Abstract
Background
Systemic chemotherapy with gemcitabine and cisplatin is standard of care for patients with metastatic urothelial bladder cancer. However, resistance formation is common after initial response. The protein Src is known as a proto-oncogene, which is overexpressed in various human cancers. Since there are controversial reports about the role of Src in bladder cancer, we evaluated the efficacy of the Src kinase inhibitor dasatinib in the urothelial bladder cancer cell line RT112 and its gemcitabine-resistant sub-line RT112rGEMCI20 in vitro and in vivo.
Methods
RT112 urothelial cancer cells were adapted to growth in the presence of 20 ng/ml gemcitabine (RT112rGEMCI20) by continuous cultivation at increasing drug concentrations. Cell viability was determined by MTT assay, cell growth kinetics were determined by cell count, protein levels were measured by western blot, and cell migration was evaluated by scratch assays. In vivo tumor growth was tested in a murine orthotopic xenograft model using bioluminescent imaging.
Results
Dasatinib exerted similar effects on Src signaling in RT112 and RT112rGEMCI20 cells but RT112rGEMCI20 cells were less sensitive to dasatinib-induced anti-cancer effects (half maximal inhibitory concentration (IC50) of dasatinib in RT112 cells: 349.2 ± 67.2 nM; IC50 of dasatinib in RT112rGEMCI20 cells: 1081.1 ± 239.2 nM). Dasatinib inhibited migration of chemo-naive and gemcitabine-resistant cells. Most strikingly, dasatinib treatment reduced RT112 tumor growth and muscle invasion in orthotopic xenografts, while it was associated with increased size and muscle-invasive growth in RT112rGEMCI20 tumors.
Conclusion
Dasatinib should be considered with care for the treatment of urothelial cancer, in particular for therapy-refractory cases.
Keywords: Acquired resistance, Cancer cell line collection, Dasatinib, Gemcitabine, Orthotopic xenograft model, Urothelial bladder cancer
Background
Bladder cancer is the second most common cancer of the genitourinary tract. First-line therapy regimens for metastatic disease include gemcitabine and cisplatin (GC) as standard of care resulting in response rates of 40–70 %. However, resistance acquisition is common and the median survival is unsatisfactory being 12–14 months [1, 2]. Effective second-line therapies are missing [3, 4].
The c-Src proto-oncogene has been strongly implicated in the development, growth, progression, and metastasis of a number of human cancers including those of colon, breast, pancreas, and brain [5, 6]. Several clinical trials showed antitumoral activity of Src family inhibitor dasatinib in different cancer entities [7, 8]. The role of Src kinase as drug target in bladder cancer is controversial. Src inhibition caused anti-cancer effects in some bladder cancer cell lines (including RT112) and animal models [9, 10]. In other studies, however, Src inhibition increased bladder cancer cell migration and metastasis formation [11, 12]. A recently published phase II trial did not demonstrate clinical benefit of single-agent dasatinib in unselected patients with muscle-invasive urothelial carcinoma of the bladder [13].
Here, we investigated the effects of dasatinib on RT112 urothelial bladder cancer cells and RT112 cells with acquired resistance to gemcitabine (RT112rGEMCI20) in cell culture and in an orthotopic xenograft model.
Methods
Drugs
Gemcitabine (Gemzar®) was obtained from Lilly (Indianapolis, IN, USA). Dasatinib was obtained from Absource Diagnostics (München, Germany).
Cell lines and lentiviral transduction
RT112 cells were obtained from the American Type Culture Collection (Manassas, VA, USA). Cells were grown in Iscove’s modified Dulbecco’s medium supplemented with 10 % fetal calf serum (FCS, Gibco, Karlsruhe, Germany). RT112 cells were adapted to growth in presence of 20 ng/ml gemcitabine by continuous cultivation in the presence of increasing drug concentrations as previously described [14] resulting in the gemcitabine-resistant subline RT112rGEMCI20. For in vivo studies, RT112 and RT112rGEMCI20 cell lines underwent transduction with a lentiviral construct carrying the luciferase firefly gene for in vivo imaging resulting in cell lines RT112luc and RT112rGEMCI20luc. The luciferase plasmid contained a blasticidin-resistance gene enabling positive selection with 10 mg/ml blasticidin (Life Technologies GmbH, Darmstadt, Germany). Cell lines were controlled for in vitro luciferase activity and cell number was correlated with bioluminescence using the Xenogen IVIS Spectrum (Caliper Lifesciences, Hopkinton, MA, USA) as previously described [15].
Viability assay
Cell viability was tested by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) dye reduction assay after 120 h incubation modified as described before [16]. All experiments were performed at least in triplicate.
Cell proliferation
At day 0, 4000 cells/ml were plated in culture flasks. Cell numbers were determined every 24 h for 6 consecutive days using the automated cell counter Countess® (Life Technologies GmbH) after trypan blue staining. Doubling time (DT) was calculated using the formula DT = culture time/cell doubling. Cell doubling = ln(Nf/Ni)/ln2, where Ni represents seeded cells number and Nf the harvested cells number [17]. All experiments were performed at least in triplicate.
Western blot
Cells were lysed in Triton X sample buffer and separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). Proteins were detected using specific antibodies against β-actin (#A5441, Sigma-Aldrich, St. Louis, MO, USA), Src (#2123, Cell Signaling, Cambridge, UK), phosphorylated Src (Tyr416, #2101, Cell Signaling), Akt (#9272, Cell Signaling), and phosphorylated Akt (Thr308, #2965, Cell Signaling and Ser473, #04-736, EMD-Millipore, Billerica, MA, USA). Protein bands were visualized by enhanced chemiluminescence using a commercially available kit (GE Healthcare, Little Chalfont, UK). Pixel density of western blots is given in percentage compared to untreated cell line (100 %). All experiments were performed at least in triplicate.
Wound healing migration assay
RT112 or RT112rGEMCI20 cells were plated onto six-well plates and allowed to form a confluent monolayer. The cell monolayer was then scratched in a straight line to make a ‘scratch wound’ with a 0.2 ml pipette tip and the cell debris was removed by washing the cells with phosphate-buffered saline. Images of the closure of the scratch were captured at 0, 6, and 24 h. Quantification of wound repair was obtained from six measurements of every treatment from three independent experiments.
Ethics statement
All animal procedures were performed according to the guidelines of the Canadian Council on Animal Care and approved by the Institutional Review Board of the University of British Columbia (approval #A10-0295).
Intramuscular orthotopic xenograft murine model
Animal experiments were performed as described previously [15, 18]. Mice were subdivided into four treatment arms: RT112luc control treatment (n = 15), RT112luc dasatinib treatment (n = 15), RT112rGEMCI20luc control treatment (n = 15), and RT112rGEMCI20luc dasatinib treatment (n = 14). Six-week-old male nude mice (Harlan Laboratories, Indianapolis, IN, USA) were anesthetized with isoflurane (2 Vol. %) and analgesia was provided by subcutaneous injection with buprenorphine and meloxicam (Boehringer Ingelheim, Burlington, ON, Canada). After disinfection of the abdominal wall with chlorhexidine, a low transverse laparotomy was made and the urinary bladder was extracorporealized. 50 μl of a cell suspension containing 5 × 105 cells were directly injected into the bladder wall. The incision was closed with suture. Bioluminescence was used to quantify tumor burden and was measured on the xenogen IVIS spectrum imaging system after intraperitoneal injection of 200 μg/kg luciferin (Caliper Lifesciences). Images were taken at 10 and 14 min after luciferin injection and the average counts were used for statistical analysis. Evaluation of bioluminescence in this orthotopic model showed that 10 × 108 photons/s correlate to 10 mm3 tumor volume [19]. Bioluminescence imaging was performed on day 5 after tumor inoculation and mice were divided into equal treatment groups based on tumor burden. Treatment started the following day and imaging was repeated every 4 days. Necropsy was performed after 4 weeks. Dasatinib was administered by oral gavage with an in vivo relevant dose of 20 mg/kg body weight (BW) twice daily [20] in sodium citrate/citric acid (Sigma-Aldrich, St. Louis, MO, USA). Vehicle treatment was prepared and administered in an identical manner, but without dasatinib. All experiments were performed at least in triplicate.
Haematoxylin and eosin staining (H&E)
All samples were fixed in buffered 4 % formalin (pH7.4) and embedded in paraffin. We used standard procedures for deparaffinization and rehydration. Slides were cut at a microtome into 3 μm slices. H&E staining were performed according to standard.
Statistical analysis
Results are expressed as mean ± standard deviation (SD) of at least three independent experiments. For statistical analysis student´s t test, analysis of variance (ANOVA), and Student–Newman–Keuls-Test were performed whenever applicable. Significance was defined at values of p ≤ 0.05.
Results
Growth characteristics and sensitivity to gemcitabine of RT112 and RT112rGEMCI20 cells
RT112 and RT112rGEMCI20 cells showed similar growth kinetics with doubling times of 23.28 ± 2.88 h in RT112 cells and 25.2 ± 3.12 h in RT112rGEMCI20 cells. Transduction with the luciferase plasmid, which is needed to monitor in vivo tumor growth, did not alter cell growth kinetics (doubling time RT112luc: 24.72 ± 5.04 h; doubling time RT112rGEMCI20luc: 27.36 ± 3.6 h) (Fig. 1a; Table 1). RT112rGEMCI20 cells (IC50 = 125.40 ± 29.78 ng/ml) displayed a 77-fold increased resistance to gemcitabine compared to parental RT112 cells (IC50 = 1.63 ± 0.55 ng/ml). There was a similar sensitivity to gemcitabine in the luciferase-transduced cell lines RT112luc and RT112rGEMCI20luc compared to RT112 and RT112rGEMCI20 [RT112luc (IC50: 1.94 ± 0.24) and RT112rGEMCI20luc (IC50: 114.39 ± 14.52)] (Table 1).
Fig. 1.
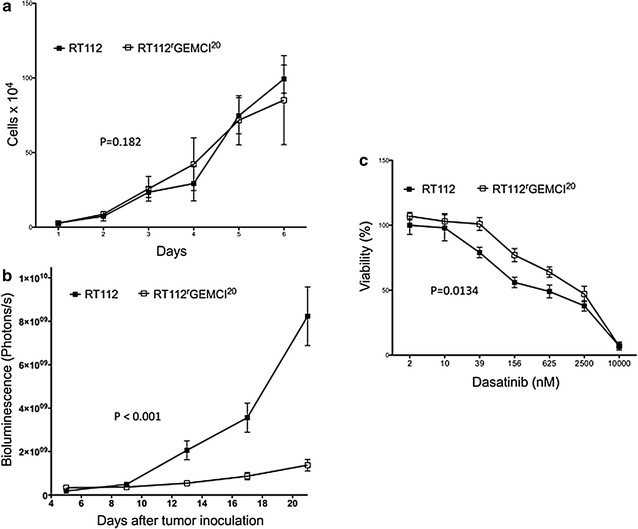
a Growth kinetics of the urothelial cancer cell line RT112 and its gemcitabine-resistant sub-line RT112rGEMCI20 in cell culture. On day 0, both cell lines started with 4000 cells per milliliter. b In vivo growth kinetics of RT112luc and RT112rGEMCI20luc tumors in an orthotopic nu/nu mouse xenograft model as determined by bioluminescence. 10 × 108 photons/s correlate to 10 mm3 tumor volume (21). c Concentration-dependent effects of dasatinib on the viability of the urothelial cancer cell line RT112 and its gemcitabine-resistant sub-line RT112rGEMCI20 as indicated by MTT assay after 120 h of incubation
Table 1.
Comparison of growth kinetics (doubling time) of RT112, RT112rGEMCI20, RT112luc, and RT112rGEMCI20luc cell lines and cell viability (IC50) after treatment with gemcitabine
| Cell line | Doubling time (h) | IC50 (ng/ml) |
|---|---|---|
| RT112 | 23.28 ± 2.88 | 1.63 ± 0.55 |
| RT112rGEMCI20 | 25.2 ± 3.12 | 125.40 ± 29.78 |
| RT112luc | 24.72 ± 5.04 | 1.94 ± 0.24 |
| RT112rGEMCI20luc | 27.36 ± 3.6 | 114.39 ± 14.52 |
Growth characteristics of RT112luc and RT112rGEMCI20luc tumors in vivo
In vivo, growth of RT112luc and RT112rGEMCI20luc tumors was investigated in an established murine orthotopic xenograft model [15, 18, 19, 21]. RT112rGEMCI20luc cells were used for the first time in this orthotopic model. Nude mice received either 5 × 105 RT112luc or RT112rGEMCI20luc cells directly injected into the bladder wall. Tumor take rates were 90 % (27 out of 30 animals) for RT112luc cells and 100 % (30 out of 30 animals) for RT112rGEMCI20luc cells. RT112rGEMCI20luc xenografts grew substantially slower than RT112luc xenografts resulting in a 6-fold smaller average tumor volume at day 25 (p < 0.0001) (Fig. 1b).
In vitro effects of dasatinib on RT112 and RT112rGEMCI20 cells
RT112rGEMCI20 cells (IC50: 1081.1 ± 239.2 nM) showed a 3.1-fold decreased sensitivity to dasatinib compared to parental RT112 cells (IC50: 349.2 ± 67.2 nM). The concentration-dependent drug response from a representative experiment is shown in Fig. 1c.
In cell culture, dasatinib caused in RT112 and in RT112rGEMCI20 cells a dose-dependent reduction of Src phosphorylation. Phosphorylation of Akt (Thr308) was also reduced in both cell lines with a stronger inhibition in the chemo-naive cell line. No inhibition of pAkt (Ser473) was detected (Fig. 2a, b).
Fig. 2.

a Representative western blots showing cellular levels of Src, pSrc, Akt, pAkt, and β-actin in RT112 and RT112rGEMCI20 cells after 24 h incubation with dasatinib. b Pixel density of western blots is given in percentage compared to untreated cell line (100 %). One of three independent experiments is shown here. *p ≤ 0.05 vs untreated cell line. c Wound healing migration assay. Effects of dasatinib (100 nM) treatment on RT112 or RT112rGEMCI20 cells were evaluated. Images of the closure of the scratch were captured at 0, 6, and 24 h. Representative images of scratch wound assays were shown (magnification ×100). d Quantification of wound repair was obtained from six measurements of every treatment from three independent experiments
Since Src inhibition was described to increase migration in urothelial cancer [12], we evaluated migratory behavior using a wound healing scratch assay in RT112 and RT112rGEMCI20 cells with and without dasatinib treatment. Results indicated a similar time-dependent migration inhibition in response to dasatinib in RT112 and RT112rGEMCI20 cells resulting in >60 % inhibition of migration in both cell lines relative to untreated control (p < 0.0001) (Fig. 2c, d).
Effects of dasatinib treatment in RT112luc and RT112rGEMCI20luc xenografts
Dasatinib treatment using 20 mg/kg twice daily [20] caused a reduction of RT112luc xenograft size by 39 % (p = 0.036) relative to untreated control at day 25. In contrast, dasatinib treatment induced a 4-fold increase in RT112rGEMCI20luc tumor size compared to vehicle treated RT112rGEMCI20luc xenografts at day 25 (p < 0.001) (Fig. 3).
Fig. 3.
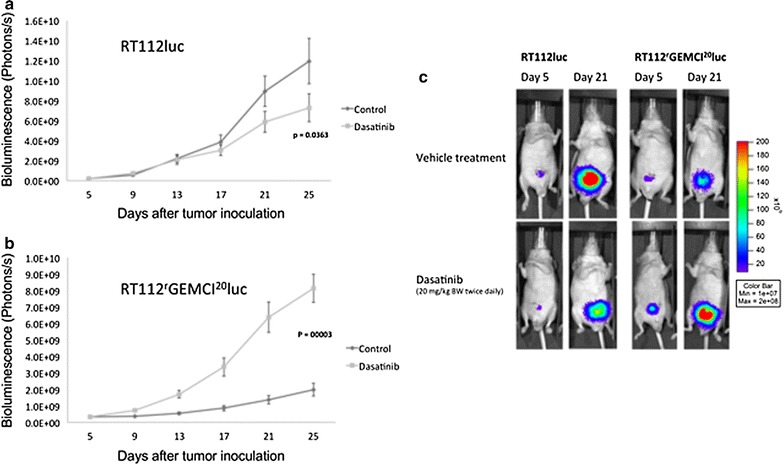
Tumor growth kinetics of murine orthotopic a RT112luc and b RT112rGEMCI20luc xenografts. Dasatinib was given per oral gavage at a dose of 20 mg/kg body weight (BW) twice daily. Control animals were vehicle-treated in the same way. 10 × 108 photons/s correlate to 10 mm3 tumor volume (21). c Representative photographs of treated and untreated RT112luc and RT112rGEMCI20luc xenografts with fluorescent bladder tumors
Tumors were sampled and H&E staining was performed at day 25. Untreated RT112luc tumors displayed muscle-invasive growth (pT2) (Fig. 4a). In contrast, no signs of muscle-invasive growth (pT1) were detected in dasatinib-treated RT112luc tumors (Fig. 4b). The opposite pattern was observed in RT112rGEMCI20luc tumors. Untreated RT112rGEMCI20luc tumors did not show muscle-invasive growth (pT1) (Fig. 4c), while dasatinib-treated RT112rGEMCI20luc xenografts displayed muscle invasive growth (pT2) (p < 0.001). In dasatinib-treated RT112rGEMCI20luc xenografts, the bladder muscle was completely infiltrated by the tumor, and the bladder wall was not clearly visible anymore (Fig. 4d).
Fig. 4.
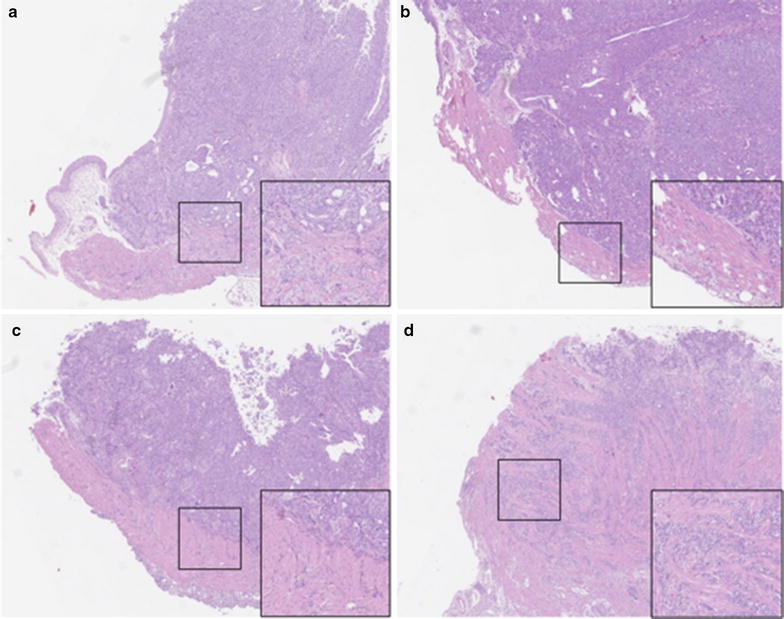
H&E staining of four representative tumors (magnification ×5). a RT112luc xenograft from a vehicle-treated mouse. b RT112luc xenograft from a dasatinib (20 mg/kg BW twice daily)-treated mouse. c RT112rGEMCI20luc xenograft from a vehicle-treated mouse. d RT112rGEMCI20luc xenograft from a dasatinib (20 mg/kg BW twice daily)-treated mouse
Discussion
In this study, we compared the effects of the Src inhibitor dasatinib on the urothelial cancer cell line RT112 and its gemcitabine-resistant sub-line RT112rGEMCI20 in cell culture and in an orthotopic bladder cancer xenograft model in mice.
In cell culture, both cell lines displayed similar growth kinetics. Dasatinib inhibited Src phosphorylation in RT112 and RT112rGEMCI20 cells at low nanomolar concentrations similar to those that had already been described to affect Src phosphorylation [9]. While dasatinib had previously been shown to interfere with the phosphorylation of Akt (Thr308 and Ser473) in squamous cell lung cancer [22], we only detected inhibition of phosphorylation of Akt (Thr308). The reasons for this may be the consequence of cell type-specific differences between the investigated models. Although dasatinib exerted similar effects on Src signaling in RT112 and RT112rGEMCI20 cells, its effects on cell viability differed between the two cell lines. The effective concentrations of dasatinib in RT112 cells (IC50 of 349.2 ± 67.2 nM) were in the range of those previously described for the treatment of urothelial cancer cells [9]. However, in RT112rGEMCI20 cells the IC50 value was about 3-fold higher (1081.1 ± 239.2 nM). This suggests that RT112rGEMCI20 cells have acquired resistance mechanisms that interfere with antitumoral effects of dasatinib downstream of Src signaling. In accordance, dasatinib treatment resulted in reduced inhibition of the phosphorylation of the Src downstream kinase Akt (Thr 308) in RT112rGEMCI20 cells compared to RT112 cells.
In nude mice, RT112rGEMCI20luc cells formed about 6-fold smaller tumors than RT112luc cells. The reasons for this remain unclear. However, it is known that different cell lines may differ in their interaction with the tissue environment in animal models resulting in discrepancies in the growth kinetics [23, 24].
The most striking differences were found after oral dasatinib treatment of mice bearing orthotopic RT112luc or RT112rGEMCI20luc xenografts. Dasatinib treatment of RT112luc bladder tumors resulted in a significant reduction of tumor size relative to untreated control. In contrast, dasatinib treatment of RT112rGEMCI20luc bladder tumors resulted in a dramatic increase of tumor growth. In addition, the dasatinib treatment prevented muscle-invasive growth of RT112luc xenografts, but strongly induced muscle-invasive growth of RT112rGEMCI20luc xenografts. The mechanistic reasons underlying this discrepancy remain elusive. Wu et al. [11] had reported that loss of Src increases metastasis formation in a bladder cancer model. Similar to this report, Thomas et al. [12] showed that Src enhances urothelial cancer cell migration and metastasis formation. However, dasatinib inhibited RT112 and RT112rGEMCI20 cell migration in a similar manner, similarly to other studies that reported on the effects of Src inhibition on cancer cell migration in models from different cancer entities [25–28]. Therefore, it seems unlikely that the dasatinib-induced increased invasive growth of RT112rGEMCI20luc cells in the xenograft model may be caused by dasatinib-induced enhanced RT112rGEMCI20luc cell migration.
Conclusions
We present the first study that investigated the effects of dasatinib on urothelial cancer cells with acquired resistance to gemcitabine. In cell culture, gemcitabine-resistant RT112 cells were less sensitive to dasatinib than parental RT112 cells. Notably, parental RT112 cells and gemcitabine-resistant RT112 cells displayed an unexpected opposite response to dasatinib in an orthotopic xenograft model in mice. While dasatinib inhibited tumor growth and muscle invasion by parental RT112 cells, it increased tumor growth and muscle invasion by gemcitabine-resistant RT112 cells. Thus, our data do not generally support the use of dasatinib for the treatment of urothelial cancer, in particular not for therapy-refractory cases after first-line chemotherapy with gemcitabine. However, further studies will need to show whether similar effects are obtained in additional models of (acquired gemcitabine resistance in) urothelial cancer. If such studies suggested that some urothelial cancer diseases may benefit from dasatinib therapy, biomarkers would need to be identified that enable the prediction of the response of individual urothelial cancer diseases to dasatinib.
Authors’ contributions
SV, KG: data collection, project development, data analysis, manuscript writing. MM, PB, JC: project development, data analysis, manuscript writing. FR: data collection, data analysis. HK: data collection, manuscript writing. RB, MB, FW: data analysis, manuscript writing. AH: data analysis, project development. All authors read and approved the final manuscript.
Acknowledgements
The work was supported by the charity Hilfe für krebskranke Kinder Frankfurt e.V., its trust Frankfurter Stiftung für krebskranke Kinder, the Patenschaftsmodell program of the University Hospital Frankfurt and the Kent Cancer Trust.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
All data and further information about “Methods” section are available via the corresponding author (Cinatl@em.uni-frankfurt.de).
Ethics
All animal procedures were performed according to the guidelines of the Canadian Council on Animal Care and approved by the Institutional Review Board of the University of British Columbia (approval #A10-0295).
Abbreviations
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- IC50
half maximal inhibitory concentration
- GC
gemcitabine and cisplatin
- DT
doubling time
- H&E
haematoxylin and eosin staining
- SD
standard deviation
- ANOVA
analysis of variance
Contributor Information
Stefan Vallo, Email: Stefan.Vallo@kgu.de.
Martin Michaelis, Email: M.Michaelis@kent.ac.uk.
Kilian M. Gust, Email: Kilian.Gust@kgu.de
Peter C. Black, Email: PBlack@mail.ubc.ca
Florian Rothweiler, Email: F.Rothweiler@kinderkrebsstiftung-frankfurt.de.
Hans-Michael Kvasnicka, Email: Hans-Michael.Kvasnicka@kgu.de.
Roman A. Blaheta, Email: Blaheta@em.uni-frankfurt.de
Maximilian P. Brandt, Email: Maximilian.Brandt@kgu.de
Felix Wezel, Email: Felix.Wezel@uniklinik-ulm.de.
Axel Haferkamp, Email: Axel.Haferkamp@kgu.de.
Jindrich Cinatl, Jr., Phone: +49 69 6301 6409, Email: Cinatl@em.uni-frankfurt.de
References
- 1.Pectasides D, Pectasides M, Economopoulos T. Systemic chemotherapy in locally advanced and/or metastatic bladder cancer. Cancer Treat Rev. 2006;32(6):456–470. doi: 10.1016/j.ctrv.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 2.von der Maase H, Sengelov L, Roberts JT, Ricci S, Dogliotti L, Oliver T, Moore MJ, Zimmermann A, Arning M. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol. 2005;23(21):4602–4608. doi: 10.1200/JCO.2005.07.757. [DOI] [PubMed] [Google Scholar]
- 3.Sonpavde G, Galsky MD, Hutson TE. Current optimal chemotherapy for advanced urothelial cancer. Expert Rev Anticancer Ther. 2008;8(1):51–61. doi: 10.1586/14737140.8.1.51. [DOI] [PubMed] [Google Scholar]
- 4.Choueiri TK, Ross RW, Jacobus S, Vaishampayan U, Yu EY, Quinn DI, Hahn NM, Hutson TE, Sonpavde G, Morrissey SC, et al. Double-blind, randomized trial of docetaxel plus vandetanib versus docetaxel plus placebo in platinum-pretreated metastatic urothelial cancer. J Clin Oncol. 2012;30(5):507–512. doi: 10.1200/JCO.2011.37.7002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Irby RB, Yeatman TJ. Role of Src expression and activation in human cancer. Oncogene. 2000;19(49):5636–5642. doi: 10.1038/sj.onc.1203912. [DOI] [PubMed] [Google Scholar]
- 6.Boyer B, Bourgeois Y, Poupon MF. Src kinase contributes to the metastatic spread of carcinoma cells. Oncogene. 2002;21(15):2347–2356. doi: 10.1038/sj.onc.1205298. [DOI] [PubMed] [Google Scholar]
- 7.Yu EY, Duan F, Muzi M, Deng X, Chin BB, Alumkal JJ, Taplin ME, Taub JM, Herman B, Higano CS, et al. Castration-resistant prostate cancer bone metastasis response measured by 18F-fluoride PET after treatment with dasatinib and correlation with progression-free survival: results from American College of Radiology Imaging Network 6687. J Nucl Med. 2015;56(3):354–360. doi: 10.2967/jnumed.114.146936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gold KA, Lee JJ, Harun N, Tang X, Price J, Kawedia JD, Tran HT, Erasmus JJ, Blumenschein GR, William WN, et al. A phase I/II study combining erlotinib and dasatinib for non-small cell lung cancer. Oncologist. 2014;19(10):1040–1041. doi: 10.1634/theoncologist.2014-0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levitt JM, Yamashita H, Jian W, Lerner SP, Sonpavde G. Dasatinib is preclinically active against Src-overexpressing human transitional cell carcinoma of the urothelium with activated Src signaling. Mol Cancer Ther. 2010;9(5):1128–1135. doi: 10.1158/1535-7163.MCT-10-0096. [DOI] [PubMed] [Google Scholar]
- 10.Saini S, Arora S, Majid S, Shahryari V, Chen Y, Deng G, Yamamura S, Ueno K, Dahiya R. Curcumin modulates microRNA-203-mediated regulation of the Src-Akt axis in bladder cancer. Cancer Prev Res (Phila) 2011;4(10):1698–1709. doi: 10.1158/1940-6207.CAPR-11-0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu Y, Moissoglu K, Wang H, Wang X, Frierson HF, Schwartz MA, Theodorescu D. Src phosphorylation of RhoGDI2 regulates its metastasis suppressor function. Proc Natl Acad Sci USA. 2009;106(14):5807–5812. doi: 10.1073/pnas.0810094106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomas S, Overdevest JB, Nitz MD, Williams PD, Owens CR, Sanchez-Carbayo M, Frierson HF, Schwartz MA, Theodorescu D. Src and caveolin-1 reciprocally regulate metastasis via a common downstream signaling pathway in bladder cancer. Cancer Res. 2011;71(3):832–841. doi: 10.1158/0008-5472.CAN-10-0730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hahn NM, Knudsen BS, Daneshmand S, Koch MO, Bihrle R, Foster RS, Gardner TA, Cheng L, Liu Z, Breen T, et al. Neoadjuvant dasatinib for muscle-invasive bladder cancer with tissue analysis of biologic activity. Urol Oncol. 2016;34(1):4.e11–7. doi: 10.1016/j.urolonc.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 14.Vallo S, Michaelis M, Rothweiler R, Bartsch G, Gust KM, Limbart DM, Rödel F, Wezel F, Haferkamp A, Cinatl J., Jr Drug-resistant urothelial cancer cell lines display diverse sensitivity profiles to potential second line therapeutics. Transl Oncol. 2015;8(3):210–216. doi: 10.1016/j.tranon.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gust KM, McConkey DJ, Awrey S, Hegarty PK, Qing J, Bondaruk J, Ashkenazi A, Czerniak B, Dinney CP, Black PC. Fibroblast growth factor receptor 3 is a rational therapeutic target in bladder cancer. Mol Cancer Ther. 2013;12(7):1245–1254. doi: 10.1158/1535-7163.MCT-12-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Michaelis M, Rothweiler F, Barth S, Cinatl J, van Rikxoort M, Löschmann N, Voges Y, Breitling R, von Deimling A, Rödel F, et al. Adaptation of cancer cells from different entities to the MDM2 inhibitor nutlin-3 results in the emergence of p53-mutated multi-drug-resistant cancer cells. Cell Death Dis. 2011;2:e243. doi: 10.1038/cddis.2011.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rutigliano L, Corradetti B, Valentini L, Bizzaro D, Meucci A, Cremonesi F, Lange-Consiglio A. Molecular characterization and in vitro differentiation of feline progenitor-like amniotic epithelial cells. Stem Cell Res Ther. 2013;4(5):133. doi: 10.1186/scrt344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Black PC, Shetty A, Brown GA, Esparza-Coss E, Metwalli AR, Agarwal PK, McConkey DJ, Hazle JD, Dinney CP. Validating bladder cancer xenograft bioluminescence with magnetic resonance imaging: the significance of hypoxia and necrosis. BJU Int. 2010;106(11):1799–1804. doi: 10.1111/j.1464-410X.2010.09424.x. [DOI] [PubMed] [Google Scholar]
- 19.Hadaschik BA, Black PC, Sea JC, Metwalli AR, Fazli L, Dinney CP, Gleave ME, So AI. A validated mouse model for orthotopic bladder cancer using transurethral tumour inoculation and bioluminescence imaging. BJU Int. 2007;100(6):1377–1384. doi: 10.1111/j.1464-410X.2007.07165.x. [DOI] [PubMed] [Google Scholar]
- 20.Wie SM, Adwan TS, DeGregori J, Anderson SM, Reyland ME. Inhibiting tyrosine phosphorylation of protein kinase Cδ (PKCδ) protects the salivary gland from radiation damage. J Biol Chem. 2014;289(15):10900–10908. doi: 10.1074/jbc.M114.551366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dinney CP, Fishbeck R, Singh RK, Eve B, Pathak S, Brown N, Xie B, Fan D, Bucana CD, Fidler IJ. Isolation and characterization of metastatic variants from human transitional cell carcinoma passaged by orthotopic implantation in athymic nude mice. J Urol. 1995;154:1532–1538. doi: 10.1016/S0022-5347(01)66923-4. [DOI] [PubMed] [Google Scholar]
- 22.Bai Y, Kim JY, Watters JM, Fang B, Kinose F, Song L, Koomen JM, Teer JK, Fisher K, Chen YA, et al. Adaptive responses to dasatinib-treated lung squamous cell cancer cells harboring DDR2 mutations. Cancer Res. 2014;74(24):7217–7228. doi: 10.1158/0008-5472.CAN-14-0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hurst RE, Hauser PJ, Kyker KD, Heinlen JE, Hodde JP, Hiles MC, Hurst RE, Kosanke SD, Dozmorov M, Ihnat MA. Suppression and activation of the malignant phenotype by extracellular matrix in xenograft models of bladder cancer: a model for tumor cell “dormancy”. PLoS ONE. 2013;8(5):e64181. doi: 10.1371/journal.pone.0064181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chopra A, Murray ME, Byfield FJ, Mendez MG, Halleluyan R, Restle DJ, Raz-Ben Aroush D, Galie PA, Pogoda K, et al. Augmentation of integrin-mediated mechanotransduction by hyaluronic acid. Biomaterials. 2013;35(1):71–82. doi: 10.1016/j.biomaterials.2013.09.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pichot CS, Hartig SM, Xia L, Arvanitis C, Monisvais D, Lee FY, Frost JA, Corey SJ. Dasatinib synergizes with doxorubicin to block growth, migration, and invasion of breast cancer cells. Br J Cancer. 2009;101(1):38–47. doi: 10.1038/sj.bjc.6605101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li YJ, He YF, Han XH, Hu B. Dasatinib suppresses invasion and induces apoptosis in nasopharyngeal carcinoma. Int J Clin Exp Pathol. 2015;8(7):7818–7824. [PMC free article] [PubMed] [Google Scholar]
- 27.McCaig AM, Cosimo E, Leach MT, Michie AM. Dasatinib inhibits CXCR4 signaling in chronic lymphocytic leukaemia cells and impairs migration towards CXCL12. PLoS ONE. 2012;7(11):e48929. doi: 10.1371/journal.pone.0048929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buettner R, Mesa T, Vultur A, Lee F, Jove R. Inhibition of Src family kinases with dasatinib blocks migration and invasion of human melanoma cells. Mol Cancer Res. 2008;6(11):1766–1774. doi: 10.1158/1541-7786.MCR-08-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data and further information about “Methods” section are available via the corresponding author (Cinatl@em.uni-frankfurt.de).


