Abstract
Currently, there is no general consensus about the management of osteoporotic vertebral fractures (OVF). In the past, conservative treatment for at least one month was deemed appropriate for the majority of vertebral fractures. When pain persisted after conservative treatment, it was necessary to consider surgical interventions including: vertebroplasty for vertebral fractures with less than 30% loss of height of the affected vertebral body and kyphoplasty for vertebral fractures with greater than 30% loss of height. Currently, this type of treatment is not feasible. Herein we review the characteristics and methods of operation of three of the most common percutaneous vertebral augmentation systems (PVAS) for the treatment of OVF: Vertebral Body Stenting® (VBS), OsseoFix® and Spine Jack®. VBS is a titanium device accompanied by a hydraulic (as opposed to mechanical) working system which allows a partial and not immediate possibility to control the opening of the device. On the other hand, OsseoFix® and Spine Jack® are accompanied by a mechanical working system which allows a progressive and controlled reduction of the vertebral fracture. Another important aspect to consider is the vertebral body height recovery. OsseoFix® has an indirect mechanism of action: the compaction of the trabecular bone causes an increase in the vertebral body height. Unlike the Vertebral Body Stenting® and Spine Jack®, the OsseoFix® has no direct lift mechanism. Therefore, for these characteristics and for the force that this device is able to provide. In our opinion, Spine Jack® is the only device also suitable for the treatment OVF, traumatic fracture (recent, old or inveterate) and primary or secondary bone tumors.
Keywords: Osteoporotic vertebral fractures (OVF), traumatic vertebral fractures, primary or secondary vertebral tumors, Spine Jack, Vertebral Body Stenting, OsseoFix
Introduction
At present there is no ‘consensus conference’ about the management of osteoporotic vertebral fractures (OVF) (1,2). The duration and indications for proceeding beyond conservative treatment of OVF remain unclear, and the surgical criteria are not distinctly defined. In the past, a conservative treatment for at least one month was deemed inappropriate for the majority of the vertebral fractures. However, when pain persisted after conservative treatment, it was necessary to consider surgical solutions including: vertebroplasty for vertebral fractures with height loss less than 30% and kyphoplasty for height loss greater than 30% (1,2). Currently, a treatment of this type is not feasible because it results in a poor quality of life, does not stop the domino effect and is associated with poor results.
Discussion
Vertebral augmentation procedures are quick and have the advantages of being performed percutaneously and under local anesthesia. The treatment of OVF has changed in recent years. The first-generation percutaneous vertebral augmentation system (PVAS), vertebroplasty, was described by Galibert et al. in 1987 (3). Although this approach produces good results in terms of pain relief, it does not restore the height of the vertebral body. Moreover, there is a risk of approximately 30% that cement leaks beyond the confines of the bone because it is necessary to inject a low viscosity cement at high pressure.
These problems prompted the development of the second-generation PVAS: the balloon kyphoplasty (4). This procedure involves the inflation of a balloon catheter inside the collapsed vertebral body which restores its height before the facture is stabilized with bone cement. The balloon creates a cavity inside the vertebral body into which a more viscous cement can be injected at lower pressure, thus considerably reducing the risk of leakage (5-10). Reducing the volume of cement as the primary stabilizer in vertebral compression fractures can also reduce the likelihood of cement leakage (11,12). Unfortunately, the recovery of vertebral body height may be only temporary as there is often a total or partial vertebral body collapse after the balloon is deflated (13-16).
This problem has prompted the development of new devices which can indefinitely restore the height of the vertebral body without using a conventional balloon with mechanical kyphoplasty, the third-generation of PVAS (17,18). Several third-generation PVASs are available but the most widely used are: the Vertebral Body Stenting System®, Spine Jack® and the OsseoFix Spinal Fracture Reduction System® (19). Although these systems have the same functions, they have differentiating technical features. The aim of this paper is to analyze these devices in order to identify the most suitable system depending on the patient’s age, etiopathogenesis, and fracture characteristics/morphology (20-23).
Vertebral Body Stenting System®
The Vertebral Body Stenting System® is an expandable, intrasomatic, titanium device for percutaneous vertebral augmentation (Figure 1). It aims to alleviate pain and restore the height of the fractured vertebra and consequently the normal curvature of the spine (24). This system uses the same balloon as in a standard kyphoplasty; this balloon is inserted and expanded into the vertebral body and can achieve an expansion ratio of 400%. By using ligamentotaxis, it is possible to produce a ‘concrete reduction of the fracture’, thus recovering the vertebral height and providing a cavity into which highly viscous poly (methylene methacrylate) (PMMA) can be injected (25,26). After the expansion phase, the balloon can be deflated and removed without risk of partial height loss because the device remains inside the vertebral body. VBS is usable for vertebral compression fractures from T5 to L5 (24-26) (Figures 2,3,4).
Figure 1.
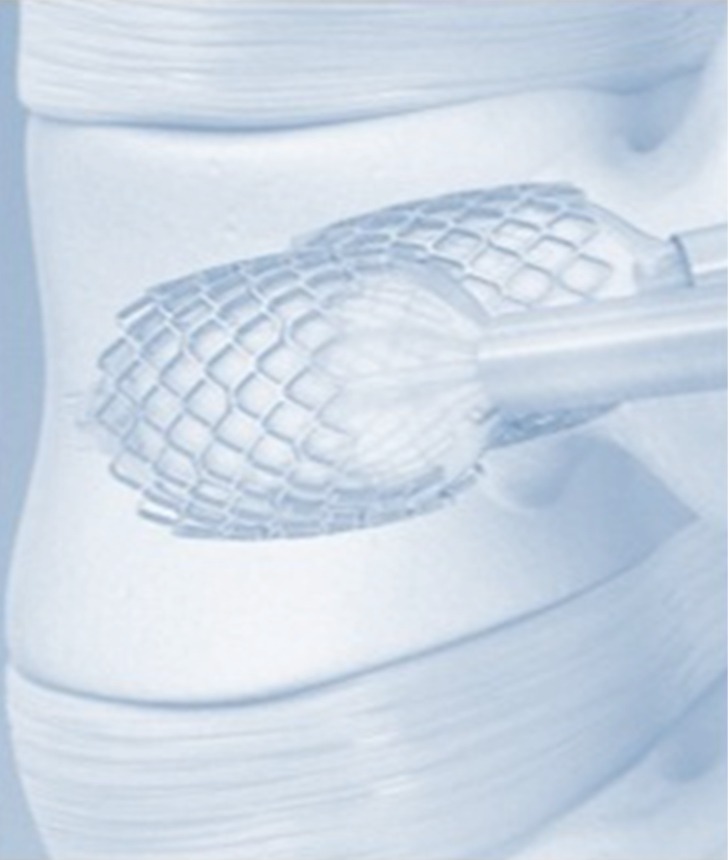
Vertebral Body Stenting®.
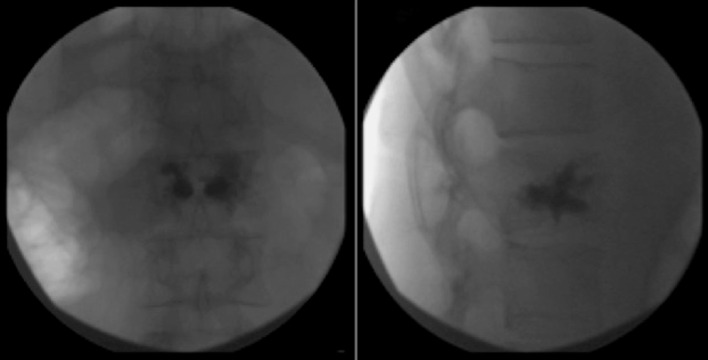
Figure 2.
Osteoporotic vertebral fractures: pre-operative image.
Figure 3.
Osteoporotic vertebral fractures: Vertebral Body Stenting® post-operative image.
Figure 4.
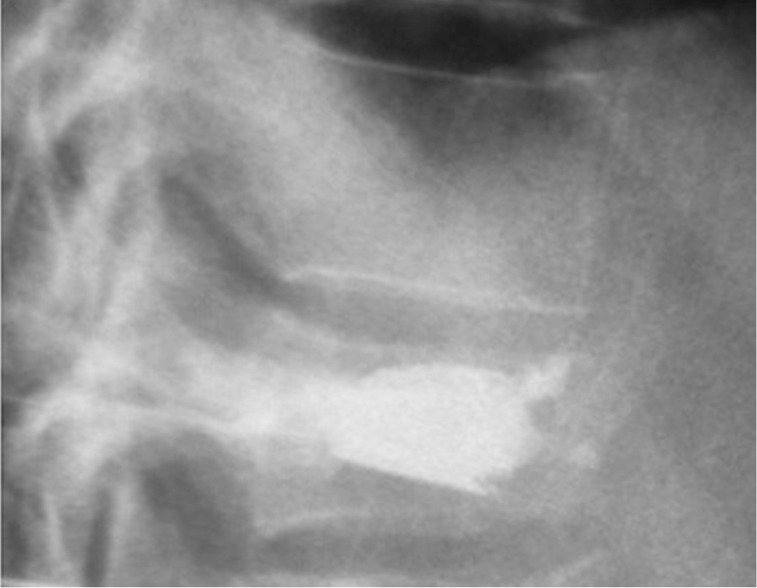
Osteoporotic vertebral fractures: Vertebral Body Stenting® post-operative image.
The indications for VBS are: osteoporotic vertebral compression fractures from T10-L5 without involvement of the posterior vertebral edge classified after Genant, grade 2 and grade 3 with a kyphotic angulation of more than 15 (21,22); fractures without involvement of the posterior vertebral edge, according to the American Orthopaedic (AO) classification (24,27,28): A1.1, A1.2, A1.3, A3.1, depending on the degree of posterior wall involvement. The contraindications are: A2, A3.3, B1.1, B2.1, B3 and C types.
Extensive mechanical and biomechanical tests have been performed to characterize the mechanical behavior and performance of the VBS (25-28). The preclinical testing of the VBS was designed to evaluate all relevant loading conditions to demonstrate a reasonable assurance of safety. These biomechanical tests demonstrated a significant reduction in height loss after balloon deflation when using the VBS as compared to balloon kyphoplasty. For example, VBS was able to maintain the pre-fracture height and avoid the loss of height which was measured with balloon deflation in kyphoplasty. In comparison to balloon kyphoplasty (28), VBS showed positive clinical results because it significantly relieved pain and improved function. At twelve months, Visual Analogue Score (VAS) and Oswestry Disability Index (ODI) were reduced by 6.4 points and 41.7%, respectively. Additionally, at twelve months a substantial improvement of the vertebral body height to 15.3% was observed, as well as a good kyphotic correction to 4.5°. The risk of adjacent vertebral fracture was estimated to be 9%, which is similar to (or slightly lower than) the rates reported in the literature for balloon kyphoplasty. We conclude that VBS can be considered a safe and effective surgical technique for the treatment of vertebral fractures and osteoporotic vertebral compression fractures with a low rate of adverse events (24-28).
Spine Jack®
Spine Jack® is a new device for mechanical kyphoplasty (Figure 5) (18,29) . It is a titanium implant designed to restore the height of the vertebral body in OVF, primary or secondary bone tumors, or traumatic fractures (A1.1, A1.2, A1.3, A3.1 and type B in selected cases, according to AO classification). Using a bilateral transpedicular approach, the Spine Jack® is inserted into the vertebral body from T10 to L5, and gradually expanded (Figures 6,7,8). The distraction caused by the device reduces the fracture by ligamentotaxis, especially when acting on the anterior longitudinal ligament. The device includes a mechanical (as opposed to hydraulic) working system which allows a progressive and controlled reduction of the vertebral fracture. This feature facilitates the recovery of the collapsed vertebra and provides 3D support to the structure which is required to mechanically stabilize the vertebrae in axial compression (30). After the reduction, PMMA is injected into the vertebral body in order to stabilize the reduction.
Figure 5.
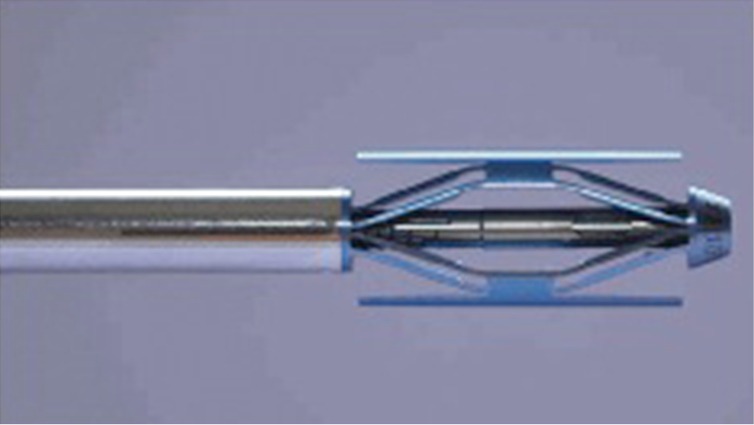
Spine Jack®.
Figure 6.
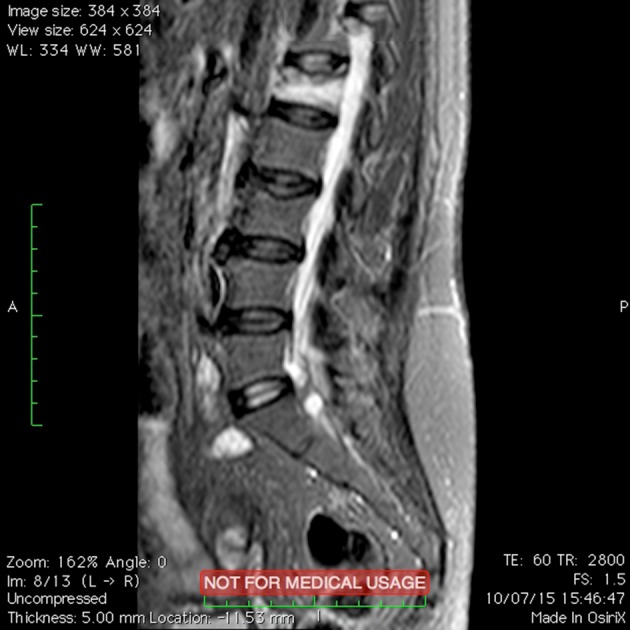
Osteoporotic vertebral fractures: pre-operative image.
Figure 7.
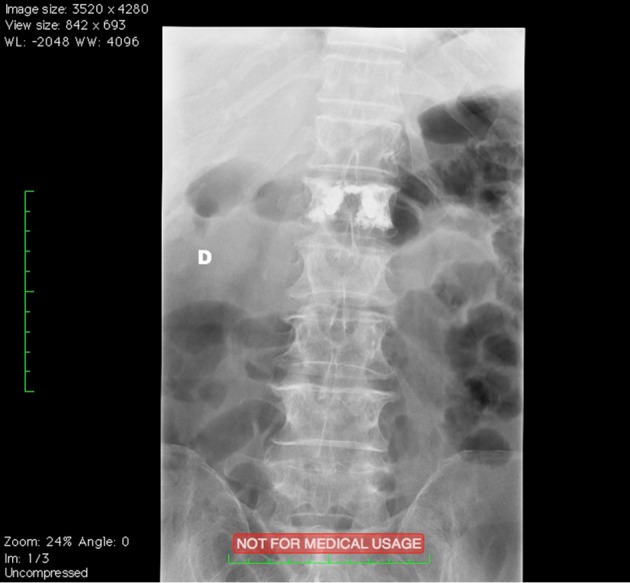
Osteoporotic vertebral fractures: Spine Jack® post-operative image.
Figure 8.
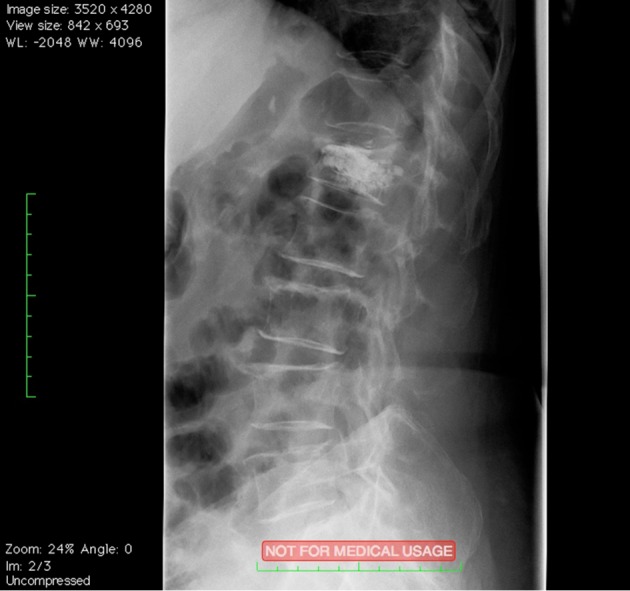
Osteoporotic vertebral fractures: Spine Jack® post-operative image.
The use of two devices symmetrically positioned inside the vertebral body allows a homogeneous spreading of the PMMA (18,29,30). The expansion of the device causes a preferential direction of the flow of the PMMA thus reducing the risk of leakage. When injected, the inter-digitation of the PMMA produces a large contact area below the midline; this is essential in order to confer stability to the vertebral body. This device also allows a reduction in the risk of leakage into intervertebral disc space, reducing the risk of the fractures of the adjacent vertebral body. The device has a ‘self-locking security system’ through which extreme load forces concentrated on the devices cause the system to automatically block itself (29,30). This restricts further expansion of the device, and the risk of vertebral endplate breakage is significantly reduced. Furthermore this reduction of the superior endplate allows a better functional recovery of the injured disc. It has been shown that Spine Jack® represents a safe and effective system for the treatment of vertebral fractures (18,29,30).
Spine Jack® and kyphoplasty were compared using two homogenous groups to evaluate the differences across age, gender distribution, fracture location, duration between injury and surgery, pre-operative VAS score, vertebral body height, and kyphotic wedge angle (Figures 4,5) (30). The radiographic parameters that were evaluated include: post-operative anterior vertebral body height, preoperative anterior vertebral body height, cephalic anterior vertebral body height and caudal anterior vertebral body height. The value of the recovery of the vertebral body height was estimated using Eq. [1] (30).
| [1] |
In order to assess the recovery of the vertebral height, vertebral height was calculated before and after the PVAS; a semi-quantitative assessment was also used by classifying the recovery in to three categories: grade 0 (no change), grade 1 (below 50%) and grade 2 (greater than 50%). It was shown that the post-operative increase in vertebral body height was greater in the Spine Jack® group than in the kyphoplasty group (P<0.05). 85% of the patients who underwent PVAS using the Spine Jack® system were classified as grade 2 (Figures 6,7), 12% as grade 1 and 3% as grade 0. 58% of the patients who underwent balloon kyphplasty were classified as grade 2, 26% as grade 1 and 16% as grade 0 (30).
It is important to note that Spine Jack® is not only useful for the treatment of OVF but also for traumatic fractures, and primary or secondary bone tumors. The system is able to produce a large force of elevation thus reducing the ‘old’ vertebral fractures (also known as ‘inveterate’ collapses) in which signs of bone marrow edema are still detectable on an MRI scan. Spine Jack® is the only third-generation device for PVAS capable of treating this type of fracture. We conclude that this device allows a safe and efficient restoration of vertebral body height in comparison to the conventional balloon kyphoplasty (30).
OsseoFix® Spine Fracture Reduction System
OsseoFix® Spine Fracture Reduction System (AlphaTec Spine Inc. Carlsbad CA, USA) is an expandable titanium device developed for the treatment of OVF (Figure 9). It is made of a titanium mesh which expands into the vertebral body with the aim of reducing the vertebral fractures and decreasing the kyphotic deformity by compacting the surrounding trabecular bone (31). OsseoFix® is usable for vertebral compression fractures from T6 to L5 in stable vertebral fractures (type A1.1 to A1.3 or A3.1, according to AO classification). This device is also useful in the treatment of acute stable traumatic vertebral fractures of the same type in young patients. The contraindications are: vertebral fractures with retropulsed fragment and dural sac or spinal cord compression, previous treatment at the same level, systemic or local infections, anaphylactic reactions to iodine-based compounds, cancer, irreversible coagulopathies, pre-existing calcium disorders, renal failure or psychiatric disorders (31,32).
Figure 9.
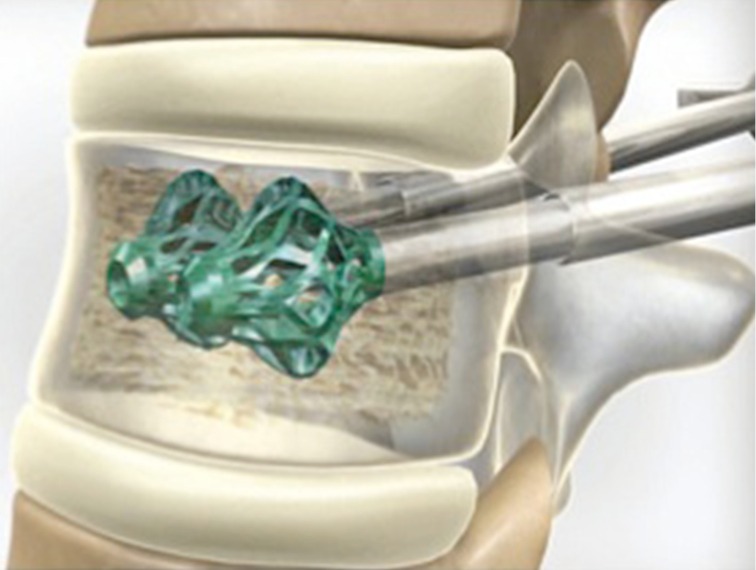
OsseoFix®.
This device is inserted into the vertebral body, targeting its tip to the anterior third of the vertebral body, and subsequently expanding the screw handle. In contrast to kyphoplasty or other balloon-assisted tenoplasty, this device remains in place so that the cavity created after the expansion is stable. The surrounding trabecular bone is compacted outward by the device (Figures 10,11) (33). This operation has two advantages: the compaction of the trabecular bone leads to an increase in the vertebral body height, thus reducing the kyphotic deformity and the interdigitation of trabecular bone into the mesh stabilizes the system itself. After this process, PMMA is injected inside the expanded device; significantly less PMMA is required in comparison to a standard kyphoplasty. Moreover the PMMA creates an interdigitation between the cells of the titanium mesh and the surrounding bone, further stabilizing the system (32). Because the trabecular bone surrounding the devices is finally compacted, this system is also able to reduce the risk of cement leakage (33). For these reasons, OsseoFix® provides both immediate and long-term effectiveness in reducing pain and kyphotic deformities secondary to vertebral compression fractures (31-33).
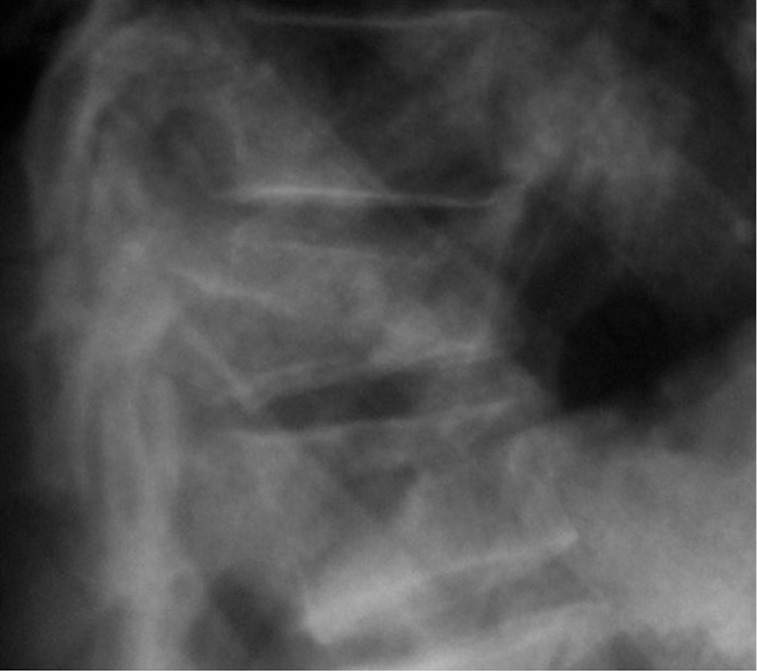
Figure 10.
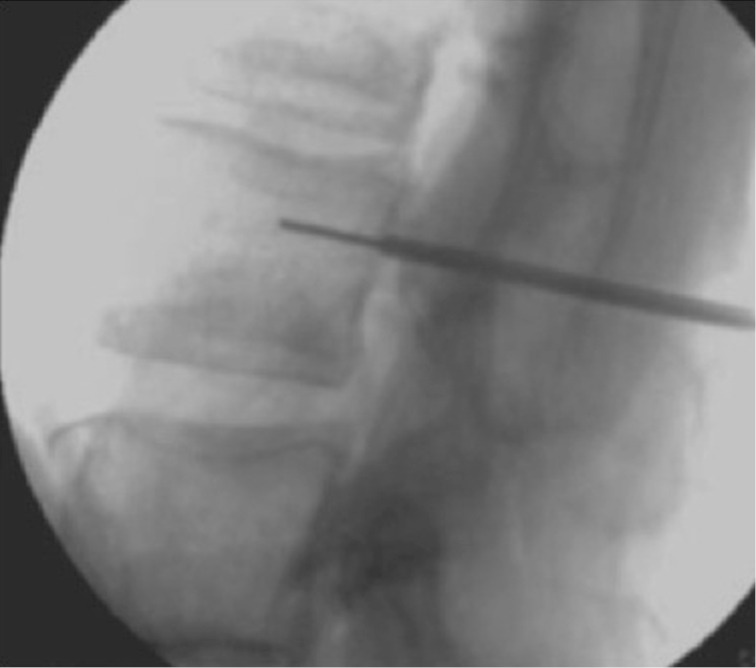
Osteoporotic vertebral fractures: intra-operative image.
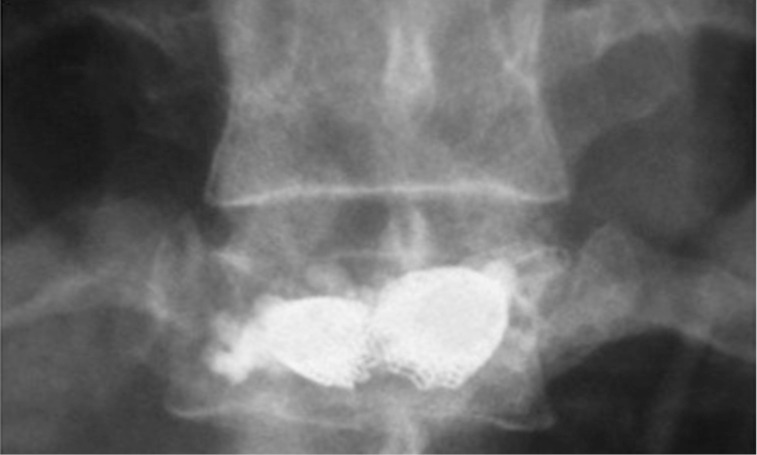
Figure 11.
Osteoporotic vertebral fractures: OsseoFix® post-operative image.
Conclusions
In conclusion, there are various devices available, but each of them has its own characteristics and its specific methods of operation. The VBS is a titanium device provided with a hydraulic (as opposed to mechanical) working system which could be responsible for a partial and not immediate possibility to control the opening of the device (19). On the other hand, OsseoFix® and Spine Jack® are provided with a mechanical working system which allows a progressive and controlled reduction of the vertebral fracture (19). Another important aspect to consider is the recovery of the vertebral body height. OsseoFix® has an indirect mechanism of action in which the compaction of the trabecular bone leads to an increase in vertebral body height (19). In this case there is no direct lift mechanism, which is the operating mode of Vertebral Body Stenting® and Spine Jack® (19). Therefore, in our opinion, Spine Jack® is the only device suitable for the treatment of both OVF, traumatic fracture (recent, old or inveterate) and primary or secondary bone tumors because it has a mechanical working system and direct lift mechanism (Table 1) (19).
Table 1. Characteristics of ‘third generation’ devices.
| Device | Pathology | Intended use | Purpose | Working | Type | Access | Material |
|---|---|---|---|---|---|---|---|
| Kyphon | Vertebral body fractures (VBF) | Recent trauma, osteoporosis, metastasis | Fracture reduction and cavity creation for cement | Balloon | Not permanent | Percutaneous | Elastomer |
| Vertebral body stenting (VBS) | VBF | Recent trauma, osteoporosis, metastasis | Fracture reduction and cavity creation for cement | Balloon + deformable metallic component | Permanent | Percutaneous | Titanium |
| Spine Jack | VBF | Recent and not recent trauma, osteoporosis metastasis | Fracture reduction and cavity creation for cement | Deformable metallic component | Permanent | Percutaneous | Titanium |
| OsseoFix | VBF | Osteoporotic collapse | Fracture reduction and cavity creation for cement | Deformable metallic component | Permanent | Percutaneous | Titanium |
VBF, vertebral body factures.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Cortet B, Blotman F, Debiais F, et al. Management of osteoporosis and associated quality of life in post menopausal women. BMC Musculoskelet Disord 2011;12:7. 10.1186/1471-2474-12-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rao RD, Singrakhia MD. Painful osteoporotic vertebral fracture. Pathogenesis, evaluation, and roles of vertebroplasty and kyphoplasty in its management. J Bone Joint Surg Am 2003;85-A:2010-22. [PubMed] [Google Scholar]
- 3.Galibert P, Deramond H, Rosat P, et al. Preliminary note on the treatment of vertebral angioma by percutaneous acrylic vertebroplasty. Neurochirurgie 1987;33:166-8. [PubMed] [Google Scholar]
- 4.McGirt MJ, Parker SL, Wolinsky JP, et al. Vertebroplasty and kyphoplasty for the treatment of vertebral compression fractures: an evidenced-based review of the literature. Spine J 2009;9:501-8. 10.1016/j.spinee.2009.01.003 [DOI] [PubMed] [Google Scholar]
- 5.Luo J, Adams MA, Dolan P. Vertebroplasty and Kyphoplasty Can Restore Normal Spine Mechanics following Osteoporotic Vertebral Fracture. J Osteoporos 2010;2010:729257. [DOI] [PMC free article] [PubMed]
- 6.Garfin SR, Yuan HA, Reiley MA. New technologies in spine: kyphoplasty and vertebroplasty for the treatment of painful osteoporotic compression fractures. Spine (Phila Pa 1976). 2001;26:1511-5. 10.1097/00007632-200107150-00002 [DOI] [PubMed] [Google Scholar]
- 7.Mathis JM, Ortiz AO, Zoarski GH. Vertebroplasty versus kyphoplasty: a comparison and contrast. AJNR Am J Neuroradiol 2004;25:840-5. [PMC free article] [PubMed] [Google Scholar]
- 8.Felder-Puig R, Piso B, Guba B, et al. Kyphoplasty and vertebroplasty for the management of osteoporotic vertebral compression fractures: a systematic review. Der Orthopade 2009,38:606-15. 10.1007/s00132-009-1446-2 [DOI] [PubMed] [Google Scholar]
- 9.Khurjekar K, Shyam AK, Sancheti PK, et al. Correlation of kyphosis and wedge angles with outcome after percutaneous vertebroplasty: a prospective cohort study. J Orthop Surg (Hong Kong) 2011;19:35-40. [DOI] [PubMed] [Google Scholar]
- 10.Bula P, Lein T, Strassberger C, et al. Balloon kyphoplasty in the treatment of osteoporotic vertebral fractures: indications - treatment strategy - complications. Z Orthop Unfall 2010;148:646-56. [DOI] [PubMed] [Google Scholar]
- 11.Minenna G, D'Amore S, D'Amore M. Complication after vertebroplasty in patient with senile osteoporosis: strategical therapy to revise? Recenti Prog Med 2006;97:389-92. [PubMed] [Google Scholar]
- 12.Al-Nakshabandi NA. Percutaneous vertebroplasty complications. Ann Saudi Med 2011;31:294-7. 10.4103/0256-4947.81542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feltes C, Fountas KN, Machinis T, et al. Immediate and early postoperative pain relief after kyphoplasty without significant restoration of vertebral body height in acute osteoporotic vertebral fractures. Neurosurg Focus 2005;18:e5. 10.3171/foc.2005.18.3.6 [DOI] [PubMed] [Google Scholar]
- 14.Rotter R, Martin H, Fuerderer S, et al. Vertebral body stenting: a new method for vertebral augmentation versus kyphoplasty. Eur Spine J 2010;19:916-23. 10.1007/s00586-010-1341-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ishiguro S, Kasai Y, Sudo A, et al. Percutaneous vertebroplasty for osteoporotic compression fractures using calcium phosphate cement. J Orthop Surg (Hong Kong) 2010;18:346-51. [DOI] [PubMed] [Google Scholar]
- 16.Heini PF, Teuscher R. Vertebral body stenting / stentoplasty. Swiss Med Wkly 2012;142:w13658. [DOI] [PubMed] [Google Scholar]
- 17.Fields AJ, Lee GL, Keaveny TM. Mechanisms of initial endplate failure in the human vertebral body. J Biomech 2010;43:3126-31. 10.1016/j.jbiomech.2010.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Noriega D, Ardura F, Beyerlein J, et al. Clinical results for the use of a new extensible cranio-caudal implant for the treatment of vertebral compression fractures results of a prospective, multicentre study after 1 year of follow-up. Eur Spine J 2012;21:1415-39. [Google Scholar]
- 19.Grillea G, Guelfi M, Levi V, et al. Third generation percutaneous vertebral augmentation procedures: update and future perspectives. 2014, Nova Science Publishers. [Google Scholar]
- 20.Ha KY, Kim YH, Chang DG, et al. Causes of late revision surgery after bone cement augmentation in osteoporotic vertebral compression fractures. Asian Spine J 2013;7:294-300. 10.4184/asj.2013.7.4.294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joseph RN, Swift AJ, Maliakal PJ. Single centre prospective study of the efficacy of percutaneous cement augmentation in the treatment of vertebral compression fractures. Br J Neurosurg 2013;27:459-64. 10.3109/02688697.2012.752431 [DOI] [PubMed] [Google Scholar]
- 22.Krüger A, Oberkircher L, Figiel J, et al. Height restoration of osteoporotic vertebral compression fractures using different intravertebral reduction devices: a cadaveric study. Spine J 2015;15:1092-8. 10.1016/j.spinee.2013.06.094 [DOI] [PubMed] [Google Scholar]
- 23.Bornemann R, Koch EM, Wollny M, et al. Treatment options for vertebral fractures an overview of different philosophies and techniques for vertebral augmentation. Eur J Orthop Surg Traumatol 2014;24 Suppl 1:S131-43. 10.1007/s00590-013-1257-3 [DOI] [PubMed] [Google Scholar]
- 24.Disch AC, Schmoelz W. Cement augmentation in a thoracolumbar fracture model: reduction and stability after balloon kyphoplasty versus vertebral body stenting. Spine (Phila Pa 1976) 2014;39:E1147-53. 10.1097/BRS.0000000000000470 [DOI] [PubMed] [Google Scholar]
- 25.Fürderer S, Anders M, Schwindling B, et al. Vertebral body stenting. A method for repositioning and augmenting vertebral compression fractures. Orthopade 2002;31:356-61. [DOI] [PubMed] [Google Scholar]
- 26.Klezl Z, Majeed H, Bommireddy R, et al. Early results after vertebral body stenting for fractures of the anterior column of the thoracolumbar spine. Injury 2011;42:1038-42. 10.1016/j.injury.2011.04.006 [DOI] [PubMed] [Google Scholar]
- 27.Diel P, Röder C, Perler G, et al. Radiographic and safety details of vertebral body stenting: results from a multicenter chart review. BMC Musculoskelet Disord 2013;14:233. 10.1186/1471-2474-14-233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Werner CM, Osterhoff G, Schlickeiser J, et al. Vertebral body stenting versus kyphoplasty for the treatment of osteoporotic vertebral compression fractures: a randomized trial. J Bone Joint Surg Am 2013;95:577-84. 10.2106/JBJS.L.00024 [DOI] [PubMed] [Google Scholar]
- 29.Krüger A, Oberkircher L, Flossdorf F, et al. Differences in the restoration of vertebral height after treatment of osteoporotic vertebra compression fractures: Cadaver study. Eur Spine J 2012;21:1415-9. [Google Scholar]
- 30.Vanni D, Pantalone A, Bigossi F, et al. New perspective for third generation percutaneous vertebral augmentation procedures: Preliminary results at 12 months. J Craniovertebr Junction Spine 2012;3:47-51. 10.4103/0974-8237.116537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eschler A, Ender SA, Ulmar B, et al. Cementless fixation of osteoporotic VCFs using titanium mesh implants (OsseoFix): preliminary results. Biomed Res Int 2014;2014:853897. [DOI] [PMC free article] [PubMed]
- 32.Ender SA, Gradl G, Ender M, et al. Osseofix® system for percutaneous stabilization of osteoporotic and tumorous vertebral compression fractures - clinical and radiological results after 12 months. Rofo 2014;186:380-7. [DOI] [PubMed] [Google Scholar]
- 33.Ender SA, Wetterau E, Ender M, et al. Percutaneous Stabilization System Osseofix® for Treatment of Osteoporotic Vertebral Compression Fractures - Clinical and Radiological Results after 12 Months. PLoS One 2013;8:e65119. 10.1371/journal.pone.0065119 [DOI] [PMC free article] [PubMed] [Google Scholar]