur J Radiol. 2011;79:e58–e62. doi: 10.1016/j.ejrad.2011.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boone J, Strauss K. Size-specific dose estimate (SSDI) in pediatric and adult body CT examinations. College Park: American Association of Physicists in Medicine; 2011. [Google Scholar]
- 16.Marin D, Nelson RC, Schindera ST, Richard S, Youngblood RS, Yoshizumi TT, Samei E. Low-tube-voltage, high-tube-current multidetector abdominal CT: improved image quality and decreased radiation dose with adaptive statistical iterative reconstruction algorithm--initial clinical experience. Radiology. 2010;254:145–153. doi: 10.1148/radiol.09090094. [DOI] [PubMed] [Google Scholar]
- 17.O’Neill SB, Mc Laughlin PD, Crush L, O’Connor OJ, Mc Williams SR, Craig O, Mc Garrigle AM, O’Neill F, Bye J, Ryan MF, et al. A prospective feasibility study of sub-millisievert abdominopelvic CT using iterative reconstruction in Crohn’s disease. Eur Radiol. 2013;23:2503–2512. doi: 10.1007/s00330-013-2858-2. [DOI] [PubMed] [Google Scholar]
- 18.Siddiki HA, Fidler JL, Fletcher JG, Burton SS, Huprich JE, Hough DM, Johnson CD, Bruining DH, Loftus EV, Sandborn WJ, et al. Prospective comparison of state-of-the-art MR enterography and CT enterography in small-bowel Crohn’s disease. AJR Am J Roentgenol. 2009;193:113–121. doi: 10.2214/AJR.08.2027. [DOI] [PubMed] [Google Scholar]
- 19.Winklehner A, Karlo C, Puippe G, Schmidt B, Flohr T, Goetti R, Pfammatter T, Frauenfelder T, Alkadhi H. Raw data-based iterative reconstruction in body CTA: evaluation of radiation dose saving potential. Eur Radiol. 2011;21:2521–2526. doi: 10.1007/s00330-011-2227-y. [DOI] [PubMed] [Google Scholar]
- 20.Bongartz G, Geleijns J, Golding S, Jurik A, Leonardi M, van Meerten E. European guidelines on quality criteria for computed tomography. European Commission. 1999. [Google Scholar]
- 21.Harrison ML, Lizotte PE, Holmes TM, Kenney PJ, Buckner CB, Shah HR. Does high body mass index obviate the need for oral contrast in emergency department patients? West J Emerg Med. 2013;14:595–597. doi: 10.5811/westjem.2013.5.12950. [DOI] [PMC free article] [PubMed] [Google Scholar]


