Abstract
Background: Observational and experimental evidence demonstrates that protein intake in infancy programs linear growth. To our knowledge, few studies have examined prenatal maternal protein intake.
Objective: Our objective was to examine associations of maternal protein intake during pregnancy with offspring linear growth.
Design: We analyzed data from 1961 mother–child pairs in Project Viva. We assessed first- and second-trimester diet with the use of food-frequency questionnaires and analyzed protein intake as grams per kilogram prepregnancy weight per day. We used research measures of offspring length at birth and in infancy (∼6 mo), early childhood (∼3 y), and midchildhood (∼7 y), as well as clinical growth measures obtained from after birth through midchildhood. We calculated sex-specific birth length z scores for gestational age with the use of international reference data. We used mixed models with repeated length measures to predict individual length gain velocities for birth to <6 mo and 6 mo to 7 y of age, then used these velocities as outcomes in adjusted linear regression models with maternal protein intake as the main predictor.
Results: Mean (range) second-trimester protein intake was 1.4 g · kg−1 · d−1 (0.3–3.1 g · kg−1 · d−1). After adjusting for maternal sociodemographics, gestational weight gain, maternal and paternal height, and (for postdelivery outcomes) child sex, gestational age, and breastfeeding duration, each 1-SD (0.36 g · kg−1 · d−1) increment in second-trimester protein intake corresponded to a −0.10 (95% CI: −0.18, −0.03) change in birth length z score, a −0.03 cm/mo (95% CI: −0.05, −0.01 cm/mo) change in slope of length growth from birth to <6mo, and a −0.09 cm/y (95% CI: −0.14, −0.05 cm/y) change in slope of length growth from 6 mo to midchildhood. Results were similar for first-trimester intake.
Conclusions: In a population with relatively high protein intake during pregnancy, higher protein intake was associated with shorter offspring birth length and slower linear growth into midchildhood. Results suggest that higher protein intake during pregnancy does not increase fetal and child growth and may even reduce early length growth. Project Viva was registered at clinicaltrials.gov as NCT02820402.
Keywords: linear growth, pregnancy, protein, cohort, maternal diet, programming
INTRODUCTION
Nutritional exposures during the fetal period and infancy contribute to determining future growth patterns. Protein and dairy milk intake in particular appear to play major roles in programming growth patterns in infancy and childhood, likely via effects on concentrations of insulin-like growth factor I (IGF-I)7 (1, 2). Infants who are fed formula, which contains higher amounts of protein than breast milk, show higher IGF-I concentrations at 3 mo of age and greater gains in body weight, length, and adiposity between 3 and 12 mo of age than do breastfed infants (3). Interestingly, there is a reversal of this association in later childhood, when children breastfed as infants exhibit higher IGF-I concentrations and faster linear growth than those who were formula-fed (2, 4, 5). In a randomized controlled trial of healthy European infants, those receiving higher-protein formula had higher weight-for-length z scores, but similar length, at 24 mo than did infants receiving lower-protein formula or breast milk (6).
Despite evidence that higher protein intake in infancy predicts more rapid early growth, studies of the effects of maternal protein intake during pregnancy on markers of fetal growth and development have been inconclusive. Animal studies of prenatal protein restriction indicate associated reductions in birth weight and persistent indicators of cardiometabolic disorder (7–9), and, in human studies, maternal low-protein diets are associated with a “thin-fat” offspring phenotype characterized by low body weight a but high proportion of adipose tissue (10). Effects of high protein intake are less clear. Higher maternal protein intake was associated with greater birth weight (11–16), head circumference (17), and placental weight (13, 14) in some studies, but not others (18, 19). Furthermore, some observational studies and supplementation trials have shown lower birth weight (17, 20–23) and ponderal index (17) with higher maternal protein intake.
Although linear growth seems to be especially sensitive to variations in protein intake during infancy, few studies have examined birth length specifically as an outcome. One study of 216 mother–infant pairs conducted in 1943 in Boston, Massachusetts, found a dose–response association between maternal protein intake in the second and third trimesters and infant birth length (r = 0.8) (16), and, in a study of pregnant Danish women, greater milk intake was associated with higher birth length (15). However, other studies of maternal milk (12) and protein (17) intake did not show direct associations with birth length. Factors such as maternal BMI, gestational weight gain (GWG), parental height, and sociodemographics may confound the association between maternal diet during pregnancy and birth outcomes and should be accounted for. Finally, studies of fetal programming must be able to determine whether effects persist into later life, and, to our knowledge there have been no studies of maternal protein intake during pregnancy extending to child growth outcomes beyond size at birth.
Our aim was to examine associations of maternal protein intake in the first and second trimesters of pregnancy with early growth in offspring, focusing on linear growth. On the basis of the literature on protein intake in infancy and linear growth, we hypothesized that higher maternal protein intake during pregnancy would be associated with greater fetal growth, more rapid linear growth in infancy and early childhood, and reduced linear growth in midchildhood.
METHODS
Subjects
We studied mother–child pairs enrolled in Project Viva (NCT02820402), a prospective cohort study examining pre- and perinatal factors in relation to pregnancy and child health outcomes. Women were recruited between 1999 and 2002 at their initial obstetric appointment from 8 offices of Atrius Health, a large multispecialty group practice in eastern Massachusetts. Exclusion criteria included multiple gestation, inability to answer questions in English, gestational age >22 wk at the time of the initial obstetric appointment, and plans to move out of the local area before delivery. Women who agreed to participate (65% of those eligible) and provided informed consent completed the first study visit after their obstetric appointment. They ranged from 4.8 to 23.7 wk gestation (mean 10.5 wk) at this first visit. The second study visit was completed at 26–28 wk for most women. Project Viva participants delivered at 1 of 2 hospitals in Boston, Massachusetts, and the third study visit was completed at the hospital 1–3 d after delivery. Detailed recruitment and retention procedures have been described previously (24).
The Project Viva cohort consisted of the 2128 women who delivered a live infant and their children. For this analysis, we included 1961 mother–child pairs who completed any 1 of the 4 postnatal in-person visits: delivery, infancy (4.9–10.6 mo; median 6.3 mo), early childhood (2.8–6.3 y; median 3.2 y) or midchildhood (6.6–10.9 y; median 7.7 y). The institutional review boards of participating institutions approved the study protocols, and participants provided written informed consent.
Measurements
Exposure: protein intake
We derived data on maternal protein intake (total and from plant and animal sources) from self-administered semiquantitative food-frequency questionnaires (FFQs) that mothers completed during the first and second study visits. The 166-item FFQ used in Project Viva was validated in other cohorts, including the Nurses’ Health Study (25), and modified for use in pregnancy (26). The reference time period for the first FFQ, administered at enrollment, was the time since the last menstrual period (LMP). The reference period for the second FFQ, administered at 26–28 wk gestation, was the previous 3 mo (roughly corresponding to the second trimester). We calculated protein intake with the Harvard nutrient composition database, which includes food composition values from the USDA and is supplemented by other sources, by multiplying a weight assigned to the reported frequency of intake of each protein-containing food by the protein content for the specified portion size (27). To address the potential measurement error often attributed to use of FFQs in assessing dietary intake, we adjusted individual nutrient estimates for total energy intake with the use of the nutrient residual method (28, 29). Adjustment for total energy intake has been shown to reduce the impact of measurement error inherent in use of FFQs for protein intake specifically (30).
The Estimated Average Requirement (EAR), or median nutrient requirement for a given life stage and sex group, may be used to assess the adequacy of an individual’s usual intake (31, 32). The EAR for protein intake in the second half of pregnancy is 67 g/d. This is based on an individual recommended intake of 0.88 g · kg−1 · d−1, calculated with the use of prepregnancy weight. The EAR for the first half of pregnancy is 38 g/d or 0.66 g · kg−1 · d−1, the same as that for nonpregnant women (33). Because protein recommendations for pregnancy are based on individual prepregnancy weight, we calculated first- and second-trimester protein intake per kilogram of prepregnancy body weight with the use of energy-adjusted protein intake and self-reported prepregnancy weights, which previously have been validated in this cohort (34). Further discussion of maternal protein intake will refer to this weight-adjusted variable. We ranked these values into quartiles separately for each trimester.
Outcome: newborn and child length and height
Research assistants (RAs) visited mother–infant pairs after delivery at the 2 primary delivery hospitals. RAs conducted postdelivery visits (n = 1205) during business hours Monday through Friday. We did not take any research measurements on babies who were not available during this time frame (including those who were in the neonatal intensive care unit), and these babies were not included in the analyses of birth length or weight. We did include these children in the growth trajectory analysis if they completed the infancy, early childhood, or midchildhood research visit. Subsequent follow-up visits were conducted in a study visit room or in another convenient location, generally the participant’s home. Trained RAs measured recumbent length at the postdelivery and infancy visits and standing height in early and midchildhood with the use of a stadiometer (Shorr Board; Weight and Measure). We also obtained clinical data on child length and height throughout the follow-up period from medical records for all participants who provided consent to access their medical records and whose pediatrician responded to our request. Both research and clinical length and height measurements had a corresponding date of measurement, which we used to calculate the child’s exact age in months at each measurement. We obtained data on birth weight for the entire cohort from hospital medical records and calculated sex-specific birth weight and length z scores by gestational age with the use of international reference data from the International Fetal and Newborn Growth Consortium for the 21st Century project (35).
The use of z scores for birth weight and length removed the contribution of gestational age. This isolated the contribution of fetal growth, our outcome of interest, to birth weight and length. We performed analyses with the use of raw birth weights and lengths, as well as z scores, as outcomes. Results were very similar, and we present only the z score results.
Covariates
Mothers completed questionnaires and interviews at the first and second prenatal visits. We obtained data through self-report on maternal age, education, race/ethnicity, parity, height, prepregnancy weight and smoking, household income, paternal height and weight, and child sex. GWG was calculated by subtracting self-reported prepregnancy weight from the last clinical weight measured within 4 wk before delivery. The child’s gestational age at delivery was derived from the delivery date and date of LMP, either reported by the mother or from a second-trimester ultrasound if the dating differed from the LMP by >10 d. Mothers self-reported breastfeeding status when the child was 6 mo old. This was categorized as breastfeeding only (feeding the baby breast milk and no infant formula), formula only (had never fed the baby breast milk), mixed (had fed the baby breast milk and was also feeding infant formula at 6 mo of age), or weaned (had fed the baby at least some breast milk but stopped by 6 mo of age).
Statistical analysis
We examined protein intake in the first and second trimesters separately for all analyses. Results of all analyses were similar for first- and second-trimester protein intake. Because fetal growth occurs primarily during the second and third trimesters, we present results of analyses while considering second-trimester protein intake as the primary exposure and include results for first-trimester intake in Supplemental Tables 1–3. We categorized protein in quartiles, and because associations were reasonably incremental across quartiles, we also examined protein intake as a continuous exposure and modeled the effect per 1-SD increment (0.36 g · kg−1 · d−1 for second-trimester intake).
We used multiple imputation methods to impute missing data. We generated 50 imputed data sets with the use of chained imputation (36), and combined estimates with the use of Rubin’s rules (37). We present results from the imputed analysis throughout the manuscript unless otherwise indicated. All 2128 participants were used in generating the imputed dataset, but only the 1961 participants who were eligible for a birth length measurement on their newborn (were visited by an RA and had ≥1 measurement taken at delivery; n = 1205) and/or completed an in-person visit with their child in infancy (n = 1697), early childhood (n = 1294), or midchildhood (n = 1116) were included in the analysis. For each analysis, we used the sample size corresponding to participants who were eligible for that outcome measurement; for analysis of birth weight z score, we further restricted the sample to participants with a birth length measurement to allow us to adjust the final model for birth length z score. We performed all analyses with both original and imputed data and results were very similar.
We examined parental and child characteristics for bivariate associations with second-trimester protein intake (as a continuous variable), and birth length and weight z scores. Next, we examined differences in length at birth and infancy (6 mo) and height in early and midchildhood across quartiles of protein intake. We used multivariable linear regression models to examine associations of protein intake (total and from animal and plant sources separately) with birth length z score. To examine whether previously reported associations between maternal protein intake and infant birth weight might be explained at least in part by effects on birth length, we also examined associations of protein intake with birth weight z score, with and without adjustment for birth length, with the use of multivariable linear regression.
We looked at the effect of maternal protein intake on growth trajectories over time with the use of a 2-step approach. First, we used mixed models to determine rate of growth over time for each individual. To obtain the best estimate of individual growth trajectories over time, we included length and height measurements obtained from research visits as well as clinical visits in these mixed models (no imputed data). This allowed us to use all available measurements for each child. In a previous measurement validation study in children aged 0 to <24 mo, we found that clinical staff systematically overestimated children’s length with a paper and pencil method compared with a length board. Thus, we used a regression correction factor to adjust for the overestimation [(clinical length in centimeters × 0.953) + 1.88 cm] (38). Each individual’s rate of growth was estimated with his or her predicted random effect or empirical Bayes estimate, if he or she had ≥2 length and height measurements. We then used linear regression to model these rates as a function of maternal protein intake, with adjustment for covariates (including imputed data). Because the slope of growth was steeper between birth and 6 mo, we performed this 2-step approach for length measurements from birth up to just <6 mo only (age 0–5.98 mo and mean age 2.1 mo; n = 1537) and then separately from 6 mo to midchildhood (age 6 mo to 10.9 y and mean age 3.0 y; n = 1,537).
We examined early growth at different time points and by using slightly different methods for quantifying the exposure and outcomes, but we did not adjust our results for multiple comparisons. We used hypothesis-driven models to look for trends and consistency of results across methods, and we interpreted all results in the context of our prespecified hypothesis. All analyses were performed with the use of SAS software, version 9.3.
RESULTS
Maternal education, race/ethnicity, household income at enrollment, smoking history, height, parity, prepregnancy BMI and GWG, and paternal height all were associated with second-trimester protein intake, birth length z score, and birth weight z score. Maternal age at enrollment was associated with birth length and weight z scores but not with protein intake (Table 1).
TABLE 1.
Association of characteristics of Project Viva participants with second-trimester protein intake and birth weight z score (N=1961) and birth length z score (N=1205)1
| Second-trimester protein intake, g · kg prepregnancy body weight−1 · d−1 |
Birth weight z score2 |
Birth length z score2 |
|||
| % | Mean ± SD | Mean ± SD | % | Mean ± SD | |
| Overall | 100 | 1.4 ± 0.4 | 0.55 ± 1.03 | 100 | 0.25 ± 1.12 |
| Maternal characteristics | |||||
| Education | |||||
| ≤High school | 11 | 1.2 ± 0.4 | 0.29 ± 1.04 | 11 | −0.00 ± 1.21 |
| Some college | 23 | 1.3 ± 0.4 | 0.50 ± 1.03 | 22 | 0.13 ± 1.15 |
| 4-y college | 36 | 1.4 ± 0.4 | 0.66 ± 1.04 | 36 | 0.33 ± 1.21 |
| Graduate school | 30 | 1.4 ± 0.3 | 0.56 ± 1.00 | 31 | 0.33 ± 1.19 |
| P3 | <0.0001 | <0.0001 | <0.01 | ||
| Race/ethnicity | |||||
| Asian | 6 | 1.6 ± 0.4 | 0.12 ± 1.01 | 5 | 0.16 ± 1.25 |
| Black | 16 | 1.2 ± 0.4 | 0.30 ± 1.05 | 17 | −0.04 ± 1.16 |
| Hispanic | 7 | 1.3 ± 0.4 | 0.42 ± 1.05 | 6 | 0.12 ± 1.26 |
| Other | 4 | 1.2 ± 0.4 | 0.49 ± 1.14 | 4 | 0.09 ± 1.30 |
| White | 67 | 1.4 ± 0.3 | 0.66 ± 1.00 | 69 | 0.35 ± 1.21 |
| P3 | <0.0001 | <0.0001 | <0.001 | ||
| Household income at enrollment | |||||
| ≤$20,000 | 5 | 1.2 ± 0.5 | 0.15 ± 1.15 | 5 | −0.17 ± 1.33 |
| $20,001–$40,000 | 12 | 1.3 ± 0.4 | 0.44 ± 1.18 | 12 | 0.13 ± 1.27 |
| $40,001–$70,000 | 24 | 1.3 ± 0.4 | 0.54 ± 1.04 | 24 | 0.26 ± 1.18 |
| >$70,000 | 59 | 1.4 ± 0.3 | 0.61 ± 1.03 | 58 | 0.31 ± 1.25 |
| P3 | <0.0001 | <0.001 | 0.02 | ||
| Smoking history | |||||
| Never | 69 | 1.4 ± 0.4 | 0.55 ± 1.02 | 69 | 0.24 ± 1.21 |
| Before pregnancy | 19 | 1.4 ± 0.3 | 0.65 ± 1.00 | 19 | 0.45 ± 1.18 |
| During pregnancy | 12 | 1.3 ± 0.4 | 0.40 ± 1.14 | 12 | 0.03 ± 1.15 |
| P3 | <0.001 | 0.01 | <0.01 | ||
| Age at enrollment, y | |||||
| <20 | 3 | 1.3 ± 0.4 | −0.06 ± 0.95 | 4 | −0.41 ± 1.03 |
| 20 to <30 | 27 | 1.3 ± 0.4 | 0.36 ± 1.04 | 26 | 0.16 ± 1.16 |
| 30 to <40 | 66 | 1.4 ± 0.4 | 0.66 ± 1.01 | 66 | 0.33 ± 1.23 |
| ≥40 | 4 | 1.4 ± 0.3 | 0.54 ± 0.94 | 4 | 0.13 ± 1.22 |
| P3 | 0.11 | <0.001 | <0.001 | ||
| Height, m | |||||
| ≤1.57 | 10 | 1.5 ± 0.4 | 0.15 ± 1.04 | 10 | −0.21 ± 0.11 |
| >1.57–1.68 | 61 | 1.4 ± 0.4 | 0.49 ± 1.01 | 60 | 0.20 ± 1.19 |
| >1.68 | 29 | 1.2 ± 0.3 | 0.82 ± 1.00 | 30 | 0.51 ± 1.18 |
| P3 | <0.0001 | <0.0001 | <0.0001 | ||
| Parity | |||||
| 0 | 48 | 1.4 ± 0.4 | 0.35 ± 1.00 | 48 | 0.12 ± 1.23 |
| ≥1 | 52 | 1.3 ± 0.4 | 0.73 ± 1.02 | 52 | 0.37 ± 1.20 |
| P3 | <0.001 | <0.0001 | <0.001 | ||
| Prepregnancy BMI, kg/m2 | |||||
| <18.5 | 3 | 1.8 ± 0.4 | 0.12 ± 0.88 | 3 | 0.18 ± 1.10 |
| 18.5–24.9 | 59 | 1.5 ± 0.3 | 0.46 ± 1.01 | 59 | 0.19 ± 1.19 |
| 25.0–29.9 | 22 | 1.2 ± 0.3 | 0.78 ± 1.02 | 22 | 0.46 ± 1.24 |
| ≥30 | 16 | 0.9 ± 0.3 | 0.67 ± 1.07 | 16 | 0.21 ± 1.19 |
| P3 | <0.0001 | <0.0001 | 0.01 | ||
| Gestational weight gain | |||||
| Inadequate | 13 | 1.4 ± 0.4 | 0.18 ± 1.04 | 12 | −0.02 ± 1.19 |
| Adequate | 29 | 1.4 ± 0.4 | 0.34 ± 0.98 | 29 | 0.16 ± 1.15 |
| Excessive | 58 | 1.3 ± 0.4 | 0.74 ± 1.01 | 59 | 0.35 ± 1.25 |
| P3 | <0.0001 | <0.0001 | <0.001 | ||
| Other characteristics | |||||
| Child sex | |||||
| F | 49 | 1.4 ± 0.4 | 0.51 ± 1.02 | 49 | 0.25 ± 1.22 |
| M | 51 | 1.3 ± 0.4 | 0.59 ± 1.03 | 51 | 0.25 ± 1.20 |
| P3 | 0.16 | 0.07 | 0.92 | ||
| Gestational age at birth, wk | |||||
| <37 | 7 | 1.4 ± 0.4 | 0.10 ± 1.06 | 4 | 0.05 ± 1.29 |
| 37–42 | 92 | 1.4 ± 0.4 | 0.58 ± 1.02 | 94 | 0.26 ± 1.22 |
| >42–43 | 1 | 1.4 ± 0.4 | 0.43 ± 1.15 | 2 | −0.09 ± 1.01 |
| P3 | 0.9 | <0.001 | 0.22 | ||
| Breastfeeding status at 6 mo | |||||
| Formula only | 12 | 1.2 ± 0.4 | 0.58 ± 1.16 | 12 | 0.30 ± 1.28 |
| Weaned | 39 | 1.3 ± 0.4 | 0.49 ± 1.10 | 38 | 0.19 ± 1.27 |
| Mixed | 24 | 1.4 ± 0.4 | 0.56 ± 1.05 | 25 | 0.28 ± 1.30 |
| Breastfeeding only | 25 | 1.4 ± 0.4 | 0.63 ± 1.01 | 26 | 0.29 ± 1.16 |
| P3 | <0.0001 | 0.14 | 0.58 | ||
| Paternal height, m | |||||
| ≤1.68 | 10 | 1.3 ± 0.4 | 0.26 ± 1.00 | 10 | 0.01 ± 1.23 |
| >1.68–1.78 | 40 | 1.4 ± 0.4 | 0.49 ± 1.05 | 40 | 0.11 ± 1.28 |
| >1.78 | 50 | 1.4 ± 0.4 | 0.65 ± 1.01 | 50 | 0.40 ± 1.15 |
| P3 | 0.02 | <0.0001 | <0.0001 | ||
Includes all mother–child pairs who completed any 1 of the 4 in-person visits from delivery to midchildhood (protein intake and birth weight z score) or who had ≥1 measurement taken on the newborn at delivery (birth length z score).
Based on International Fetal and Newborn Growth Consortium for the 21st Century growth standards; sex-specific by gestational age.
Global (type 3) P value testing for difference in outcome across categories of maternal and other characteristics.
Of 1961 Project Viva participants included in our analytic sample, estimated usual protein intake was at or above the weight-based EAR for 98% of women in the first trimester and 91% in the second trimester. This indicated that only a small percentage of our sample (2% in the first trimester and 9% in the second trimester) likely did not have a sufficient intake of protein (31).
The mean and range of total protein intake by quartile are shown in Supplemental Table 1 (first trimester) and Table 2 (second trimester). First- and second-trimester protein intake was highly correlated (Spearman r = 0.79, P < 0.0001); 40% of women were in the first or second quartile of intake in both trimesters, and 41% were in the third or fourth quartile in both trimesters. Mean (range) intake of animal protein was 60.2 g/d (3.5–124.7 g/d) and 0.93 g · kg−1 · d−1 (0.04–2.56 g · kg−1 · d−1) in the first trimester and 61.5 g/d (1.78–137.0 g/d) and 0.95 g · kg−1 · d−1 (0.02–2.60 g · kg−1 · d−1) in the second. Mean (range) intake of plant protein was 26.8 g/d (9.11–77.5 g/d) and 0.42 g · kg−1 · d−1 (0.06–1.62 g · kg−1 · d−1) in the first trimester and 26.1 g/d (7.95–66.9 g/d) and 0.41 g · kg−1 · d−1 (0.07–1.22 g · kg−1 · d−1) in the second.
TABLE 2.
Length at birth and in infancy and height in early childhood and midchildhood by quartile of second-trimester maternal protein intake1
| Quartile of second-trimester protein intake, g · kg prepregnancy body weight−1 · d−1 |
|||||||
| n | All | 1 | 2 | 3 | 4 | P2 | |
| Protein intake | 19613 | ||||||
| g · kg prepregnancy body weight− · d− | 1.4 (0.3–3.1) | 0.9 (0.3–1.1) | 1.2 (1.1–1.3) | 1.5 (1.3–1.6) | 1.8 (1.6–3.1) | ||
| g/d | 88 (42–153) | 77 (42–131) | 84 (52–131) | 89 (58–132) | 100 (67–153) | ||
| Birth length, cm | 12054 | 49.8 | 49.9 | 49.8 | 49.7 | 49.5 | <0.05 |
| Infant (6-mo) length, cm | 16975 | 66.8 | 67.2 | 67.0 | 66.6 | 66.5 | 0.0001 |
| Early childhood height, cm | 12946 | 97.6 | 98.9 | 97.9 | 96.7 | 96.9 | <0.0001 |
| Midchildhood height, cm | 11167 | 128.8 | 130.7 | 129.7 | 127.2 | 127.5 | <0.0001 |
Values are means or means (ranges).
From a test for linear trend.
Includes all participants eligible for a length measurement at any time point.
Includes all participants eligible for a newborn length measurement (had either newborn anthropometric measurements, length, or blood pressure).
Includes all participants eligible for an infant length measurement (completed an in-person visit in infancy).
Includes all participants eligible for an early childhood height measurement (completed an in-person visit in early childhood).
Includes all participants eligible for a midchildhood height measurement (completed an in-person visit in midchildhood).
In bivariate analyses (Table 2), we observed an inverse relation of protein intake with length at birth and at later time points through midchildhood. For example, mothers in the top quartile of protein intake had babies who were 0.4 cm shorter at birth than mothers in the bottom quartile of protein intake. In addition, children of mothers who were in the top 2 quartiles of protein intake were shorter at the infancy, early childhood, and midchildhood visits than were children of mothers who were in the bottom 2 quartiles.
In multivariable regression analyses (Table 3), we observed a negative association between protein intake in the second trimester and birth length z score. In unadjusted analysis, each 1-SD (0.36 g · kg−1 · d−1) increment in maternal protein intake during the second trimester corresponded to a −0.10 (95% CI: −0.17, −0.03) change in birth length z score. This association strengthened after we adjusted in addition for maternal education, age at enrollment, race/ethnicity, and household income (−0.15; 95% CI: −0.22, −0.08) but attenuated back to the effect estimate observed in the first model after further adjustment for GWG and maternal and paternal height (−0.10; 95% CI: −0.18, −0.03). Further adjustment for maternal smoking and parity did not change the effect estimates, and we did not retain these variables in the final model. Results of analyses comparing quartiles of protein intake were similar: in the fully adjusted model, being in the top quartile of second-trimester maternal protein intake was associated with a change of −0.23 (95% CI: −0.43, −0.03) in birth length z score compared with the bottom quartile. Results were very similar for first-trimester protein intake (Supplemental Table 2).
TABLE 3.
Association of second-trimester maternal protein intake, presented as a continuous variable and by quartile, with birth length z score1
| Birth length z score2 (n = 1205) |
|||||
| Second-trimester maternal protein intake | Mean birth length, cm | Mean birth length z score | Model 13 | Model 24 | Model 35 |
| Continuous 1-SD (0.36 g · kg prepregnancy body weight− · d−) increment | −0.10 (−0.17, −0.03) | −0.15 (−0.22, −0.08) | −0.10 (−0.18, −0.03) | ||
| Quartile (median intake, g · kg prepregnancy body weight− · d−) | |||||
| 1 (0.94) | 49.9 | 0.35 | Ref | Ref | Ref |
| 2 (1.23) | 49.8 | 0.32 | −0.03 (−0.22, 0.17) | −0.10 (−0.29, 0.10) | −0.07 (−0.26, 0.13) |
| 3 (1.46) | 49.7 | 0.20 | −0.15 (−0.35, 0.05) | −0.25 (−0.45, −0.05) | −0.19 (−0.39, 0.01) |
| 4 (1.75) | 49.5 | 0.12 | −0.23 (−0.43, −0.04) | −0.35 (−0.54, −0.15) | −0.23 (−0.43, −0.03) |
Based on International Fetal and Newborn Growth Consortium for the 21st Century growth standards; sex-specific by gestational age. Ref, reference.
Values are β (95% CI), based on multivariable linear regression models. Protein intake is adjusted for total energy intake.
Unadjusted.
Adjusted for maternal education, maternal race/ethnicity, household income at enrollment, and maternal age at enrollment.
Adjusted for covariates in Model 2 plus gestational weight gain and maternal and paternal height.
We examined the intake of protein from animal and plant sources separately; both showed negative associations with birth length z score similar to that of total protein intake. In models fully adjusted for maternal education, maternal race/ethnicity, household income and maternal age at enrollment, GWG, and maternal and paternal height, a 1-SD (0.30 g) increase in second-trimester animal protein intake corresponded to a −0.08 (95% CI: −0.15, −0.01) change in birth length z score, and the effect was similar for a 1-SD (0.13 g) increase in plant protein (−0.08; 95% CI: −0.15, −0.01).
In the model adjusted for maternal education, maternal race/ethnicity, household income, maternal age at enrollment, GWG, and maternal and paternal height, each 1-SD (0.36 g · kg−1 · d−1) increase in second-trimester protein intake corresponded to a −0.12 (95% CI: −0.18, −0.06) change in birth weight z score (Table 4). Further adjustment for birth length z score attenuated the regression coefficient to −0.06 (95% CI: −0.11, −0.01). We saw similar results when protein intake was analyzed in quartiles: birth weight z score decreased with increasing protein intake after we adjusted for all covariates, including birth length, although estimates were not statistically significant after adjusting for birth length. Results also were similar for first-trimester protein intake (Supplemental Table 3).
TABLE 4.
Association of second-trimester maternal protein intake, presented as a continuous variable and by quartile, with birth weight z score1
| Birth weight z score2 (n = 1205) |
||||||
| Second-trimester maternal protein intake | Mean birth weight, kg | Mean birth weight z score | Model 13 | Model 24 | Model 35 | Model 46 |
| Continuous 1-SD (0.36 g · kg prepregnancy body weight− · d−) increment | −0.15 (−0.21, −0.09) | −0.17 (−0.23, −0.11) | −0.12 (−0.18, −0.06) | −0.06 (−0.11, −0.01) | ||
| Quartile (median intake, g · kg prepregnancy body weight− · d−) | ||||||
| 1 (0.94) | 3.6 | 0.72 | Ref | Ref | Ref | Ref |
| 2 (1.23) | 3.5 | 0.62 | −0.11 (−0.28, 0.07) | −0.16 (−0.33, 0.02) | −0.13 (−0.30, 0.04) | −0.10 (−0.23, 0.04) |
| 3 (1.46) | 3.5 | 0.55 | −0.17 (−0.34, 0.00) | −0.23 (−0.40, −0.06) | −0.16 (−0.33, 0.01) | −0.06 (−0.19, 0.08) |
| 4 (1.75) | 3.4 | 0.38 | −0.34 (−0.51, −0.17) | −0.39 (−0.56, −0.21) | −0.25 (−0.43, −0.08) | −0.13 (−0.27, 0.02) |
Based on International Fetal and Newborn Growth Consortium for the 21st Century growth standards; sex-specific by gestational age. Ref, reference.
Values are β (95% CI), based on multivariable linear regression models. Protein intake is adjusted for total energy intake.
Unadjusted.
Adjusted for maternal education, maternal race/ethnicity, household income at enrollment, and maternal age at enrollment.
Adjusted for covariates in Model 2 plus gestational weight gain and maternal and paternal height.
Adjusted for covariates in Model 3 plus birth length.
Stratification by maternal race/ethnicity for the fully adjusted models examining associations of second trimester maternal protein intake presented as a continuous variable with birth length and weight z scores demonstrated similar associations for all race/ethnicity groups (data not shown).
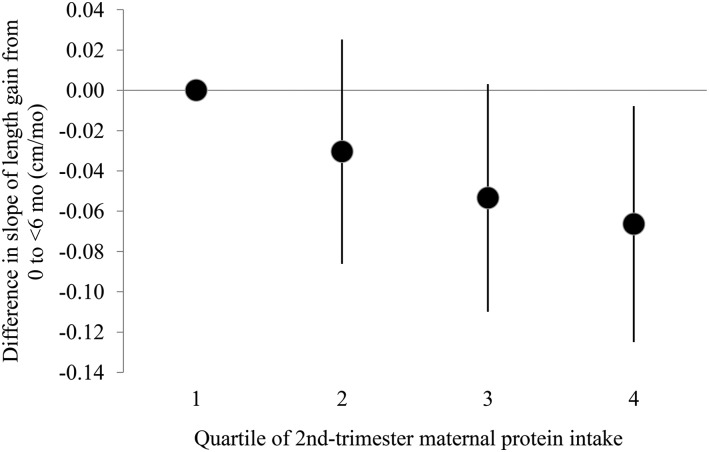
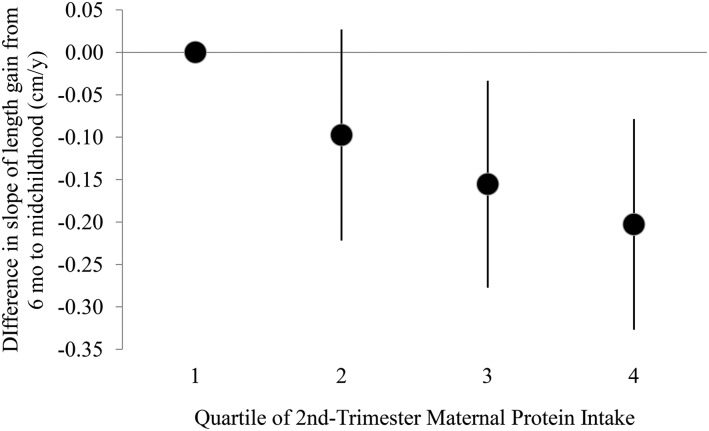
Because the slope of growth tended to be steepest in the first 6 mo, we modeled the slope of growth from birth up to (but not including) 6 mo and again from 6 mo to midchildhood (age ∼7 y) as a function of second-trimester maternal protein intake. In models adjusted for child sex and gestational age at delivery, a 1-SD increase in second-trimester protein intake corresponded to a −0.04 cm/mo change (95% CI: −0.06, −0.02 cm/mo) in slope of growth from birth to <6 mo. Adding maternal education, race/ethnicity and age at enrollment, household income at enrollment, GWG, and maternal and paternal height to the model slightly attenuated this association (−0.03 cm/mo; 95% CI: −0.05, −0.01 cm/mo); this estimate did not change after further adjustment for duration of breastfeeding. In the model fully adjusted for all of these covariates, including breastfeeding duration, a 1-SD increase in second-trimester protein intake corresponded to a −0.09 cm/y change (95% CI: −0.14, −0.05 cm/y) in slope of growth from 6 mo to midchildhood. We also examined slope of length gain during both of these time intervals as a function of protein intake categorized into quartiles, and the results suggested an inverse association between protein intake and early growth (Figures 1 and 2).
FIGURE 1.
Difference in slope of length gain from 0 to <6 mo of age (0–5.98 mo of age; mean age 2.1 mo) by quartile of maternal protein intake (grams per kilogram per day) compared with the lowest quartile of intake. Dots indicate the difference from the lowest quartile in the mean predicted random effect (i.e., mean slope) for subjects in that protein intake group, with 95% CIs indicated by error bars. From multivariable linear regression models adjusted for child sex and gestational age at delivery, maternal education, maternal race/ethnicity, household income at enrollment, maternal age at enrollment, gestational weight gain, maternal and paternal height, and duration of breastfeeding (months). Includes 1537 mother–child pairs.
FIGURE 2.
Difference in slope of length gain from 6 mo to midchildhood (6 mo to 10.9 y of age; mean age 3.0 y) by quartile of maternal protein intake (grams per kilogram per day) compared with the lowest quartile of intake. Dots indicate the difference from the lowest quartile in the mean predicted random effect (i.e., mean slope) for subjects in that protein intake group, with 95% CIs indicated by error bars. From multivariable linear regression models adjusted for child sex and gestational age at delivery, maternal education, maternal race/ethnicity, household income at enrollment, maternal age at enrollment, gestational weight gain, maternal and paternal height, and duration of breastfeeding (months). Includes 1537 mother–child pairs.
DISCUSSION
In a large cohort of mother–child pairs with data on protein intake at 2 time points in pregnancy over 8 y of follow-up with multiple measures of growth and information on many potential confounders, we observed a consistent negative association between maternal protein intake during pregnancy and markers of offspring growth measured at birth through midchildhood. This finding was contrary to our hypothesis that there would be a direct relation between maternal protein intake and child growth during the fetal, infancy, and early childhood periods and a reversal of this association in midchildhood.
The few studies examining associations of maternal intake of protein during pregnancy with offspring growth have focused primarily on birth weight (11, 13, 14, 20, 21, 23). Our main focus was on linear growth, and we also examined associations with birth weight (with and without adjustment for birth length) for comparison with other studies in the literature, and to determine whether previously observed associations with birth weight might be due to effects on birth length. Our results are consistent with the inverse association with birth weight observed in some studies (20, 21), and are in contrast to the direct relation seen in others (11–16). These discrepancies in part may result from differences in the specific type of protein examined (total, animal, plant, or dairy protein), timing of exposure assessment [evidence from animal studies and natural human experiments suggests that outcomes depend on the timing of alterations in maternal nutrient status (39)], and mean protein intake in the population. In addition, we used a slightly different approach to categorize the exposure in considering protein intake in grams per kilogram of prepregnancy weight, which aligns with the weight-specific dietary guidelines for pregnancy. This approach also helps to minimize any confounding effect of the mother’s weight status.
To our knowledge, few previous studies have examined relations with birth length (15–18), a proxy measure of fetal length growth, although it is possible that associations of maternal protein intake with birth weight are a result of effects on fetal linear growth. In contrast to our results, Burke et al. (16) observed a direct dose–response relation between the protein content of the mother’s diet and infant birth length. However, that was a small study conducted in 1943, and 68% of the women in this study consumed <70 g protein/d in the fourth through ninth months of pregnancy. Over 91% of women in our sample had an estimated protein intake above the late-pregnancy EAR of 0.88 g · kg−1 · d−1 in their second trimester. Our results may be reconciled with those of Burke et al. (16) by considering the possibility that maternal protein intake has a U-shaped relation with fetal growth: growth is optimized at a moderate intake but is restricted by both inadequate and very high intake.
This U-shaped relation was observed by Sloan et al. (21) in a cohort of low-income urban women and their babies. In comparison with women with intermediate protein intake during pregnancy, those with either high (≥85 g) or low (<50 g) protein intake had babies with lower birth weight. Quadratic models indicated that birth weight increased with protein intake ≤69.5 g/d and declined with higher intake. In our cohort, intake was above this threshold for 88% of women in the first trimester and 90% in the second trimester, which may explain the observed negative relation between protein intake and measures of fetal growth. Watson et al. (20) also observed a quadratic relation between protein intake and birth weight, but with a higher quadratic curve maximum of 108 g/d.
Maternal diet during pregnancy is a proxy measure of fetal nutrient exposure, with the fetus at the end of a complex nutrient supply line. Delivery of nutrients to the fetus is modulated by maternal metabolism and partitioning of nutrients, the intrauterine environment, placental characteristics affecting the efficiency of nutrient transfer, and the fetal endocrine environment and metabolism (39, 40). Normal fetal growth requires an adequate supply of amino acids and effective transport across the placenta. Compromised maternal nutritional status appears to impair placental amino acid transport (41), and pregnancies complicated by intrauterine growth restriction are characterized by reductions in both cord plasma amino acid concentrations and placental amino acid transporter activity (42, 43). Although reduced amino acid availability to the fetus can result from inadequate maternal concentrations, placental transport and umbilical uptake of certain amino acids may also be impaired by high maternal concentrations because of competition for transporters (43). Thus, the quadratic relation between maternal protein intake and measures of fetal growth may be explained at least in part by insufficient transport of the amino acids required for fetal growth at both low and high maternal concentrations. This also may explain the discrepancy in results between our study, which showed an inverse association between maternal protein and fetal and infant growth, and other studies indicating that greater maternal protein intake is associated with higher measures of early growth.
Our study has several limitations. First, we do not have data on birth length for the entire cohort. Measurements were missing for higher proportions of Asian and Hispanic women and women with lower education levels, and babies who did not have a birth length measurement had a lower gestational age at delivery (39.1 compared with 39.7 wk; P < 0.0001) and lower birth weight (3379 compared with 3524 g; P < 0.0001) than did babies who did have their birth length measured. Maternal protein intake did not differ between observations with and without a birth length measurement. In addition, few participants had data from all 4 research visits, but the use of linear mixed models allows for inclusion of observations with missing data in modeling growth over time, assuming data were missing at random. We also included clinical measurements obtained from medical records to increase the number of observations and available data points for the mixed models, and we used multiple imputation methods, a commonly accepted statistical technique for increasing the number of observations available for analysis while maintaining the uncertainty inherent in the imputed values and thus allowing valid statistical inference (44, 45), to increase the number of observations included in our analyses.
We used dietary data obtained from FFQs administered during the first and second trimesters only. Although the FFQ used in this study has been validated extensively in several populations, FFQs in general are subject to several limitations in measuring nutritional exposures. Absolute nutrient intake is estimated with some degree of measurement error, but we have used several methods to address this error. First, results were consistent when we ranked participants into quartiles of protein intake and when we examined protein intake as a continuous variable. This approach is accepted as a valid method for analyzing data obtained from FFQs, and has been used in several analyses of maternal diet variables in Project Viva specifically (46, 47). In addition, one common technique for improving the validity of FFQs is adjusting for total energy intake (28, 29), and our nutrient intake estimates were energy adjusted with the use of the nutrient residual method.
Residual confounding remains a possibility, particularly given the influence of genetic factors on length growth. We accounted for many potential confounders, including both maternal and paternal height, in multivariable models, but we did not have results of genetic analyses available to fully account for genetic contributions to growth. Finally, our sample is not representative of pregnant women in the general US population. All women in the Project Viva cohort resided in the greater Boston area and had health insurance at the time of recruitment. Many were college educated, and the cohort was ∼67% white. Protein intake in our sample may have been higher than that in other populations of women of childbearing age (48, 49). Our results may not be generalizable to all populations of pregnant women.
In conclusion, in a cohort of pregnant women with relatively high protein intake, higher intake was associated with a lower offspring birth length z score, slower linear growth through 6 mo of age and into midchildhood, and a lower birth weight z score after adjusting for a variety of confounding factors. Although adequate protein intake during pregnancy is important for fetal growth and development, our results suggest the possibility that the high dietary intake observed in well-nourished women may slightly impair fetal and early growth.
Acknowledgments
We thank Nolwenn Regnault for her work in preparing the clinical length/height data for analysis. The authors’ responsibilities were as follows—KMS: designed and conducted the research, analyzed the data, drafted the manuscript, and had primary responsibility for the final content; PFJ, AM, MWG, and EO: provided study oversight; KPK: provided guidance on statistical analysis; and all authors: provided critical intellectual contributions and read and approved the final manuscript. None of the authors reported a conflict of interest related to the study.
Footnotes
Abbreviations used: EAR, Estimated Average Requirement; FFQ, food-frequency questionnaire; GWG, gestational weight gain; IGF-I, insulin-like growth factor I; LMP, last menstrual period; RA, research assistant.
REFERENCES
- 1.Larnkjaer A, Ingstrup HK, Schack-Nielsen L, Hoppe C, Molgaard C, Skovgaard IM, Juul A, Michaelsen KF. Early programming of the IGF-I axis: negative association between IGF-I in infancy and late adolescence in a 17-year longitudinal follow-up study of healthy subjects. Growth Horm IGF Res 2009;19:82–6. [DOI] [PubMed] [Google Scholar]
- 2.Martin RM, Holly JM, Gunnell D. Milk and linear growth: programming of the IGF-I axis and implication for health in adulthood. Nestle Nutr Workshop Ser Pediatr Program 2011;67:79–97. [DOI] [PubMed] [Google Scholar]
- 3.Ong KK, Langkamp M, Ranke MB, Whitehead K, Hughes IA, Acerini CL, Dunger DB. Insulin-like growth factor I concentrations in infancy predict differential gains in body length and adiposity: the Cambridge Baby Growth Study. Am J Clin Nutr 2009;90:156–61. [DOI] [PubMed] [Google Scholar]
- 4.Baumann MU, Deborde S, Illsley NP. Placental glucose transfer and fetal growth. Endocrine 2002;19:13–22. [DOI] [PubMed] [Google Scholar]
- 5.Madsen AL, Larnkjaer A, Molgaard C, Michaelsen KF. IGF-I and IGFBP-3 in healthy 9 month old infants from the SKOT cohort: breastfeeding, diet, and later obesity. Growth Horm IGF Res 2011;21:199–204. [DOI] [PubMed] [Google Scholar]
- 6.Koletzko B, von Kries R, Closa R, Escribano J, Scaglioni S, Giovannini M, Beyer J, Demmelmair H, Gruszfeld D, Dobrzanska A, et al. . Lower protein in infant formula is associated with lower weight up to age 2 y: a randomized clinical trial. Am J Clin Nutr 2009;89:1836–45. [DOI] [PubMed] [Google Scholar]
- 7.Rehfeldt C, Lang IS, Gors S, Hennig U, Kalbe C, Stabenow B, Brussow KP, Pfuhl R, Bellmann O, Nurnberg G, et al. . Limited and excess dietary protein during gestation affects growth and compositional traits in gilts and impairs offspring fetal growth. J Anim Sci 2011;89:329–41. [DOI] [PubMed] [Google Scholar]
- 8.Han R, Li A, Li L, Kitlinska JB, Zukowska Z. Maternal low-protein diet up-regulates the neuropeptide Y system in visceral fat and leads to abdominal obesity and glucose intolerance in a sex- and time-specific manner. FASEB J 2012;26:3528–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zohdi V, Lim K, Pearson JT, Black MJ. Developmental programming of cardiovascular disease following intrauterine growth restriction: findings utilising a rat model of maternal protein restriction. Nutrients 2014;7:119–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blumfield ML, Collins CE. High-protein diets during pregnancy: healthful or harmful for offspring? Am J Clin Nutr 2014;100:993–5. [DOI] [PubMed] [Google Scholar]
- 11.Cucó G, Arija V, Iranzo R, Vila J, Prieto MT, Fernandez-Ballart J. Association of maternal protein intake before conception and throughout pregnancy with birth weight. Acta Obstet Gynecol Scand 2006;85:413–21. [DOI] [PubMed] [Google Scholar]
- 12.Heppe DH, van Dam RM, Willemsen SP, den Breeijen H, Raat H, Hofman A, Steegers EA, Jaddoe VW. Maternal milk consumption, fetal growth, and the risks of neonatal complications: the Generation R Study. Am J Clin Nutr 2011;94:501–9. [DOI] [PubMed] [Google Scholar]
- 13.Godfrey K, Robinson S, Barker DJ, Osmond C, Cox V. Maternal nutrition in early and late pregnancy in relation to placental and fetal growth. BMJ 1996;312:410–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moore VM, Davies MJ, Willson KJ, Worsley A, Robinson JS. Dietary composition of pregnant women is related to size of the baby at birth. J Nutr 2004;134:1820–6. [DOI] [PubMed] [Google Scholar]
- 15.Olsen SF, Halldorsson TI, Willett WC, Knudsen VK, Gillman MW, Mikkelsen TB, Olsen J. NUTRIX Consortium. Milk consumption during pregnancy is associated with increased infant size at birth: prospective cohort study. Am J Clin Nutr 2007;86:1104–10. [DOI] [PubMed] [Google Scholar]
- 16.Burke BS, Harding VV, Stuart HC. Nutrition studies during pregnancy; relation of protein content of mother’s diet during pregnancy to birth length, birth weight, and condition of infant at birth. J Nutr 1943;23:506–15. [Google Scholar]
- 17.Andreasyan K, Ponsonby AL, Dwyer T, Morley R, Riley M, Dear K, Cochrane J. Higher maternal dietary protein intake in late pregnancy is associated with a lower infant ponderal index at birth. Eur J Clin Nutr 2007;61:498–508. [DOI] [PubMed] [Google Scholar]
- 18.Lagiou P, Tamimi RM, Mucci LA, Adami HO, Hsieh CC, Trichopoulos D. Diet during pregnancy in relation to maternal weight gain and birth size. Eur J Clin Nutr 2004;58:231–7. [DOI] [PubMed] [Google Scholar]
- 19.Mathews F, Yudkin P, Neil A. Influence of maternal nutrition on outcome of pregnancy: prospective cohort study. BMJ 1999;319:339–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Watson PE, McDonald BW. The association of maternal diet and dietary supplement intake in pregnant New Zealand women with infant birthweight. Eur J Clin Nutr 2010;64:184–93. [DOI] [PubMed] [Google Scholar]
- 21.Sloan NL, Lederman SA, Leighton J, Himes JH, Rush D. The effect of prenatal dietary protein intake on birth weight. Nutr Res 2001;21:129–39. [Google Scholar]
- 22.Rush D, Stein Z, Susser M. A randomized controlled trial of prenatal nutritional supplementation in New York City. Pediatrics 1980;65:683–97. [PubMed] [Google Scholar]
- 23.Viegas OA, Scott PH, Cole TJ, Eaton P, Needham PG, Wharton BA. Dietary protein energy supplementation of pregnant Asian mothers at Sorrento, Birmingham. II: Selective during third trimester only. Br Med J (Clin Res Ed) 1982;285:592–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oken E, Baccarelli AA, Gold DR, Kleinman KP, Litonjua AA, De Meo D, Rich-Edwards JW, Rifas-Shiman SL, Sagiv S, Taveras EM, et al. . Cohort profile: Project Viva. Int J Epidemiol 2015;44:37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol 1992;135:1114–26. [DOI] [PubMed]
- 26.Fawzi WW, Rifas-Shiman SL, Rich-Edwards JW, Willett WC, Gillman MW. Calibration of a semi-quantitative food frequency questionnaire in early pregnancy. Ann Epidemiol 2004;14:754–62. [DOI] [PubMed] [Google Scholar]
- 27.US Department of Agriculture, Agricultural Research Service. USDA national nutrient database for standard reference, release 14. Washington (DC): USDA; 2001. [Google Scholar]
- 28.Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr 1997;65(4 Suppl):1220S–28S; discussion 1229S–31S. [DOI] [PubMed]
- 29.Mackerras D. Energy adjustment: the concepts underlying the debate. J Clin Epidemiol 1996;49:957–62. [DOI] [PubMed] [Google Scholar]
- 30.Kipnis V, Subar AF, Midthune D, Freedman LS, Ballard-Barbash R, Troiano RP, Bingham S, Schoeller DA, Schatzkin A, Carroll RJ. Structure of dietary measurement error: results of the OPEN biomarker study. Am J Epidemiol 2003;158:14–21. [DOI] [PubMed]
- 31.Murphy SP, Poos MI. Dietary reference intakes: summary of applications in dietary assessment. Public Health Nutr 2002;5:843–9. [DOI] [PubMed] [Google Scholar]
- 32.Institute of Medicine (US) Subcommittee on Interpretation and Uses of Dietary Reference Intakes, Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. DRI Dietary reference intakes: applications in dietary assessment. Washington (DC): National Academies Press; 2000. [PubMed] [Google Scholar]
- 33.National Research Council. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids (macronutrients). Washington (DC): The National Academies Press; 2005. [Google Scholar]
- 34.Oken E, Taveras EM, Kleinman KP, Rich-Edwards JW, Gillman MW. Gestational weight gain and child adiposity at age 3 years. Am J Obstet Gynecol 2007;196:322.e1–322.e8. [DOI] [PMC free article] [PubMed]
- 35.Villar J, Cheikh Ismail L, Victora CG, Ohuma EO, Bertino E, Altman DG, Lambert A, Papageorghiou AT, Carvalho M, Jaffer YA, et al. . International standards for newborn weight, length, and head circumference by gestational age and sex: the Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet 2014;384:857–68. [DOI] [PubMed] [Google Scholar]
- 36.White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med 2011;30:377–99. [DOI] [PubMed] [Google Scholar]
- 37.Rubin D. Multiple imputation for nonresponse in surveys. Hoboken (NJ): Wiley-Interscience; 2004. [Google Scholar]
- 38.Rifas-Shiman SL, Rich-Edwards JW, Scanlon KS, Kleinman KP, Gillman MW. Misdiagnosis of overweight and underweight children younger than 2 years of age due to length measurement bias. MedGenMed 2005;7:56. [PMC free article] [PubMed] [Google Scholar]
- 39.Kind KL, Moore VM, Davies MJ. Diet around conception and during pregnancy–effects on fetal and neonatal outcomes. Reprod Biomed Online 2006;12:532–41. [DOI] [PubMed] [Google Scholar]
- 40.Harding JE. The nutritional basis of the fetal origins of adult disease. Int J Epidemiol 2001;30:15–23. [DOI] [PubMed] [Google Scholar]
- 41.Hayward CE, Greenwood SL, Sibley CP, Baker PN, Challis JR, Jones RL. Effect of maternal age and growth on placental nutrient transport: potential mechanisms for teenagers’ predisposition to small-for-gestational-age birth? Am J Physiol Endocrinol Metab 2012;302:E233–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Iruloh CG, D’Souza SW, Fergusson WD, Baker PN, Sibley CP, Glazier JD. Amino acid transport systems beta and A in fetal T lymphocytes in intrauterine growth restriction and with tumor necrosis factor-alpha treatment. Pediatr Res 2009;65:51–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Regnault TR, de Vrijer B, Battaglia FC. Transport and metabolism of amino acids in placenta. Endocrine 2002;19:23–41. [DOI] [PubMed] [Google Scholar]
- 44.Walani SR, Cleland CM. The multiple imputation method: a case study involving secondary data analysis. Nurse Res 2015;22:13–9. [DOI] [PubMed] [Google Scholar]
- 45.Barnard J, Meng XL. Applications of multiple imputation in medical studies: from AIDS to NHANES. Stat Methods Med Res 1999;8:17–36. [DOI] [PubMed] [Google Scholar]
- 46.Camargo CA Jr, Rifas-Shiman SL, Litonjua AA, Rich-Edwards JW, Weiss ST, Gold DR, Kleinman K, Gillman MW. Maternal intake of vitamin D during pregnancy and risk of recurrent wheeze in children at 3 y of age. Am J Clin Nutr 2007;85:788–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Radesky JS, Oken E, Rifas-Shiman SL, Kleinman KP, Rich-Edwards JW, Gillman MW. Diet during early pregnancy and development of gestational diabetes. Paediatr Perinat Epidemiol 2008;22:47–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.U.S. Department of Agriculture, Agricultural Research Service. Nutrient intakes from food: mean amounts and percentages of calories from protein, carbohydrate, fat, and alcohol, one day, 2005-2006. 2008.
- 49.Halkjaer J, Olsen A, Bjerregaard LJ, Deharveng G, Tjonneland A, Welch AA, Crowe FL, Wirfalt E, Hellstrom V, Niravong M, et al. . Intake of total, animal and plant proteins, and their food sources in 10 countries in the European Prospective Investigation into Cancer and Nutrition. Eur J Clin Nutr 2009;63(Suppl 4):S16–36. [DOI] [PubMed] [Google Scholar]