Abstract
Background: The utility of glycemic index (GI) values for chronic disease risk management remains controversial. Although absolute GI value determinations for individual foods have been shown to vary significantly in individuals with diabetes, there is a dearth of data on the reliability of GI value determinations and potential sources of variability among healthy adults.
Objective: We examined the intra- and inter-individual variability in glycemic response to a single food challenge and methodologic and biological factors that potentially mediate this response.
Design: The GI value for white bread was determined by using standardized methodology in 63 volunteers free from chronic disease and recruited to differ by sex, age (18–85 y), and body mass index [BMI (in kg/m2): 20–35]. Volunteers randomly underwent 3 sets of food challenges involving glucose (reference) and white bread (test food), both providing 50 g available carbohydrates. Serum glucose and insulin were monitored for 5 h postingestion, and GI values were calculated by using different area under the curve (AUC) methods. Biochemical variables were measured by using standard assays and body composition by dual-energy X-ray absorptiometry.
Results: The mean ± SD GI value for white bread was 62 ± 15 when calculated by using the recommended method. Mean intra- and interindividual CVs were 20% and 25%, respectively. Increasing sample size, replication of reference and test foods, and length of blood sampling, as well as AUC calculation method, did not improve the CVs. Among the biological factors assessed, insulin index and glycated hemoglobin values explained 15% and 16% of the variability in mean GI value for white bread, respectively.
Conclusions: These data indicate that there is substantial variability in individual responses to GI value determinations, demonstrating that it is unlikely to be a good approach to guiding food choices. Additionally, even in healthy individuals, glycemic status significantly contributes to the variability in GI value estimates. This trial was registered at clinicaltrials.gov as NCT01023646.
Keywords: glycemic index, variability, healthy volunteers, insulin index, glycated hemoglobin
See corresponding editorial on page 955.
INTRODUCTION
Consumption of carbohydrate-containing foods elicits varied postprandial blood glucose responses. To better define this effect, Jenkins et al. (1) proposed the glycemic index (GI)6 method to classify foods according to their postingestion plasma glucose response. The GI is defined as the “incremental area under the blood glucose response curve (AUC) of a 50-g available carbohydrate portion of both a test and reference food consumed by the same individual over a 2-h period, expressed as a percentage” (1). Glycemic load (GL) is GI adjusted for serving size of the food (2). This concept was originally developed as a food selection guide for diabetic individuals to improve their glycemic control by classifying foods into low (<55), medium (56-69) and high (>70) GI categories (3). However, use of GI has gone beyond this original intent and is now being endorsed for use as a labeling tool to guide food choices to reduce chronic disease risk (4) and serves as the basis for many popular diets (e.g., South Beach and Zone). Although there are some clinical data to support a modest benefit of low GI and GL diets in the management of diabetes (5, 6), in the nondiabetic population the evidence is mixed (7). Meta-analysis of prospective studies supports a positive association between high GI diets and increased chronic disease risk (8), but data from randomized clinical trials indicate an inconsistent relation between dietary GI and GL values and insulin sensitivity (9), cardiovascular disease (8, 10), weight loss (11, 12), and cancer (13, 14). Some of this discrepancy in the literature has been attributed to methodologic issues with its measurement (15–18).
The GI value is considered to represent the inherent property of the food and not the metabolic response of an individual to the food (11). Thus, theoretically GI values should be reproducible within and among individuals. However, a review of the published GI tables, which is a compilation of the GI values of individual foods generated by several laboratories (3, 19), indicates different estimates for the GI value within a category and even for the same food (11, 20, 21). This could result in a food being ranked as low by one laboratory and high by another (22). To reconcile these differences, several methodologic factors have been evaluated (23) and in an attempt to standardize GI value determination of foods an International Standard (24) was established based on the Joint FAO/WHO Expert Consultation (18, 25) and other recommendations (21, 23). However, evidence is still limited on sources of variability that affect the reliability of GI value estimates. The objective of the present study was to determine the intra-individual and interindividual variability in glycemic response to a single food challenge and potential methodologic and biological factors that could mediate responses among healthy adults.
METHODS
Study participants
Healthy men and women with wide age (18–85 y) and BMI ranges [(in kg/m2) 20–35] were recruited from the Greater Boston area (Supplemental Figure 1). Individuals who were morbidly obese, were diagnosed with type 1 or 2 diabetes, had a fasting glucose ≥125 mg/dL, or reported use of medications or supplements known to affect glucose metabolism (insulin, sulfonylureas, metformin, glucosidase inhibitors, thiazolidinedione, insulin sensitizers), gastrointestinal motility (prokinetic agents), or lipid metabolism (bile acid sequestrants, fibrates, cholesterol absorption and 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase inhibitors, nicotinic acids, anabolic steroids, fish oil) were excluded. Also excluded were individuals with renal, liver, pancreatic, or cardiovascular disease; uncontrolled hypertension; inflammatory bowel disease; disorders of esophageal and gastrointestinal motility; previous esophageal or gastric resection; autoimmune or connective tissue disorders; hypo- or hyperthyroidism; and anemia or poor venous access. Other exclusion criteria included smoking, weight gain or loss ≥5 kg within the prior 6 mo, consuming >7 alcoholic drinks/wk, pregnant or breastfeeding women, and those unwilling to adhere to study protocol. Seventy volunteers were enrolled in the study. Seven of these volunteers did not complete the study for the following reasons: time constraints (n = 4), change in medical status (n = 2), and noncompliance (n = 1). All volunteers were informed about the nature and risks of the experimental procedures before their written, informed consent was obtained. The study was approved by the Tufts University/Tufts Medical Center Institutional Review Board.
Study design
Volunteers underwent 6 sessions in our Metabolic Research Unit, and each session was 6–7 h in duration. The study protocol, including environment, procedures, and timing, was standardized to minimize introduction of unaccounted-for variability. Sessions took place 1–2 times/wk, with a maximum of 12 wk to complete all sessions. Individuals underwent 3 sets of food challenges in random order, and each set included glucose or bread. The statistician (LMA) was responsible for generation of the randomization sequence using the random-number generator in Microsoft Excel, and numbers were assigned to the volunteers in sequential order by enrollment date by the study coordinator. The principle investigator (AHL), co-investigator (NRM), and all laboratory personnel were blinded to the testing order. The reference, glucose drink [500 mL (100 g/L)], and the test food, white bread (Pepperidge Farm Original White Bread; 96.25 g white bread, 2.4 g dietary fiber), both provided 50 g of available carbohydrates. On the days that volunteers were challenged with white bread, they also consumed 500 mL water to match the volume of the glucose solution. Before testing, volunteers fasted overnight for 12 h and refrained from consuming alcohol and exercising for 72 h.
Blood sampling and testing protocol
Fifteen to 30 min before each session, a retrograde intravenous catheter was inserted into the lower cephalic or superficial dorsal veins of the hand to allow sampling of arterialized venous blood. This approach allowed an adequate volume of blood to be collected for the biochemical measures proposed and avoided the variability introduced when venous blood is sampled (23, 26, 27). A continuous normal saline infusion was used to maintain the blood sampling line during the course of the challenge. In addition, 15 min before each blood draw time point, volunteers were asked to place their hand in a moderately heated box (44°C–46°C) to avoid the inconsistencies associated with temperature control when using heated pads. After collection of a baseline blood sample, volunteers consumed the test or reference food within a 10-min period. Additional blood samples were obtained at 15, 30, 45, 60, 90, 120, 150, 180, 210, 240, 270, and 300 min. Volunteers remained in the Metabolic Research Unit and were restricted to sedentary activities (watching television, reading, etc.) to avoid displacement of the intravenous line. During one of the sessions, habitual physical activity patterns were assessed by using the Community Healthy Activities Program questionnaire (28). Volunteers also underwent a body composition scan by using dual-energy X-ray absorptiometry (DXA).
Biochemical analysis
At each time point, serum was immediately separated by centrifugation at 1100 × g at 4°C. Glucose was measured by using a commercially available kit (Cobas MIRA; Roche Diagnostics). Insulin was measured by using a solid-phase, 2-site chemiluminescent immunoassay (Diagnostic Products Corporation). Interassay CVs were 1.7 and 6.0 for glucose and insulin, respectively. Homeostatic model assessment (HOMA), an indicator of insulin resistance and β cell function, was calculated according to the following formula: glucose (mg/dL) × immunoreactive insulin (μU/mL)/22.5. Total cholesterol (TC), LDL cholesterol, HDL cholesterol, and triglyceride concentrations were measured at baseline and 60, 120, 180, and 240 min on a Hitachi 911 automated analyzer (Roche Diagnostics) by using enzymatic or immunoturbidimetric reagents. VLDL cholesterol was estimated as the difference between TC minus LDL cholesterol and HDL cholesterol. The assays were standardized through the Lipid Standardization Program of the Centers for Disease Control, Atlanta, Georgia. Nonesterified free fatty acids (NEFAs) were monitored over the entire 5-h period by using an in vitro enzymatic colorimetric method (Wako Chemicals). C-reactive protein (CRP) was measured by using the Tina-quant CRP high sensitive immunoturbidimetric assay, whereas glycated hemoglobin (HbA1c), a measure of longer-term glucose control (6-wk half-life), and fructosamine, a measure of shorter-term glucose control (12-d half-life), were measured by using commercially available reagents (Roche Diagnostics). Body composition was measured by using a Hologic QDR 4500 densitometer (Hologic Inc.). Measures included total fat, lean muscle mass (LM), and lean plus bone mineral content (LM+BMC), calculated for whole body, trunk, and abdominal regions by using DXA. Body-fat content was expressed as a percentage of total weight. Standard anthropometric measurements (weight, height, and circumferences of the waist, hip, and midthigh) were made on the same day that body composition was determined by DXA, in triplicate, by using standard techniques.
Calculation of glycemic variables
Consistent with the recommended protocol for nondiabetic individuals, the incremental glucose AUC (AUCi) was calculated geometrically as the sum of the areas of the triangles and trapezoids over 2 h, excluding the area below the initial fasting glucose concentration (18, 25). The GI for each individual was calculated by dividing the serum glucose AUC for white bread by the mean serum glucose AUC for the reference glucose drink, measured 3 times, and multiplied by 100. These values were then averaged to obtain the mean GI value for each individual. Similarly, the insulin index was calculated by dividing the serum insulin AUC for white bread by the mean serum insulin AUC for the reference glucose drink, multiplied by 100, and then averaged for each individual.
Statistical analysis
Statistical analyses were performed on data from the 63 volunteers who completed the study by using SAS 9.3 (SAS Institute Inc.). Descriptive data are expressed as means ± SDs. PROC VARCOMP was used to derive the interindividual (square root of subject variance divided by the mean GI value) and intra-individual (square root of error term divided by the mean GI value) CV. Comparison of baseline characteristics between sexes was done by using unpaired t test. PROC MIXED was used to determine differences in serum glucose, insulin, NEFAs, TC, LDL cholesterol, HDL cholesterol, VLDL cholesterol, and triglyceride concentrations between glucose and white bread over the course of the study. When the food challenges × time interaction was significant at P < 0.05, multiple comparisons at each time point were carried out by using Tukey’s post hoc test.
Given that current recommendations stipulate that the reference food should be tested 2 or 3 times in each subject, and a minimum of 10 subjects should be studied, we determined the effect of sample size and replication of the test and reference by calculating the mean GI value for the first 2 of the 3 trials, or all 3 trials for sequential numbers of volunteers in groups of 10, up to 63. We also calculated the GI values at 30, 45, 60, 90, 120, 150, 180, 210, 240, 270, and 300 min by using the AUCi method. In addition, 3 other approaches were used to calculate the AUC for the glucose drink and white bread, and corresponding GI values at 120, 150, 180, 240, and 300 min. The cut AUC (AUCcut) was calculated as the incremental area until serum glucose concentrations first returned to the initial fasting glucose concentration. The minimum AUC (AUCmin) was calculated as the incremental area using the lowest serum glucose concentration as baseline. The net AUC (AUCnet) was calculated by subtracting the sum of the negative areas of the triangles and trapezoids from the positive areas. In each case, the intra- and interindividual CVs were calculated as described above. Correlations between GI value of white bread and serum glucose AUC following consumption of the reference glucose drink were determined by Spearman correlation. PROC GLM was used to measure the association between mean GI value for each individual and body composition variables adjusted by age and sex. Finally, we fitted a random-intercept mixed-model by using PROC MIXED with a normal distribution for the error term to determine which biological factors contributed to the total variation in GI at 120 min, assuming equal correlation among the 3 GI values for each individual. The random intercept was used to decompose the total variance in GI into the sum of the interindividual and the intra-individual variance.
RESULTS
Baseline characteristics
The mean ± SD age and BMI for all volunteers were 49 ± 16 y and 27 ± 4, respectively (Table 1). When analyzed by sex, women were younger with lower waist circumference; waist-to-hip ratio; blood pressure; and glucose, fructosamine, and LDL cholesterol concentrations; and higher HDL cholesterol and NEFA concentrations than men. Additionally, differences in body composition, primarily higher percentage of whole body and trunk total fat, and lower LM content and BMC were observed in the women, who also reported being less physically active. No sex difference was observed for BMI or for triglyceride and CRP concentrations.
TABLE 1.
Baseline characteristics of the study participants1
| All (n = 63) | Men (n = 33) | Women (n = 30) | P2 | |
| Age, y | 49.3 ± 15.5 | 53.0 ± 14.0 | 45.2 ± 16.3 | 0.045 |
| Body composition | ||||
| BMI, kg/m2 | 27.3 ± 4.3 | 27.4 ± 4.1 | 27.1 ± 4.6 | 0.791 |
| Waist circumference, cm | 90.3 ± 12.5 | 95.0 ± 11.6 | 85.1 ± 11.5 | 0.001 |
| Hip circumference, cm | 103.3 ± 8.6 | 101.8 ± 7.5 | 105.0 ± 9.4 | 0.138 |
| Waist-to-hip ratio | 0.87 ± 0.09 | 0.93 ± 0.08 | 0.81 ± 0.06 | <0.0001 |
| Abdominal region, % | ||||
| Total fat | 31.0 ± 9.1 | 28.8 ± 8.5 | 33.1 ± 9.2 | 0.066 |
| LM | 68.3 ± 8.9 | 70.5 ± 8.4 | 66.1 ± 9.0 | 0.060 |
| LM+BMC | 69.0 ± 9.1 | 71.2 ± 8.5 | 66.9 ± 9.2 | 0.066 |
| Trunk region, % | ||||
| Total fat | 30.3 ± 8.9 | 24.6 ± 7.1 | 36.2 ± 6.4 | <0.0001 |
| LM | 67.2 ± 8.6 | 72.8 ± 6.9 | 61.5 ± 6.0 | <0.0001 |
| LM+BMC | 69.7 ± 8.9 | 75.4 ± 7.1 | 63.8 ± 6.4 | <0.0001 |
| Whole body, % | ||||
| Total fat | 29.7 ± 8.4 | 24.3 ± 6.7 | 35.2 ± 6.1 | <0.0001 |
| LM | 67.3 ± 8.1 | 72.6 ± 6.5 | 61.9 ± 5.7 | <0.0001 |
| LM+BMC | 70.3 ± 8.4 | 75.7 ± 6.7 | 64.7 ± 6.0 | <0.0001 |
| Blood pressure, mm Hg | ||||
| Systolic | 117.9 ± 13.1 | 123.3 ± 11.9 | 112.7 ± 14.2 | 0.002 |
| Diastolic | 73.7 ± 9.0 | 76.8 ± 11.2 | 69.0 ± 16.0 | 0.028 |
| Fasting plasma lipid and inflammatory profile | ||||
| Total cholesterol, mmol/L | 4.8 ± 0.8 | 4.9 ± 0.8 | 9.9 ± 1.8 | 0.243 |
| LDL cholesterol, mmol/L | 3.0 ± 0.7 | 3.2 ± 0.7 | 6.0 ± 1.5 | 0.036 |
| HDL cholesterol, mmol/L | 1.3 ± 0.3 | 1.2 ± 0.3 | 3.0 ± 0.6 | 0.004 |
| VLDL cholesterol, mmol/L | 0.5 ± 0.3 | 0.5 ± 0.3 | 0.4 ± 0.2 | 0.314 |
| Triglycerides, mmol/L | 1.0 ± 0.6 | 1.1 ± 0.6 | 0.9 ± 0.5 | 0.318 |
| Total cholesterol:HDL cholesterol | 3.8 ± 1.1 | 4.2 ± 1.1 | 3.4 ± 0.9 | 0.002 |
| Nonesterified fatty acids, μmol/L | 563 ± 193 | 507 ± 144 | 625 ± 223 | 0.015 |
| C-reactive protein, mg/L | 2.4 ± 3.9 | 2.2 ± 4.6 | 2.6 ± 3.0 | 0.665 |
| Glycemic profile | ||||
| Glucose, mmol/L | 5.0 ± 0.5 | 5.1 ± 0.6 | 4.8 ± 0.4 | 0.042 |
| Insulin, pmol/L | 58.2 ± 22.2 | 60.0 ± 23.4 | 55.8 ± 21.6 | 0.471 |
| Fructosamine, μmol/L | 2.4 ± 0.4 | 2.5 ± 0.4 | 2.2 ± 0.4 | 0.031 |
| Glycated hemoglobin, % | 5.6 ± 0.5 | 5.6 ± 0.5 | 5.5 ± 0.4 | 0.155 |
| Homeostatic model assessment | 2.2 ± 1.0 | 2.3 ± 1.0 | 2.0 ± 0.9 | 0.238 |
| Insulin index | 78.8 ± 26.3 | 85.1 ± 27.4 | 71.6 ± 23.5 | 0.043 |
| Physical activity, 1000 kcal/wk | 3.4 ± 3.4 | 4.3 ± 4.5 | 2.4 ± 1.3 | 0.032 |
Values are means ± SDs. LM, lean muscle; LM+BMC, lean muscle + bone mineral content.
Between men and women, derived by using unpaired t test.
Glycemic and lipid response
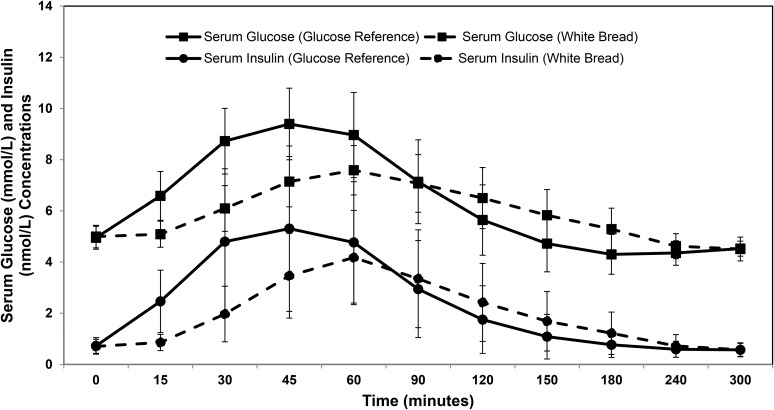
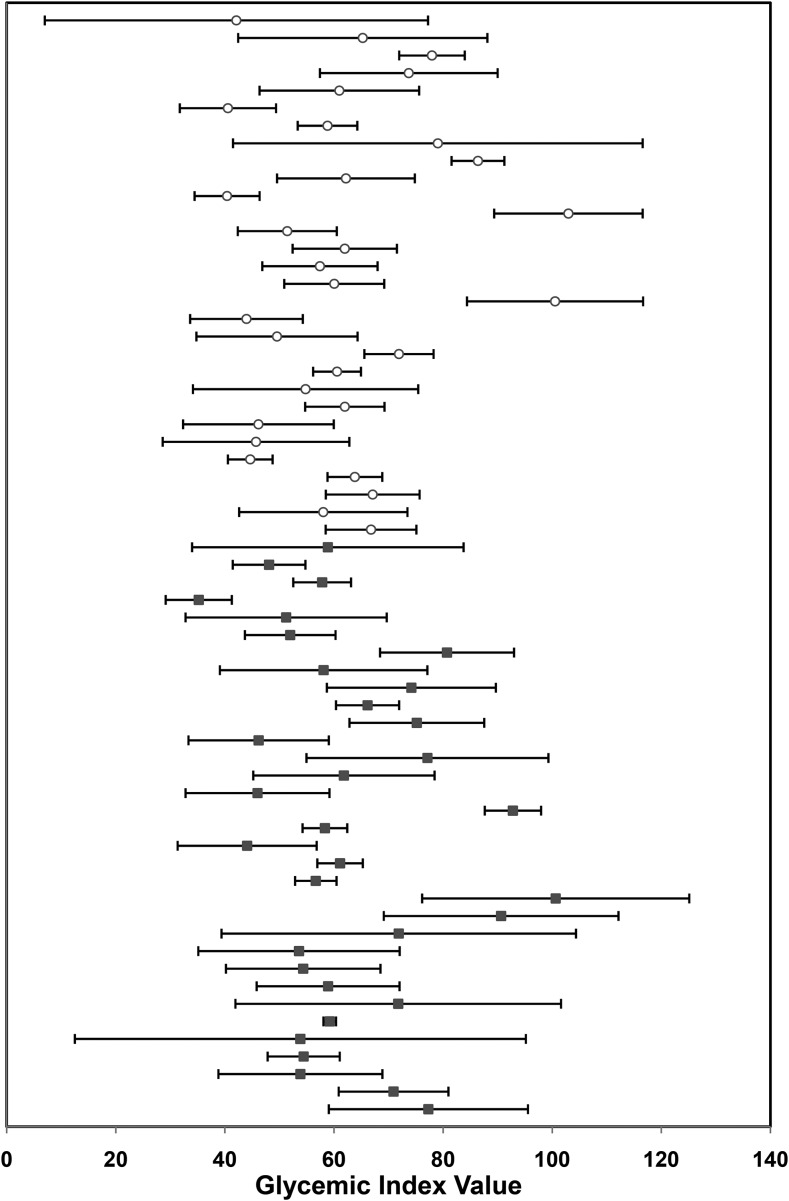
The serum glucose and insulin response to the glucose drink (reference) and white bread (test) was measured over the course of the 5-h study period (Figure 1). To be consistent with the recommended protocol for calculating the GI value (24), we initially used the 2-h data to calculate the AUCi. The glucose AUCi was 5755 ± 1054 and 3510 ± 731 and insulin AUCi was 4624 ± 924 and 3427 ± 818 for the glucose reference and white bread, respectively. The mean GI value for white bread was 62.4 ± 15.3. However, there was marked variability in the GI values among volunteers (Figure 2). The intra-individual CV was 20%, and the interindividual CV was 25%, ranging from 2% to 77%. Interestingly, volunteers with a low GI value for white bread had a similar SD to that of volunteers with a high GI value (48 ± 14 compared with 82 ± 17), which suggests that the relation between the mean GI value and SD is not linear.
FIGURE 1.
Serum glucose and insulin response after consumption of the reference glucose drink and white bread. The symbol at each time point represents the mean ± SD for all subjects (n = 63).
FIGURE 2.
Intra-individual and interindividual variability in glycemic index values for white bread. Each symbol represents the mean of 3 glycemic index value determinations in men [▪] (n = 33) and women [○] (n = 30). Horizontal bars depict the SDs.
An initial decline was observed in serum NEFA concentrations, which was significantly different between the glucose drink and white bread at 45, 60, and 90 min, but this rebounded starting at 150 min and remained significantly higher from 180 through 300 min (Supplemental Figure 2). There was no significant effect on postprandial TC, LDL cholesterol, and HDL cholesterol concentrations between the glucose drink and white bread (Supplemental Figure 3A). An increase in triglyceride concentrations after consumption of white bread relative to the glucose drink was observed at 120 and 180 min (Supplemental Figure 3B). The differences in NEFA and triglyceride concentrations were modest, and their clinical relevance is unclear.
Methodologic variables
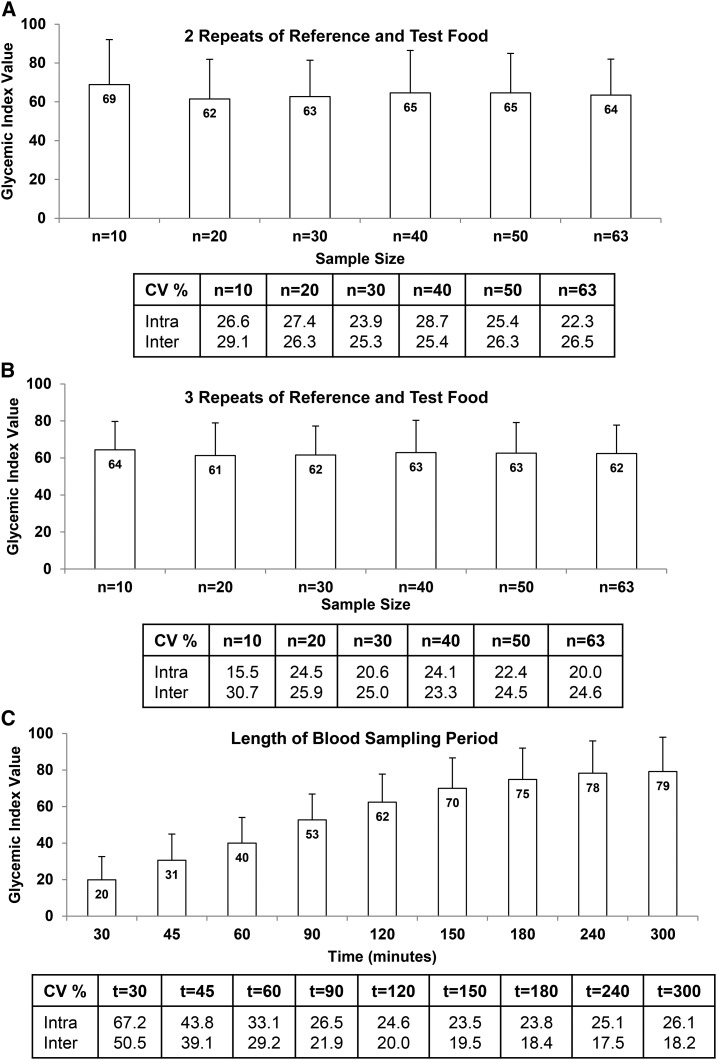
Increasing sample size as well as number of repeat measures of the reference and test food from 2 to 3 did not have a significant effect on the mean GI value for white bread (Figure 3A, B), or on the intra- and interindividual CVs. The GI value for white bread increased whereas the interindividual variability decreased as the length of sampling time increased from 120 to 300 min (Figure 3C). The intra-individual variability also decreased with increased sampling time, being lowest at 150 min and then increasing slightly at 240 and 300 min.
FIGURE 3.
Methodologic variables contributing to intra- and interindividual variability in glycemic index values for white bread including (A) 2 repeats of the reference and test food, (B) 3 repeats of the reference and test food, and (C) length of blood sampling period. For panels A and B, the bar at each time point represents the glycemic index mean ± SD for 10–63 subjects. For panel C, the bar at each time point represents the glycemic index mean ± SD for 63 subjects. The table below each figure shows the mean intra- and interindividual CV%, derived by using PROC VARCOMP. The square root of the subject variance divided by the mean glycemic index value yields the interindividual CV, whereas the square root of the error term divided by the mean glycemic index value yields the intra-individual CV.
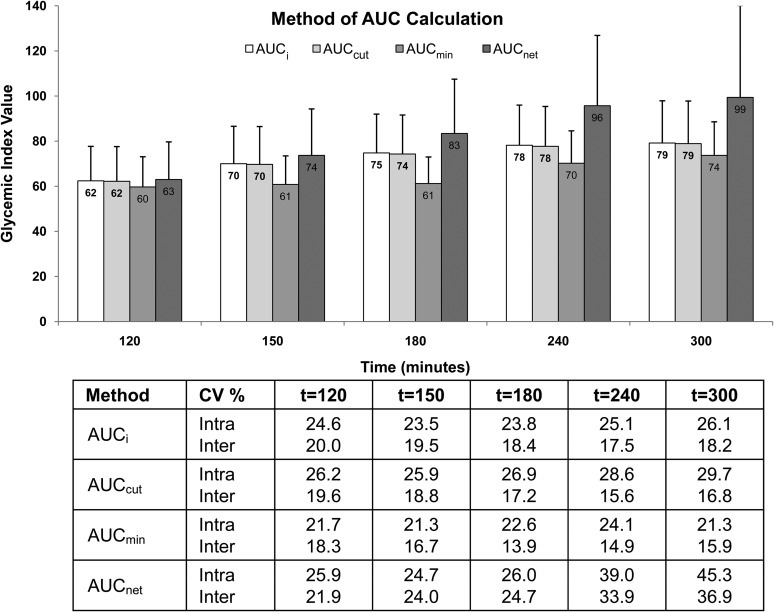
The method of AUC calculation had a substantial effect on the GI value as well as the variability (Figure 4). The AUCmin method consistently resulted in the lowest mean GI value as well as lowest intra- and interindividual CVs, whereas the AUCnet method resulted in the highest mean GI value. The recommended AUCi method and the AUCcut method resulted in similar mean GI values at all time points. Of note, with the exception of the AUCmin method, significant correlations were observed between the GI value for white bread and the plasma glucose AUC after consumption of the glucose reference at all time points when the AUCnet method was used, and starting from the 150-min time point when the AUCi method was used and at the 240-min time point for the AUCcut method (Table 2).
FIGURE 4.
Effect of method of AUC calculation on the intra- and interindividual variability in glycemic index values for white bread. The bar at each time point represents the glycemic index mean ± SD for all subjects (n = 63) calculated by using one of the following methods: AUCi, incremental AUC calculated geometrically as the sum of the areas of the triangles and trapezoids over 2 h, excluding the area below the initial fasting glucose concentration; AUCcut, cut AUC measured until the serum glucose concentrations first returned to the initial fasting glucose concentration; AUCmin, minimum AUC calculated by using the lowest serum glucose concentration as baseline; AUCnet, net AUC calculated by subtracting the sum of the negative areas of the triangles and trapezoids from the positive areas. The table below the figure shows the intra- and interindividual CV%, derived by using PROC VARCOMP. The square root of the subject variance divided by the mean glycemic index value yields the interindividual CV, whereas the square root of the error term divided by the mean glycemic index value yields the intra-individual CV.
TABLE 2.
Correlation between glycemic index value for white bread and serum glucose AUC after consumption of the glucose reference calculated by the different methods1
| Time point, min |
||||||
| AUC method | Correlation coefficient2 | 120 | 150 | 180 | 240 | 300 |
| AUCi | r2 | −0.221 | −0.280 | −0.309 | −0.353 | −0.326 |
| P | 0.082 | 0.026* | 0.014* | 0.005* | 0.009* | |
| AUCcut | r2 | −0.175 | −0.239 | −0.246 | −0.293 | −0.298 |
| P | 0.169 | 0.059 | 0.052 | 0.020* | 0.018* | |
| AUCmin | r2 | −0.214 | −0.233 | −0.186 | 0.013 | −0.121 |
| P | 0.092 | 0.066 | 0.145 | 0.921 | 0.343 | |
| AUCnet | r2 | −0.257 | −0.396 | −0.504 | −0.583 | −0.503 |
| P | 0.042* | 0.001* | <0.0001* | <0.0001* | <0.0001* | |
*Significant correlation between glycemic index value for white bread and serum glucose AUC, suggesting that the method is influenced by an individual's glycemic status. AUCcut, cut AUC measured until serum glucose concentrations first return to the initial fasting glucose concentration; AUCi, incremental AUC calculated geometrically as sum of the areas of the triangles and trapezoids over 2 h, excluding the area below the initial fasting glucose concentration; AUCmin, minimum AUC, calculated by using the lowest serum glucose concentration as baseline; AUCnet, net AUC, calculated by subtracting the sum of the negative areas of the triangles and trapezoids from the positive areas.
Determined by using Spearman correlation.
Biological variables
By using univariate analysis there was no significant effect of sex (63 ± 15 for men compared with 62 ± 16 for women; P = 0.81) or BMI [67 ± 18, 62 ± 14, and 58 ± 15 for BMI categories 18.5–24.9 (normal), 25–29.9 (overweight) and 30–35 (obese), respectively, P = 0.17] on the GI value for white bread. The GI value for white bread was negatively associated with total and trunk fat and positively associated with LM and LM+BMC. However, after controlling for age and sex, these associations were no longer significant (Supplemental Figure 4).
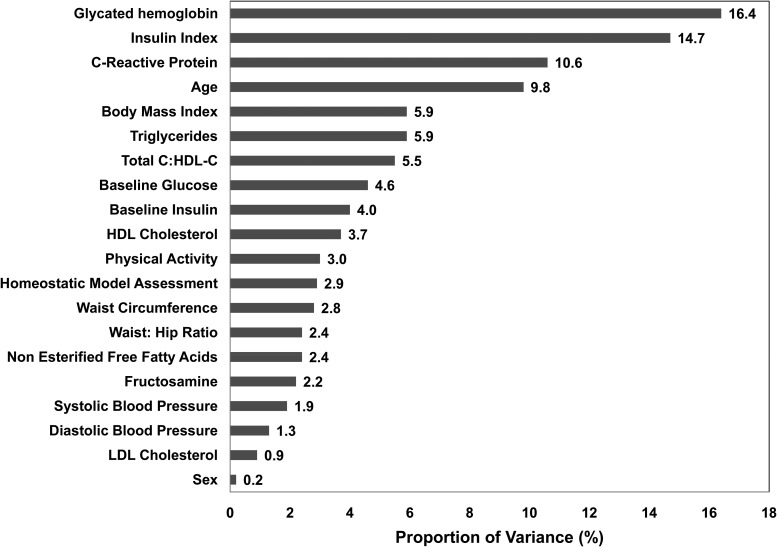
Using mixed models, we then examined the proportion of interindividual variation in GI value for white bread (Figure 5) that was contributed by biological variables. Sex; waist-to-hip ratio; blood pressure; NEFAs; LDL cholesterol; HDL cholesterol; baseline serum glucose, insulin, and fructosamine concentrations; HOMA; and physical activity contributed <5% to the variability. Age, BMI, and serum triglyceride and CRP concentrations, and TC:HDL cholesterol ratio explained between 5% and 11% of the variability, whereas 15% and 16% of the variability in GI value for white bread was explained by insulin index and HbA1c values, respectively. The mean insulin index was 78.8 ± 26.3 with significant differences between men and women (85 ± 27 and 72 ± 24, respectively; P = 0.04).
FIGURE 5.
Biological variables contributing to interindividual variability in glycemic index values for white bread (n = 63). PROC MIXED with a normal distribution for the error term was used to fit a random-intercept mixed-model to determine which biological factors contributed to the total variation in glycemic index at 120 min, assuming equal correlation among the 3 glycemic index values for each individual. HDL-C, high-density lipoprotein cholesterol; total C, total cholesterol.
DISCUSSION
The usefulness of the GI concept for dietary guidance has been questioned because of limitations with methodology. In this study, despite using recommended GI methodology, we documented substantial variability in the mean intra-individual (20%) and interindividual (25%) CVs for a single food, white bread. Addressing methodologic variables, such as sample size, test replication, and length of sampling as well as AUC calculation method, did not improve the CVs. Among the biological variables assessed, age, BMI, TC:HDL cholesterol ratio, and serum triglyceride and CRP concentrations accounted for 5–11% of the variability. A novel finding of this work was that insulin index and HbA1c values accounted for 15% and 16% of the interindividual variability in GI value for white bread, respectively, indicating that even in healthy individuals, glycemic status significantly contributes to the variability in GI estimates, which limits its clinical and public health applicability.
The substantial variation in GI value observed in our study is consistent with prior studies that have reported CVs. Intra-individual CVs for white bread in healthy volunteers have ranged from 17% (29) to 31% (27, 30), whereas the interindividual CVs have ranged from 12% to 50%, averaging between 20% and 25% (27, 31–33). This high variability suggests greater potential for misclassification of foods. As demonstrated in our volunteers, the white bread tested had a mean GI value of 62, classifying it in the medium category. However, the range of individual GI values was large, so it would be classified as having a low value for 22 individuals (GI: 35–55), intermediate for 23 individuals (GI: 57–67), and high for 18 individuals (GI: 70–103).
Studies have identified methodologic factors that can affect GI value determinations (22, 23, 27, 34), but efforts to address them have been met with varying degrees of success (16). To minimize the intra-individual CV, it is recommended that the GI value of the test food be derived from the ratio of the glycemic response it elicits over the average of 2, preferably 3, glycemic responses to the reference (24). Similar to previous studies, we found intra-individual variability was not reduced with this testing strategy (22, 33). Of note, we also controlled for factors, such as physical activity and alcohol consumption, before testing days that have been shown to contribute to the day-to-day fluctuations in GI values (30). However, our volunteers were not required to consume a standardized evening meal before testing, which has been recommended in the updated International Standards Organization methodology. Although this has been proposed as a potential source of variability (35), other studies (36, 37) have not documented differences in glucose AUC after either a standardized or nonstandardized meal.
Among methodologic variables that can potentially influence the interindividual variability in GI values, we addressed sample size, length of blood sampling, and AUC calculation method. The standardized methodology recommends testing a minimum of 10 subjects, although it is suggested that a larger sample size would improve precision (22, 23). Increasing the sample size to 63 individuals did not reduce the interindividual CV. With regard to the length of blood sampling period, at early time points (30–90 min) the variability was high, presumably reflecting differences in absorption rates among volunteers. Extending the time to 300 min proportionally increased the GI value but decreased both the intra- and interindividual CVs. Method of AUC calculation also affected the mean GI value and CVs (34). Based on the hypothesis that the glucose tolerance status of an individual would not affect the GI value of the food being tested, we calculated the correlation between the GI value and glucose AUC after consumption of the reference. Only the AUCmin method met these criteria at all time points tested. The AUCi and AUCcut methods were valid only at 120 min. The AUCnet method did not meet the criteria at any of the time points tested.
The GI value is derived by testing the reference and test food in the same volunteer, which theoretically should control for the variability among people that can be caused by biological factors. Consequently, guidelines do not include sex- or age-specific criteria, although it is recommended to study healthy individuals, with the International Standards Organization standards excluding individuals with diseases or taking drugs that affect digestion and glucose metabolism, as we did. In our study, sex; body composition variables; lipid profile; blood pressure; NEFA; baseline glucose, insulin, and fructosamine, concentrations; HOMA; and physical activity were not significant contributors to the variability in GI values. Age and inflammatory status, as reflected by CRP concentrations, explained ∼10% of the variability in GI value for white bread. This age effect has been observed in one study (38), but was not seen in another (39), probably because of the smaller differential in mean age in the latter compared with the former study (20 compared with 46 y), as well as our study (67 y).
Our results document that the insulinemic response as reflected by the insulin index, and longer-term glycemic control as reflected by HbA1c values, were important contributors to the variability in GI. It has been suggested that the insulin index might be a better variable than the GI value because postprandial insulin responses are not always proportional to blood glucose concentrations or to the carbohydrate load of some foods (40). However, we observed similarly high intra-individual (16%) and interindividual (30%) CVs for the insulin index. Based on prior studies (41, 42) in normal compared with diabetic individuals and those with impaired glucose tolerance, it has been concluded that glycemic status does not significantly affect the mean GI value, although it has been acknowledged that the variability differs among groups. This has led to the recommendation that GI values be determined in normoglycemic individuals. Our study indicates that glycemic status is a significant contributor to the variability in GI response, even in normoglycemic individuals. Additionally, given the positive relation between postprandial glycemic response with HbA1c and CRP concentrations (43, 44), the inflammatory status of the individual might also be an important mediating factor.
Blood glucose response is affected by several additional factors: differing physical structure of apparently similar foods, food processing or preparation method (45), and meal consumption patterns (single or mixed meals) (11, 22, 46), which can contribute to the marked inter- and intra-individual variation observed in the GI value of foods. These factors, together with those described in this paper, document that there are far too many exceptions that influence the accuracy and precision of GI values calculations for them to be used as a basis for making food-based recommendations. Additionally, GI values do not reflect the nutritional quality of the food, further questioning their clinical relevance.
In summary, our data indicate substantial variability in GI value determinations for white bread despite the use of standardized methodology and multiple testing in a large number of healthy volunteers. The high degree of variability demonstrates that there is potential to misclassify foods into the 3 commonly used GI categories (low, medium, and high), which would result in the inability to distinguish between foods, thus invalidating the practical applicability of the GI value. Additionally, our results indicate that the variability was explained in part by differences in baseline HbA1c and insulin index, suggesting that longer-term glycemic control and insulin response, even in normoglycemic individuals, affect the GI value. The impact of these findings on GI estimates and subsequent associations with chronic disease risk needs to be considered.
Acknowledgments
We thank the study coordinator Janey Ronxhi.
The authors’ responsibilities were as follows—NRM: conducted the research, wrote the initial draft of the manuscript, and had primary responsibility for the final content; AHL: designed and conducted the research; LMA, HM, and HT: were responsible for analyzing the data and performing the statistical analysis; and all authors: contributed to critically reviewing the manuscript. None of the authors reported a conflict of interest related to this study.
Footnotes
Abbreviations used: AUCcut, cut AUC; AUCi, incremental glucose AUC; AUCmin, minimum AUC; AUCnet, net AUC; BMC, bone mineral content; CRP, C-reactive protein; DXA, dual-energy X-ray absorptiometry; GI, glycemic index; GL, glycemic load; HbA1c, glycated hemoglobin; HOMA, homeostatic model assessment; LM, lean muscle; NEFA, nonesterified free fatty acid; TC, total cholesterol.
REFERENCES
- 1.Jenkins DJ, Wolever TM, Taylor RH, Barker H, Fielden H, Baldwin JM, Bowling AC, Newman HC, Jenkins AL, Goff DV. Glycemic index of foods: a physiological basis for carbohydrate exchange. Am J Clin Nutr 1981;34:362–6. [DOI] [PubMed] [Google Scholar]
- 2.Salmerón J, Ascherio A, Rimm EB, Colditz GA, Spiegelman D, Jenkins DJ, Stampfer MJ, Wing AL, Willett WC. Dietary fiber, glycemic load, and risk of NIDDM in men. Diabetes Care 1997;20:545–50. [DOI] [PubMed] [Google Scholar]
- 3.Atkinson FS, Foster-Powell K, Brand-Miller JC. International tables of glycemic index and glycemic load values: 2008. Diabetes Care 2008;31:2281–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glycemic Index Foundation. The GI Symbol Program [Internet]. [cited 2016 Jul 21]. Available from: http://www.gisymbol.com.
- 5.Livesey G, Taylor R, Livesey H, Liu S. Is there a dose-response relation of dietary glycemic load to risk of type 2 diabetes? Meta-analysis of prospective cohort studies. Am J Clin Nutr 2013;97:584–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dong JY, Zhang YH, Wang P, Qin LQ. Meta-analysis of dietary glycemic load and glycemic index in relation to risk of coronary heart disease. Am J Cardiol 2012;109:1608–13. [DOI] [PubMed] [Google Scholar]
- 7.Hare-Bruun H, Nielsen BM, Grau K, Oxlund AL, Heitmann BL. Should glycemic index and glycemic load be considered in dietary recommendations? Nutr Rev 2008;66:569–90. [DOI] [PubMed] [Google Scholar]
- 8.Mirrahimi A, Chiavaroli L, Srichaikul K, Augustin LS, Sievenpiper JL, Kendall CW, Jenkins DJ. The role of glycemic index and glycemic load in cardiovascular disease and its risk factors: a review of the recent literature. Curr Atheroscler Rep 2014;16:381. [DOI] [PubMed] [Google Scholar]
- 9.Sacks FM, Carey VJ, Anderson CA, Miller ER 3rd, Copeland T, Charleston J, Harshfield BJ, Laranjo N, McCarron P, Swain J, et al. . Effects of high vs low glycemic index of dietary carbohydrate on cardiovascular disease risk factors and insulin sensitivity: the OmniCarb randomized clinical trial. JAMA 2014;312:2531–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kristo AS, Matthan NR, Lichtenstein AH. Effect of diets differing in glycemic index and glycemic load on cardiovascular risk factors: review of randomized controlled-feeding trials. Nutrients 2013;5:1071–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pi-Sunyer FX. Glycemic index and disease. Am J Clin Nutr 2002;76:290S–8S. [DOI] [PubMed] [Google Scholar]
- 12.Karl JP, Roberts SB, Schaefer EJ, Gleason JA, Fuss P, Rasmussen H, Saltzman E, Das SK. Effects of carbohydrate quantity and glycemic index on resting metabolic rate and body composition during weight loss. Obesity (Silver Spring) 2015;23:2190–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi Y, Giovannucci E, Lee JE. Glycaemic index and glycaemic load in relation to risk of diabetes-related cancers: a meta-analysis. Br J Nutr 2012;108:1934–47. [DOI] [PubMed] [Google Scholar]
- 14.Terry PD, Jain M, Miller AB, Howe GR, Rohan TE. Glycemic load, carbohydrate intake and risk of colorectal cancer in women: a prospective cohort study. J Natl Cancer Inst 2003;95:914–6. [DOI] [PubMed] [Google Scholar]
- 15.US Department of Health and Human Services and US Department of Agriculture. Dietary Guidelines for Americans 2015–2020: eighth edition [Internet]. [cited 2016 Jul 21]. Available from: http://health.gov/dietaryguidelines/2015/guidelines/.
- 16.Aziz A, Dumais L, Barber J. Health Canada’s evaluation of the use of glycemic index claims on food labels. Am J Clin Nutr 2013;98:269–74. [DOI] [PubMed] [Google Scholar]
- 17.European Food Safety Authority. Scientific opinion on dietary reference values for carbohydrates and dietary fibre. EFSA J 2010;8:1462. [Google Scholar]
- 18.Mann J, Cummings JH, Englyst HN, Key T, Liu S, Riccardi G, Summerbell C, Uauy R, van Dam RM, Venn B, et al. . FAO and WHO scientific update on carbohydrates in human nutrition: conclusions. Eur J Clin Nutr 2007;61(Suppl 1):S132–37. [DOI] [PubMed] [Google Scholar]
- 19.Foster-Powell K, Holt SH, Brand-Miller JC. International table of glycemic index and glycemic load values. Am J Clin Nutr 2002;76:5–56. [DOI] [PubMed] [Google Scholar]
- 20.Kirpitch AR, Maryniuk MD. The 3 R’s of glycemic index: recommendations, research, and the real world. Clin Diabetes 2011;29:155–9. [Google Scholar]
- 21.Wolever TM, Vorster HH, Bjorck I, Brand-Miller J, Brighenti F, Mann JI, Ramdath DD, Granfeldt Y, Holt S, Perry TL, et al. . Determination of the glycaemic index of foods: interlaboratory study. Eur J Clin Nutr 2003;57:475–82. [DOI] [PubMed] [Google Scholar]
- 22.Venn BJ, Green TJ. Glycemic index and glycemic load: measurement issues and their effect on diet–disease relationships. Eur J Clin Nutr 2007;61(Suppl 1):S122–31. [DOI] [PubMed] [Google Scholar]
- 23.Brouns F, Bjorck I, Frayn KN, Gibbs AL, Lang V, Slama G, Wolever TM. Glycaemic index methodology. Nutr Res Rev 2005;18:145–71. [DOI] [PubMed] [Google Scholar]
- 24.International Standards Organization. ISO 26642: food products—determination of the glycaemic index (GI) and recommendation for food classification. Geneva (Switzerland): ISO; 2010. [Google Scholar]
- 25.Food and Agriculture Organization. Carbohydrates in human nutrition: a summary of the Joint FAO and WHO Expert Consultation. Geneva (Switzerland): WHO; 1997. [Google Scholar]
- 26.Hätönen KA, Similä ME, Virtamo JR, Eriksson JG, Hannila M-L, Sinkko HK, Sundvall JE, Mykkänen HM, Valsta LM. Methodologic considerations in the measurement of glycemic index: glycemic response to rye bread, oatmeal porridge, and mashed potato. Am J Clin Nutr 2006;84:1055–61. [DOI] [PubMed] [Google Scholar]
- 27.Vrolix R, Mensink RP. Variability of the glycemic response to single food products in healthy subjects. Contemp Clin Trials 2010;31:5–11. [DOI] [PubMed] [Google Scholar]
- 28.Stewart AL, Mills KM, King AC, Haskell WL, Gillis D, Ritter PL. CHAMPS physical activity questionnaire for older adults: outcomes for interventions. Med Sci Sports Exerc 2001;33:1126–41. [DOI] [PubMed] [Google Scholar]
- 29.Vega-López S, Ausman LM, Griffith JL, Lichtenstein AH. Interindividual variability and intra-individual reproducibility of glycemic index values for commercial white bread. Diabetes Care 2007;30:1412–7. [DOI] [PubMed] [Google Scholar]
- 30.Wolever TM, Brand-Miller JC, Abernethy J, Astrup A, Atkinson F, Axelsen M, Bjorck I, Brighenti F, Brown R, Brynes A, et al. . Measuring the glycemic index of foods: interlaboratory study. Am J Clin Nutr 2008;87:247S–57S. [DOI] [PubMed] [Google Scholar]
- 31.Williams SM, Venn BJ, Perry T, Brown R, Wallace A, Mann JI, Green TJ. Another approach to estimating the reliability of glycaemic index. Br J Nutr 2008;100:364–72. [DOI] [PubMed] [Google Scholar]
- 32.Wolever TMS, Campbell JE, Geleva D, Anderson GH. High-fiber cereal reduces postprandial insulin responses in hyperinsulinemic but not normoinsulinemic subjects. Diabetes Care 2004;27:1281–5. [DOI] [PubMed] [Google Scholar]
- 33.Wolever TM, Csima A, Jenkins DJ, Wong GS, Josse RG. The glycemic index: variation between subjects and predictive difference. J Am Coll Nutr 1989;8:235–47. [DOI] [PubMed] [Google Scholar]
- 34.Wolever TM. Effect of blood sampling schedule and method of calculating the area under the curve on validity and precision of glycaemic index values. Br J Nutr 2004;91:295–301. [DOI] [PubMed] [Google Scholar]
- 35.Wolever TM, Jenkins DJ, Ocana AM, Rao VA, Collier GR. Second-meal effect: low-glycemic-index foods eaten at dinner improve subsequent breakfast glycemic response. Am J Clin Nutr 1988;48:1041–7. [DOI] [PubMed] [Google Scholar]
- 36.Wallace AJ, Eady SL, Willis JA, Scott RS, Monro JA, Frampton CM. Variability in measurements of blood glucose response to foods in human subjects is not reduced after a standard breakfast. Nutr Res 2009;29:238–43. [DOI] [PubMed] [Google Scholar]
- 37.Campbell JE, Glowczewski T, Wolever TMS. Controlling subjects’ prior diet and activities does not reduce within-subject variation of postprandial glycemic responses to foods. Nutr Res 2003;23:621–9. [Google Scholar]
- 38.Venn BJ, Williams SM, Perry T, Richardson S, Cannon A, Mann JI. Age-related differences in postprandial glycaemia and glycaemic index. Age Ageing 2011;40:755–8. [DOI] [PubMed] [Google Scholar]
- 39.Wolever TM, Jenkins AL, Vuksan V, Campbell J. The glycaemic index values of foods containing fructose are affected by metabolic differences between subjects. Eur J Clin Nutr 2009;63:1106–14. [DOI] [PubMed] [Google Scholar]
- 40.Holt SHA, Brand Miller JC, Petocz P. An insulin index of foods: the insulin demand generated by 1000-kJ portions of common foods. Am J Clin Nutr 1997;66:1264–76. [DOI] [PubMed] [Google Scholar]
- 41.Wolever TMS, Chiasson J-L, Hunt JA, Palmason C, Ross SA, Ryn EA. Similarity of relative glycaemic but not relative insulinaemic responses in normal, IGT and diabetic subjects. Nutr Res 1998;18:1667–76. [Google Scholar]
- 42.Wolever TM, Jenkins DJ, Josse RG, Wong GS, Lee R. The glycemic index: similarity of values derived in insulin-dependent and non-insulin-dependent diabetic patients. J Am Coll Nutr 1987;6:295–305. [DOI] [PubMed] [Google Scholar]
- 43.Zeevi D, Korem T, Zmora N, Israeli D, Rothschild D, Weinberger A, Ben-Yacov O, Lador D, Avnit-Sagi T, Lotan-Pompan M, et al. . Personalized nutrition by prediction of glycemic responses. Cell 2015;163:1079–94. [DOI] [PubMed] [Google Scholar]
- 44.Sander D, Schulze-Horn C, Bickel H, Gnahn H, Bartels E, Conrad B. Combined effects of hemoglobin A1c and C-reactive protein on the progression of subclinical carotid atherosclerosis: the INVADE study. Stroke 2006;37:351–7. [DOI] [PubMed] [Google Scholar]
- 45.Boers HM, Seijen ten Hoorn J, Mela DJ. A systematic review of the influence of rice characteristics and processing methods on postprandial glycaemic and insulinaemic responses. Br J Nutr 2015;114:1035–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Flint A, Moller BK, Raben A, Pedersen D, Tetens I, Holst JJ, Astrup A. The use of glycaemic index tables to predict glycaemic index of composite breakfast meals. Br J Nutr 2004;91:979–89. [DOI] [PubMed] [Google Scholar]