Abstract
Background: Some previous studies have indicated that a low basal metabolic rate (BMR) is an independent predictor of future weight gain, but low rates of follow-up and highly select populations may limit the ability to generalize the results.
Objective: We assessed whether adults with a low BMR gain more weight than do adults with a high BMR who are living in a typical Western environment.
Design: We extracted BMR, body-composition, demographic, and laboratory data from electronic databases of 757 volunteers who were participating in our research protocols at the Mayo Clinic between 1995 and 2012. Research study volunteers were always weight stable, had no acute illnesses and no confounding medication use, and were nonsmokers. The top and bottom 15th percentiles of BMR, adjusted for fat-free mass (FFM), fat mass, age, and sex, were identified. Follow-up electronic medical record system data were available for 163 subjects, which allowed us to determine their subsequent weight changes for ≥3 y (mean: ∼9.7 y).
Results: By definition, the BMR was different in the high-BMR group (2001 ± 317 kcal/d; n = 86) than in the low-BMR group (1510 ± 222 kcal/d; n = 77), but they were comparable with respect to age, body mass index, FFM, and fat mass. Rates of weight gain were not greater in the bottom BMR group (0.3 ± 1.0 kg/y) than in the top BMR group (0.5 ± 1.5 kg/y) (P = 0.17).
Conclusion: Adults with low BMRs did not gain more weight than did adults with high BMRs, implying that habitual differences in food intake or activity counterbalance variations in BMR as a risk factor for weight gain in a typical Western population.
Keywords: body composition, body-weight change, clinical research, energy expenditure, free-living adults, indirect calorimetry, longitudinal follow-up, obesity, Western population
INTRODUCTION
Obesity is a consequence of a persistent imbalance between energy intake and energy expenditure (EE).3 Body size is a main determinant of 24-h EE and the basal metabolic rate (BMR), which is likely to be somewhat stable over relatively brief periods of time, whereas energy intake can vary widely from day to day. For many adults in Western cultures, the BMR is the single largest component of daily EE. Therefore, the BMR is of interest in the context of energy balance. Much of the interindividual variability in the BMR is predicted by body composition, age, sex, and ethnicity (1, 2). Some (3–6) but not all (7, 8) investigators report that a low BMR, adjusted for body composition, sex, and age, is an independent predictor of future weight gain. However, the participants in the prominent, positive studies were somewhat selected populations (3–5). Our objective was to understand whether a reduced resting metabolic rate is an independent predictor of weight gain in a more typical Western population. We collated our well-controlled sets of BMR and body-composition data from studies that were conducted between 1995 and 2012 in Rochester, Minnesota. We used the electronic medical record (EMR) system to track the subsequent body weight of the research volunteers. These 2 resources allowed us to address whether a low BMR independently predisposes individuals to a weight gain.
METHODS
We extracted the BMR, body-composition, demographic, and laboratory data from our databases of volunteers who participated in 30 different institutional review board-approved protocols that were conducted between 1995 and 2012 at the Mayo Clinic. If volunteers participated in >1 protocol, we used data from the first study. From this database, we identified individuals in the top and bottom 15th percentiles of BMR after adjustment for fat-free mass (FFM), fat mass, age, and sex. We used the Mayo Clinic EMR to assess subsequent body-weight changes. Enrollment criteria for all studies were such that the volunteers were free of acute illnesses, weight stable, and nonsmokers. The studies systematically excluded volunteers who regularly took sympathomimetic medications or who had thyroid disease (unless rendered euthyroid with a normal thyroid-stimulating hormone). We included the available follow-up weight data only if volunteers had ≥1 weight recorded ≥3 y after the date of the research study in which they participated. If >1 weight/y was available, we recorded the first weight in each year after the original research study for as many years as were available. All participants provided informed written consent, and we collected medical record data only if patients consented that their records could be used for research.
Data collection
The quantitative variables we collected from the research-study data included age, sex, height, weight, BMI (in kg/m2), BMR, and body composition. Age, sex, fat mass, and FFM were collected because these variables contribute to most of the variance in the BMR. We also collected data on fasting plasma glucose and insulin when available. All patient scales at the Mayo Clinic undergo regular calibrations that include an annual visual inspection and verification of the overall operation of the scale; every 2 y, scales are calibrated with the use of Grip-handle and Nesting Slab Weights (Rice Lake Weighing Systems). The calibration weights were at least three-quarters of the maximum capacity of the scale.
BMR
The BMR was always measured in the overnight postabsorptive state with the use of calorimetry with a ventilated hood (DeltaTrack Metabolic Monitor; Sensor Medics). The extensive calibration of these metabolic carts has been reported (9). In brief, the metabolic carts were calibrated each morning and underwent extra quality control including monthly pressure and gas calibrations together with biannual alcohol-burn test calibrations. The test-retest difference was <3% for duplicate measures of oxygen uptake for adults in the same environment on sequential days with the use of our instruments. In addition, each day, we first checked the calibration with a known gas mixture, and if the variance from the known mixture was >1.25%, the instrument was reset. If the ambient CO2 concentrations exceeded those that are known to interfere with CO2 measurements, the room ventilation was increased to lower ambient CO2 to acceptable concentrations. We also measured oxygen uptake and CO2 each month in one of our personnel; if the O2 consumption rate was >20-mL/min different from the mean, we remeasured that person with another instrument to test for biological compared with instrument issues.
All volunteers were admitted as inpatients to the Mayo Clinic General Clinical Research Center in the evening before the study day and consumed their evening meals at a standardized time (1800). An indirect calorimetry measurement was performed in the fasting state before the participant arose from bed the next morning, which was typically between 0700 and 0800.
Body composition
Total body fat and FFM were measured with the use of dual-energy X-ray absorptiometry (DXA). All DXA measurements were performed with the use of Lunar/GE equipment. To ensure consistency over time and between instruments, we used 4 independent calibration phantoms that were composed of a range of known fat and nonfat contents (Hormel Institute). Each instrument was calibrated to the phantoms such that we could identify any discrepancies between DXA-predicted and -known percentages of fat. The DXA-reported body fat was corrected for any calibration variations as assessed by the phantoms. Every new DXA instrument was tested with the use of an institutional review board–approved protocol whereby volunteers and the meat-block phantoms (not just company phantoms) were scanned on old and new DXA instruments. Software updates were tested by analyzing scan data with the use of both old and new versions to correct for possible variations that could have been introduced by software changes. These procedures assured that we maintained consistent body-composition measures over long periods of time and with different instruments.
Assays
Fasting insulin concentrations were measured with the use of chemiluminescent sandwich assays (Sanofi Diagnostics Pasteur).
Calculations and statistical analysis
All values are given as means ± SDs. The approach to identifying subjects in the lowest and highest 15th percentiles of BMR is depicted in Supplemental Figure 1. The predicted BMR for our population was determined with the use of the measured BMR as the dependent variable and each individual’s FFM, fat mass, age, and sex as independent variables in a multiple linear regression formula. Each individual’s variation from the predicted BMR (observed minus predicted) was used to understand the total variation in our population and, thus, to identify subjects in the top 15% and bottom 15% of BMR. We identified follow-up weight data from 86 and 77 volunteers in the high- and low-BMR groups, respectively. The a priori hypothesis was that there would be greater rates of body-weight gain in the low-BMR group than in the high-BMR group. Statistical power calculations were based on the following information: 1) publications have indicated that the mean annual weight gain of US adults is ∼0.6 (10); 2) we and other authors (5) have shown that the SD of weight gain averages 1.2 kg/y; and 3) our low- and high-BMR groups differed by ∼500 kcal/d, which, according to Piaggi et al. (5), should have resulted in a mean greater weight gain of 1 kg/y in the low-BMR group. This estimate was based on an observed inverse relation between the sleeping metabolic rate and 24-h EE (both of which were adjusted for age, sex, ethnicity, FFM, and FM) and the rate of the percentage of weight change (5). With the use of this data, we showed that, if we had 75 persons/group, we would have had a power of 0.99 to detect a difference of 1 kg/y of weight gain between the high-and low-BMR groups with the use of a 2-sided t test and P = 0.05.
To compare high- and low-BMR groups, including the difference in rates of body-weight changes (kilograms per year) and percentages of body-weight changes per year, an unpaired Student’s t test was performed. The rate of body-weight change (kilograms and percentage) per year for each individual was obtained with the use of a linear regression model for all collected weight and date time points. For all analyses, 2-tailed P values were reported. The difference was considered significant at P < 0.05 for the primary outcome variables. For secondary outcome variables that were not part of our a priori hypothesis, we reported the unadjusted P values but also noted which values would not have been <0.05 if the values had been adjusted with the use of a Bonferroni test. Statistical analyses were performed with JMP statistical software (version 10.0.0).
RESULTS
Data from a total of 757 unique subjects were identified to create the database. From this database, we identified cohorts in the top and bottom 15th percentiles of BMR (adjusted for FFM, fat mass, age, and sex) who had BMRs that were >169 and <−173 kcal/d above and below predicted BMRs, respectively (n = 114 for the top group, and n = 113 for the bottom group). We were able to collect ≥1 (mean: 7) EMR weight for 163 of 227 volunteers. Baseline data from subjects from whom we were able to collect follow-up weight are provided in Table 1. The 2 groups were comparable with respect to age, BMI, FFM, fat mass, and fasting plasma glucose and insulin concentrations. By definition, subjects in the top-15% group had BMRs that were much higher than those of subjects in the bottom-15% group (2001 ± 317 compared with 1510 ± 222 kcal/d); because the BMR was not a random variable, these data were not appropriate for statistical testing.
TABLE 1.
Characteristics of study participants1
| Baseline data | Total population | Top 15% of BMR | Bottom 15% of BMR | P |
| n | 757 | 86 | 77 | |
| Male sex, % | 49 | 59 | 62 | 0.70 |
| Age, y | 40 ± 172 | 39 ± 15 | 43 ± 17 | 0.16 |
| Height, cm | 171.9 ± 9.8 | 174.0 ± 9.2 | 175.0 ± 9.7 | 0.53 |
| Weight, kg | 80.2 ± 17.3 | 83.3 ± 18.4 | 86.4 ± 16.0 | 0.26 |
| BMI, kg/m2 | 27.1 ± 5.1 | 27.4 ± 5.2 | 28.3 ± 5.4 | 0.28 |
| FFM, kg | 54.3 ± 12.4 | 56.9 ± 11.9 | 59.1 ± 11.2 | 0.23 |
| Fat mass, kg | 25.5 ± 12.3 | 25.7 ± 12.1 | 27.1 ± 13.5 | 0.51 |
| BMR, kcal/d | 1665 ± 322 | 2001 ± 317 | 1510 ± 222 | — |
| Fasting glucose, mg/dL | 91 ± 10 [615]3 | 92 ± 18 [70] | 91 ± 7 [62] | 0.71 |
| Insulin, μU/mL | 6.2 ± 8.1 [715] | 6.3 ± 5.2 [82] | 5.3 ± 3.5 [74] | 0.17 |
P values are nonpaired t test comparisons between the top 15% and bottom 15% of BMR. Because BMR was the criteria for selecting the populations, it was not a random variable and thus not subject to statistical testing. BMR, basal metabolic rate; FFM, fat-free mass.
Mean ± SD (all such values).
Mean ± SD; n in brackets (all such values).
The 64 participants for whom we could not collect follow-up weight data differed from subjects with whom we could follow up in that they were younger (32 ± 13 compared with 41 ± 16 y; P < 0.0001). Otherwise, there were no differences in the characteristics of subjects for whom we could and could not obtain follow-up weight data. There were no significant differences in the characteristics of subjects who were lost to follow-up between low- and high-BMR groups.
The duration of follow-up was 8.9 ± 5.0 and 10.7 ± 3.8 y for the groups in the top and bottom 15th percentiles, respectively (P = 0.08; Bonferroni correction). Rates of weight change were not different between subjects in the top and bottom 15th percentiles of BMR (0.5 ± 1.5 compared with 0.3 ± 1.0 kg/y, respectively; P = 0.16) (Figure 1). Likewise, there were no differences in the percentage of weight change per year between groups (0.7% ± 1.7%/y in the top 15th percentile of BMR, and 0.3% ± 1.0%/y in the bottom 15th percentile of BMR; P = 0.08). To provide data in a manner that was consistent with previous reports (4), we also identified participants who gained >10 kg by 3 y after the initial study date. There was no significant difference in the percentages of subjects who gained 10 kg between top- and bottom-BMR groups (4.7% compared with 3.9%, respectively). We also tested post hoc whether subjects with and without obesity might differ with regard to the relation between BMRs and rates of weight gain. For subjects with normal BMI, the high-BMR group (n = 61) had a greater rate of weight gain than did the low-BMR group (n = 52) (0.67 ± 1.04 compared with 0.23 ± 0.76 kg/y, respectively; P = 0.04 with Bonferroni adjustment). For subjects with BMI ≥30, there were no significant differences between weight changes in the high-BMR group (n = 25) and low-BMR group (n = 25) (0.20 ± 2.19 compared with. 0.31 ± 1.33 kg/y, respectively; P = 0.70 with Bonferroni adjustment).
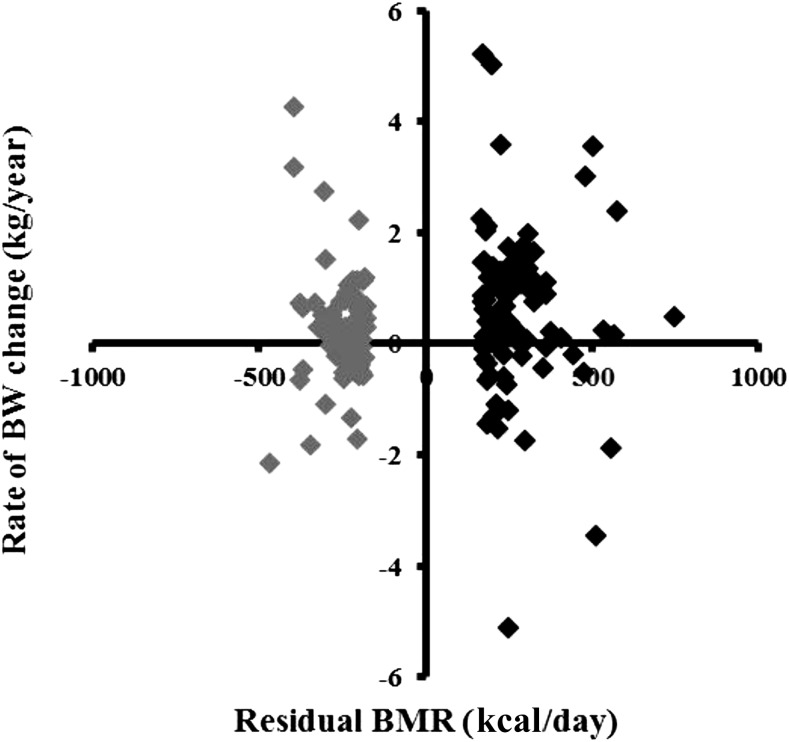
FIGURE 1.
Relation between initial residual BMR (measured minus predicted) and the rate of BW change (ρ = 0.12, P = 0.13; nonpaired t test) is depicted. Black diamonds represent data from subjects in the top 15% of residual BMR, and gray diamonds represent data from subjects in the bottom 15% of residual BMR. BMR, basal metabolic rate; BW, body weight.
To assess the combined biological and technical variation in these measurements, we queried our entire database of 757 volunteers for subjects who had participated in 2 different studies. We found 33 subjects who had participated in 2 studies with separate body-composition and BMR measurements. The mean time between the 2 studies was 1.0 ± 0.9 y and occurred between 1996 and 2009. For each participant in each study, we assessed the deviation of the BMR from that predicted for the entire cohort on the basis of FFM, fat mass, age, and sex. We compared the deviation from the predicted BMR between the first and second studies with the use of a paired t test. For these 33 participants, the BMR deviation from the predicted BMR was −33 ± 143 kcal/d (−2% ± 9%) and −61 ± 190 kcal/d (−4% ± 11%) for the first and second studies, respectively (P = 0.40).
We also examined rates of weight gain in the top and bottom 10th percentiles of BMR. We had 56 and 50 participants with follow-up data in the top and bottom groups, respectively. The groups were well matched for body composition, age, and sex; the groups differed by ∼600 kcal/d in BMRs (2061 ± 316 compared with 1480 ± 204 kcal/d, respectively), and the rates of weight gain were likewise not different between groups (0.5 ± 1.5 compared with. 0.3 ± 1.0 kg/y, respectively). The smaller groups of top and bottom fifth percentiles (<40 subjects/group but with 670-kcal/d between-group differences in BMRs) also did not differ with respect to weight gain. Although between-group difference in BMRs was greater, the number of subjects with satisfactory follow-up was less, such that the statistical power to detect differences was not improved.
DISCUSSION
Because a BMR is a substantial portion of daily EE for many adults in modern societies, we assessed whether adults with low BMRs (bottom 15% adjusted for body composition, age, and sex) are more predisposed to gain weight than are adults with high BMRs (top 15%). By collating data from 757 volunteers studied in the Mayo Clinic General Clinical Research Center under strict protocol conditions, we identified 2 cohorts of adults with mean BMRs that differed by 500 kcal/d. With the use of comprehensive EMR data at our institution, we were able to collect follow-up weight data on ∼72% of these research participants at time points ≥3 y after the original study. We showed that the rates of body-weight change (kilograms per year and percentage per year) were not greater and were numerically lower in adults with low BMRs than in adults with high BMRs. These findings indicate that adults with low BMRs are not uniquely predisposed to future weight gains.
A number of previous studies have shown that the BMR, sleeping metabolic rate, and 24-h EE, adjusted for FFM, fat mass, age, and sex, are independently predictive of future weight gain (3–6). Although one of the studies reported a significant relation between low BMR and weight gain in Italian Caucasians (6), the other statistically significant associations were shown in studies of the Pima Indian population (3–5). In addition to the somewhat homogeneous genetic background of the Pima Indian population, it is possible that the environment in the Indian communities is such that they are more sensitive to a reduced BMR as a predisposition to weight gain than are inhabitants of Southwestern Minnesota. Most studies enrolled obese participants (mean BMI: 32–36) (3–5), and some of studies included relatively small numbers of subjects (6) or had shorter durations of follow-up (3, 4). The percentage of subjects in whom we were able to collect follow-up weight data was comparable with that in other studies (3, 5) and much greater than the 30% follow-up reported in a study of Italian adults (6). One study of lean Nigerians showed that higher resting energy expenditures (REEs) were positively associated with weight gain (8), but the REE was not measured under as carefully controlled conditions, and body composition was measured by bioelectrical impedance, either of which could have affected the results. The authors suggested that there may be a differential regulation of body-weight gain between lean and overweight populations (8). The previous studies that showed no relation between the REE and weight change included relatively small numbers of subjects (11) or used skinfold thicknesses to measure body composition (which is a suboptimal body-composition approach to adjusting BMR) in men only (7). For these reasons, neither study definitively addressed the question of BMR and weight gain.
The strength of our study is our access to data from a large number of adults in a typical Western population who had robust body-composition assessments and BMRs measured under very standardized conditions. We included participants who were lean and obese, young and old adults, metabolically normal and insulin resistant. We excluded acutely ill participants and participants with illnesses or who were taking medications that could have affected their metabolic phenotypes. These people are exactly the population who are most likely to be characterized as at risk of weight gain because of their constitutionally low BMRs. To exaggerate potential differences, we compared these adults with a group of high-BMR adults who were supposedly protected from weight gain. Over the years, we have used rigorous methods to maintain accurate and consistent indirect calorimetry and body-composition measurements, thereby ensuring that the between-subject differences in BMRs were biological and not technical. We tested this association by examining data from subjects who participated in 2 different studies over the course of 13 y to better define the combined biological and technical variability of our methods. The absolute differences in intraindividual test-retest BMRs from predicted BMRs was small (mean of 7%), and there was no trend for positive or negative changes over the years of the study. In contrast, BMRs were a mean of 33% greater in the high-BMR group than in the low-BMR group. Finally, our sample size was more than sufficient to detect the previously reported associations (5). We suggest that our efforts to ensure consistent BMR and body-composition measurements under standardized conditions, our practical approach to real-world follow-up with multiple weight observations for most subjects, and the large sample size of both men and women clearly addressed whether BMR is an independent predictor of future body-weight gain in free-living adults.
However, there are limitations to this study. The retrospective cohort study design meant that we could not collect data from people who moved away. However, our follow-up rates were comparable with or better than those of studies that reported significant associations between BMR and subsequent weight gain (3, 5, 6). Because it was not possible to collect comprehensive information regarding the diet and activity habits for the ∼9 y after the original studies, we cannot know why adults with low BMRs gained no more weight than did adults with high BMRs. However, note that previous investigators concluded that a low BMR is an independent predictor of greater weight gain (e.g., persons with a low BMR will gain more weight irrespective of lifestyle factors). Although some participants were given information regarding their BMR results, we did not indicate to them whether their BMRs deviated from expected BMRs. Thus, it is unlikely that participation in our studies resulted in substantial, lifelong changes in diet or activity habits. None of our participants with follow-up EMR data developed cancer, and thus we do not believe that weight changes that could have been due to cancer or cancer treatments confounded our results.
In conclusion, we show that adults with BMRs that are well below predicted BMRs do not gain more weight than do adults with BMRs that are well above predicted BMRs despite a 500-kcal/d difference between the 2 groups. These data indicate that, in a typical majority Caucasian, Western population, variations in BMR are not responsible for tendencies toward weight gain. In the real world, people start and stop diets, exercise programs, and medications that may affect weight. We suggest that these factors are far more effective than BMR is in predisposing individuals to weight gain. The implication of our findings is that adults with low BMRs either eat less or expend more energy in physical activity than do those with high BMRs under free-living conditions.
Acknowledgments
The authors’ responsibilities were as follows—MDJ: was the guarantor of this work and, as such, had full access to all of the data in the study and took responsibility for the integrity of the data and accuracy of the data analysis; and both authors: designed and performed the research, analyzed the data, wrote the manuscript, and read and approved the final manuscript. Neither author reported a conflict of interest related to the study.
Footnotes
Abbreviations used: BMR, basal metabolic rate; DXA, dual-energy X-ray absorptiometry; EE, energy expenditure; EMR, electronic medical record; FFM, fat-free mass; REE, resting energy expenditure.
REFERENCES
- 1.Weyer C, Snitker S, Rising R, Bogardus C, Ravussin E. Determinants of energy expenditure and fuel utilization in man: effects of body composition, age, sex, ethnicity and glucose tolerance in 916 subjects. Int J Obes Relat Metab Disord 1999;23:715–22. [DOI] [PubMed] [Google Scholar]
- 2.Toubro S, Sorensen TI, Ronn B, Christensen NJ, Astrup A. Twenty-four-hour energy expenditure: the role of body composition, thyroid status, sympathetic activity, and family membership. J Clin Endocrinol Metab 1996;81:2670–4. [DOI] [PubMed] [Google Scholar]
- 3.Tataranni PA, Harper IT, Snitker S, Del Parigi A, Vozarova B, Bunt J, Bogardus C, Ravussin E. Body weight gain in free-living Pima Indians: effect of energy intake vs expenditure. Int J Obes Relat Metab Disord 2003;27:1578–83. [DOI] [PubMed] [Google Scholar]
- 4.Ravussin E, Lillioja S, Knowler WC, Christin L, Freymond D, Abbott WG, Boyce V, Howard BV, Bogardus C. Reduced rate of energy expenditure as a risk factor for body-weight gain. N Engl J Med 1988;318:467–72. [DOI] [PubMed] [Google Scholar]
- 5.Piaggi P, Thearle MS, Bogardus C, Krakoff J. Lower energy expenditure predicts long-term increases in weight and fat mass. J Clin Endocrinol Metab 2013;98:E703–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buscemi S, Verga S, Caimi G, Cerasola G. Low relative resting metabolic rate and body weight gain in adult Caucasian Italians. Int J Obes (Lond) 2005;29:287–91. [DOI] [PubMed] [Google Scholar]
- 7.Seidell JC, Muller DC, Sorkin JD, Andres R. Fasting respiratory exchange ratio and resting metabolic rate as predictors of weight gain: the Baltimore Longitudinal Study on Aging. Int J Obes Relat Metab Disord 1992;16:667–74. [PubMed] [Google Scholar]
- 8.Luke A, Durazo-Arvizu R, Cao G, Adeemo A, Tayo B, Cooper R. Positive association between resting energy expenditure and weight gain in a lean adult population. Am J Clin Nutr 2006;83:1076–81. [DOI] [PubMed] [Google Scholar]
- 9.Jensen MD, Bajnarek J, Lee SY, Nielsen S, Koutsari C. Relationship between postabsorptive respiratory exchange ratio and plasma free fatty acid concentrations. J Lipid Res 2009;50:1863–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kahn HS, Cheng YJ. Longitudinal changes in BMI and in an index estimating excess lipids among white and black adults in the United States. Int J Obes (Lond) 2008;32:136–43. [DOI] [PubMed] [Google Scholar]
- 11.Weinsier RL, Nelson KM, Hensrud DD, Darnell BE, Hunter GR, Schutz Y. Metabolic predictors of obesity. Contribution of resting energy expenditure, thermic effect of food, and fuel utilization to four-year weight gain of post-obese and never-obese women. J Clin Invest 1995;95:980–5. [DOI] [PMC free article] [PubMed] [Google Scholar]