Abstract
Background
Patient dissatisfaction with donor site morbidity has led to the search for alternative grafting options and techniques. This report compares patient satisfaction rates between autograft and graft substitutes for anterior cervical discectomy and fusion (ACDF).
Methods
This study was performed with the approval of the local area health network ethics committee. Over a 9-year period, the author performed 574 ACDF procedures (697 levels). Of these, 22 patients had previous surgery with autograft, with a subsequent ACDF procedure performed using a graft substitute. Patients rated their satisfaction with pain, recovery, and preference of autograft versus a bone graft substitute. Graft substitutes used include: tricalcium phosphate/hydroxyapatite (TCP/HA) composite and iFactor placed within a polyetheretherketone (PEEK) cage.
Results
Results demonstrated that 21/22 patients achieved a solid fusion with the graft substitute. A total of 20/22 patients rated the autograft incision more painful than the anterior cervical incision, and 21/22 preferred the graft substitute. Three patients had adjacent segment disc replacement performed after autograft/ACDF. All patients reported preference for the total disc replacement (TDR) procedure in terms of recovery and postoperative pain.
Conclusions
Patient satisfaction with bone graft substitutes is very high compared with autograft with all but one (21/22) preferring the graft substitute option. The author questions the traditional recommendation that autograft is the “gold standard” for ACDF. In modern age of graft substitutes, autograft should not be considered the gold standard, but an index option between other options for comparison.
Keywords: Anterior cervical fusion, biphasic calcium phosphate, polyetheretherketone (PEEK), plating, synthetic bone graft
Introduction
Interbody fusion following anterior cervical decompression is a widely accepted procedure in patients suffering degenerative or posttraumatic conditions of the cervical spine comprising of: spondylosis, stenosis, herniated intervertebral discs and ossification of the posterior longitudinal ligament (1-3). Due to the debilitating nature of such conditions, patients often suffer from significant reductions in quality of life in addition to functional disability. Consequently, the goals of surgical intervention are decompression of neural elements through removal of the pathological intervertebral disc and restoration of spinal alignment and stability.
The advantages of an anterior approach are plentiful including: minimal soft tissue injury, direct visualisation of the pathology and decompression site, and increased accessibility facilitating easier removal of damaged disk and overall fusion (4-6). Further, although some complications may be severe, reported rates remain extremely minimal—with most due to surgical error or graft harvesting (1).
As evidenced in the Cochrane library, autograft remains the traditional gold standard in anterior cervical discectomy and fusion (ACDF) to achieve a solid fusion, though competitive options are rising throughout available literature (5,7-12). Currently, the graft harvesting procedure can result in a range of complications and both short- and long-term morbidity, namely donor site pain, haematoma, lateral cutaneous nerve palsy and infection (13,14). As a comparison, allograft, which gained popularity in the effort to circumvent the need for autograft, has its own associated complications including the risk of disease transmission, infection and histocompatibility differences (15). Graft collapse and pseudoarthrosis has been seen in autograft fusion without plating (16). The impetus behind the creation of intervertebral cages with bone graft substitute technologies has been to minimise or eliminate autograft use with the aim of improving recovery time and clinical outcomes (14,17).
In this study, we evaluate a cohort of patients whom have had a previous ACDF procedure with autograft, and have subsequently required a further ACDF procedure due to adjacent segment degeneration. To maintain a common set of indications and follow-up criteria, the data was collected from a single surgeon series (RJM). The second procedure was performed with a bone graft substitute, made of either iFactor or tricalcium phosphate (TCP) (both Mastergraft TCP and KG Bone). The former represents a newly used allograft comprised of anorganic bone matrix and small peptide-15, demonstrated to play a beneficial role in the setting of spinal surgery (11,17). Patient satisfaction was inquired with regards to the harvest of bone graft versus a substitute, length of stay in hospital and overall patient preference of autograft versus a substitute were also recorded.
Methods
Study approval
Ethics approval was obtained from the South Eastern Sydney Local Health District–Northern Sector (SESLHD-NS) ethics committee under the code HREC 11/183. Written informed consent for participation in the study was obtained from all participants undergoing surgery with the senior author (RJM).
Patient data
Over a 108-month time period (2004–2012), from a single surgeon series of anterior cervical surgery performed for all indications, 574 patients (697 levels) were operated with data prospectively collected. A total of 22 patients were identified as having had a previous ACDF procedure with autograft. The author performed 2 of the original 22 autograft/ACDF procedures with the other 20 performed elsewhere. Seven patients had revision ACDF without anterior cervical plating. Length of stay was documented for the second procedure, while patients were questioned as to the length of stay from the original procedure when this information was not available via previous records.
Inclusion criteria were patients having a revision ACDF on a background of a previous fused ACDF using autograft, with the revision procedure performed using an available bone graft substitute.
Surgical procedure
All revision ACDF patients were operated (RJM) on using interbody grafting with either a TCP/HA composite or iFactor by the same surgeon. A modified Smith-Robinson technique was employed under general anaesthesia for all operations. Following a right antero-lateral incision, Caspar retracting pins were positioned in the adjacent vertebral bodies for adequate distraction. If a previous anterior plate was present it was removed prior to the revision. Under the direct observation of an operating microscope, removal of pathological disc was performed using rongeurs, curettes and a high-speed drill. Osteophytes were removed and the posterior longitudinal ligament divided. In all cases, complete decompression and visualization of the dura and nerve roots was achieved. Decortication of the vertebral endplates was performed to optimize the bone-graft interface.
A trial cage was inserted to confirm the height of the disc space. Either a biphasic calcium phosphate (KG Bone, Kasios Biomaterials or Mastergraft, Medtronic) or iFactor (P15/ABM) was packed into the center of a polyetheretherketone (PEEK) cage. The interbody implant was inserted using forceps and tapped into place in a standard fashion.
With the implant in place, anterior plate fixation was inserted for the plated group. Antero-posterior and lateral plain radiographs were obtained intra-operatively to check correct positioning before wound closure. All non-plated were advised to wear a cervical orthosis postoperatively for a period of 6 weeks.
Interbody graft
There were 3 types of bone graft substitutes used: Mastergraft TCP with Bone Marrow Aspirate (BMA) (Medtronic/USA)—3 patients, KG Bone with BMA (Kasios Biomaterials, France)—16 patients, and iFactor (Cerapedics, USA)—3 patients. The biology and fusion rates of bone graft substitutes is discussed in detail elsewhere and will not be discussed here (18,19).
Outcome measures
A prospective review of patient files and imaging was performed to determine clinical and radiographic outcome following anterior cervical spine surgery. The author questioned all patients at 3 months following the revision procedure to assess their satisfaction with the procedure and comparisons with their prior ACDF. The questions and data are summarised in the results.
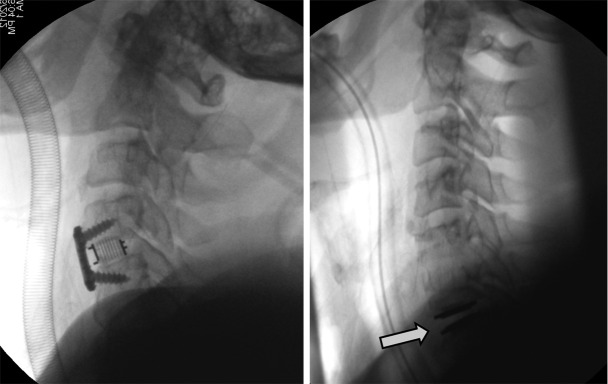
Radiographic fusion was assessed at every follow-up by an independent radiologist. Plain radiographs were the first choice of modality for radiographic assessment. Ethics board approval for fusion assessment was for X-ray studies, including flexion/extension radiographs. Approval for CT scan was given only if there was the suggestion or potential for non-union. Radiographs were routinely taken intra-operatively then at 1 day, 6 weeks, 3 months, 6 months and 1 year postoperatively. Fusion was considered successful if bridging bone incorporating the graft and adjoining endplates was apparent (see Figure 1), with additional loss of radiolucency, restoration of interbody space and no hardware failure. Lack of movement on flexion/extension X-rays were also used to confirm status. If required, computed tomography (CT) was performed to verify the fusion status.
Figure 1.
(A) Adjacent segment degeneration with ACDF at C4/5 above a previous C5/6 fusion; (B) C6/7 Total Disc Arthoplasty (M6C, Spinal Kinetics/USA) below a previous C5/6 fusion.
Statistical analysis
Descriptive data are represented as means ± standard deviation (range, minimum–maximum). All data sets were tested for normality with the D’Agostino and Pearson omnibus normality test. Nonparametric data was analyzed using the Mann-Whitney U test and parametric unrelated data with the unpaired t-test for comparison of the results between the Plated and Non-Plated Groups. A paired t-test was used for comparison between pre- and postoperative continuous variables within patient groups. Statistical significance was set at level of P<0.05. All analyses and graphs were generated using a commercial software package (GraphPad Prism version 5.01, GraphPad Software, Inc., USA).
Results
From 579 patients in the original prospective dataset, 22 patients were identified as having an initial ACDF operation with autograft, then a subsequent ACDF procedure with a bone graft substitute. A further 3 patients had an adjacent segment revision procedure with a disc arthroplasty.
There were 16 males and 6 females, with a mean age of 64 years (range, 37–81 years). This represents an older mean age than most ACDF studies would quote due to this patient population being a revision surgical population rather than as an initial procedure. There were 3 smokers, 2 diabetics and 3 workers compensation cases. This data is summarized in Table 1.
Table 1. Demographic data of patients included in the study.
| Variables | Plated group |
|---|---|
| Total number | 22 |
| Age (years) | 64 [37–81] |
| Sex (M:F) | 16:6 |
| Tobacco smokers | 3 |
| Diabetics | 2 |
| Workers compensation | 3 |
| TCP/HA (KG Bone & Mastergraft) | 19/22 |
| iFactor (P15/ABM) | 3/22 |
| Fusion at 9 months (F/E X-ray) | 21/22 (%) |
| Preference: autograft/graft substitute | 1/22 vs. 21/22 |
| Length of stay—Initial procedure | 4.7 [2–13] days |
| Length of stay—Graft substitute | 1.8 [0–6] days |
TCP/HA, tricalcium phosphate/hydroxyapatite.
Radiological outcomes
Of the 22 patients studied, 21 patients had achieved fusion by 9 months with one non-union in a patient who had received a TCP graft (Mastergraft). All patients whom had the iFactor and KG-Bone TCP/hydroxyapatite (HA) graft achieved fusion based on either Flexion/Extension films or CT scan. Of the 3 patients who had a disc replacement adjacent to a previous autograft ACDF fusion, one patient had failure of the prosthesis at 15 months and required a posterior revision fusion procedure.
Patient satisfaction
Patients were asked a series of simple questions at the 3 months follow-up visit:
“Which incision was more painful in your recollection of the initial procedure, the hip or the neck incision?” A total of 20/22 (91%) patients rated the autograft incision more painful than the anterior cervical approach incision. Of the remaining 2 patients, one patient had a postoperative hematoma requiring drainage and stated that this experience was more concerning than the graft harvest incision. The remaining 1 patient had a poor recollection of the initial surgery;
“Would you rather the graft taken from your hip, or the synthetic graft that you had with your most recent surgery?” A total of 21/22 (96%) preferred the graft substitute option over the autograft option. A further 3 patients had an adjacent segment total disc replacement (TDR) performed after autograft/ACDF with all these 3 patients reporting a strong preference for the TDR procedure in terms of recovery and postoperative pain, based on the absence of hip pain with the TDR intervention;
“Which operation was more comfortable; the initial or the most recent?” A total of 24/25 (96%) preferred the second of the procedures, inclusive of the 3 patients who had a TDR procedure. The author accepts that the technical aspects of the ACDF/TDR procedure may be advanced as compared with the initial surgery due to advances in instrumentation and techniques and this may skew the results of this question.
Discussion
Autograft is still widely considered as the gold standard in ACDF (7). However donor site morbidity associated with autograft has fuelled the growing interest in alternative materials (6), namely ceramics, as fusion substrates for anterior cervical arthrodesis. Ceramics provide a safe option with demonstrated biocompatibility, osteoconductive potential, abundant and affordable supply, and a means of avoiding morbidity at the iliac crest. In this study the interbody spacer used in all cases was a PEEK cage as this provides a hard frame able to resist spinal loading and has an elastic modulus similar to that of bone, minimising graft subsidence and shrinkage (20). It is also able to maintain spinal alignment despite remodeling of the bone graft substitute within the cage cavity.
Amongst studies that have compared TCP vs. autograft, Cho et al. (20) conducted a prospective study of 40 patients who underwent one-level ACDF with a PEEK/TCP cage versus iliac crest autograft. The PEEK/TCP group achieved 100% solid fusion, increased cervical lordosis and increased height and cross-sectional area of foramina. Additionally, a minimal complication rate (2.5% experienced pharyngitis) was noted in the PEEK group compared with 17.5% complication rate in the autograft group (including graft collapse, dislodgement and donor site morbidity) (20). Similar outcomes were achieved in a study comparing PEEK cage filled with BCP to autograft, with the authors deeming this graft combination a suitable alternative to autograft, with shorter hospital stay, decreased operative time, less blood loss and no donor site complications (21,22).
The role of a PEEK/TCP combination without plating was also compared to autograft with and without plate fixation in multilevel ACDF (23). By 12 months the PEEK/TCP cage option and autograft with plate fixation demonstrated 100% and 98% fusion rates respectively whereas autograft alone achieved 87% fusion. Complication rates of autograft alone were also much higher at 50% (owing to graft collapse, pseudoarthrosis, dislodged graft) compared with 0% in the PEEK/TCP group and 4% in autograft with plating. Overall, the authors indicated preference for the PEEK/TCP cage in treating multilevel cervical degenerative disease due to its significantly lower complication rate.
Limitations
A chief limitation of this study is the relatively small numbers involved, with only 22 patients having had revision surgery out of a cohort of 576 patients that met the inclusion criteria. A review conducted by Hilibrand and Robbins concluded that the prevalence of adjacent segment disease (ASD) is 13.6% at 5 years follow up, with the annual incidence of ASD requiring additional surgery is between 1.5–4% (24). Although the figures for adjacent segment surgery in this study is effectively 25/576=4.3%, this likely represents the length of time the author has been in clinical practice rather than a true representation of the actual figure of symptomatic ASD.
Assessment of interbody fusion remains a challenge, across both autograft and allograft patients. As there are no universally accepted criteria for determining radiological fusion, it is often difficult to arrive at a true assessment of fusion based on plain radiography alone particularly when synthetic cages are utilized. Fine-cut CT scans with reconstructions have been shown to be more reliable and sensitive for the detection of pseudarthrosis than plain radiography, however subjecting patients to CT scanning at regular intervals purely for an assessment of fusion was deemed to be unnecessary, costly and potentially harmful to patients (25,26). We have only utilized CT scanning where there has been a query regarding fusion status or pseudoarthrosis in the context of unexpected/poor clinical outcomes and recurrence of symptoms at follow up (27). Future large sized multi-center prospective studies and registries (28-30) are required to further confirm the results presented in this study.
Conclusions
This is the first publication in the spine literature that reports on patient satisfaction comparing two bone grafting options in the same patient: autograft versus synthetic graft substitutes. Based on this single surgeon data, there is no doubt that patients prefer synthetic graft substitutes over the traditional gold standard of autograft. The author questions the concept that autograft is considered the “gold standard” graft option for ACDF. Autograft is not the preferred option for bone grafting based on the results presented here, with acceptable fusion rates between both options. Autograft should remain as the index graft option which other bone compares grafting options, however in clinical practice it should not be considered the “gold standard”.
Acknowledgements
None.
Ethical Statements: Ethics approval was obtained from the South Eastern Sydney Local Health District–Northern Sector (SESLHD-NS) ethics committee under the code HREC 11/183. Written informed consent for participation in the study was obtained from all participants undergoing surgery with the senior author (RJM).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Fountas KN, Kapsalaki EZ, Nikolakakos LG, et al. Anterior cervical discectomy and fusion associated complications. Spine (Phila Pa 1976) 2007;32:2310-7. 10.1097/BRS.0b013e318154c57e [DOI] [PubMed] [Google Scholar]
- 2.Fraser JF, Härtl R. Anterior approaches to fusion of the cervical spine: a metaanalysis of fusion rates. J Neurosurg Spine 2007;6:298-303. 10.3171/spi.2007.6.4.2 [DOI] [PubMed] [Google Scholar]
- 3.Moreland DB, Asch HL, Clabeaux DE, et al. Anterior cervical discectomy and fusion with implantable titanium cage: initial impressions, patient outcomes and comparison to fusion with allograft. Spine J 2004;4:184-91; discussion 191. 10.1016/j.spinee.2003.05.001 [DOI] [PubMed] [Google Scholar]
- 4.Jacobs WC, Anderson PG, Limbeek J, et al. Single or double-level anterior interbody fusion techniques for cervical degenerative disc disease. Cochrane Database Syst Rev 2004;(4):CD004958. [DOI] [PubMed] [Google Scholar]
- 5.Jacobs W, Willems PC, van Limbeek J, et al. Single or double-level anterior interbody fusion techniques for cervical degenerative disc disease. Cochrane Database Syst Rev 2011;(1):CD004958. [DOI] [PubMed] [Google Scholar]
- 6.Thomas KA, Toth JM, Crawford NR, et al. Bioresorbable polylactide interbody implants in an ovine anterior cervical discectomy and fusion model: three-year results. Spine (Phila Pa 1976) 2008;33:734-42. 10.1097/BRS.0b013e3181695716 [DOI] [PubMed] [Google Scholar]
- 7.Chau AM, Mobbs RJ. Bone graft substitutes in anterior cervical discectomy and fusion. Eur Spine J 2009;18:449-64. 10.1007/s00586-008-0878-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chau AM, Xu LL, Wong JH, et al. Current status of bone graft options for anterior interbody fusion of the cervical and lumbar spine. Neurosurg Rev 2014;37:23-37. 10.1007/s10143-013-0483-9 [DOI] [PubMed] [Google Scholar]
- 9.Chen F, He W, Mahaney K, et al. Alternative grafts in anterior cervical fusion. Clin Neurol Neurosurg 2013;115:2049-55. 10.1016/j.clineuro.2013.07.013 [DOI] [PubMed] [Google Scholar]
- 10.Dai LY, Jiang LS. Anterior cervical fusion with interbody cage containing beta-tricalcium phosphate augmented with plate fixation: a prospective randomized study with 2-year follow-up. Eur Spine J 2008;17:698-705. 10.1007/s00586-008-0643-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lauweryns P, Raskin Y. Prospective analysis of a new bone graft in lumbar interbody fusion: results of a 2- year prospective clinical and radiological study. Int J Spine Surg 2015;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller LE, Block JE. Safety and effectiveness of bone allografts in anterior cervical discectomy and fusion surgery. Spine (Phila Pa 1976) 2011;36:2045-50. 10.1097/BRS.0b013e3181ff37eb [DOI] [PubMed] [Google Scholar]
- 13.Schnee CL, Freese A, Weil RJ, et al. Analysis of harvest morbidity and radiographic outcome using autograft for anterior cervical fusion. Spine (Phila Pa 1976) 1997;22:2222-7. 10.1097/00007632-199710010-00005 [DOI] [PubMed] [Google Scholar]
- 14.Silber JS, Anderson DG, Daffner SD, et al. Donor site morbidity after anterior iliac crest bone harvest for single-level anterior cervical discectomy and fusion. Spine (Phila Pa 1976) 2003;28:134-9. 10.1097/00007632-200301150-00008 [DOI] [PubMed] [Google Scholar]
- 15.Samartzis D, Shen FH, Goldberg EJ, et al. Is autograft the gold standard in achieving radiographic fusion in one-level anterior cervical discectomy and fusion with rigid anterior plate fixation? Spine (Phila Pa 1976) 2005;30:1756-61. 10.1097/01.brs.0000172148.86756.ce [DOI] [PubMed] [Google Scholar]
- 16.Hacker RJ, Cauthen JC, Gilbert TJ, et al. A prospective randomized multicenter clinical evaluation of an anterior cervical fusion cage. Spine (Phila Pa 1976) 2000;25:2646-54; discussion 2655. 10.1097/00007632-200010150-00017 [DOI] [PubMed] [Google Scholar]
- 17.Mobbs RJ, Maharaj M, Rao PJ. Clinical outcomes and fusion rates following anterior lumbar interbody fusion with bone graft substitute i-FACTOR, an anorganic bone matrix/P-15 composite. J Neurosurg Spine 2014;21:867-76. 10.3171/2014.9.SPINE131151 [DOI] [PubMed] [Google Scholar]
- 18.Dusseldorp JR, Mobbs RJ. Iliac crest reconstruction to reduce donor-site morbidity: technical note. Eur Spine J 2009;18:1386-90. 10.1007/s00586-009-1108-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mobbs RJ, Chau AM, Durmush D. Biphasic calcium phosphate contained within a polyetheretherketone cage with and without plating for anterior cervical discectomy and fusion. Orthop Surg 2012;4:156-65. 10.1111/j.1757-7861.2012.00185.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cho DY, Liau WR, Lee WY, et al. Preliminary experience using a polyetheretherketone (PEEK) cage in the treatment of cervical disc disease. Neurosurgery 2002;51:1343-49; discussion 1349-50. [PubMed] [Google Scholar]
- 21.Cho DY, Lee WY, Sheu PC, et al. Cage containing a biphasic calcium phosphate ceramic (Triosite) for the treatment of cervical spondylosis. Surg Neurol 2005;63:497-503; discussion 503-4. 10.1016/j.surneu.2004.10.016 [DOI] [PubMed] [Google Scholar]
- 22.Chou YC, Chen DC, Hsieh WA, et al. Efficacy of anterior cervical fusion: comparison of titanium cages, polyetheretherketone (PEEK) cages and autogenous bone grafts. J Clin Neurosci 2008;15:1240-5. 10.1016/j.jocn.2007.05.016 [DOI] [PubMed] [Google Scholar]
- 23.Cho DY, Lee WY, Sheu PC. Treatment of multilevel cervical fusion with cages. Surg Neurol 2004;62:378-85, discussion 385-6. 10.1016/j.surneu.2004.01.021 [DOI] [PubMed] [Google Scholar]
- 24.Hilibrand AS, Robbins M. Adjacent segment degeneration and adjacent segment disease: the consequences of spinal fusion? Spine J 2004;4:190S-194S. 10.1016/j.spinee.2004.07.007 [DOI] [PubMed] [Google Scholar]
- 25.Carreon LY, Glassman SD, Djurasovic M. Reliability and agreement between fine-cut CT scans and plain radiography in the evaluation of posterolateral fusions. Spine J 2007;7:39-43. 10.1016/j.spinee.2006.04.005 [DOI] [PubMed] [Google Scholar]
- 26.Santos ER, Goss DG, Morcom RK, et al. Radiologic assessment of interbody fusion using carbon fiber cages. Spine (Phila Pa 1976) 2003;28:997-1001. 10.1097/01.BRS.0000061988.93175.74 [DOI] [PubMed] [Google Scholar]
- 27.Phan K, Kim JS, Lee NJ, et al. Relationship Between ASA Scores and 30-Day Readmissions in Patients Undergoing Anterior Cervical Discectomy and Fusion (ACDF). Spine (Phila Pa 1976) 2016. [Epub ahead of print]. 10.1097/BRS.0000000000001680 [DOI] [PubMed] [Google Scholar]
- 28.Phan K, Tian DH, Cao C, et al. Systematic review and meta-analysis: techniques and a guide for the academic surgeon. Ann Cardiothorac Surg 2015;4:112-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Phan K, Mobbs RJ. Systematic reviews and meta-analyses in spine surgery, neurosurgery and orthopedics: guidelines for the surgeon scientist. J Spine Surg 2015;1:19-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mobbs RJ, Phan K, Malham G, et al. Lumbar interbody fusion: techniques, indications and comparison of interbody fusion options including PLIF, TLIF, MI-TLIF, OLIF/ATP, LLIF and ALIF. J Spine Surg 2015;1:2-18. [DOI] [PMC free article] [PubMed] [Google Scholar]