Abstract
Each year millions of pregnant woman are exposed to caffeine, which acts to antagonize adenosine action. The long-term consequences of this exposure on the developing fetus are largely unknown, although in animal models we have found adverse effects on cardiac function. To assess if these effects are transmitted transgenerationally, we exposed pregnant mice to caffeine equivalent to 2–4 cups of coffee at two embryonic stages. Embryos (F1 generation) exposed to caffeine early from embryonic (E) day 6.5–9.5 developed a phenotype similar to dilated cardiomyopathy by 1 year of age. Embryos exposed to caffeine later (E10.5–13.5) were not affected. We next examined the F2 generation and F3 generation of mice exposed to caffeine from E10.5–13.5, as this coincides with germ cell development. These F2 generation adult mice developed a cardiac phenotype similar to hypertrophic cardiomyopathy. The F3 generation exhibited morphological changes in adult hearts, including increased mass. This report shows that in utero caffeine exposure has long-term effects into adulthood and that prenatal caffeine exposure can exert adverse transgenerational effects on adult cardiac function.
It is well recognized that disruption of the intrauterine environment by nutritional or chemical factors may influence the fetus, resulting in long-term adverse effects after birth and into adulthood1,2,3,4. By disrupting normal prenatal development, environmental factors and chemical exposures may lead to the fetal programming of adult disease, including cardiovascular disease5,6,7. It is also recognized that prenatal insults can have transgenerational effects8,9.
One of the most common chemicals that fetuses are exposed to is caffeine, which is a non-selective adenosine receptor antagonist10,11. Dietary caffeine comes from many sources including food, drinks and medication. Caffeine is present in foods such as chocolate, gum, beef jerky, and other foods including coffee-flavored yogurt12. Caffeinated beverages are widely consumed including tea, soft drinks, energy drinks, and coffee13. At serum concentrations achieved with human consumption, the physiological effects of caffeine are due to antagonism of adenosine action through competitive inhibition at the receptor level10,11. In addition, caffeine readily crosses the placenta to reach the fetus, where the half-life of caffeine is longer (12–24 hours) than in adults (2–4 hours) due to the absence of the enzyme CYP1A2 in placenta and fetus11,14,15.
Average caffeine intake is between 150 and 200 mg per day among women of child bearing age14. Caffeine consumption during the first month of pregnancy is reported by 60% of women, who often do not know that they are pregnant14. Caffeine consumption is associated with low birth weight and increased rates of spontaneous abortions16,17,18,19,20,21,22,23,24. Although, the notion that prenatal caffeine exposure is associated with low birth weight and other adverse effects is not accepted by all14,25, it is concerning enough that Nordic countries, the United Kingdoms, and the United States recommend that pregnant women limit their caffeine intake to less than 200 mg per day26,27.
Several reports indicate that in utero caffeine exposure can have long-term negative effects. In mice, maternal exposure to caffeine is associated with altered brain development and behavioral changes in neonatal and adult offspring28,29. In addition, in utero caffeine exposure is associated with high blood pressure, altered cardiac morphology, and reduced cardiac function in adult mice30,31,32. In humans, caffeine has long-lasting effects as well including reduced birth weight and growth in neonates and adverse behavior at 18 months33,34,35.
Increasing evidence shows that epigenetic processes can mediate heritable changes in gene expression without altering the DNA sequence and include DNA methylation, microRNA (miRNA), and histone modification36. Epigenetics also plays a critical role in mediating the fetal programming of adult disease3,36,37,38. Alterations in DNA methylation patterns are recognized to transduce in utero environmental stress into an increased risk for adult disease39. DNA methylation inhibits gene expression through suppressing transcription factor binding or recruiting histone deacetylases that cause chromatin condensation and gene inactivation40.
The DNA methylation state of the genome is changing throughout embryogenesis and two important DNA methylation events were targeted in our experiments. First, genomic DNA in the inner cell mass undergoes a rapid wave of demethylation after fertilization and this DNA methylation is re-established during embryogenesis from E3.5–9.5 in mice41,42,43. DNA methylation patterns can be altered by nutritional and environmental factors during this remethylation process resulting in long-lasting effects43,44,45,46. Second, primordial germ cells (PGCs) in mice go through specific DNA methylation remodeling events during embryogenesis47. PGCs migrate along the developing hindgut to the genital ridges47. During and after this migration (E10.5–13.5), PGC DNA actively undergoes a wave of demethylation47. This process is important for removing genomic imprints from the previous generation47,48. These epigenetic events can be affected by alteration within the intrauterine environment, including chemical exposures, nutritional deprivation, and oxygen availability. These effects are associated with adult diseases and transgenerational effects on health8,9,49,50,51,52,53.
We previously demonstrated that in utero caffeine exposure leads to long-term changes in the DNA methylation pattern in adult hearts31. In addition, we observed changes in expression of important DNA methylation enzymes, including DNA methyltransferases 1, 3a, 3b, in embryonic hearts exposed to caffeine in utero54. The timing of in utero caffeine exposure is important in this process, as it determines which DNA methylation process may be affected, and can determine which generations are affected, as well as the type of phenotypic changes induced in adulthood7.
Based on these data, we hypothesized that in utero caffeine exposure alters DNMT activity leading to altered DNA methylation patterns in adult tissue. We postulated that this altered DNA methylation pattern would lead to alter gene expression and cardiac function in adult hearts. In addition, we hypothesized that based on the timing of caffeine exposure and therefore which DNA methylation process is targeted, we would expect that different generations would be affected.
Our results confirmed our hypotheses as the results outlined in this report indicate in utero caffeine exposure leads to long-term altered effects on cardiac function, morphology, gene expression, and sensitivity to β-adrenergic stimulation in adult offspring. In addition, we demonstrate that in utero caffeine exposure leads to transgenerational effects on the heart in the F2 and F3 generations.
Results
The timing of in utero caffeine exposure influences long-term effects on cardiac function and morphology
At 12 weeks of age, F1 generation offspring of dams treated from E6.5–9.5 with caffeine did not have altered cardiac morphology or function (Table 1).
Table 1. Echocardiography analysis of adult F1 generation mice exposed to caffeine or vehicle as embryos in utero from E6.5–9.5.
| Treatment | Age | IVS;d | LVID;d | LVPW;d | LV Vol;d | IVS;s | LVID;s | LVPW;s | LV Vol;s | % EF | % FS | LV Mass | Heart Rate | Weight | N |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E6.5–9.5 | mm | mm | mm | ul | mm | mm | mm | ul | % | % | mg | BPM | g | ||
| Vehicle | 12 Weeks | 0.74 | 4.77 | 0.84 | 106.50 | 1.01 | 3.56 | 1.17 | 53.69 | 49.90 | 25.53 | 124.70 | 481.57 | 39.85 | 4 |
| Caffeine | 12 Weeks | 0.80 | 4.60 | 0.89 | 97.92 | 1.10 | 3.29 | 1.23 | 45.23 | 54.76 | 28.69 | 128.07 | 475.44 | 42.98 | 4 |
| P-value | 0.07 | 0.08 | 0.18 | 0.08 | 0.10 | 0.06 | 0.24 | 0.09 | 0.09 | 0.08 | 0.36 | 0.42 | 0.08 | ||
| E6.5–9.5 | |||||||||||||||
| Vehicle | 22 Weeks | 0.84 | 4.63 | 0.93 | 98.97 | 1.15 | 3.25 | 1.30 | 43.26 | 56.58 | 29.81 | 137.03 | 503.31 | 53.54 | 3 |
| Caffeine | 22 Weeks | 0.92* | 4.81* | 0.92 | 108.31* | 1.25 | 3.50* | 1.26 | 51.65* | 52.72 | 27.35 | 154.51* | 499.41 | 53.86 | 4 |
| P-value | 0.02 | 0.01 | 0.46 | 0.01 | 0.06 | 0.04 | 0.28 | 0.05 | 0.14 | 0.15 | 0.04 | 0.45 | 0.46 | ||
| E6.5–9.5 | |||||||||||||||
| Vehicle | 1 year | 0.95 | 4.68 | 1.07 | 102.53 | 1.33 | 3.30 | 1.49 | 44.87 | 56.47 | 29.66 | 169.77 | 495.54 | 62.13 | 4 |
| Caffeine | 1 year | 0.96 | 4.94 | 1.06 | 115.91 | 1.30 | 3.70* | 1.32* | 59.77* | 48.89* | 25.28* | 183.34 | 469.46 | 59.36 | 4 |
| P-value | 0.45 | 0.11 | 0.42 | 0.12 | 0.35 | 0.04 | 0.02 | 0.04 | 0.02 | 0.02 | 0.26 | 0.18 | 0.34 | ||
IVS, interventricular septum; LVID, left ventricle internal diameter; LVPW, left ventricular posterior wall; LV, left ventricle; FS, fraction shortening; Vol, volume; EF, ejection fraction; d, diastole; s, systole. Units: IVS, LVID, LVPW are measured in millimeters (mm); LV vol is measured in milliliters (ml); LV mass is measured in milligrams (mg). *P-value ≤ 0.05. P-values were calculated by Student’s t-test.
At 22 weeks after birth, F1 generation offspring of dams treated from E6.5–9.5 with caffeine had altered cardiac morphology, including increased interventricular septum (IVS) thickness during both systole and diastole, and increased left ventricle (LV) mass compared to vehicle controls (Table 1). In addition, we observed increased left ventricle internal diameter (LVID) and LV volume during both diastole and systole in the caffeine-treated group (Table 1).
At one year of age, F1 generation mice exposed to caffeine in utero from E6.5–9.5 showed altered cardiac morphology, as well as altered cardiac function that was characteristic of dilated cardiomyopathy. Hearts from the caffeine-treated group displayed systolic dysfunction including a 12.6% decrease in the left ventricular posterior wall thickness during systole (LVPW;s) and a 10.9% increase in LVID during systole (LVID;s), which led to a 24.9% increase in LV volume during systole (Table 1). These effects on cardiac morphology lead to reduced cardiac function, including a 17.3% decrease in % fractional shortening (%FS) and a 15.5% decrease in % ejection fraction (%EF; Table 1).
Indicating that the timing of caffeine exposure is critical for the observed effects on cardiac function and morphology, F1 adult offspring of dams exposed to caffeine from E10.5–13.5 did not display adverse effects on cardiac morphology or function in adulthood at 22 weeks or at 1 year of age (Table 2).
Table 2. Echocardiography analysis of F1, F2, and F3 generation adult offspring of F0 dams exposed to vehicle or caffeine.
| Age/Generation | IVS;d | LVID;d | LVPW;d | LV Vol;d | IVS;s | LVID;s | LVPW;s | LV Vol;s | % EF | % FS | LV Mass | Heart Rate | Weight | N | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E10.5-13.5 | 22 Weeks | mm | mm | mm | ul | mm | mm | mm | ul | % | % | mg | BPM | g | |
| Vehicle | F1 | 1.00 | 4.47 | 0.94 | 92.01 | 1.37 | 3.24 | 1.24 | 43.55 | 53.71 | 28.02 | 147.37 | 521.75 | 50.83 | 5 |
| Caffeine | F1 | 0.94 | 4.61 | 0.98 | 99.24 | 1.26 | 3.49 | 1.24 | 52.95 | 47.11 | 24.11 | 150.62 | 493.81 | 50.97 | 4 |
| P-value | 0.21 | 0.25 | 0.11 | 0.21 | 0.14 | 0.20 | 0.50 | 0.16 | 0.15 | 0.16 | 0.42 | 0.16 | 0.49 | ||
| E10.5-13.5 | 1 year | ||||||||||||||
| Vehicle | F1 | 1.03 | 4.58 | 0.98 | 96.47 | 1.34 | 3.26 | 1.34 | 43.46 | 55.47 | 28.94 | 157.57 | 496.03 | 52.51 | 5 |
| Caffeine | F1 | 0.90 | 4.84 | 1.01 | 110.90 | 1.25 | 3.49 | 1.38 | 52.46 | 54.03 | 28.19 | 164.50 | 473.44 | 61.33 | 4 |
| P-value | 0.08 | 0.14 | 0.27 | 0.13 | 0.16 | 0.21 | 0.29 | 0.20 | 0.37 | 0.39 | 0.32 | 0.15 | 0.08 | ||
| E10.5–13.5 | 22 Weeks | ||||||||||||||
| Vehicle | F2 | 0.88 | 4.62 | 0.94 | 98.84 | 1.21 | 3.36 | 1.25 | 46.72 | 53.01 | 27.42 | 142.55 | 494.17 | 52.16 | 5 |
| Caffeine | F2 | 0.84 | 4.59 | 0.95 | 97.20 | 1.16 | 3.29 | 1.29 | 44.35 | 54.29 | 28.36 | 137.13 | 471.52 | 50.06 | 4 |
| P-value | 0.23 | 0.42 | 0.48 | 0.41 | 0.23 | 0.31 | 0.33 | 0.32 | 0.37 | 0.36 | 0.28 | 0.20 | 0.30 | ||
| E10.5–13.5 | 1 year | ||||||||||||||
| Vehicle | F2 | 0.92 | 5.00 | 1.00 | 118.72 | 1.29 | 3.66 | 1.34 | 57.24 | 52.09 | 26.89 | 173.76 | 523.84 | 60.10 | 5 |
| Caffeine | F2 | 0.89 | 4.75* | 1.11* | 105.32* | 1.25 | 3.32* | 1.50* | 45.54* | 57.01* | 30.07* | 169.31 | 526.52 | 59.62 | 7 |
| P-value | 0.22 | 0.03 | 0.01 | 0.03 | 0.24 | 0.02 | 0.01 | 0.02 | 0.04 | 0.04 | 0.33 | 0.44 | 0.47 | ||
| E10.5–13.5 | 22 Weeks | ||||||||||||||
| Vehicle | F3 | 0.92 | 4.60 | 0.94 | 97.37 | 1.28 | 3.32 | 1.25 | 45.24 | 53.67 | 27.75 | 145.25 | 518.58 | 51.71 | 5 |
| Caffeine | F3 | 0.92 | 4.77 | 0.96* | 106.35 | 1.28 | 3.40 | 1.33* | 48.24 | 54.95 | 28.66 | 157.30* | 498.39 | 51.35 | 4 |
| P-value | 0.31 | 0.09 | 0.04 | 0.09 | 0.37 | 0.33 | 0.01 | 0.31 | 0.23 | 0.21 | 0.05 | 0.39 | 0.31 | ||
| E10.5–13.5 | 1 year | ||||||||||||||
| Vehicle | F3 | 1.00 | 4.70 | 1.06 | 102.75 | 1.42 | 3.28 | 1.48 | 44.50 | 57.26 | 30.39 | 174.27 | 554.53 | 62.73 | 5 |
| Caffeine | F3 | 0.95 | 5.07 | 1.15 | 125.27 | 1.42 | 3.28 | 1.48 | 67.29 | 49.37 | 25.72 | 201.70* | 552.16 | 63.04 | 4 |
| P-value | 0.26 | 0.18 | 0.08 | 0.15 | 0.27 | 0.20 | 0.16 | 0.18 | 0.26 | 0.28 | 0.04 | 0.44 | 0.35 | ||
Only the F1 generation embryos were exposed to vehicle or caffeine in utero from E10.5-13.5. For abbreviations please see Table 1. *P-value ≤ 0.05. P-values were calculated by Student’s t-test.
In utero caffeine exposure leads to impaired β-adrenergic responsiveness in adulthood
At 12 weeks of age, caffeine-treated mice showed only a slight difference in morphology compared to vehicle controls after 20 minutes of dobutamine exposure, including an increase in IVS thickness during diastole (Table 3). At 12 weeks, dobutamine treatment had no effect on cardiac function in mice exposed to caffeine in utero (Table 3).
Table 3. Echocardiography analysis 20 minutes after dobutamine treatment of CD-1 mice exposed to caffeine or vehicle in utero from E6.5–9.5.
| Treatment | Age | IVS;d | LVID;d | LVPW;d | LV Vol;d | IVS;s | LVID;s | LVPW;s | LV Vol;s | % EF | % FS | LV Mass | Heart Rate | N |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E6.5–9.5 | mm | mm | mm | ul | mm | mm | mm | ul | % | % | mg | BPM | ||
| Vehicle | 12 Weeks | 0.82 | 4.22 | 0.99 | 80.06 | 1.35 | 2.35 | 1.63 | 20.62 | 75.31 | 44.59 | 122.21 | 489.36 | 4 |
| Caffeine | 12 Weeks | 0.89* | 4.03 | 1.02 | 71.98 | 1.42 | 2.21 | 1.58 | 17.54 | 76.68 | 45.67 | 121.04 | 503.48 | 4 |
| P-value | 0.01 | 0.09 | 0.31 | 0.08 | 0.07 | 0.15 | 0.33 | 0.14 | 0.29 | 0.31 | 0.43 | 0.17 | ||
| E6.5–9.5 | ||||||||||||||
| Vehicle | 22 Weeks | 0.92 | 4.25 | 1.09 | 81.43 | 1.59 | 2.11 | 1.78 | 15.38 | 81.65 | 50.53 | 142.17 | 485.50 | 3 |
| Caffeine | 22 Weeks | 0.95 | 4.15 | 0.99* | 77.45 | 1.45* | 2.44 | 1.54* | 22.24 | 72.42* | 41.53* | 130.98 | 529.10* | 3 |
| P-value | 0.15 | 0.33 | 0.01 | 0.35 | 0.01 | 0.12 | 0.001 | 0.15 | 0.03 | 0.02 | 0.20 | 0.01 | ||
For abbreviations please see Table 1. *P-value ≤ 0.05. P-values were calculated by Student’s t-test.
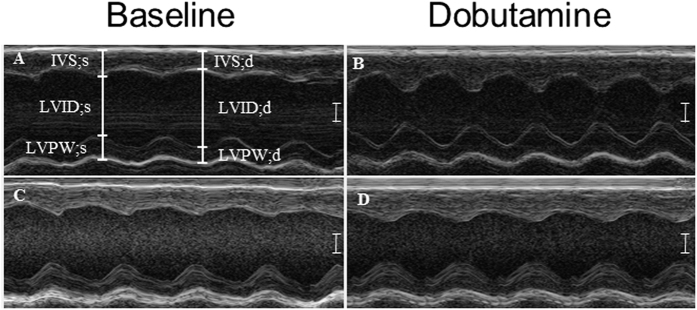
At 22 weeks, hearts from the caffeine-treated group exhibited impaired responses to dobutamine treatment at the morphological and functional levels. We observed altered morphology in caffeine-treated hearts including decreased LVPW thickness (15.6%) during systole compared to controls (Fig. 1; Table 3). Changes in morphology were associated with reduced function including, decreased % EF (12.7%) and % FS (21.7%) compared to controls in response to dobutamine (Table 3). These data show that in utero caffeine exposure induces long-term adverse effects on the hearts ability to respond to β-adrenergic stimulation.
Figure 1. M-mode measurements before and after dobutamine treatment in 22 week old mice.
For this analysis F1 generation mice from dams treated with vehicle or caffeine from E6.5–9.5 were used. Baseline echocardiograms were taken of (A) vehicle- and (C) caffeine-treated mice. 20 minutes after dobutamine another set of echocardiograms were taken of (B) vehicle- and (D) caffeine-treated mice. Data indicate that caffeine-exposed hearts have impaired response to β-adrenergic stimulation during systole and diastole. Measurements that were altered include interventricular septum (IVS;s); left ventricular posterior wall (LVPW;s, d); d, diastole; s, systole. N = 3–4, P ≤ 0.05. Scale bar = 1 mm.
In utero caffeine exposure leads to adverse transgenerational effects on adult cardiac morphology and function
In the F2 caffeine-treated group, we did not observe changes in cardiac morphology or function at 22 weeks of age, but there were significant adverse effects at 1 year of age (Table 2). At 1 year of age, the caffeine-treated group exhibited significant changes in cardiac morphology including decreased LVID, decreased LV volume and increased LVPW thickness during both systole and diastole (Table 2). These changes in morphology were associated with changes in cardiac function including a 10.6% increase in % FS (10.4%) and an 8.6% increase in % EF (Table 2). These changes in the caffeine-treated group are similar to those seen in hypertrophic cardiomyopathy with hyperdynamic function in humans55,56.
We also examined the F3 generation offspring, which were never directly exposed to caffeine. At 22 weeks, the F3 caffeine group displayed effects on morphology including increased LVPW thickness during systole and diastole compared to controls and a 7.7% increase in LV mass (Table 2). At 1 year of age, we observed that hearts of the F3 generation caffeine group had a 13.6% increase in LV mass compared to controls that did not coincide with an increase in body weight (Table 2).
In utero caffeine exposure leads to altered cardiac gene expression in adulthood
We first assessed cardiac gene expression in F1 adult mice exposed to caffeine in utero from E6.5–9.5. At 1 year of age, we observed significant changes in several cardiac genes including a 4-fold increase in the structural gene Myh7, increases in transcription factors Mef2c and Nfatc1-a, and a 2-fold increase in the hormone ANP (Fig. 2). In F1 hearts exposed to caffeine from E10.5–13.5, we also observed changes in gene expression including Tnni3, ANP, Mef2d, and Nfatc1-c (Fig. 2). Other than ANP, the different exposure periods of caffeine caused long-term changes in expression of a different set of genes in F1 adult hearts (Fig. 2).
Figure 2. In utero caffeine exposure leads to altered cardiac gene expression in the F1 generation at 1 year of age.
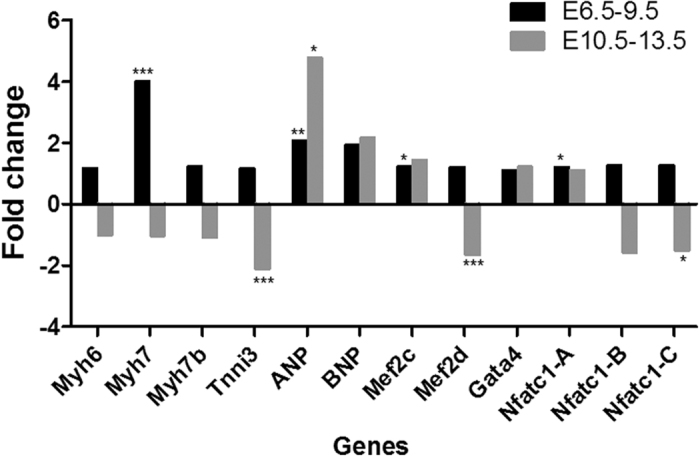
Early in utero exposure to caffeine from E6.5–9.5 caused increased RNA expression of Myh7, ANP, Mef2c, and Nfatc-1A in the left ventricle. Late in utero exposure from E10.5–13.5 caused altered expression of Tnni3, ANP, Mef2d, and Nfatc1-C. Fold change in gene expression between vehicle and caffeine treated mice was calculated with the 2−∆∆CT method using Gapdh as the control gene. Significance was determined by Student’s t-test and a P ≤ 0.05 was determined to be significant. N = 4, *P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.001.
We examined gene expression in the F2 and F3 generation of offspring to determine if in utero caffeine exposure induces transgenerational changes in cardiac gene expression. In the F2 generation at 1 year, we observed significant changes in the structural cardiac genes Myh7, Tnni3, and Tnnc1, the transcription factor Camta1, and the hormone BNP (Fig. 3). In the F2 caffeine-treated hearts at 1 year, Myh7 was down-regulated 2.2-fold (Fig. 3). In the F3 caffeine group, the only significant change in gene expression observed at 1 year of age was with Gata4 (Fig. 3).
Figure 3. In utero caffeine exposure leads to altered cardiac gene expression in F2 and F3 generation at 1 year of age.
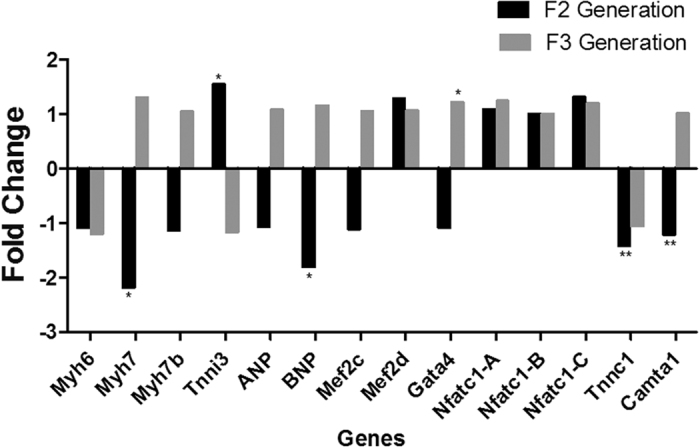
qPCR analysis revealed that in utero caffeine treatment during E10.5–13.5 altered gene expression in the left ventricles of F2 generation offspring, including Myh7, Tnni3, BNP, Tnnc1, and Camta1. In utero caffeine treatment during E10.5–13.5 increased Gata4 expression in the left ventricles of F3 generation offspring. Fold-change in gene expression between vehicle and caffeine treated mice was calculated with the 2−∆∆CT method using Gapdh as the control gene. Significance was determined by Student’s t-test and a P ≤ 0.05 was determined to be significant. N = 5, *P ≤ 0.05, **P ≤ 0.01.
Next, we performed transcriptomic mRNA-sequencing on left ventricles for a more comprehensive analysis of gene expression in the F1 generation adult hearts at 1 year of age. The mapping efficiency of sequencing reads was about 80%, and the samples were well clustered by treatments (Supplementary Figure 1). Differential gene expression (DE) analysis by EdgeR software revealed that 85 genes were up-regulated, and 31 genes were down-regulated significantly (Supplementary Table 1).
Diseases and Bio Functions analysis by Ingenuity Pathway Analysis (IPA) revealed that pathways related to cell death and survival, cell morphology, cellular function and maintenance, and cell signaling were affected by in utero caffeine exposure from E6.5–9.5 (Table 4). In addition, many cardiovascular disease pathways were significantly enriched (Table 5). In particular, the myocardium infarction and permeability of vascular system pathways were inhibited (Table 5), possibly serving as a compensation mechanisms in response to the adverse effects caused by in utero caffeine treatment.
Table 4. Altered cellular morphology and function pathways in adult F1 mice treated with caffeine during E6.5–10.5.
| p-value | Activation z-scorea | # of molecules | |
|---|---|---|---|
| Cell death and survival | |||
| Cytolysis | 6.79E-04 | −1.034 | 7 |
| Proliferation of cells | 2.11E-03 | −0.513 | 37 |
| Cell death | 2.40E-03 | −0.323 | 34 |
| Cell morphology, cellular function and maintenance | |||
| Nucleation of filaments | 9.91E-04 | — | 3 |
| Cellular homeostasis | 3.38E-03 | 0.297 | 19 |
| Morphology of cells | 5.15E-03 | — | 22 |
| Generation of filaments | 6.22E-03 | — | 2 |
| Cell signaling | |||
| Activation of cells | 2.76E-05 | −0.500 | 18 |
| Quantity of Ca2+ | 8.31E-03 | −0.240 | 7 |
aA positive z-score means the pathway is activated, and a negative z-score indicates the pathway is inhibited.
Table 5. Altered cardiovascular disease pathways in adult F1 mice treated with caffeine during E6.5–9.5.
| Pathways | p-value | Activation z-scorea | Genes |
|---|---|---|---|
| Infarction | 3.47E-06 | −2.750 | ACTA1,APOE,C1QA,CD55,FSTL1,GDF15,GPX1,HLA-A,PTGDS,STAT1,TNFRSF12A |
| Myocardial infarction | 8.08E-04 | −1.992 | ACTA1,APOE,CD55,FSTL1,GDF15,STAT1 |
| Permeability of vascular system | 8.44E-03 | −1.969 | APOE,AQP4,FGFBP3,TNFRSF12A |
| Size of infarct | 3.31E-06 | −1.673 | APOE,C1QA,GDF15,GPX1,HLA-A,PTGDS,STAT1,TNFRSF12A |
| Vasculogenesis | 6.05E-03 | −1.109 | ABRA,APOE,C1QA,GPX1,HLA-DQB1,KLK3,SEMA3B,STAT1,THBS4,TNFRSF12A,WARS |
| Adhesion of endothelial cells | 6.33E-03 | −0.293 | C1QA,CD274,GDF15,STAT1 |
| Lesioning of aorta | 9.10E-07 | APOE,GPX1,NPC1 | |
| Peripheral vascular disease | 8.48E-03 | APOE,AQP4,CFH,GPX1,MARK1,SEMA3B,TNFRSF12A | |
| Size of vascular lesion | 7.61E-03 | APOE,C1QA,GPX1 | |
| Advanced stage peripheral arterial disease | 6.89E-03 | APOE,CFH,MARK1,SEMA3B | |
| Thickness of heart ventricle | 6.70E-03 | APOE,KLK3 | |
| Cellularity of atherosclerotic lesion | 2.05E-05 | APOE,NPC1 | |
| Function of cardiovascular system | 1.18E-03 | ABRA,APOE,AQP4,CD55,CFP,GDF15,GPX1,KLK3 | |
| Thrombosis | 1.16E-02 | APOE,C1QA,CFH,GDF15 | |
| Mass of left ventricle | 1.05E-02 | KLK3,THBS4 |
aA positive z-score means the pathway is activated, and a negative z-score indicates the pathway is inhibited.
Further functional ontology analysis of genes altered by in utero caffeine exposure was conducted using the Database for Annotation, Visualization and Integrated Discovery (DAVID). DAVID analysis revealed that pathways related to immune response, antigen processing and presentation, GTP binding, and GTPase activity were affected by prenatal caffeine treatment (Supplementary Table 2). Also, mRNA expression of many cell surface molecules and signaling peptides were altered in caffeine-treated hearts (Supplementary Table 2). DAVID analysis of differentially expressed genes also identified sarcomere genes, including KAT2B (−1.68 fold), ACTA1 (2.69 fold), ABRA (1.76 fold), and MYOT (1.55 fold); genes involved in blood vessel development and blood circulation, including NPPB (4.28 fold), WARS (1.58 fold), GPX1 (1.55 fold), TNFRSF12A (1.93 fold), APOE (1.58 fold), and FGFBP3 (−117.24 fold); and genes related to membrane channel activity, including CACNG6 (−37.69 fold; calcium channel), KCNJ14 (2.39 fold; potassium channel), GRIN2C (3.06 fold; ionotropic glutamate receptor NMDA2C), and AQP4 (−15.92 fold; water channel).
Discussion
We find that the timing of in utero caffeine exposure determines which generation is effected and the cardiac phenotype induced. Early embryonic exposure to caffeine leads to a dilated cardiomyopathy phenotype in the 1st generation of offspring, and caffeine exposure later in development induces hypertrophic cardiomyopathy in the 2nd generation and altered morphology in the 3rd generation (Fig. 4). Collectively, these data show that caffeine exposure during fetal development has lasting effects on the heart, including reduced cardiac function and altered gene expression.
Figure 4. Timing of in utero caffeine exposure leads to different cardiac phenotypes in adult offspring.
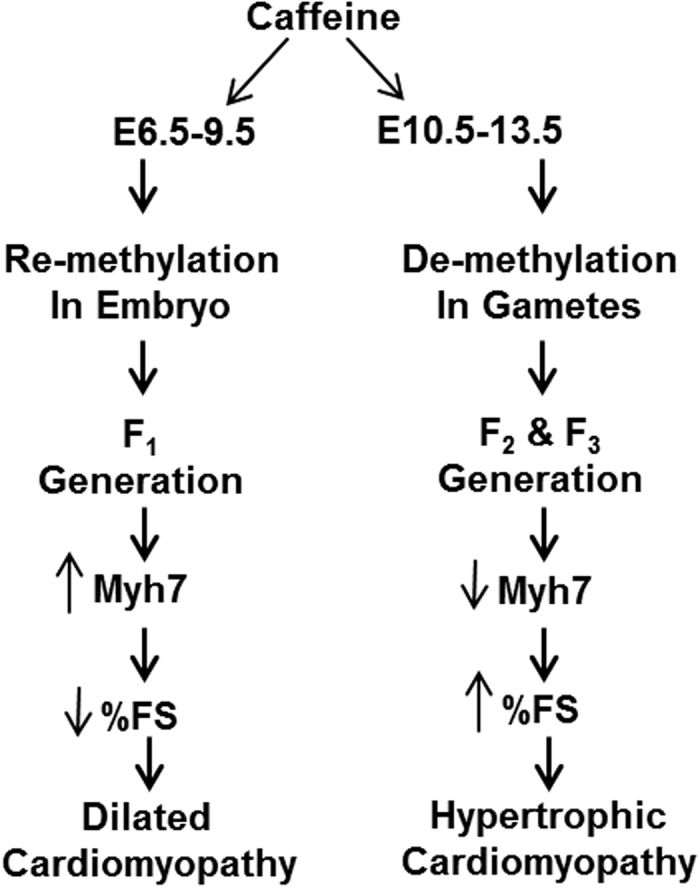
The timing of in utero caffeine treatment leads to differences in adult cardiac function, gene expression, and phenotype. Exposure to caffeine from E6.5–9.5 leads the F1 generation to develop dilated cardiomyopathy with decreased % FS and increased Myh7 expression. In utero caffeine exposure from E10.5–13.5 leads to a hypertrophic cardiomyopathy in the F2 generation along with increased % FS and decreased Myh7 expression.
Although caffeine exposure is not a teratogen considered harmful to the developing fetus by many14,25, a recent Norwegian Mother and Child Cohort Study (MoBa) involving 59,123 pregnant women in Norway strongly supports the notion that caffeine consumption is associated with decreased birth weight, even at levels below recommended limits35. The decrease in birth weight associated with fetal caffeine exposure is small35, but studies demonstrate that reduced fetal growth is associated with higher instances of adult disease later in life, including cardiovascular disease57,58. Data from this report and our previous results31,32 support the notion that in utero caffeine exposure induces long-term effects on cardiac gene expression and function into adulthood.
In these studies, we examined CD-1 mice because they have more severe phenotypes compared to inbred strains in transgenerational studies9. We exposed CD-1 mice to caffeine early in gestation (E6.5–9.5), as before31,32, and observed a phenotype similar to that found in the inbred C57Bl6 strain32. The changes in cardiac morphology and function induced by in utero caffeine exposure in the F1 generation CD-1 mice are characteristics seen in dilated cardiomyopathy in humans including dilated ventricular cavity and systolic dysfunction59. The effects of caffeine on cardiac function in CD-1 mice were not observed until 1 year of age compared to the inbred C57Bl/6 strain that exhibited reduced cardiac function at 10 weeks of age32. Thus, the CD-1 strain develops cardiac dysfunction later in adulthood compared to the inbred strain, but the effects are more severe31,32.
Based on our previous data31,32, we hypothesized that in utero caffeine exposure disrupts the DNA re-methylation event that occurs from E3.5–9.5 throughout the embryo43. Our results support this hypothesis as exposure to caffeine later in development from E10.5–13.5 had no effect on cardiac function or morphology in the F1 generation at either 22 weeks or 1 year of age. These results indicate that the timing of caffeine exposure is critical to inducing long-term effects in adult hearts of the F1 generation.
During the E10.5–13.5 developmental window, primordial germ cells are migrating, maturing, and going through a process of demethylation43. Thus, we hypothesized that caffeine could interfere with this demethylation process, which could lead to altered cardiac function in the F2 (germ cells at the time of exposure) and possibly the F3 (not directly exposed to caffeine) generations.
The effects of in utero caffeine exposure on adult cardiac function and morphology in the F2 generation offspring were opposite to those seen in the F1 generation exposed to caffeine from E6.5–9.5. The F2 caffeine hearts at 1 year displayed decreased LVID;s, increased LVPW;s thickness, and increased FS, which are characteristic of hypertrophic cardiomyopathy, including asymmetric left ventricular thickening, increased LV cavity, and hyperdynamic function55,56.
The F3 generation caffeine hearts had altered morphology and increased mass. Although caffeine induces effects on the heart in the F3 generation, they are less severe and they do not lead to altered cardiac function.
In addition, we observed that in utero treated (from E6.5–9.5) caffeine hearts had impaired responses to dobutamine stimulation, indicating that caffeine treated hearts may have a reduced ability to respond to or sense β-adrenergic stimulation. This impaired response could have a significant clinical impact as β-adrenergic agonists are given to patients with failing hearts to increase cardiac output.
We find that in utero caffeine exposure has long-lasting effects on cardiac gene expression in adult offspring of the F1, F2, and F3 generation. The most significant change in gene expression observed was with the Myh7 gene. MYH7 is generally an embryonic and prenatal form of cardiac myosin that is greatly reduced in adult murine hearts, but Myh7 expression is activated in adult hearts during cardiac stress and heart failure in mice60,61. In F1 generation mice exposed to caffeine from E6.5–9.5, we observed an increase in Myh7 expression. The increase in Myh7 expression is indicative of the phenotype induced by caffeine as increased Myh7 expression is associated with cardiomyopathy and heart failure61,62. Two other genes, Tnni3 and Mef2c, were also altered in adult F1 hearts when exposed to caffeine early in embryogenesis. These genes are associated with dilated cardiomyopathy63,64,65 and we have demonstrated previously that their expression is sensitive to caffeine exposure54. In addition, we observe increased ANP expression in adult F1 hearts which is also increased in human idiopathic dilated cardiomyopathy66. From our RNAseq data we identify new genes that we had not previously associated with caffeine exposure and cardiomyopathy, including Nppb which is up regulated in F1 hearts at one year and in dilated cardiomyopathy67, and Mylk4 which is down regulated in our caffeine exposed F1 hearts and in human dilated cardiomyopathy patient left ventricle tissue68.
In contrast, we observed that F2 generation mice exposed to in utero caffeine from E10.5–13.5 had reduced expression of Myh7. This opposite effect on Myh7 gene expression compared to the F1 generation mice exposed to caffeine from E6.5–9.5 correlates with the opposite effect that caffeine has on adult heart function in the F2 generation. These data indicate that Myh7 expression is sensitive to caffeine exposure and may be predictive of cardiac dysfunction in adult hearts (Fig. 4).
In conclusion, we find that in utero caffeine exposure has long-term effects on cardiac morphology, function, and gene expression. We find that long-term effects of caffeine exposure in utero are determined in part by the timing of exposure (Fig. 4). We show that caffeine may interfere with the hearts ability to respond to stress, as caffeine treated mice had an impaired response to β-adrenergic stimulation. In addition, we demonstrate that caffeine has transgenerational effects on the F2 generation including altered cardiac morphology, function, and gene expression. Transgenerational effects of caffeine are also observed in the F3 generation. As such, these observations are of potential major clinical significance to the thousands of women who consume caffeine during pregnancy.
Methods
Animals
All animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Florida and all methods were carried out in accordance with approved guidelines. Pregnant CD-1 dams (F0 generation) were treated with 0.9% NaCl (vehicle) or 20 mg/kg caffeine in vehicle via i.p. injection. CD-1 mice were used as they have been demonstrated to have more severe transgenerational effects on health in adulthood than inbred strains when exposed to endocrine disruptors in utero9. This dose was chosen because we previously demonstrated that it leads to long-term effects on cardiac function and results in a serum level equivalent to 2–4 cups of coffee in humans31,32. Dams were treated once daily for four days either from E6.5–9.5 or E10.5–13.5. We chose these exposure times because they coincide with two important DNA methylation transition events during embryogenesis, including a large scale DNA remethylation of the genome that occurs from E3.5–9.5, and genome-wide demethylation that occurs during PGC development from E10.5–13.5 43.
Our previous analysis indicated that there was no difference in the effects of in utero caffeine exposure on cardiac function between males and females32. In addition, we observed elevated % body fat levels in males exposed to caffeine in utero32, therefore we only examined male offspring in this study.
The F1 generation (embryos exposed in utero), F2 generation (exposed gametes within the F1 generation embryos) and F3 generation (naïve to caffeine exposure) were examined up to one year of age (Tables 6 and 7). We generated the F2 generation by mating F1 generation mice at 10–12 weeks of age that had been similarly treated with either caffeine or vehicle, taking care not to mate siblings or cousins. Next, we generated the F3 generation of mice, which were naïve to caffeine exposure, by mating F2 generation mice that were similarly treated.
Table 6. Timing of analysis performed on F1 offspring exposed to vehicle or caffeine in utero from E6.5–9.5.
| Dam | F1 Offspring | ||
|---|---|---|---|
| Experimental Analysis | |||
| Treatment/Timing | 12 Weeks | 22 Weeks | 52 Weeks |
| Vehicle/E6.5–9.5 Caffeine/E6.5–9.5 | 1) Echocardiography | 1) Echocardiography | 1) Echocardiography |
| 2) Dobutamine Treatment | 2) Dobutamine Treatment | 2) Gene Expression | |
| 4) RNA Seq. | |||
| 5) Histology | |||
Table 7. Timing of analysis performed on F1, F2, and F3 offspring exposed to vehicle or caffeine in utero from E10.5–13.5.
| Dam | F1 Offspring | F2 Offspring | F3 Offspring | |||
|---|---|---|---|---|---|---|
| Treatment/Timing | Analysis | Analysis | Analysis | |||
| 22 Weeks | 52 Weeks | 22 Weeks | 52 Weeks | 22 Weeks | 52 Weeks | |
| Vehicle/E10.5–13.5 Caffeine/E10.5–13.5 | 1) Echos | 1) Echos 2) Gene Expression 3) Histology | 1) Echos | 1) Echos 2) Gene Expression 3) Histology | 1) Echos | 1) Echos 2) Gene Expression 3) Histology |
Statistical Analysis
Data are presented as mean ± the standard error of the mean (SEM). Analysis was performed with the statistics software package included with Microsoft Excel (Microsoft, Redmond, WA, USA) and GraphPad Prism 6.0 (GraphPad Software Inc., La Jolla, CA, USA).
Echocardiography
Cardiac function of male offspring was assessed using echocardiography, as described31,32,69. Briefly, offspring were anesthetized with a continuous flow of isoflurane administered via nosecone and anesthesia levels were regulated to maintain heart rates between 400 and 500 beats per minute. Transthoracic 2D M-mode echocardiography was performed using a 30-MHz probe with the Vevo 770 (Visualsonics, Toronto, ON, Canada) small animal ultrasound device32.
Ultrasound analysis of cardiac function was performed after administration of dobutamine, a β-adrenergic agonist, in order to stress the heart. Dobutamine, an inotropic agent, induces a more forceful contraction of heart muscle, which increases the LVPW thickness during systole leading to increased % FS and % EF70. After baseline echocardiograms were obtained, mice were injected with 1.5 mg/kg dobutamine via i.p. Echocardiograms were retaken 20 minutes after dobutamine injection. Echocardiography and analysis of results were performed blinded. Analyses of the effects of dobutamine were determined by comparing echocardiograms at baseline and 20 minutes. Based on a pilot experiment that examined cardiac function 5, 10, 15, and 20 minutes after dobutamine injection, we determined that the results at 20 minutes were the most significant compared to baseline.
For echocardiography, 1–5 offspring from 3–6 dams were analyzed. Statistical comparisons between groups for echocardiography were performed with Student’s t-test assuming equal variance. For Tables 1, 2, and 3 N = number of dams treated. For Tables 1, 2, and 3 measurements for offspring from one dam were averaged and the averages for each litter were used to calculate significance. For Table 3, N = the number of mice treated with dobutamine. P ≤ 0.05 was considered to be statistically significant.
Quantitative real-time PCR (qPCR) analysis
For qPCR, 1–2 offspring were examined from 4–5 different dams. Results from offspring of the same dam were averaged before determining significance and fold change. Total RNA from left ventricles of adult male offspring was extracted with RNeasy Plus Mini Kit (Qiagen), according to the manufacturer’s protocol. cDNA was synthesized using iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA, USA). For most cardiac genes, published primer pairs were used71. Camta1 forward: TCCTTATCCAGAGCAAATTCCGA; Camta1 reverse: CACACTTCTTGTAACTCCGGTAG. Myh6 (PPM04500A) and Myh7 (PPM67019A) primers were proprietary sequences designed by Qiagen SABiosciences. Gapdh primers were used as an internal control. SYBR®Green PCR Master Mix (Life Technologies Applied Biosystems®) was used to perform qPCR analysis in a GeneAmp 7300 Real Time PCR System71. Each sample was measured in two separate reactions on the same plate. Amplification efficiencies of the target genes and Gapdh primer pairs were tested to ensure that they were not statistically different. Differences in expression between the treatment groups were calculated with the 2−∆∆CT method54. Statistical significance was determined by Student’s t-test analysis on the ΔCT values, thus error bars are not included on the gene expression graphs (Figs 2 and 3).
Illumina transcriptomic RNA sequencing (RNA-Seq)
Total RNA was isolated from the left ventricles of male 1 year-old mice treated with 20 mg/kg caffeine at E6.5–9.5 by using the RNeasy Plus Mini kit (Qiagen). mRNA was isolated from total RNA using NEXTflex™ Poly(A) Beads (Bioo Scientific, Austin, TX, USA). Sequencing libraries were prepared with the NEBNext® mRNA Library Prep Master Mix Set for Illumina (NEB, Ipswich, MA, USA) and the NEBNext Multiplex Oligos for Illumina (NEB). Illumina-adapted libraries (n = 3/treatment) were pooled at equal molar ratio and sequenced with one High Output 1 × 75 cycles run on a NextSeq500 sequencer (Illumina, San Diego, CA, USA). All RNA-Seq data were uploaded to the Gene Expression Omnibus (GEO), and the accession number is GSE79013.
RNA-Seq data analysis for differential gene expression
The fastq files generated from RNA-Seq were uploaded to the UF Research Computing Galaxy instance developed by the University of Florida72. The data were cleaned with the FastQC program and mapped to the mouse genome (mm10) with the Tophat2 tool. Counting of RNA-seq reads were performed with HTSeq73. Differential expression (DE) of genes between treatments was analyzed using R package EdgeR74, with Ensembl Mus_GRCm38.79.gtf as the reference annotation. Genes with false discovery rate (FDR) less than 0.05 and absolute fold change greater than 1.5 were considered as significant. EdgeR was also used to calculate the principle component (x-axis) analysis to determine the variance between the vehicle- and caffeine-treated groups (Supplementary Figure 1). Functional ontology was conducted using the Database for Annotation, Visualization and Integrated Discovery (DAVID) and IPA (Qiagen), and the significance was determined as described for each software package75,76. The significance criterion for pathway analysis is p ≤ 0.05 with Benjamini Hochberg correction.
Additional Information
How to cite this article: Fang, X. et al. In Utero Caffeine Exposure Induces Transgenerational Effects on the Adult Heart. Sci. Rep. 6, 34106; doi: 10.1038/srep34106 (2016).
Supplementary Material
Acknowledgments
This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development at the National Institutes of Health (R01 HD058086 to S.A. Rivkees and C.C. Wendler). We thank Daniel Freeman, Olivia Shi, Olivia Donnelly, Randall Autrey, and Greg Shoemaker for technical assistance.
Footnotes
Author Contributions All authors contributed to the design of the study and interpretation of the data. R.R.P. and C.C.W. performed and analyzed echocardiography. X.F. performed and analyzed gene expression and pathway analysis. C.C.W. wrote the first and last draft of the manuscript with all authors providing critical revisions. X.F. prepared Tables 4, 5, S2 and Figure S1. R.R.P. prepared Tables 1, 2, 3 and Figure 2. C.C.W. prepared Figures 1, 3, 4, 5 and Table S1. All authors reviewed the final draft of the manuscript.
References
- Cetin I., Mando C. & Calabrese S. Maternal predictors of intrauterine growth restriction. Current opinion in clinical nutrition and metabolic care 16, 310–319, doi: 10.1097/MCO.0b013e32835e8d9c (2013). [DOI] [PubMed] [Google Scholar]
- Gluckman P. D., Cutfield W., Hofman P. & Hanson M. A. The fetal, neonatal, and infant environments-the long-term consequences for disease risk. Early Hum Dev 81, 51–59 (2005). [DOI] [PubMed] [Google Scholar]
- Vo T. & Hardy D. B. Molecular mechanisms underlying the fetal programming of adult disease. Journal of cell communication and signaling 6, 139–153, doi: 10.1007/s12079-012-0165-3 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T., Kikkawa R., Yamada H. & Horii I. Identification of oxidative stress-related proteins for predictive screening of hepatotoxicity using a proteomic approach. The Journal of toxicological sciences 30, 213–227 (2005). [DOI] [PubMed] [Google Scholar]
- Barker D. J. Fetal origins of cardiovascular disease. Ann Med 31 Suppl 1, 3–6 (1999). [PubMed] [Google Scholar]
- Barker D. J. The fetal and infant origins of adult disease. BMJ 301, 1111 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluckman P. D., Hanson M. A., Buklijas T., Low F. M. & Beedle A. S. Epigenetic mechanisms that underpin metabolic and cardiovascular diseases. Nature reviews. Endocrinology 5, 401–408, doi: 10.1038/nrendo.2009.102 (2009). [DOI] [PubMed] [Google Scholar]
- Clementi C., Miller K., Mero A., Satchi-Fainaro R. & Pasut G. Dendritic poly(ethylene glycol) bearing paclitaxel and alendronate for targeting bone neoplasms. Molecular pharmaceutics 8, 1063–1072, doi: 10.1021/mp2001445 (2011). [DOI] [PubMed] [Google Scholar]
- Guerrero-Bosagna C. et al. Epigenetic transgenerational inheritance of vinclozolin induced mouse adult onset disease and associated sperm epigenome biomarkers. Reprod Toxicol 34, 694–707, doi: 10.1016/j.reprotox.2012.09.005 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredholm B. B., Battig K., Holmen J., Nehlig A. & Zvartau E. E. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol Rev 51, 83–133 (1999). [PubMed] [Google Scholar]
- Fredholm B. B. Astra Award Lecture. Adenosine, adenosine receptors and the actions of caffeine. Pharmacol.Toxicol. 76, 93–101 (1995). [DOI] [PubMed] [Google Scholar]
- Rudolph E., Farbinger A. & Konig J. Determination of the caffeine contents of various food items within the Austrian market and validation of a caffeine assessment tool (CAT). Food additives & contaminants. Part A, Chemistry, analysis, control, exposure & risk assessment 29, 1849–1860, doi: 10.1080/19440049.2012.719642 (2012). [DOI] [PubMed] [Google Scholar]
- Heckman M. A., Weil J. & Gonzalez de Mejia E. Caffeine (1, 3, 7-trimethylxanthine) in foods: a comprehensive review on consumption, functionality, safety, and regulatory matters. Journal of food science 75, R77–R87, doi: 10.1111/j.1750-3841.2010.01561.x (2010). [DOI] [PubMed] [Google Scholar]
- Browne M. L. Maternal exposure to caffeine and risk of congenital anomalies: a systematic review. Epidemiology 17, 324–331 (2006). [DOI] [PubMed] [Google Scholar]
- Aldridge A., Aranda J. V. & Neims A. H. Caffeine metabolism in the newborn. Clinical pharmacology and therapeutics 25, 447–453 (1979). [DOI] [PubMed] [Google Scholar]
- Clausson B. et al. Effect of caffeine exposure during pregnancy on birth weight and gestational age. Am J Epidemiol 155, 429–436 (2002). [DOI] [PubMed] [Google Scholar]
- Cnattingius S. et al. Caffeine intake and the risk of first-trimester spontaneous abortion. N Engl J Med 343, 1839–1845 (2000). [DOI] [PubMed] [Google Scholar]
- Vlajinac H. D., Petrovic R. R., Marinkovic J. M., Sipetic S. B. & Adanja B. J. Effect of caffeine intake during pregnancy on birth weight. Am J Epidemiol 145, 335–338 (1997). [DOI] [PubMed] [Google Scholar]
- Matijasevich A., Barros F. C., Santos I. S. & Yemini A. Maternal caffeine consumption and fetal death: a case-control study in Uruguay. Paediatr Perinat Epidemiol 20, 100–109 (2006). [DOI] [PubMed] [Google Scholar]
- Higdon J. V. & Frei B. Coffee and health: a review of recent human research. Crit Rev Food Sci Nutr 46, 101–123 (2006). [DOI] [PubMed] [Google Scholar]
- Bech B. H., Nohr E. A., Vaeth M., Henriksen T. B. & Olsen J. Coffee and fetal death: a cohort study with prospective data. Am J Epidemiol 162, 983–990 (2005). [DOI] [PubMed] [Google Scholar]
- Weng X., Odouli R. & Li D. K. Maternal caffeine consumption during pregnancy and the risk of miscarriage: a prospective cohort study. Am J Obstet Gynecol (2008). [DOI] [PubMed] [Google Scholar]
- Sulter G., Elting J. W., Maurits N., Luijckx G. J. & De Keyser J. Acetylsalicylic acid and acetaminophen to combat elevated body temperature in acute ischemic stroke. Cerebrovasc Dis 17, 118–122, doi: 10.1159/000075779 (2004). [DOI] [PubMed] [Google Scholar]
- Greenwood D. C. et al. Caffeine intake during pregnancy, late miscarriage and stillbirth. Eur J Epidemiol 25, 275–280, doi: 10.1007/s10654-010-9443-7 (2010). [DOI] [PubMed] [Google Scholar]
- Peck J. D., Leviton A. & Cowan L. D. A review of the epidemiologic evidence concerning the reproductive health effects of caffeine consumption: a 2000–2009 update. Food and chemical toxicology: an international journal published for the British Industrial Biological Research Association 48, 2549–2576, doi: 10.1016/j.fct.2010.06.019 (2010). [DOI] [PubMed] [Google Scholar]
- NNR Project group: In Nordic nutrition recommendations 2004, Integrating nutrition and physical activity. Volume Nord 2004. 4th edition. Copenhagen, Denmark: Nordic Council of Ministers, 2004(13), (2004). [Google Scholar]
- Goodman Y. & Mattson M. P. K+ channel openers protect hippocampal neurons against oxidative injury and amyloid beta-peptide toxicity. Brain Res 706, 328–332 (1996). [DOI] [PubMed] [Google Scholar]
- Silva C. G. et al. Adenosine receptor antagonists including caffeine alter fetal brain development in mice. Science translational medicine 5, 197ra104, doi: 10.1126/scitranslmed.3006258 (2013). [DOI] [PubMed] [Google Scholar]
- Bjorklund O., Kahlstrom J., Salmi P. & Fredholm B. B. Perinatal caffeine, acting on maternal adenosine A(1) receptors, causes long-lasting behavioral changes in mouse offspring. PLoS One 3, e3977, doi: 10.1371/journal.pone.0003977 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serapiao-Moraes D. F., Souza-Mello V., Aguila M. B., Mandarim-de-Lacerda C. A. & Faria T. S. Maternal caffeine administration leads to adverse effects on adult mice offspring. European journal of nutrition 52, 1891–1900, doi: 10.1007/s00394-012-0490-6 (2013). [DOI] [PubMed] [Google Scholar]
- Clementi M. et al. Treatment of hyperthyroidism in pregnancy and birth defects. J Clin Endocrinol Metab 95, E337–E341, doi: 10.1210/jc.2010-0652 (2010). [DOI] [PubMed] [Google Scholar]
- Wendler C. C. et al. Embryonic caffeine exposure induces adverse effects in adulthood. FASEB J 23, 1272–1278, doi: 10.1096/fj.08-124941 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekkhus M., Skjothaug T., Nordhagen R. & Borge A. I. Intrauterine exposure to caffeine and inattention/overactivity in children. Acta paediatrica 99, 925–928, doi: 10.1111/j.1651-2227.2010.01744.x (2010). [DOI] [PubMed] [Google Scholar]
- Bakker R. et al. Maternal caffeine intake from coffee and tea, fetal growth, and the risks of adverse birth outcomes: the Generation R Study. The American journal of clinical nutrition 91, 1691–1698, doi: 10.3945/ajcn.2009.28792 (2010). [DOI] [PubMed] [Google Scholar]
- Sengpiel V. et al. Maternal caffeine intake during pregnancy is associated with birth weight but not with gestational length: results from a large prospective observational cohort study. BMC medicine 11, 42, doi: 10.1186/1741-7015-11-42 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirodkar A. V. & Marsden P. A. Epigenetics in cardiovascular disease. Current opinion in cardiology 26, 209–215, doi: 10.1097/HCO.0b013e328345986e (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Sullivan L., Combes A. N. & Moritz K. M. Epigenetics and developmental programming of adult onset diseases. Pediatr Nephrol 27, 2175–2182, doi: 10.1007/s00467-012-2108-x (2012). [DOI] [PubMed] [Google Scholar]
- Ordovas J. M. & Smith C. E. Epigenetics and cardiovascular disease. Nature reviews. Cardiology 7, 510–519, doi: 10.1038/nrcardio.2010.104 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joubert B. R. et al. Maternal smoking and DNA methylation in newborns: in utero effect or epigenetic inheritance? Cancer Epidemiol Biomarkers Prev 23, 1007–1017, doi: 10.1158/1055-9965.EPI-13-1256 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Tian X. B., Fang H., Liu P. & Meng Q. Y. [Study on the expression of uncoupling protein 2 in the kidney of the rats with hypothyroidism]. Zhongguo wei zhong bing ji jiu yi xue = Chinese critical care medicine = Zhongguo weizhongbing jijiuyixue 22, 729–732 (2010). [PubMed] [Google Scholar]
- Sanz L. A., Kota S. K. & Feil R. Genome-wide DNA demethylation in mammals. Genome biology 11, 110, doi: 10.1186/gb-2010-11-3-110 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller A., Rozin A., Rigler O. & Sack J. Neurobehavioral development of neonatal rats after in-utero hypothyroxinemia: efficacy of prenatal thyroxine treatment. Early human development 46, 63–76 (1996). [DOI] [PubMed] [Google Scholar]
- He X. J., Chen T. & Zhu J. K. Regulation and function of DNA methylation in plants and animals. Cell Res 21, 442–465, doi: 10.1038/cr.2011.23 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolinoy D. C., Weidman J. R. & Jirtle R. L. Epigenetic gene regulation: linking early developmental environment to adult disease. Reprod Toxicol 23, 297–307, doi: 10.1016/j.reprotox.2006.08.012 (2007). [DOI] [PubMed] [Google Scholar]
- Waterland R. A. & Jirtle R. L. Transposable elements: targets for early nutritional effects on epigenetic gene regulation. Mol Cell Biol 23, 5293–5300 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho S. M., Tang W. Y., Belmonte de Frausto J. & Prins G. S. Developmental exposure to estradiol and bisphenol A increases susceptibility to prostate carcinogenesis and epigenetically regulates phosphodiesterase type 4 variant 4. Cancer Res 66, 5624–5632, doi: 10.1158/0008-5472.CAN-06-0516 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajkova P. Epigenetic reprogramming in the germline: towards the ground state of the epigenome. Philos Trans R Soc Lond B Biol Sci 366, 2266–2273, doi: 10.1098/rstb.2011.0042 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trasler J. M. Epigenetics in spermatogenesis. Mol Cell Endocrinol 306, 33–36, doi: 10.1016/j.mce.2008.12.018 (2009). [DOI] [PubMed] [Google Scholar]
- Clementi N. et al. A human monoclonal antibody with neutralizing activity against highly divergent influenza subtypes. PLoS One 6, e28001, doi: 10.1371/journal.pone.0028001 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heindel J. J. Role of exposure to environmental chemicals in the developmental basis of reproductive disease and dysfunction. Seminars in reproductive medicine 24, 168–177, doi: 10.1055/s-2006-944423 (2006). [DOI] [PubMed] [Google Scholar]
- LeBaron M. J. et al. Epigenetics and chemical safety assessment. Mutat Res 705, 83–95, doi: 10.1016/j.mrrev.2010.04.003 (2010). [DOI] [PubMed] [Google Scholar]
- Manikkam M., Tracey R., Guerrero-Bosagna C. & Skinner M. K. Dioxin (TCDD) induces epigenetic transgenerational inheritance of adult onset disease and sperm epimutations. PLoS One 7, e46249, doi: 10.1371/journal.pone.0046249 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurberg P. & Andersen S. L. Antithyroid drug use in early pregnancy and birth defects. Time windows of relative safety and high risk? European journal of endocrinology/European Federation of Endocrine Societies, doi: 10.1530/EJE-14-0135 (2014). [DOI] [PubMed] [Google Scholar]
- Fang X., Mei W., Barbazuk W. B., Rivkees S. A. & Wendler C. C. Caffeine Exposure Alters Cardiac Gene Expression in Embryonic Cardiomyocytes. Am J Physiol Regul Integr Comp Physiol, ajpregu 00307, 02014, doi: 10.1152/ajpregu.00307.2014 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gersh B. J. et al. 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 124, 2761–2796, doi: 10.1161/CIR.0b013e318223e230 (2011). [DOI] [PubMed] [Google Scholar]
- Parthiban A. & Shirali G. Echocardiographic evaluation of the failing heart. Cardiology in the young 25 Suppl 2, 87–93, doi: 10.1017/S1047951115000876 (2015). [DOI] [PubMed] [Google Scholar]
- Wang K. C. et al. Fetal growth restriction and the programming of heart growth and cardiac insulin-like growth factor 2 expression in the lamb. J Physiol 589, 4709–4722, doi: 10.1113/jphysiol.2011.211185 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross M. G. & Beall M. H. Adult sequelae of intrauterine growth restriction. Semin Perinatol 32, 213–218, doi: 10.1053/j.semperi.2007.11.005 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura A. Molecular genetics and pathogenesis of cardiomyopathy. Journal of human genetics, doi: 10.1038/jhg.2015.83 (2015). [DOI] [PubMed] [Google Scholar]
- Kim Y. et al. The MEF2D transcription factor mediates stress-dependent cardiac remodeling in mice. J Clin Invest 118, 124–132, doi: 10.1172/JCI33255 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey N. & Olson E. N. Cardiac hypertrophy: the good, the bad, and the ugly. Annual review of physiology 65, 45–79, doi: 10.1146/annurev.physiol.65.092101.142243 (2003). [DOI] [PubMed] [Google Scholar]
- Kawaji T. et al. Clinical impact of left ventricular outflow tract obstruction in takotsubo cardiomyopathy. Circ J 79, 839–846, doi: 10.1253/circj.CJ-14-1148 (2015). [DOI] [PubMed] [Google Scholar]
- Zhao Y. et al. Targeted next-generation sequencing of candidate genes reveals novel mutations in patients with dilated cardiomyopathy. Int J Mol Med 36, 1479–1486, doi: 10.3892/ijmm.2015.2361 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marston S. et al. OBSCN Mutations Associated with Dilated Cardiomyopathy and Haploinsufficiency. PLoS One 10, e0138568, doi: 10.1371/journal.pone.0138568 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Oort R. J. et al. MEF2 activates a genetic program promoting chamber dilation and contractile dysfunction in calcineurin-induced heart failure. Circulation 114, 298–308, doi: 10.1161/CIRCULATIONAHA.105.608968 (2006). [DOI] [PubMed] [Google Scholar]
- Ardizzone N. et al. Atrial natriuretic peptide and CD34 overexpression in human idiopathic dilated cardiomyopathies. APMIS: acta pathologica, microbiologica, et immunologica Scandinavica 115, 1227–1233, doi: 10.1111/j.1600-0643.2007.00663.x (2007). [DOI] [PubMed] [Google Scholar]
- Lombardi R. et al. Differential interactions of thin filament proteins in two cardiac troponin T mouse models of hypertrophic and dilated cardiomyopathies. Cardiovasc Res 79, 109–117, doi: 10.1093/cvr/cvn078 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrer I. et al. RNA-sequencing analysis reveals new alterations in cardiomyocyte cytoskeletal genes in patients with heart failure. Lab Invest 94, 645–653, doi: 10.1038/labinvest.2014.54 (2014). [DOI] [PubMed] [Google Scholar]
- Jacoby J. J. et al. Cardiomyocyte-restricted knockout of STAT3 results in higher sensitivity to inflammation, cardiac fibrosis, and heart failure with advanced age. Proc Natl Acad Sci USA 100, 12929–12934 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesmann F. et al. Dobutamine-stress magnetic resonance microimaging in mice : acute changes of cardiac geometry and function in normal and failing murine hearts. Circ Res 88, 563–569 (2001). [DOI] [PubMed] [Google Scholar]
- Fang X., Mei W., Barbazuk W. B., Rivkees S. A. & Wendler C. C. Caffeine exposure alters cardiac gene expression in embryonic cardiomyocytes. American journal of physiology. Regulatory, integrative and comparative physiology 307, R1471–R1487, doi: 10.1152/ajpregu.00307.2014 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goecks J., Nekrutenko A. & Taylor J. Galaxy: a comprehensive approach for supporting accessible, reproducible, and transparent computational research in the life sciences. Genome biology 11, R86, doi: 10.1186/gb-2010-11-8-r86 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders S., Pyl P. T. & Huber W. HTSeq–a Python framework to work with high-throughput sequencing data. Bioinformatics 31, 166–169, doi: 10.1093/bioinformatics/btu638 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson M. D., McCarthy D. J. & Smyth G. K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140, doi: 10.1093/bioinformatics/btp616 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang da W., Sherman B. T. & Lempicki R. A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nature protocols 4, 44–57, doi: 10.1038/nprot.2008.211 (2009). [DOI] [PubMed] [Google Scholar]
- Fang X. et al. Transcriptomic Changes in Zebrafish Embryos and Larvae Following Benzo[a]pyrene Exposure. Toxicological sciences: an official journal of the Society of Toxicology, doi: 10.1093/toxsci/kfv105 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.