Abstract
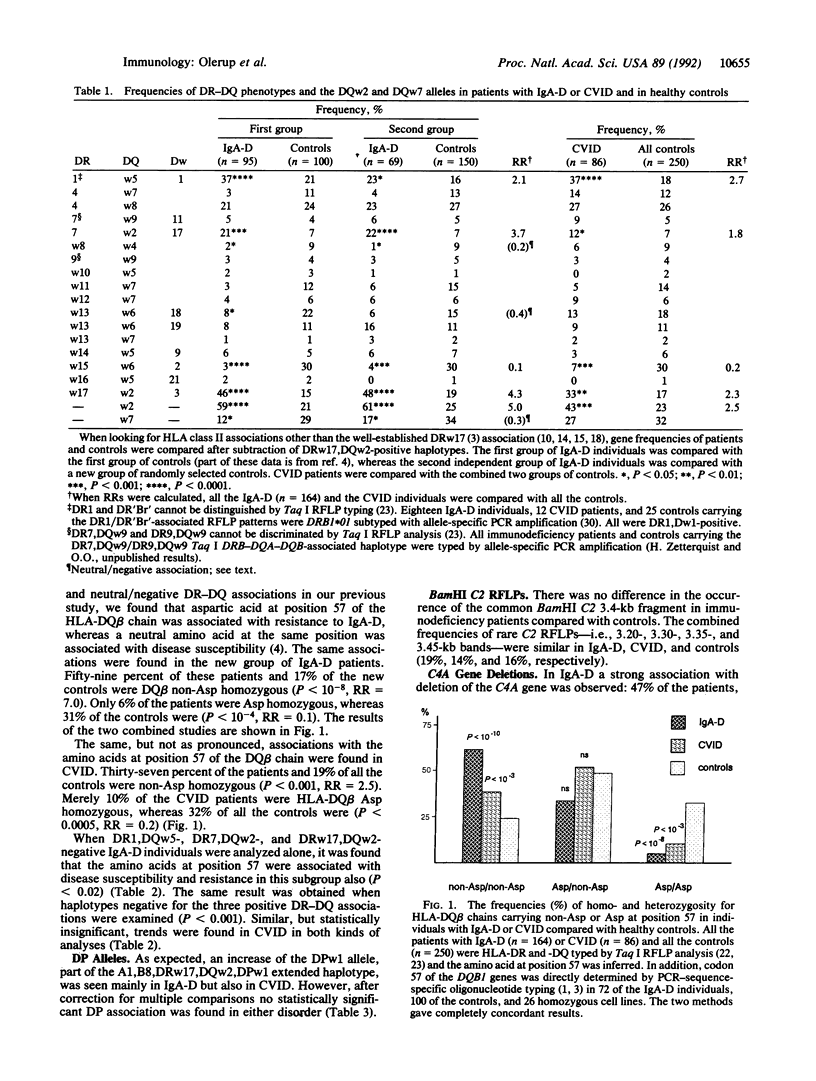
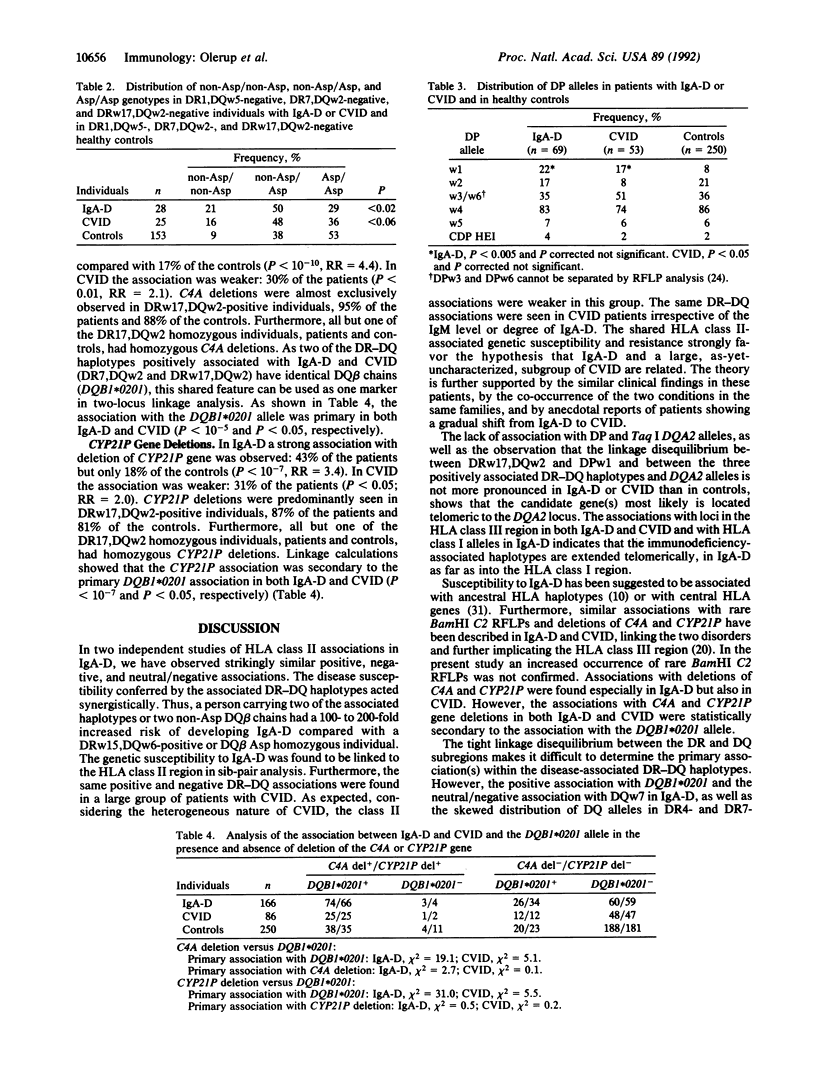
Most cases of selective IgA deficiency (IgA-D) and common variable immunodeficiency (CVID) occur sporadically. However, familial clustering is not uncommon, and the two disorders can occur within the same family. We have previously described positive associations with three DR-DQ haplotypes as well as a strong negative association with DRw15,DQw6,Dw2 in IgA-D. Different amino acids at position 57 of the HLA-DQ beta chain were found to be related to susceptibility and resistance to IgA-D. Now we have found identical, although somewhat weaker, positive and negative DR-DQ associations in a large group of CVID patients (n = 86), as well as the same associations with codon 57 of the DQB1 gene. In addition, we have confirmed our earlier observations in an independent group of IgA-D individuals (n = 69), and in sib-pair analysis we have found linkage of the genetic susceptibility to IgA-D to the HLA class II region. In IgA-D individuals not carrying the three overrepresented DR-DQ haplotypes, the same positive association with a non-aspartic acid residue at position 57 of the HLA-DQ beta chain was seen. The previously reported associations with deletions of the HLA class III genes C4A (fourth component of complement) and CYP21P (steroid 21-hydroxylase pseudogene) were, in our groups of immunodeficient individuals, statistically secondary to the association with the DQB1 allele 0201. The shared HLA class II associations in the two humoral immunodeficiencies support the hypothesis that IgA-D and CVID are related disorders. Disease susceptibility and resistance are most closely associated with a gene(s) within the DR-DQ region, alleles of the DQB1 locus being candidate genes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambrus M., Hernádi E., Bajtai G. Prevalence of HLA-A1 and HLA-B8 antigens in selective IgA deficiency. Clin Immunol Immunopathol. 1977 May;7(3):311–314. doi: 10.1016/0090-1229(77)90062-9. [DOI] [PubMed] [Google Scholar]
- Belt K. T., Carroll M. C., Porter R. R. The structural basis of the multiple forms of human complement component C4. Cell. 1984 Apr;36(4):907–914. doi: 10.1016/0092-8674(84)90040-0. [DOI] [PubMed] [Google Scholar]
- Bidwell J. L., Bidwell E. A., Savage D. A., Middleton D., Klouda P. T., Bradley B. A. A DNA-RFLP typing system that positively identifies serologically well-defined and ill-defined HLA-DR and DQ alleles, including DRw10. Transplantation. 1988 Mar;45(3):640–646. doi: 10.1097/00007890-198803000-00027. [DOI] [PubMed] [Google Scholar]
- Carlsson B., Wallin J., Böhme J., Möller E. HLA-DR-DQ haplotypes defined by restriction fragment analysis. Correlation to serology. Hum Immunol. 1987 Oct;20(2):95–113. doi: 10.1016/0198-8859(87)90025-5. [DOI] [PubMed] [Google Scholar]
- Cobain T. J., French M. A., Christiansen F. T., Dawkins R. L. Association of IgA deficiency with HLA A28 and B14. Tissue Antigens. 1983 Aug;22(2):151–154. doi: 10.1111/j.1399-0039.1983.tb01181.x. [DOI] [PubMed] [Google Scholar]
- Conley M. E., Cooper M. D. Immature IgA B cells in IgA-deficient patients. N Engl J Med. 1981 Aug 27;305(9):495–497. doi: 10.1056/NEJM198108273050905. [DOI] [PubMed] [Google Scholar]
- Cooper M. D., Lawton A. R., Bockman D. E. Agammaglobulinaemia with B lymphocytes. Specific defect of plasma-cell differentiation. Lancet. 1971 Oct 9;2(7728):791–794. doi: 10.1016/s0140-6736(71)92742-5. [DOI] [PubMed] [Google Scholar]
- Cuccia-Belvedere M., Monafo V., Martinetti M., Plebani A., De Paoli F., Burgio G. R. Recurrent extended HLA haplotypes in children with selective IgA deficiency. Tissue Antigens. 1989 Aug;34(2):127–132. doi: 10.1111/j.1399-0039.1989.tb01725.x. [DOI] [PubMed] [Google Scholar]
- French M. A., Dawkins R. L. Central MHC genes, IgA deficiency and autoimmune disease. Immunol Today. 1990 Aug;11(8):271–274. doi: 10.1016/0167-5699(90)90110-u. [DOI] [PubMed] [Google Scholar]
- Gregersen P. K., Silver J., Winchester R. J. The shared epitope hypothesis. An approach to understanding the molecular genetics of susceptibility to rheumatoid arthritis. Arthritis Rheum. 1987 Nov;30(11):1205–1213. doi: 10.1002/art.1780301102. [DOI] [PubMed] [Google Scholar]
- Hammarström L., Axelsson U., Björkander J., Hanson L. A., Möller E., Smith C. I. HLA antigens in selective IgA deficiency: distribution in healthy donors and patients with recurrent respiratory tract infections. Tissue Antigens. 1984 Jul;24(1):35–39. doi: 10.1111/j.1399-0039.1984.tb00395.x. [DOI] [PubMed] [Google Scholar]
- Hammarström L., Smith C. I. HLA-A, B, C and DR antigens in immunoglobulin A deficiency. Tissue Antigens. 1983 Jan;21(1):75–79. doi: 10.1111/j.1399-0039.1983.tb00375.x. [DOI] [PubMed] [Google Scholar]
- Hoddinott S., Dornan J., Bear J. C., Farid N. R. Immunoglobulin levels, immunodeficiency and HLA in type 1 (insulin-dependent) diabetes mellitus. Diabetologia. 1982 Oct;23(4):326–329. doi: 10.1007/BF00253738. [DOI] [PubMed] [Google Scholar]
- Horiuchi T., Macon K. J., Kidd V. J., Volanakis J. E. cDNA cloning and expression of human complement component C2. J Immunol. 1989 Mar 15;142(6):2105–2111. [PubMed] [Google Scholar]
- Horn G. T., Bugawan T. L., Long C. M., Erlich H. A. Allelic sequence variation of the HLA-DQ loci: relationship to serology and to insulin-dependent diabetes susceptibility. Proc Natl Acad Sci U S A. 1988 Aug;85(16):6012–6016. doi: 10.1073/pnas.85.16.6012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe H. S., So A. K., Farrant J., Webster A. D. Common variable immunodeficiency is associated with polymorphic markers in the human major histocompatibility complex. Clin Exp Immunol. 1991 Mar;83(3):387–390. doi: 10.1111/j.1365-2249.1991.tb05648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyldig-Nielsen J. J., Morling N., Odum N., Ryder L. P., Platz P., Jakobsen B., Svejgaard A. Restriction fragment length polymorphism of the HLA-DP subregion and correlations to HLA-DP phenotypes. Proc Natl Acad Sci U S A. 1987 Mar;84(6):1644–1648. doi: 10.1073/pnas.84.6.1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemola T., Savilahti E., Koskimies S., Pelkonen P. HLA antigens in IgA deficient paediatric patients. Tissue Antigens. 1988 Oct;32(4):218–223. doi: 10.1111/j.1399-0039.1988.tb01659.x. [DOI] [PubMed] [Google Scholar]
- Lakhanpal S., O'Duffy J. D., Homburger H. A., Moore S. B. Evidence for linkage of IgA deficiency with the major histocompatibility complex. Mayo Clin Proc. 1988 May;63(5):461–465. doi: 10.1016/s0025-6196(12)65643-2. [DOI] [PubMed] [Google Scholar]
- Lanchbury J. S., Hall M. A., Welsh K. I., Panayi G. S. Sequence analysis of HLA-DR4B1 subtypes: additional first domain variability is detected by oligonucleotide hybridization and nucleotide sequencing. Hum Immunol. 1990 Feb;27(2):136–144. doi: 10.1016/0198-8859(90)90110-b. [DOI] [PubMed] [Google Scholar]
- Oen K., Petty R. E., Schroeder M. L. Immunoglobulin A deficiency: genetic studies. Tissue Antigens. 1982 Mar;19(3):174–182. doi: 10.1111/j.1399-0039.1982.tb01437.x. [DOI] [PubMed] [Google Scholar]
- Olerup O., Hillert J., Fredrikson S., Olsson T., Kam-Hansen S., Möller E., Carlsson B., Wallin J. Primarily chronic progressive and relapsing/remitting multiple sclerosis: two immunogenetically distinct disease entities. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7113–7117. doi: 10.1073/pnas.86.18.7113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olerup O., Smith C. I., Hammarström L. Different amino acids at position 57 of the HLA-DQ beta chain associated with susceptibility and resistance to IgA deficiency. Nature. 1990 Sep 20;347(6290):289–290. doi: 10.1038/347289a0. [DOI] [PubMed] [Google Scholar]
- Olerup O., Zetterquist H. HLA-DRB1*01 subtyping by allele-specific PCR amplification: a sensitive, specific and rapid technique. Tissue Antigens. 1991 May;37(5):197–204. doi: 10.1111/j.1399-0039.1991.tb01872.x. [DOI] [PubMed] [Google Scholar]
- Rønningen K. S., Iwe T., Halstensen T. S., Spurkland A., Thorsby E. The amino acid at position 57 of the HLA-DQ beta chain and susceptibility to develop insulin-dependent diabetes mellitus. Hum Immunol. 1989 Nov;26(3):215–225. doi: 10.1016/0198-8859(89)90040-2. [DOI] [PubMed] [Google Scholar]
- Schaffer F. M., Palermos J., Zhu Z. B., Barger B. O., Cooper M. D., Volanakis J. E. Individuals with IgA deficiency and common variable immunodeficiency share polymorphisms of major histocompatibility complex class III genes. Proc Natl Acad Sci U S A. 1989 Oct;86(20):8015–8019. doi: 10.1073/pnas.86.20.8015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith W. I., Jr, Rabin B. S., Huellmantel A., Van Thiel D. H., Drash A. Immunopathology of juvenile-onset diabetes mellitus. I. IgA deficiency and juvenile diabetes. Diabetes. 1978 Nov;27(11):1092–1097. doi: 10.2337/diab.27.11.1092. [DOI] [PubMed] [Google Scholar]
- Sollid L. M., Markussen G., Ek J., Gjerde H., Vartdal F., Thorsby E. Evidence for a primary association of celiac disease to a particular HLA-DQ alpha/beta heterodimer. J Exp Med. 1989 Jan 1;169(1):345–350. doi: 10.1084/jem.169.1.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd J. A., Bell J. I., McDevitt H. O. HLA-DQ beta gene contributes to susceptibility and resistance to insulin-dependent diabetes mellitus. Nature. 1987 Oct 15;329(6140):599–604. doi: 10.1038/329599a0. [DOI] [PubMed] [Google Scholar]
- Todd J. A. Genetic control of autoimmunity in type 1 diabetes. Immunol Today. 1990 Apr;11(4):122–129. doi: 10.1016/0167-5699(90)90049-f. [DOI] [PubMed] [Google Scholar]
- White P. C., New M. I., Dupont B. Structure of human steroid 21-hydroxylase genes. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5111–5115. doi: 10.1073/pnas.83.14.5111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilton A. N., Cobain T. J., Dawkins R. L. Family studies of IgA deficiency. Immunogenetics. 1985;21(4):333–342. doi: 10.1007/BF00430799. [DOI] [PubMed] [Google Scholar]
- Wordsworth B. P., Lanchbury J. S., Sakkas L. I., Welsh K. I., Panayi G. S., Bell J. I. HLA-DR4 subtype frequencies in rheumatoid arthritis indicate that DRB1 is the major susceptibility locus within the HLA class II region. Proc Natl Acad Sci U S A. 1989 Dec;86(24):10049–10053. doi: 10.1073/pnas.86.24.10049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C. Y., Campbell R. D. Definitive RFLPs to distinguish between the human complement C4A/C4B isotypes and the major Rodgers/Chido determinants: application to the study of C4 null alleles. Immunogenetics. 1987;25(6):383–390. doi: 10.1007/BF00396104. [DOI] [PubMed] [Google Scholar]





