Abstract
Background
Medicare claims have been used to study lipid-lowering medication (LLM) use among US adults.
Methods
We analyzed the agreement between Medicare claims for LLM and LLM use indicated by self-report during a telephone interview and, separately, by a medication inventory performed during an in-home study visit upon enrollment into the REasons for Geographic And Racial Differences in Stroke (REGARDS) study. We included REGARDS participants ≥65 years enrolled in 2006–2007 with Medicare pharmacy benefits (Part D) from 120 days before their telephone interview through their medication inventory (n=899).
Results
Overall, 39.2% and 39.5% of participants had a Medicare claim for a LLM within 120 days prior to their interview and medication inventory, respectively. Also, 42.7% of participants self-reported using LLMs, and 41.8% had a LLM in their medication inventory. The Kappa statistic (95% confidence interval [95%CI]) for agreement of Medicare claims with self-report and medication inventory was 0.68 (0.63–0.73) and 0.72 (0.68–0.77), respectively. No Medicare claims for LLMs were present for 22.1% (95%CI: 18.1%–26.6%) of participants who self-reported taking LLMs and 18.9% (15.1%–23.3%) with LLMs in their medication inventory. Agreement between Medicare claims and self-report was lower among black males (Kappa=0.34 [95%CI: 0.14–0.54]) compared with black females (0.70 [0.61–0.79]), white males (0.65 [0.56–0.75]) and white females (0.79 [0.72–0.86]). Agreement between Medicare claims and the medication inventory was also low among black males (Kappa=0.48 [95%CI: 0.29–0.66]).
Conclusions
Although substantial agreement exists, many Medicare beneficiaries who self-report LLM use or have LLMs in a medication inventory have no claims for these medications.
Introduction
The determination of medication use in epidemiology studies that include primary data collection is often performed through participant self-report or a medication inventory. Although these approaches are considered accurate, they rely on participant recall or their ability to correctly provide the medications they are taking during an inventory.1,2
Large administrative databases are increasingly being used to estimate the prevalence of disease and medication utilization, assess quality of care, and conduct comparative effectiveness research and pharmacovigilance studies.3–5 Medication utilization in epidemiology studies using administrative databases is usually ascertained through pharmacy claims. Pharmacy claims capture prescription medications filled when a reimbursement request is submitted. However, claims only provide information on whether individuals fill their prescriptions and not on whether the medication is taken.6 Additionally, claims will not identify prescriptions if medications are purchased out-of-pocket (i.e., when no insurance claim is filed) or free samples are provided.4
Lipid-lowering medication (LLM) use, particularly the use of statins, has increased substantially over the past 15 years in the United States.7,8 Also, some LLMs are now available as low-cost generic drugs which may result in many people paying out-of-pocket since the use of pharmacy benefits is not mandatory in the US and the incentive to submit a claim for a small reimbursement amount may be low for some individuals. Few studies have compared LLM use determined through primary data collection and pharmacy claims. Disagreement in LLM use when assessed through primary data collection and pharmacy claims may indicate the presence of misclassification and lead to biased study results.9,10 To better understand the presence of potential misclassification, we investigated the agreement between LLM use ascertained through primary data collection as part of a population-based cohort study and LLM use determined from Medicare pharmacy claims. Medicare constitutes the primary health insurance program for people ≥65 years old in the US, and since 2006 it has provided a pharmacy benefit (i.e., Medicare Part D).
Methods
Study population
We analyzed data from REasons for Geographic And Racial Differences in Stroke (REGARDS) study participants linked to Medicare pharmacy claims. As described elsewhere, 30,239 black and white men and women ≥45 years of age from all 48 contiguous US states and the District of Columbia were enrolled into the REGARDS study between January 2003 and October 2007.11 Data from REGARDS study participants were linked to Medicare claims data using social security numbers with matches confirmed using sex and birthdate.12 The REGARDS study protocol, including linkage with Medicare claims, was approved by the Institutional Review Board at participating institutions and all participants provided written informed consent.
Only REGARDS study participants enrolled in 2006–2007 were eligible for the present analysis because LLM use was ascertained at baseline in the REGARDS study and the Medicare Part D program started on January 1, 2006. We included REGARDS participants linked to Medicare who completed the study’s baseline computer-assisted telephone interview (CATI) and, subsequently, an in-home study visit and were ≥65 years of age 120 days prior to completing the CATI. We excluded participants who did not have continuous Medicare Part D coverage from 120 days prior to their CATI through their in-home study visit. Medicare Part D coverage was defined on a monthly basis by being enrolled in a stand-alone Medicare Prescription Drug Plan, or in a Medicare Health Maintenance Organization or a Medicare Preferred Provider Organization with prescription drug benefits, or by having ≥1 pharmacy claim. Additionally, participants without complete data on LLM use in the REGARDS study database (described below) were excluded from the current analyses.
Baseline assessment
The CATI was administered by trained staff and used to collect information on participants’ age, sex, race, education, income, co-morbid conditions, cognitive impairment, depressive symptoms, medication adherence and use of LLMs, antihypertensive and antidiabetes medications. After the interview, trained health professionals conducted in-home examinations following standardized protocols. Procedures included blood pressure measurements, an electrocardiogram and collection of blood samples. Participants were instructed to collect all containers for medications that they took within the two weeks prior to their in-home study visit for a medication inventory. During the in-home study visit, trained health professionals reviewed these containers and recorded medication names which were subsequently coded into drug classes. A description of the REGARDS study variables used in this analysis are provided in Supplemental table 1.
For each Medicare pharmacy claim, we abstracted the generic name, national drug code (NDC), dispensing date, days of medication supplied, total cost, amount paid by the beneficiary, the pharmacy benefit plan (which defines the formulary covered and cost-sharing requirements),13,14 and the benefit phases (which define the medication cost paid by Medicare and by the beneficiary).15 We calculated the total number of medications filled, total medication costs, and total amount paid by the beneficiary across all of their pharmacy claims. Having a coverage gap, also known as being in the ‘donut hole’, was defined by a Medicare Part D benefit phase ‘initial coverage limit’.15 We used a State buy-in entitlement in Medicare as a marker of socioeconomic deprivation. A State buy-in entitlement identifies Medicare beneficiaries with low socioeconomic resources who receive supplementary medical insurance from their state of residence to reduce the costs they are responsible for paying.16,17
LLM identification
In the REGARDS study, LLM use was ascertained during the CATI and through the medication inventory. For many participants, the REGARDS CATI and the medication inventory were conducted several weeks apart (median duration: 28 days; 25th–75th percentiles: 21–42 days). Therefore, we conducted analyses defining LLM use in the REGARDS study by self-report and, separately, through the medication inventory. LLM use by self-report was defined by answering ‘yes’ to the following two questions during the CATI “Have you even been told by a doctor that you have high cholesterol or an abnormal level of fats in your blood?” and “Are you now taking any medicine to treat it?” LLM use through the medication inventory was defined by the identification of a container for a LLM. Also, we conducted analyses among participants who completed the CATI and study visit within 45 days wherein we required participants to both self-report LLM use and have a LLM identified in their medication inventory to be categorized as taking LLM in the REGARDS study. For these analyses, participants who did not both self-report LLM use and have a LLM in their medication inventory were categorized as not taking LLM in the REGARDS study.
The presence of LLMs in Medicare was ascertained by reviewing NDCs from pharmacy claims within 120 days (i.e., the look-back period) prior to (1) the CATI for comparison with LLM use defined by self-report in REGARDS, and separately, (2) the in-home visit for comparison with LLM use in REGARDS defined through the medication inventory and through both self-report and medication inventory. A 120 day look-back period was considered appropriate for this analysis because >99.9% of Medicare pharmacy claims for LLMs in the study population were for ≤100 days of supply.
LLMs identified through the REGARDS medication inventory and, separately, through Medicare claims included statins, ezetimibe, niacin, fibrates and bile acid sequestrants (Supplemental table 2). Statins were classified as generic if there was at least one generic formulation approved by the US Food and Drug Administration in 2006–2007.
Statistical analysis
Characteristics of participants and their Medicare Part D utilization were calculated by the cross-tabulation of LLM use identified through the REGARDS medication inventory and Medicare claims. We analyzed the overall agreement and agreement above that expected by chance, the Kappa statistic, between LLM use identified through the medication inventory, by self-report, and through both self-report and medication inventory, each versus Medicare claims.18 Agreement was determined for the full population and in subgroups defined by age, race-sex, education, income, State buy-in entitlement, cognitive status, depressive symptoms and year of enrollment in REGARDS. The agreement between the REGARDS medication inventory and Medicare claims was also calculated for statins, overall and for branded and generic statins, separately. Agreement for ezetimibe, niacin, fibrates and bile acid sequestrants was not calculated separately as few participants were taking these medications according to either the medication inventory or Medicare claims. Although no consensus exists, a Kappa statistic between 0.41–0.60, 0.61–0.80 and >0.80 is considered moderate, substantial and almost perfect agreement, respectively.19 We tested differences in Kappa statistics across subgroups using the approach described by Fleiss et al.20 We evaluated the agreement between LLM use in REGARDS and Medicare claims in a series of six sensitivity analyses as described in the Supplemental methods. In a secondary analysis, we evaluated the effect of using a 90-day and 182-day look-back period to ascertain the presence of LLMs in Medicare claims on the agreement with REGARDS medication inventory and self-report.
We calculated the percentage of participants without Medicare claims for a LLM among participants with a LLM in REGARDS through the medication inventory, by self-report, and through both self-report and medication inventory. We also calculated the percentage of participants without a LLM in REGARDS among participants with a Medicare claim for LLM. Finally, we calculated the percentage of participants with a Medicare claim for a LLM among participants without a LLM in REGARDS, and the percentage of participants with a LLM in REGARDS among participants without a Medicare claim for a LLM.
Participant characteristics (age, race-sex, education, income, State buy-in entitlement, cognitive status, depressive symptoms and year of enrollment in REGARDS) associated with not having LLMs in Medicare claims among participants with LLMs in the REGARDS medication inventory were determined by calculating multivariable-adjusted prevalence ratios using Poisson regression models with robust variance estimation.21,22 We also calculated prevalence ratios for not having LLMs in the REGARDS medication inventory among participants with LLMs in Medicare claims. Prevalence ratios were also calculated comparing Medicare claims for LLMs versus LLM use by self-report in REGARDS. All analyses were conducted in SAS (SAS Institute Inc., Cary, NC) version 9.3 using a two-sided level of significance alpha <0.05.
Results
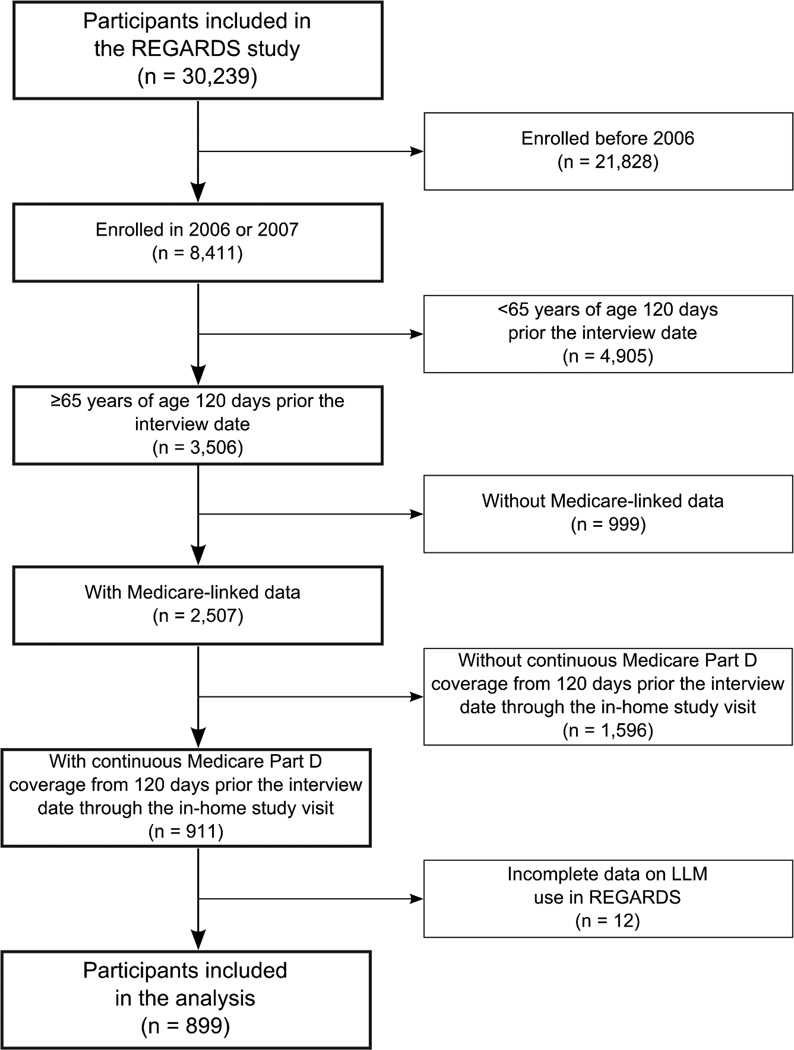
There were 899 REGARDS study participants who met the inclusion criteria for this analysis (Figure 1). Using Medicare data, 355 (39.5%) and 352 (39.2%) participants had a pharmacy claim for a LLM within 120 days prior to their medication inventory and CATI, respectively. Using REGARDS data, 376 (41.8%) and 384 (42.7%) participants were defined as using LLM through the medication inventory and by self-report, respectively. Participants with a LLM in the REGARDS medication inventory and/or in Medicare claims were more likely to have diabetes and a history of CHD, more medications recorded in the REGARDS medication inventory and lower levels of total and LDL cholesterol when compared to their counterparts without a LLM in either the REGARDS medication inventory or Medicare claims (Table 1).
Figure 1.
Flow-chart of participants included in the analysis of the agreement between lipid-lowering medication use assessed in the REGARDS study and Medicare claims.
LLM: lipid-lowering medications; REGARDS: REasons for Geographic And Racial Differences in Stroke.
Table 1.
Characteristics of participants according to the presence of lipid-lowering medications in the REGARDS medication inventory and in Medicare claims.
| No LLM in the REGARDS medication inventory |
LLM in the REGARDS medication inventory |
|||
|---|---|---|---|---|
| No LLM in Medicare claims* (n=473) |
LLM in Medicare claims* (n=50) |
No LLM in Medicare claims* (n=71) |
LLM in Medicare claims* (n=305) |
|
| Baseline characteristics | ||||
| Age in years, mean (SD) | 73.0 (6.0) | 73.8 (6.6) | 72.5 (5.7) | 72.9 (5.8) |
| Men, n (%) | 166 (35.1%) | 25 (50.0%) | 30 (42.3%) | 119 (39.0%) |
| Blacks, n (%) | 183 (38.7%) | 22 (44.0%) | 32 (45.1%) | 104 (34.1%) |
| Less than high school, n (%) | 84 (17.8%) | 12 (24.0%) | 14 (19.7%) | 56 (18.4%) |
| Annual income lower than 20,000 US$, n (%) | 102 (26.8%) | 10 (24.4%) | 20 (31.8%) | 75 (29.3%) |
| State buy-in entitlement, n (%)† | 77 (16.3%) | 10 (20.0%) | 14 (19.7%) | 56 (18.4%) |
| Region of residence, n (%) | - | - | - | - |
| Stroke belt (buckle states) | 118 (24.9%) | 9 (18.0%) | 18 (25.4%) | 88 (28.9%) |
| Stroke belt (non-buckle states) | 138 (29.2%) | 16 (32.0%) | 25 (35.2%) | 83 (27.2%) |
| Other contiguous US states | 217 (45.9%) | 25 (50.0%) | 28 (39.4%) | 134 (43.9%) |
| Cognitive impairment, n (%) | 49 (10.4%) | 3 (6.0%) | 13 (18.3%) | 35 (11.5%) |
| Depressive symptoms, n (%) | 57 (12.1%) | 5 (10.0%) | 10 (14.1%) | 37 (12.2%) |
| Diabetes, n (%) | 64 (14.0%) | 20 (40.0%) | 26 (37.7%) | 112 (37.7%) |
| Hypertension, n (%) | 294 (64.9%) | 34 (72.3%) | 58 (82.9%) | 226 (76.4%) |
| History of CHD, n (%) | 66 (14.3%) | 19 (38.0%) | 21 (30.0%) | 99 (33.7%) |
| History of stroke, n (%) | 35 (7.4%) | 6 (12.0%) | 10 (14.1%) | 28 (9.4%) |
| Total cholesterol (mg/dL), mean (SD) | 200.9 (41.1) | 170.0 (43.6) | 181.1 (36.4) | 169.7 (32.0) |
| LDL cholesterol (mg/dL), mean (SD) | 125.3 (35.3) | 96.8 (32.1) | 106.9 (31.9) | 97.4 (27.0) |
| HDL cholesterol (mg/dL), mean (SD) | 52.9 (15.7) | 53.1 (19.2) | 47.9 (11.5) | 49.9 (13.2) |
| Triglycerides (mg/dL), median (25th–75th pctl) | 101 (75–142) | 99 (68–135) | 114 (83–167) | 106 (79–143) |
| High medication adherence, overall, n (%)‡ | 300 (71.1%) | 31 (66.0%) | 42 (66.7%) | 191 (66.1%) |
| Number of medications in the medication inventory, median (25th–75th pctl) |
5 (3–8) | 7 (5–10) | 8 (6–11) | 8 (6–11) |
| Medicare Part D utilization* | ||||
| Number of medications, median (25th–75th pctl) | 3 (1–6) | 7 (4–9) | 3 (1–6) | 7 (4–10) |
| Total medication cost in US$, median (25th–75th pctl) | 270 (50–582) | 558 (407–1,109) | 200 (13-–567) | 755 (407–1,308) |
| Total amount paid by the beneficiary in US$, median (25th–75th pctl) |
49 (10–140) | 127 (46–277) | 36 (0–150) | 135 (50–289) |
| At least 1 claim in the ‘donut hole’, n (%)§ | 38 (8.0%) | 11 (22.0%) | 3 (4.2%) | 82 (26.9%) |
Numbers in table are sample size (column percent), mean (standard deviation) or median (25th – 75th percentile).
Stroke buckle includes coastal North Carolina, South Carolina and Georgia. Stroke belt (non-buckle) includes the remaining parts of the stroke buckle states and Tennessee, Mississippi, Alabama, Louisiana and Arkansas.
CATI: computer-assisted telephone interview; CHD: coronary heart disease; HDL: high-density lipoprotein; LDL: low-density lipoprotein; LLM: lipid-lowering medication; pctl: percentile; REGARDS: REasons for Geographic And Racial Differences in Stroke; SD: standard deviation; US$: US dollars.
Within 120 days prior in-home study visit.
A State buy-in entitlement identifies Medicare beneficiaries with low socioeconomic resources who receive supplementary medical insurance from their state of residence to reduce the costs they are responsible for paying.
High medication adherence was defined by a score of 0 on the 4-item Morisky scale administered during the CATI.38
At least 1 claim in the benefit phase ‘initial coverage limit’.
Agreement between REGARDS data and Medicare claims for LLMs
There was substantial agreement in LLM use identified through the medication inventory, by self-report, and through both self-report and medication inventory, each versus Medicare claims (Table 2). Results were similar when branded and generic statins were analyzed separately. Agreement did not change substantially in most sensitivity analyses, but it was lower when LLM use in Medicare was defined based on having a day of supply for LLM on the date of the CATI or medication inventory in REGARDS (Supplemental table 3). Agreement of LLM use in the REGARDS medication inventory with Medicare claims was similar across pre-specified sub-groups with the exception of race-sex groups (Supplemental table 4). In this analysis, the agreement was lower among black males (Kappa 0.48; 95% confidence interval [CI] 0.29–0.66) compared with black females (0.73; 0.65–0.82), white males (0.73; 0.65–0.82) and white females (0.77; 0.70–0.84). Agreement of LLM use by self-report in REGARDS with Medicare claims was also lower among black males (Kappa 0.34; 95% CI 0.14–0.54) compared with black females (0.70; 0.61–0.79), white males (0.65; 0.56–0.75) and white females (0.79; 0.72–0.86; Supplemental table 5). Participants with lower education or State buy-in entitlement also had a lower agreement between LLM use by self-report and Medicare claims. The Kappa statistic for the agreement of LLM use defined using medication inventory and separately, self-report, with Medicare claims was higher with a longer look-back period (Supplemental table 6).
Table 2.
Agreement between lipid-lowering medication use in REGARDS and Medicare claims.
| No LLM in REGARDS | LLM in REGARDS | |||||
|---|---|---|---|---|---|---|
| No LLM in Medicare claims* N (%)† |
LLM in Medicare claims* N (%)† |
No LLM in Medicare claims* N (%)† |
LLM in Medicare claims* N (%)† |
Agreement % (95% CI) |
Kappa (95% CI) | |
| REGARDS medication inventory vs Medicare claims | ||||||
| Any LLM class | 473 (52.6) | 50 (5.6) | 71 (7.9) | 305 (33.9) | 86.5 (84.1–88.7) | 0.72 (0.68–0.77) |
| Statins | 506 (56.3) | 51 (5.7) | 61 (6.8) | 281 (31.3) | 87.5 (85.2–89.6) | 0.73 (0.69–0.78) |
| Branded statins‡ | 759 (84.4) | 21 (2.3) | 28 (3.1) | 91 (10.1) | 94.6 (92.9–95.9) | 0.76 (0.69–0.82) |
| Generic statins‡ | 640 (71.2) | 36 (4.0) | 34 (3.8) | 189 (21.0) | 92.2 (90.3–93.9) | 0.79 (0.75–0.84) |
| REGARDS self-report vs Medicare claims | ||||||
| Any LLM class | 462 (51.4) | 53 (5.9) | 85 (9.4) | 299 (33.3) | 84.7 (82.1–86.9) | 0.68 (0.63–0.73) |
| Both REGARDS self-report and medication inventory vs Medicare claims§ | ||||||
| Any LLM class | 367 (55.1) | 65 (9.8) | 37 (5.6) | 197 (29.6) | 84.7 (81.7–87.3) | 0.67 (0.62–0.73) |
CATI: computer-assisted telephone interview; CI: confidence intervals; LLM: lipid-lowering medication; REGARDS: REasons for Geographic And Racial Differences in Stroke.
Within 120 days prior the in-home visit for comparison with the REGARDS medication inventory and both self-report and medication inventory in REGARDS. Within 120 days prior the CATI for comparison with REGARDS self-report.
Row percentages.
Branded statins included atorvastatin, rosuvastatin and fluvastatin. Generic statins included simvastatin, pravastatin and lovastatin.
For this analysis, LLM use in REGARDS was defined by both having a LLM in the medication inventory and self-reporting taking a LLM during the CATI.
Participants who reported taking LLM but did not have a LLM in their medication inventory or had a LLM in their medication inventory but did not self-report taking LLM were included in the “No LLM in REGARDS” category. This analysis was limited to participants who completed the REGARDS CATI and medication inventory within 45 days (n=666).
Discordance between REGARDS data and Medicare claims for LLMs
Among participants defined as taking LLM through the medication inventory, by self-report, and through both self-report and medication inventory, 18.9%, 22.1 %, and 15.8%, respectively, did not have a Medicare claim for a LLM (Supplemental figure 1, Panel A). Among participants with a Medicare claim for a LLM, 14.1%, 15.1% and 24.8% were defined as not taking LLM through the medication inventory, by self-report, and through both self-report and medication inventory, respectively (Supplemental figure 1, Panel B). Between 9% and 15% of participants not taking LLM according to REGARDS data had a Medicare claim for a LLM (Supplemental figure 1, Panel C) and a similar percentage of participants without a Medicare claim for a LLM were taking LLM when defined based on data in REGARDS (Supplemental figure 1, Panel D). Using a longer look-back period resulted in a lower percentage of participants with a LLM in the REGARDS medication inventory or self-report who did not have Medicare claims for LLMs, but a higher percentage of participants without a LLM in REGARDS who had a Medicare claim for a LLM (Supplemental figures 2 and 3).
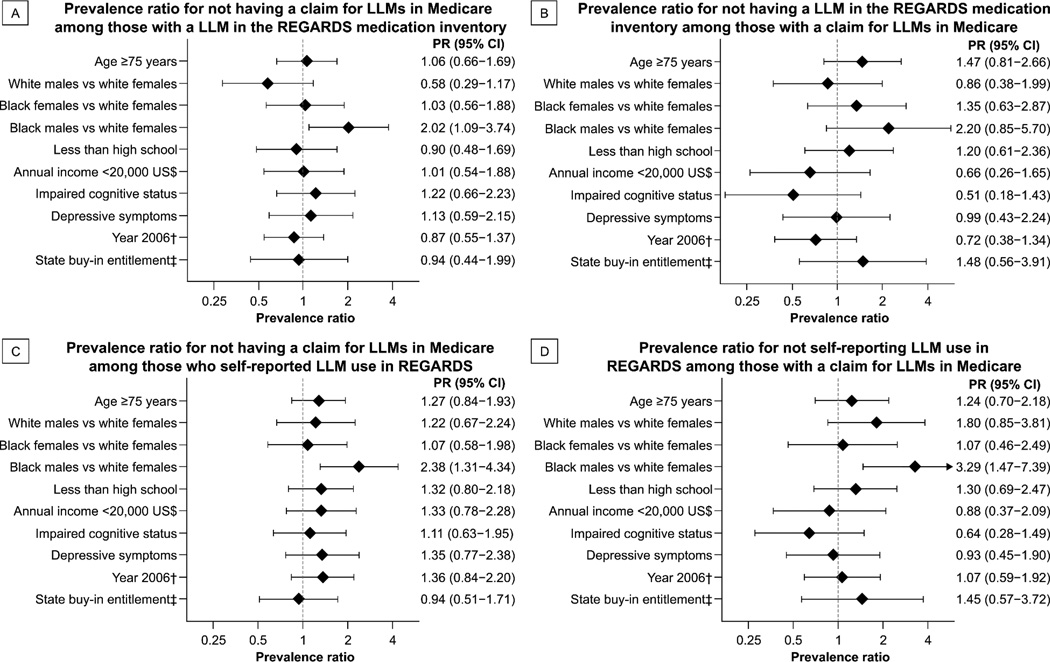
After multivariable adjustment, black males were more likely to have a discordance between LLM use defined through the REGARDS medication inventory and Medicare claims as compared with white females (Figure 2, Panels A and B). Results were similar for the discordance between LLM use by self-report in REGARDS and Medicare claims (Figure 2, Panels C and D).
Figure 2.
Multivariable adjusted prevalence ratios for discordance between the presence of lipid-lowering medications in REGARDS data and Medicare claims.*
Note: Models include adjustment for age, sex, race, education, income, cognitive impairment, depressive symptoms, year of enrollment and State buy-in entitlement.
CATI: computer-assisted telephone interview; CI: confidence interval; LLM: lipid-lowering medication; PR: prevalence ratio; REGARDS: REasons for Geographic And Racial Differences in Stroke; US$: US dollars.
* Presence of LLMs in Medicare claims was ascertained within 120 days prior the in-home study visit for comparison with the REGARDS medication inventory, and within 120 days prior the CATI for comparison with REGARDS self-report.
† Compared with 2007.
‡ A State buy-in entitlement identifies Medicare beneficiaries with low socioeconomic resources who receive supplementary medical insurance from their state of residence to reduce the costs they are responsible for paying.
Discussion
In the current analysis of participants ≥65 years of age in a nationwide population-based cohort, there was substantial agreement between LLM use assessed by self-report or through a medication inventory with Medicare pharmacy claims. However, about one in five participants using LLM by self-report or through a medication inventory did not have a Medicare claim for LLM. Also, many participants with a Medicare claim for a LLM did not report taking this medication or did not have this medication in their inventory. Diabetes and history of CHD were more common among participants with a LLM in the medication inventory or with Medicare claims for a LLM compared with those without a LLM in either data source. Additionally, those with a LLM in either data source had lower total and LDL cholesterol. These results suggest that both primary data collection in REGARDS and Medicare claims were identifying individuals taking LLM.
Claims data, including those for prescription medications, are increasingly being used in epidemiology research.23,24 Agreement between medication use identified through pharmacy claims and participant report has varied across medication classes in prior studies.25–29 LLMs are a cornerstone for cardiovascular disease risk reduction and according to current guidelines, about 56 million US adults 40 to 75 years of age are eligible for statin therapy.30,31 LLM use is a common exposure in cardiovascular research studies but few data have been published on the agreement of LLM use assessed through pharmacy claims versus self-report or a medication inventory. Paired percent agreement between atorvastatin use defined by self-report and by having a day of supply available to take based on pharmacy claims was 72.7% among 780 Atherosclerosis Risk In Communities (ARIC) study participants with Medicare drug coverage benefits in 2009.32 Also, 24.5% of ARIC participants who self-reported taking atorvastatin were defined as non-users based on Medicare claims.32 This is consistent with the current study, 22.1% and 18.9% of participants taking LLM when assessed by self-report and through the medication inventory, respectively, did not have a Medicare claim for LLMs within 120 days prior to their assessment in REGARDS.
Using pharmacy claims to determine medication use may lead to misclassification as they are not collected for research purposes. Participants who fill LLMs may not take the medication. Also, participants may not submit a claim for their LLM to Medicare for reimbursement if they use other pharmacy benefits (e.g., Veteran Administration), received free samples or paid out-of-pocket.23,32–34 Major retail pharmacy chains offering generic drug discount programs and medical centers which treat low-income patients (i.e., Federally Qualified Health Centers or Medicare disproportionate share hospitals) could provide low-cost prescription medications without submitting a claim.4,24,33–36 Also, pharmacy claims cannot identify over-the-counter LLMs including fish oil. Misclassification when using pharmacy claims to determine LLM use could have implications for epidemiology research, including the categorization of those not taking medication as users, those taking medication as non-users, prevalent users as new users, and those with good medication adherence as having low adherence.9
Using self-report and medication inventory to identify medication use are not without limitations and misclassification could also arise. Self-report may be inaccurate due to recall errors or social desirability.1 During a medication inventory, individuals may present containers for medications they have stopped taking or could fail to present containers for medications that they are currently taking.2 Also, both self-report and a medication inventory provide a single time-point assessment of medication use, which limits the ability to evaluate medication adherence or changes in medication use over time. Misclassification when using self-report or a medication inventory to determine LLM use could also lead to the categorization of those not taking this medication as users and those taking this medication as non-users, reducing the agreement with pharmacy claims.
Misclassification of LLM use, whether through pharmacy claims or primary data collection, could bias an association between LLM use and outcomes towards or away from the null.10,37 While the current study indicates that primary data collection and Medicare claims have high agreement and could both be used to identify individuals taking LLM, results suggest that misclassification of LLM use with either method could be differential across race-sex groups. This could have implications for cardiovascular epidemiology research that need to be addressed in future studies. Specifically, future studies should investigate the mechanisms leading to the lower agreement between Medicare claims and primary data collection among black males compared with other race-sex groups. Also, future studies should investigate the impact of misclassification through Medicare pharmacy claims and primary data collection on epidemiology research, including the estimation of the association between LLM use and cardiovascular outcomes.
Strengths of the current study include the availability of LLM use defined by self-report and through a medication inventory for comparison with Medicare claims, along with a concomitant lipid profile. Also, data for the current study were derived from a nationwide sample of community-dwelling black and white adults with a high degree of generalizability to Medicare beneficiaries ≥65 years of age with pharmacy benefits.12 The current study has known and potential limitations. The present study lacked a true gold standard to define LLM use and the discordance observed between REGARDS and Medicare claims could be attributed to misclassification using either data source. Most REGARDS study participants did not have Medicare pharmacy coverage at the time of their enrollment in 2003 to 2007 as Medicare Part D started in 2006. Analyses using Medicare claims, self-report and medication inventory assessed a different time period to determine LLM use (i.e., within the prior 120 days for Medicare claims, ‘now’ for self-report, and within the prior two weeks for the medication inventory). Therefore, some of the discordance between Medicare claims and primary data collection in REGARDS may be attributable to participants discontinuing their medication before their CATI or medication inventory. The agreement between Medicare claims and primary data collection to determine LLM use may have changed since 2006–2007 because more types of statins became generic or due to changes in the characteristics of Medicare beneficiaries with pharmacy benefits. Finally, the number of participants included in some subgroup analyses was low, which led to small cell sizes being used to calculate agreement.
In conclusion, substantial agreement exists between LLM use identified through Medicare claims and primary data collection. However, between 15% and 25% of participants taking LLM based on primary data collection did not have Medicare claims for LLMs, and a similar percentage of participants with Medicare claims for LLM did not take this medication based on primary data collection. The misclassification of LLM use by either method may have consequences for epidemiology research.
Supplementary Material
Acknowledgments
This research project is supported by a cooperative agreement U01-NS041588 from the National Institute of Neurological Disorders and Stroke, National Institutes of Health, Department of Health and Human Service. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the National Institutes of Health. Representatives of the funding agency have been involved in the review of the manuscript but not directly involved in the collection, management, analysis or interpretation of the data. Additional funding was provided by grants R01-HL080477 and K24-HL111154 from the National, Heart, Lung and Blood Institute to Dr. Safford, and grant R01-HS-8517 from the Agency for Healthcare Research and Quality to Dr. Curtis. The authors thank the other investigators, the staff, and the participants of the REGARDS study for their valuable contributions. A full list of participating REGARDS investigators and institutions can be found at http://www.regardsstudy.org.
STK, MK, MMS and PM have received grant support from Amgen Inc. LDC, ED, JRC and GH have no disclosures.
References
- 1.Schneeweiss S, Avorn J. A review of uses of health care utilization databases for epidemiologic research on therapeutics. Journal of clinical epidemiology. 2005;58:323–337. doi: 10.1016/j.jclinepi.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 2.Sjahid SI, van der Linden PD, Stricker BH. Agreement between the pharmacy medication history and patient interview for cardiovascular drugs: the Rotterdam elderly study. British journal of clinical pharmacology. 1998;45:591–595. doi: 10.1046/j.1365-2125.1998.00716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sorlie PD, Bild DE, Lauer MS. Cardiovascular epidemiology in a changing world--challenges to investigators and the National Heart, Lung, and Blood Institute. American journal of epidemiology. 2012;175:597–601. doi: 10.1093/aje/kws138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roberto PN, Stuart B. Out-of-plan medication in Medicare Part D. The American journal of managed care. 2014;20:743–748. [PubMed] [Google Scholar]
- 5.Curtis JR, Westfall AO, Allison J, Freeman A, Kovac SH, Saag KG. Agreement and validity of pharmacy data versus self-report for use of osteoporosis medications among chronic glucocorticoid users. Pharmacoepidemiology and drug safety. 2006;15:710–718. doi: 10.1002/pds.1226. [DOI] [PubMed] [Google Scholar]
- 6.Krousel-Wood M, Holt E, Joyce C, et al. Differences in cardiovascular disease risk when antihypertensive medication adherence is assessed by pharmacy fill versus self-report: the Cohort Study of Medication Adherence among Older Adults (CoSMO) Journal of hypertension. 2015;33:412–420. doi: 10.1097/HJH.0000000000000382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mann D, Reynolds K, Smith D, Muntner P. Trends in statin use and low-density lipoprotein cholesterol levels among US adults: impact of the 2001 National Cholesterol Education Program guidelines. The Annals of pharmacotherapy. 2008;42:1208–1215. doi: 10.1345/aph.1L181. [DOI] [PubMed] [Google Scholar]
- 8.Muntner P, Levitan EB, Brown TM, et al. Trends in the prevalence, awareness, treatment and control of high low density lipoprotein-cholesterol among United States adults from 1999–2000 through 2009–2010. The American journal of cardiology. 2013;112:664–670. doi: 10.1016/j.amjcard.2013.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lauffenburger JC, Balasubramanian A, Farley JF, et al. Completeness of prescription information in US commercial claims databases. Pharmacoepidemiology and drug safety. 2013;22:899–906. doi: 10.1002/pds.3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yood MU, Campbell UB, Rothman KJ, et al. Using prescription claims data for drugs available over-the-counter (OTC) Pharmacoepidemiology and drug safety. 2007;16:961–968. doi: 10.1002/pds.1454. [DOI] [PubMed] [Google Scholar]
- 11.Howard VJ, Cushman M, Pulley L, et al. The REasons for Geographic And Racial Differences in Stroke Study: objectives and design. Neuroepidemiology. 2005;25:135–143. doi: 10.1159/000086678. [DOI] [PubMed] [Google Scholar]
- 12.Xie F, Colantonio LD, Curtis JR, et al. Linkage of a population-based cohort with primary data collection to Medicare claims: The Reasons for Geographic and Racial Differences in Stroke (REGARDS) study. American journal of epidemiology. 2016 doi: 10.1093/aje/kww077. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dismuke CE, Egede LE. Medicare part D prescription drug program: benefits, unintended consequences and impact on health disparities. Journal of general internal medicine. 2013;28:860–861. doi: 10.1007/s11606-013-2423-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gellad WF, Donohue JM, Zhao X, et al. Brand-name prescription drug use among Veterans Affairs and Medicare Part D patients with diabetes: a national cohort comparison. Annals of internal medicine. 2013;159:105–114. doi: 10.7326/0003-4819-159-2-201307160-00664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chronic Condition Data Warehouse. Version 1.1. West Des Moines, IA: Chronic Condition Data Warehouse; 2014. Technical Guidance for Researchers Summarizing and Describing Prescription Drug Utilization. [Google Scholar]
- 16.Medicare and Medicaid; eligibility for premium hospital insurance; state buy-in agreements--HCFA. Final rule with comment period. Fed Regist. 1991;56:38074–38082. [PubMed] [Google Scholar]
- 17.Medicare general information, eligibility, and entitlement manual. Chapter 2–Hospital insurance and supplementary medical insurance. [Accessed Nov 26, 2015];Centers for Medicare & Medicaid Services (CMS) 2002 at https://www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/Internet-Only-Manuals-IOMs-Items/CMS050111.html?DLPage=1&DLEntries=10&DLSort=0&DLSortDir=ascending.
- 18.Cohen J. A coefficient of agreement for nominal scales. Educational and psychological measurement. 1960;20:37–46. [Google Scholar]
- 19.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 20.Fleiss JL, Levin B, Paik MC. Statistical methods for rates and proportions. 3rd. Hoboken, N.J: J. Wiley; 2003. [Google Scholar]
- 21.Barros AJ, Hirakata VN. Alternatives for logistic regression in cross-sectional studies: an empirical comparison of models that directly estimate the prevalence ratio. BMC medical research methodology. 2003;3:21. doi: 10.1186/1471-2288-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. American journal of epidemiology. 2005;162:199–200. doi: 10.1093/aje/kwi188. [DOI] [PubMed] [Google Scholar]
- 23.Avorn J. Medicaid-based pharmacoepidemiology: claims and counterclaims. Epidemiology (Cambridge, Mass) 1990;1:98–100. [PubMed] [Google Scholar]
- 24.Tungol A, Starner CI, Gunderson BW, Schafer JA, Qiu Y, Gleason PP. Generic drug discount programs: are prescriptions being submitted for pharmacy benefit adjudication? Journal of managed care pharmacy : JMCP. 2012;18:690–700. doi: 10.18553/jmcp.2012.18.9.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lau HS, de Boer A, Beuning KS, Porsius A. Validation of pharmacy records in drug exposure assessment. Journal of clinical epidemiology. 1997;50:619–625. doi: 10.1016/s0895-4356(97)00040-1. [DOI] [PubMed] [Google Scholar]
- 26.Allin S, Bayoumi AM, Law MR, Laporte A. Comparability of self-reported medication use and pharmacy claims data. Health reports. 2013;24:3–9. [PubMed] [Google Scholar]
- 27.Pit SW, Byles JE, Cockburn J. Accuracy of telephone self-report of drug use in older people and agreement with pharmaceutical claims data. Drugs & aging. 2008;25:71–80. doi: 10.2165/00002512-200825010-00008. [DOI] [PubMed] [Google Scholar]
- 28.Yun H, Curtis JR, Saag K, et al. Generic alendronate use among Medicare beneficiaries: are Part D data complete? Pharmacoepidemiology and drug safety. 2013;22:55–63. doi: 10.1002/pds.3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Richardson K, Kenny RA, Peklar J, Bennett K. Agreement between patient interview data on prescription medication use and pharmacy records in those aged older than 50 years varied by therapeutic group and reporting of indicated health conditions. Journal of clinical epidemiology. 2013;66:1308–1316. doi: 10.1016/j.jclinepi.2013.02.016. [DOI] [PubMed] [Google Scholar]
- 30.Pencina MJ, Navar-Boggan AM, D'Agostino RB, Sr, et al. Application of new cholesterol guidelines to a population-based sample. The New England journal of medicine. 2014;370:1422–1431. doi: 10.1056/NEJMoa1315665. [DOI] [PubMed] [Google Scholar]
- 31.Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S1–S45. doi: 10.1161/01.cir.0000437738.63853.7a. [DOI] [PubMed] [Google Scholar]
- 32.Zhou L, Stearns SC, Thudium EM, Alburikan KA, Rodgers JE. Assessing Medicare Part D claim completeness using medication self-reports: the role of veteran status and generic drug discount programs. Medical care. 2015;53:463–470. doi: 10.1097/MLR.0000000000000341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Castellon YM, Bazargan-Hejazi S, Masatsugu M, Contreras R. The impact of patient assistance programs and the 340B Drug Pricing Program on medication cost. The American journal of managed care. 2014;20:146–150. [PubMed] [Google Scholar]
- 34.Harding J. Medicare Part D Enrollees' Use of Out-of-Plan Discounted Generic Drugs, Revisited. Journal of the American Geriatrics Society. 2013;61:309–310. doi: 10.1111/jgs.12076. [DOI] [PubMed] [Google Scholar]
- 35.Patel HK, Dwibedi N, Omojasola A, Sansgiry S. Impact of generic drug discount programs on managed care organizations. The American Journal of Pharmacy Benefits. 2011;3:45–53. [Google Scholar]
- 36.Clark BL, Hou J, Chou CH, Huang ES, Conti R. The 340B discount program: outpatient prescription dispensing patterns through contract pharmacies in 2012. Health affairs (Project Hope) 2014;33:2012–2017. doi: 10.1377/hlthaff.2014.0833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Staa TP, Abenhaim L, Leufkens H. A study of the effects of exposure misclassification due to the time-window design in pharmacoepidemiologic studies. Journal of clinical epidemiology. 1994;47:183–189. doi: 10.1016/0895-4356(94)90023-x. [DOI] [PubMed] [Google Scholar]
- 38.Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Medical care. 1986;24:67–74. doi: 10.1097/00005650-198601000-00007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.